Abstract
Purpose
We aimed to analyze the effectiveness and safety of low-dose midazolam and ketamine combination for upper gastrointestinal endoscopy (UGIE) in children.
Methods
The study included the children (n=425, 10.78±3.81 years) who underwent UGIE for diagnostic purpose during 1 year period. All children were sedated with low dose midazolam (0.1 mg/kg) and ketamine (0.5 mg/kg) intravenously. Effectiveness of the sedation and complications during the procedure and recovery period were recorded.
Results
Endoscopic procedure was successfully completed in 414 patients (97.4%; 95% confidence interval, 95.8-98.9). Mean±standard deviation (SD) duration of procedure was 6.36±1.64 minutes (median, 6.0 minutes; range, 4-12 minutes). Minor complications occurred during the procedure in 39.2% of the patients. The most common complication was increased oral secretion (33.1%). No major complications were observed in any patient. Age and Ramsay sedation scores of patients with complications during the procedure were lower than the others (9.49±4.05 years vs. 11.61±3.43 years, p=0.002 and 2.10±1.46 vs. 4.37±1.16, p=0.001). Mean recovery time was 22 minutes (range, 10-90 minutes; mean±SD, 25±12.32 minutes). Minor complications developed during recovery in 60.1% of the patients. The most common complication was transient double vision (n=127, 30.7%). Emergence reaction was observed in 5 patients (1.2%).
Conclusion
The procedure was completed with high level of success without any major complication in our study. Combination of low-dose midazolam and ketamine is a suitable sedation protocol for pediatric endoscopists in UGIE.
Keywords: Endoscopy, Child, Conscious sedation
INTRODUCTION
A large number of sedative or anesthetic drugs are used by anesthetists for appropriate sedation in upper gastrointestinal endoscopy (UGIE) in children. However, the number of drugs that can safely be used in children by pediatric endoscopists is limited [1]. These drugs are benzodiazepines (e.g., midazolam), opioids (e.g., fentanil) and sedative-hypnotics (e.g., ketamine) [1,2].
The most frequently employed sedatives are benzodiazepines [3]. These enhance the inhibitor effect of gamma-amino butyric acid (GABA) by binding between alpha and gamma subunits on GABA receptors. All benzodiazepines have anxiolytic and sedative effects. The anxiolytic effects appear at low doses, and the dose range is quite wide. Midazolam is a benzodiazepine with very short-term effects. It has sedative, hypnotic, anxiolytic and anticonvulsant properties. It causes anterograde amnesia and has muscle relaxing effects. It does not establish analgesia, but causes mild respiratory depression. It may cause apnea when administered rapidly at high doses [4,5]. Although it can be safely used in UGIE in children, it is of inadequate efficacy by itself and is therefore used together with other drugs [6,7].
Ketamine is one of the most popular drugs used in combination with midazolam. It interacts with various receptors in the brain, such as N-methyl-D-aspartate, opiate, muscarinic, cholinergic and nicotinic receptors [8]. It is a short-acting agent (reaching a peak IV concentration in 1 min), is short-lived (15-30 min) and has a broad safety range [9,10]. It causes dissociative amnesia characterized by sedation/anesthesia, amnesia and analgesia [11]. In contrast to benzodiazepine/narcotic drugs, ketamine has a sitimulative effect on the pulmonary systems (e.g., bronchodilation) [12]. However, sideeffects such as aspiration, stridor, laryngospasm and post-sedation agitation have been reported [13,14]. Late side-effects such as the unpleasant dreams known as emergence reaction, delirium, excitation and physical aggression may also rarely be seen [15].
Effective and safety dosages for ketamine and midazolam combination have not been fully determined in children, and the majority of these studies have been retrospective [15]. The safety (low side effects) and efficacy of the sedative drugs is important in children for the complete endoscopy. Anesthetists are not always available in many pediatric endoscopy centers, and sedative drugs are mainly performed by the supervision of pediatric endoscopists. Therefore, we aimed to analyze the effectiveness and safety of low-dose midazolam and ketamine combination in children used by pediatric endoscopists.
MATERIALS AND METHODS
The study was performed prospectively at the Department of Pediatric Gastroenterology Hepatology and Nutrition, Faculty of Medicine, Karadeniz Technical University. Patients receiving UGIE for diagnostic purposes were included to the study between January 2013 and 2014. In our unit, endoscopy was performed by pediatric gastroenterologist and endoscopy nurse. Additionally, a pediatric assistant and intern were also present during the procedure. All patients were in classes 1 or 2 as defined by the American Society of Anesthesiologists [16]. Patients with respiratory system infection, glaucoma, psychosis, porphyria, hypertension, metabolic or neurological disease, increased intracranial pressure and intracranial mass or with known allergy to midazolam or ketamine were excluded, and anesthesia was performed on these patients accompanied by anesthetist. Patients undergoing endoscopy for therapeutic purposes or receiving emergency endoscopy were also excluded. All of other patients were included to the study (n=425).
Topical pharyngeal anesthesia was performed on all patients before sedation using 10% lidocaine spray (10 mg/puff, Xylocaine Spray; AstraZeneca Ltd., Silk Road, UK). Once venous access had been secured, midazolam (1 mg/mL, 5 mL vial, Dormicum; Deva Holding Ltd., Istanbul, Turkey) 0.1 mg/kg (maximum 4 mg) was slowly injected (2 min) intravenously. Two minutes later, 0.5 mg/kg ketamine (50 mg/mL, 10 mL vial, Ketalar; Pfizer Ltd., Sandwich, UK) diluted with saline solution was slowly (2 min) injected intravenously. The patient's response to verbal and tactile stimuli was assessed 2 min after ketamine administration. If no response was obtained, the procedure was initiated by the endoscopist. No antidote was given to any patient when waking after the procedure.
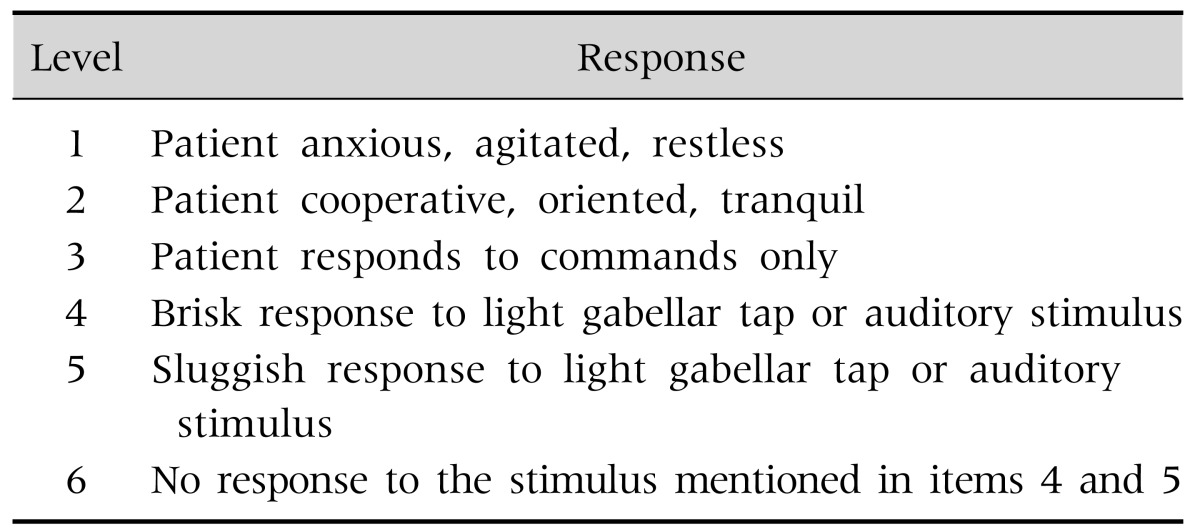
All UGIE procedures were performed on a Pentax EPK-100 device (HOYA Corporation, Tokyo, Japan). Patients were fasted at least 3 hours prior the sedation as the endoscopy protocol. Esophageal, gastric or duodenal biopsies were taken when necessary during the procedure. Effectiveness of sedation during the procedure was assessed using a modified Ramsay sedation score (RSS) [3] (Table 1). Under this system, patients are classified from 1 to 6 on the basis of their response to stimuli.
Table 1. Ramsay Scale for the Assessment of the Level of Sedation.
Patients were monitored during the procedure, and peripheral oxygen saturation, heart rate and respiration rate by the endoscopist or nurse or other physician were recorded. All patients were given oxygen by nasal cannula (2 L/min) during the procedure. Duration of procedure was recorded. Side effect and complications during the procedure and recovery were classified as follows; hypoxia (peripheral oxygen saturation <92%), increased oral secretion (copious oral secretions requiring suctioning), bradycardia (heart rate less than 30% for age), tachycardia (heart rate in excess of 30% for age), respiratory (e.g., apnea, laryngospasm) and cardiac complications (e.g., cardiac arrest), retching/vomiting, flushing/urticaria, recovery reactions including agitation or crying, visual problems including double or blurred vision and nystagmus, vertigo, emerge reactions and others. Emerge reaction is defined as severe recovery agitations such as noisy and pointless hallucinatory responses, nightmares or unpleasant dreams. Major complications were considered as prolonged apnea, bradycardia or cardiac arrest [17].
Time of recovery was assessed using REACT score. This is scored between 0 and 10 depending on the patient's activity, body temperature, and state of consciousness, respiration and circulation [18]. Patients with a REACT score of 10 were discharged from the endoscopy unit. Time from end of the procedure to discharged from the endoscopy unit (recovery time) was recorded. Complications were recorded as patients left the unit.
Verbal and inscriptive informed consent was taken from the parents of the children before the endoscopy procedure.
Data were analyzed on SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA) software. Data are presented as mean±standard deviation (SD). or percentage where appropriate. The independent two samples t-test was used to analyze normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. The chi-square test was used to compare categorical variables. p<.0.05 was regarded as significant.
RESULTS
Patient characteristics
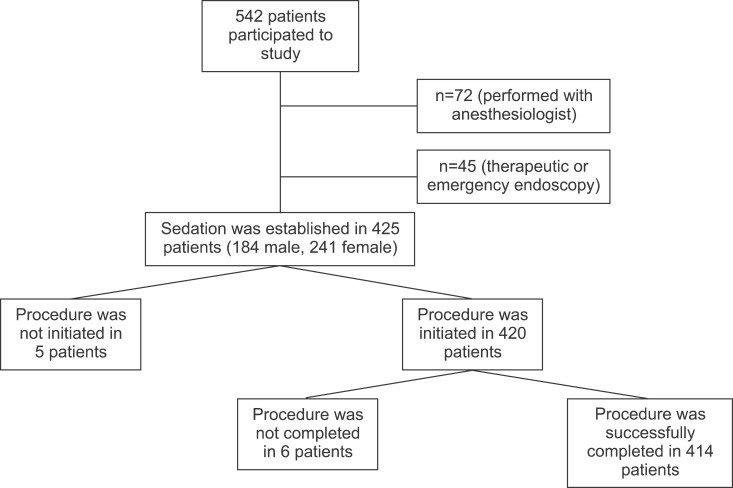
UGIE had been performed on 542 patients in during the study period. It was performed together with special anesthetists in 72 patients (especially patients younger than 1 years old), and for therapeutic purposes or as emergency endoscopy in 45 patients. Sedation was established with midazolam and ketamine in 425 patients (184 male, 241 female) receiving endoscopy for diagnostic purposes (Fig. 1). Median age of patients was 11 years (range, 1-18 years; mean±SD, 10.78±3.81 years), and median weight 35 kg (range, 10-90 kg; mean±SD, 36.46±15.43 kg). Indication of UIGE is shown in Table 2.
Fig. 1. Study protocol.
Table 2. The Demographic Characteristics of Patients (n=425).
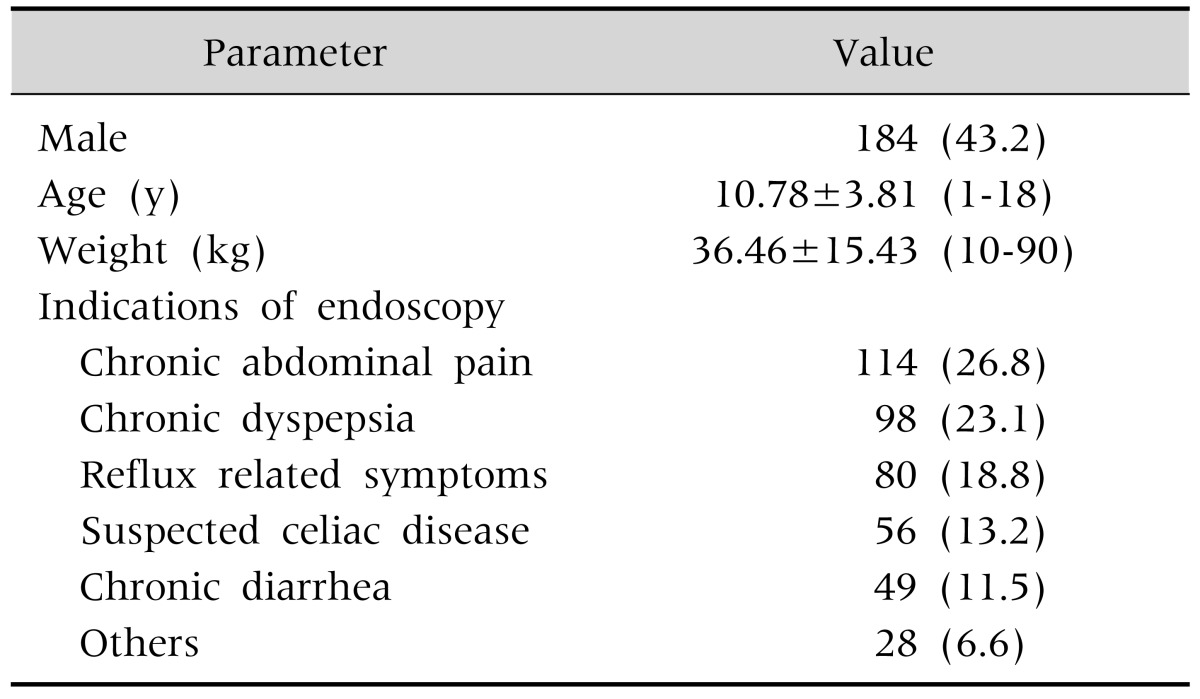
Values are presented as number (%) or mean±standard deviation (range).
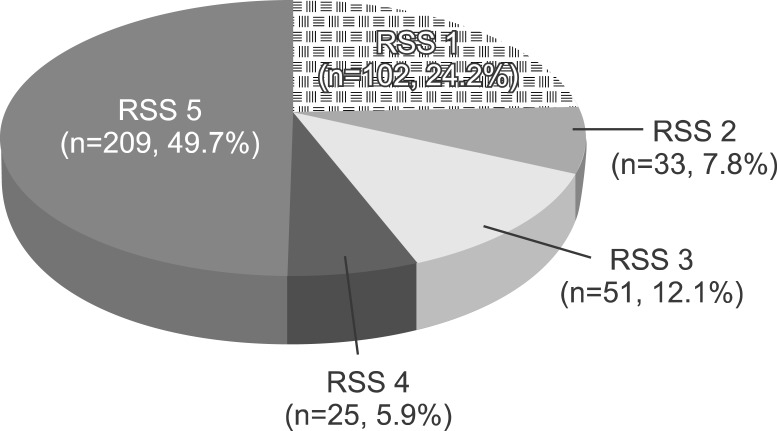
The procedure could not be initiated in 5 patients (1.1%) due to agitation of the patient. RSS was 5 in 49.7% of 420 patients (Fig. 2). Median duration of procedure was 6.0 min (range, 4-12 min; mean±SD, 6.36±1.64 min) (Table 3).
Fig. 2. Ramsay sedation score (RSS) of the patients.
Table 3. Evaluation of Patients during and after Endoscopy (n=425).
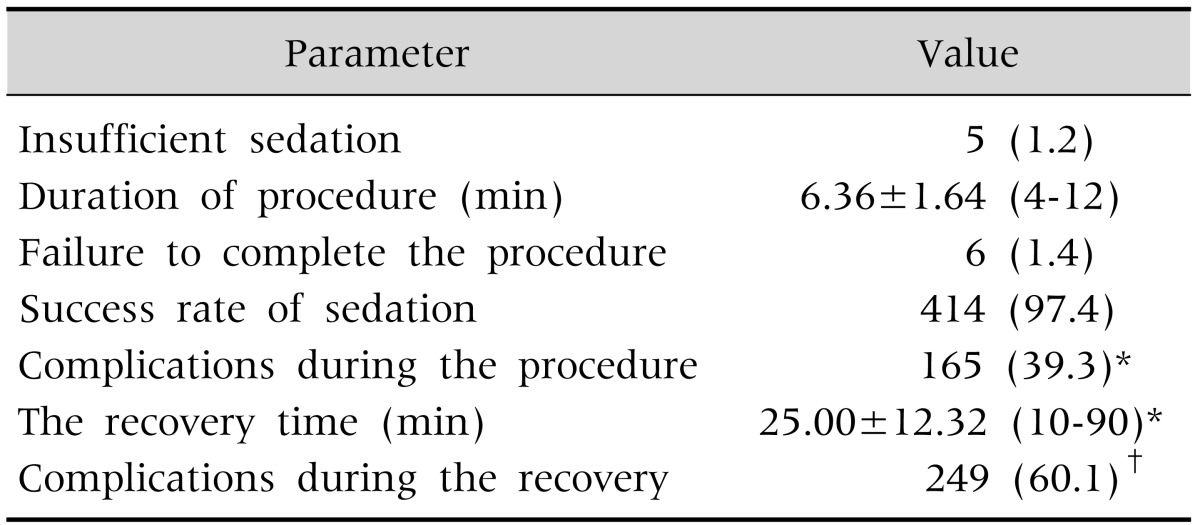
Values are presented as number (%) or mean±standard deviation (range).
*n=420, †n=414.
Complications during procedure
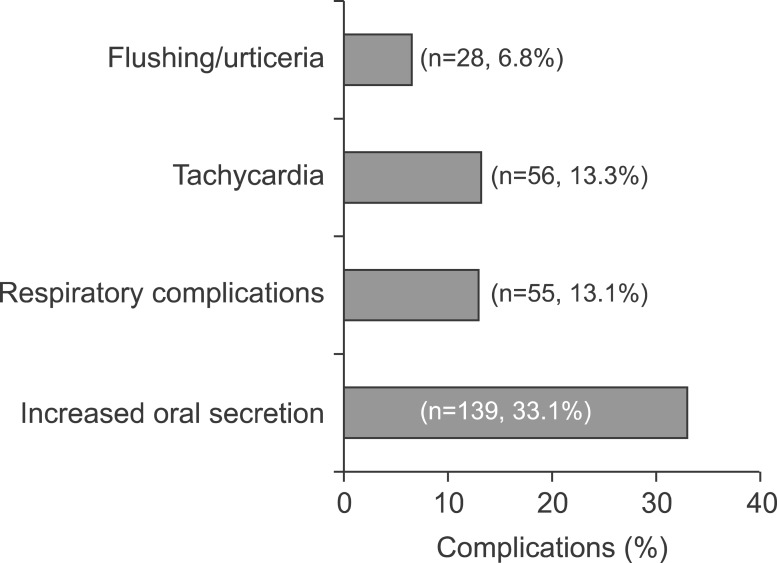
Minor complications occurred during the procedure in 165 of 420 patients (39.3%; 95% confidence interval [CI], 34.5-43.8). The most common complication was increased oral secretion (139 patients, 33.1%). However, oral secretion was not so severe as to terminate the procedure in any patient. Respiratory complications were seen in 55 patients (13.1%); 18 patients (4.3%) had hypoxia and 37 patients (8.8%) had transient cough attack. Fifty-six patients (13.3%) had mild tachycardia during the procedure. Flushing was observed in 27 patients (6.4%), urticaria in 1 (0.2%) and hiccupping in 1 (0.2%) patient (Fig. 3). No major complications such as apnea, bradycardia or cardiac arrest were observed in any patient. Saturation was corrected with the administration of additional oxygen (4-6 L/min by nasal cannula) in 15 (83.3%) of the 18 patients developing hypoxia.
Fig. 3. Frequency of complications during the procedure.
The procedure was terminated in three patients in whom saturation did not improve and additionally in three exhibiting agitation during the procedure (6 patients, 1.4%). Saturation was improved by repositioning the airway and with additional oxygen support. No intubation or transfer to intensive care was required in any patient. An 11-year-old girl who developed urticaria was given antihistaminics during the procedure. The urticaria improved, and the procedure was concluded successfully, albeit with difficulty (RSS was 1).
There was no difference between the patients with or without complications in terms of gender or length of procedure. Age and RSS of patients with complications during the procedure were lower than the others (9.49±4.05 years vs. 11.61±3.43 years, p=0.002 and 2.10±1.46 vs. 4.37±1.16, p=0.001).
Post-procedural complications
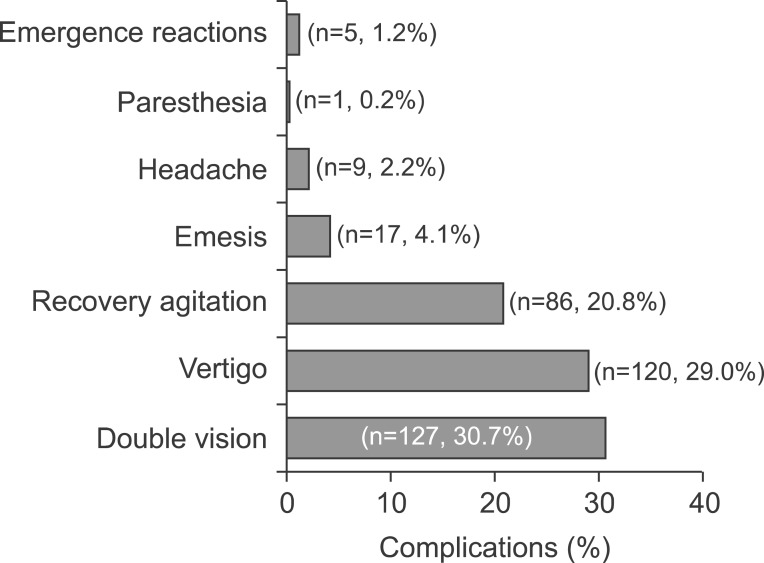
Totally, endoscopic procedure was successfully completed in 414 patients (97.4%; 95% CI, 95.8-98.9) (the procedure could not be initiated in 1.1% of patients due to failure to establish sufficient sedation, while the procedure was terminated in 1.4%). Mean recovery time was 22 min (range, 10-90 min; mean±SD, 25.00±12.32 min) (Table 3). Complications developed during recovery in 249 of the 414 patients (60.1%; 95% CI, 55.3-64.8). The most common complication was double vision (n=127, 30.7%). Vertigo was observed in 120 patients (29.0%), recovery agitation in 86 patients (20.8%), emesis in 17 patients (4.1%), headache in 9 patients (2.2%) and paresthesia 1 patient (0.2%) (Fig. 4). There was no motor loss in the 11-year-old female patient with paresthesia in the hands and feet, and deep tendon reflexes were observed. The patient was monitored and the paresthesia resolved within 4 h. There was no difference in terms of gender, age and duration of procedure between the patients with complication during recovery and those without. However, RSS was lower in patients developing complications during recovery (mean±SD, 3.40±1.76 vs. 3.59±1.61; p=0.002).
Fig. 4. Frequency of complications during the recovery.
Emergence reaction was observed in 5 patients (1.2%; 95% CI, 0.15-2.2). All emerge reactions were observed in the hospital during the recovery and all the patients were seen by the anesthetists. Two had nightmares and others had noisy and pointless hallucinations. All were given intravenous midazolam. Patients were discharged from the endoscopy unit within two hours. Parents were recalled by the telephone one day after and all had uneventful sleeping.
DISCUSSION
In this study, we found that the success rate of endoscopy with low-dose midazolam and ketamine combination was 97.4%. Minor complications during the procedure were seen in 39.2% of the patients, and no major complication was seen. Minor complications rate during the recoverywere 60.1%, while emergence reactions were seen in 0.9% of cases. Our study shows that low dose midazolam and ketamine combination is a suitable sedation protocol for pediatric endoscopists in UGIE.
Since the majority of previous studies have been retrospective and employed different methods and drugs, their success and complication rates were differ from ours. Miqdady et al. [19] used 0.01-0.02 mg/kg intravenously atropine, a mean 0.16 mg/kg (0.07-0.39 mg/kg) intravenously midazolam and 1.06 mg/kg (0.31-2.67 mg/kg) intravenously ketamine and reported an endoscopy success rate of 97.7%. Gilger et al. [9] reported an insufficient sedation level of 3.1% using 0.05-0.2 mg/kg intravenously midazolam and 0.75-2.0 mg/kg intravenously ketamine. Motamed et al. [11] determined an insufficient sedation level of 3.9% when they administered a mean 0.13 mg/kg intravenously midazolam and 5 mg/kg oral ketamine. Brecelj et al. [20] showed that the initial ketamine dose should be 1-1.5 mg/kg, and that the drug is not sufficiently effective at lower doses. Although we used low dose ketamine and midazolam, our procedural success rates were similar with previous studies.
We used to lower dose ketamine due to decrease complications. So the major complication were not observed in our study in contrast to Miqdady et al.'s [19] and Motamed et al.'s [11] study. However the rate of minor complications during the procedure and recovery were high in our study compared to previous studies. Because our study was prospective and reported all of the complications. All of the complications were managed successfully; the produce was completed in the majority of the patients. On the other hand, if only major complications were considered, complication rate was zero in our study.
The most common complication during the procedure was increased oral secretion (33.1%). Increased oral secretions may cause upper respiratory tract problems and laryngospasm especially in oropharyngeal procedures. Anticholinergics were commonly used to mitigate the side effect of hypersalivation (65%), but it was not recommended in meta-analysis due to increased risk of ketamine-related airway and respiratory adverse events. In a recent study, Chong et al. [21] showed that prophylactic atropine did not decrease the frequency of hypersalivation in children given ketamine sedation. The routine use of atropine for the upper endoscopy did not recommended in previous studies [22]. We did not use anticholinergic in our study, because co-administration of atropine with ketamine and midazolam combination may increase the risk of medication errors and takes up more time before the sedation. Additionally; hypersalivation was not so severe as to terminate the procedure in any patient in our study. In our opinion; anticholinergics may be used with the sedation in neurology impaired patients or may be used for the treatment of patients with massive hypersalivation during the procedure.
Studies have reported a mean prevalence of ketamine-related airway and respiratory difficulties of 3.9% [23]. Administration of rapid and high dose ketamine intravenously (initial dose >2.5 mg/kg or total dose >5 mg/kg), age of <2 years and ≥13 years old and use of co-administered anticholinergics and benzodiazepines are increased the risk of respiratory complications. The most common respiratory complications are upper airway obstruction (hypoventilation, or oxygen desaturation that resolved with repositioning of the airway), apnea (0.8%), persistent abnormal oxygen saturation and laryngospasm (0.3%). In our study, we did not observe laryngospasm or apnea in any patient. A decrease in oxygen saturation was observed in 4.2% of the patients and most of them improved with the administration of additional oxygen. Our study showed that respiratory complications with low dose ketamine and midazolam combination are rare and transient in children despite oropharyngeal procedure.
Although the ketamine dosage was lower than that previous studies and was co-administered with midazolam in our study, the level of complications during recovery were high [9,20,24]. The most common complication during the recovery was transient double vision (30.7%). It was reported 27.8% (5 of the 18 children) in a group of children who received ketamine and midazolam sedation with spinal anesthesia for orthopedic surgery [25]. As in double vision, recovery agitation was high in our study group (20.8%). It was reported 7.6% in previous studies. We found that complications during the recovery were higher in patients with low periprocedural RSS. Patients may exhibit greater recovery agitation or reaction to verbal and visual stimuli due to periprocedural pain associated with insufficient sedation. In meta-analysis, recovery reactions were found to be associated with especially subdissociative (<3 mg/kg intramuscular) dosing (odds ratio [OR], 2.9). Other factors were unusual high intravenous dose (OR, 2.1) and procedures other than oropharyngeal procedure (OR, 2.2) [23].
Emergence reactions during recovery are important ketamine-related side-effects. Although emergence reactions are seen at levels up to 30% in adults, a level of 1.4% in children has been reported in meta-analyses [23]. It was seen in 1.2% in our study. They are particularly common when ketamine is used alone, when given in high doses, when administered quickly (<1 min) or in the presence of excessive visual and verbal stimuli during recovery [9,11]. Fewer emergence reactions could be achieved with the oral administration of midazolam 30 min before ketamine or intravenous administration 15 min before ketamine [20]. It is unclear whether lower ketamine doses reduce the incidence or severity of emergence reactions. As recovery reaction, it may be more frequent with the lower dose due to incomplete effacement of external stimuli.
In conclusion, the procedure was completed with high level of success without any major complication in our study, despite the use of ketamine at the low dose of 0.5 mg/kg. Combination with low-dose midazolam and ketamine can be one of the effective and safe sedation protocols without major complication for UGIE in children. Further studies are needed to analyze efficiency of this sedation protocol in small infants and urgent procedures.
References
- 1.Schwarz SM, Lightdale JR, Liacouras CA. Sedation and anesthesia in pediatric endoscopy: one size does not fit all. J Pediatr Gastroenterol Nutr. 2007;44:295–297. doi: 10.1097/MPG.0b013e31802f6435. [DOI] [PubMed] [Google Scholar]
- 2.Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet. 2006;367:766–780. doi: 10.1016/S0140-6736(06)68230-5. [DOI] [PubMed] [Google Scholar]
- 3.Martinez JL, Sutters KA, Waite S, Davis J, Medina E, Montano N, et al. A comparison of oral diazepam versus midazolam, administered with intravenous meperidine, as premedication to sedation for pediatric endoscopy. J Pediatr Gastroenterol Nutr. 2002;35:51–58. doi: 10.1097/00005176-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Malamed SF. Pharmacology. In: Malamed SF, editor. Sedation A guide to patient management. Missouri: Mosby Company; 1989. pp. 330–379. [Google Scholar]
- 5.Reves JG, Glass PSA, Lubarsky DA. Nonbarbiturate intravenous anesthetics. In: Miller RD, Cucchiara RF, editors. Anesthesia. 5th ed. Philadelphia: Churchill Livingstone; 2000. pp. 228–272. [Google Scholar]
- 6.Verhage J, Mulder CJ, Willekens FL. Intravenous midazolam sedation in pediatric diagnostic upper digestive endoscopy. A prospective study in a general hospital. Rom J Gastroenterol. 2003;12:273–276. [PubMed] [Google Scholar]
- 7.Mamula P, Markowitz JE, Neiswender K, Zimmerman A, Wood S, Garofolo M, et al. Safety of intravenous midazolam and fentanyl for pediatric GI endoscopy: prospective study of 1578 endoscopies. Gastrointest Endosc. 2007;65:203–210. doi: 10.1016/j.gie.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Moore KA, Sklerov J, Levine B, Jacobs AJ. Urine concentrations of ketamine and norketamine following illegal consumption. J Anal Toxicol. 2001;25:583–588. doi: 10.1093/jat/25.7.583. [DOI] [PubMed] [Google Scholar]
- 9.Gilger MA, Spearman RS, Dietrich CL, Spearman G, Wilsey MJ, Jr, Zayat MN. Safety and effectiveness of ketamine as a sedative agent for pediatric GI endoscopy. Gastrointest Endosc. 2004;59:659–663. doi: 10.1016/s0016-5107(04)00180-4. [DOI] [PubMed] [Google Scholar]
- 10.Kirberg A, Sagredo R, Montalva G, Flores E. Ketamine for pediatric endoscopic procedures and as a sedation complement for adult patients. Gastrointest Endosc. 2005;61:501–502. doi: 10.1016/s0016-5107(04)02724-5. [DOI] [PubMed] [Google Scholar]
- 11.Motamed F, Aminpour Y, Hashemian H, Soltani AE, Najafi M, Farahmand F. Midazolam-ketamine combination for moderate sedation in upper GI endoscopy. J Pediatr Gastroenterol Nutr. 2012;54:422–426. doi: 10.1097/MPG.0b013e3182323c75. [DOI] [PubMed] [Google Scholar]
- 12.White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119–136. doi: 10.1097/00000542-198202000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36:186–197. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- 14.Green SM, Johnson NE. Ketamine sedation for pediatric procedures: part 2, review and implications. Ann Emerg Med. 1990;19:1033–1046. doi: 10.1016/s0196-0644(05)82569-7. [DOI] [PubMed] [Google Scholar]
- 15.Green SM, Roback MG, Kennedy RM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med. 2011;57:449–461. doi: 10.1016/j.annemergmed.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Lowrie L, Weiss AH, Lacombe C. The pediatric sedation unit: a mechanism for pediatric sedation. Pediatrics. 1998;10:E30. doi: 10.1542/peds.102.3.e30. [DOI] [PubMed] [Google Scholar]
- 17.Green SM, Li J. Ketamine in adults: what emergency physicians need to know about patient selection and emergence reactions. Acad Emerg Med. 2000;7:278–281. doi: 10.1111/j.1553-2712.2000.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 18.Tolia V, Peters JM, Gilger MA. Sedation for pediatric endoscopic procedures. J Pediatr Gastroenterol Nutr. 2000;30:477–485. doi: 10.1097/00005176-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Miqdady MI, Hayajneh WA, Abdelhadi R, Gilger MA. Ketamine and midazolam sedation for pediatric gastrointestinal endoscopy in the Arab world. World J Gastroenterol. 2011;17:3630–3635. doi: 10.3748/wjg.v17.i31.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brecelj J, Trop TK, Orel R. Ketamine with and without midazolam for gastrointestinal endoscopies in children. J Pediatr Gastroenterol Nutr. 2012;54:748–752. doi: 10.1097/MPG.0b013e31824504af. [DOI] [PubMed] [Google Scholar]
- 21.Chong JH, Chew SP, Ang AS. Is prophylactic atropine necessary during ketamine sedation in children? J Paediatr Child Health. 2013;49:309–312. doi: 10.1111/jpc.12149. [DOI] [PubMed] [Google Scholar]
- 22.Hofley MA, Hofley PM, Keon TP, Gallagher PR, Poon C, Liacouras CA. A placebo-controlled trial using intravenous atropine as an adjunct to conscious sedation in pediatric esophagogastroduodenoscopy. Gastrointest Endosc. 1995;42:457–460. doi: 10.1016/s0016-5107(95)70050-1. [DOI] [PubMed] [Google Scholar]
- 23.Green SM, Roback MG, Krauss B, Brown L, McGlone RG, Agrawal D, et al. Emergency Department Ketamine Meta-Analysis Study Group. Predictors of emesis and recovery agitation with emergency department ketamine sedation: an individual-patient data meta-analysis of 8,282 children. Ann Emerg Med. 2009;54:171–180. doi: 10.1016/j.annemergmed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Rafeey M, Ghojazadeh M, Feizo Allah Zadeh H, Majidi H. Use of oral midazolam in pediatric upper gastrointestinal endoscopy. Pediatr Int. 2010;52:191–195. doi: 10.1111/j.1442-200X.2009.02936.x. [DOI] [PubMed] [Google Scholar]
- 25.Piraccini E, Albarello R, Biagini C, Novi A, Agnoletti V, Gambale G. Spinal anesthesia plus ketamine-midazolam sedation for pediatric orthopedic surgery in a developing country. HSR Proc Intensive Care Cardiovasc Anesth. 2012;4:176–178. [PMC free article] [PubMed] [Google Scholar]