Abstract
Purpose
Measurement of serum ceruloplasmin level is the first step in screening for Wilson's disease (WD). Despite the rarity of WD in the general population, ceruloplasmin levels are routinely measured through hepatitis screening in both adults and children. Herein, we evaluated the diagnostic value of ceruloplasmin for the diagnosis of WD among children with hepatitis.
Methods
We retrospectively reviewed data on serum ceruloplasmin levels measured as a serologic marker for patients with hepatitis at Asan Medical Center (Seoul, Korea) between from January 2004 to November 2013. The diagnosis of WD was confirmed by the identification of pathogenic variants in the ATP7B gene. To determine the diagnostic accuracy of ceruloplasmin, receiver operation characteristic (ROC) curves were constructed and the area under curve (AUC) were calculated.
Results
Measurements of serum ceruloplasmin were performed in 2,834 children who had hepatitis. Among these, 181 (6.4%) children were diagnosed with WD. The sensitivity, specificity, and accuracy of a ceruloplasmin level of <20 mg/dL in the discrimination of WD were 93.4%, 84.2%, and 84.8%, respectively. In this study, 418 (14.7%) false-positive cases and 12 (0.4%) false-negative cases were noted. Using a ROC curve, a ceruloplasmin level of ≤16.6 mg/dL showed the highest AUC value (0.956) with a sensitivity of 91.2%, a specificity of 94.9%, and an accuracy of 94.7%.
Conclusion
The measurement of serum ceruloplasmin was frequently used for the screening of WD in children, despite a low positive rate. The diagnostic value of ceruloplasmin may be strengthened by adopting a new lower cut-off level.
Keywords: Ceruloplasmin, Genetic diagnosis, Child, Hepatitis, Accuracy
INTRODUCTION
Wilson's disease (WD; MIM #277900) is a genetic disorder with copper metabolism disturbances leading to copper accumulation in many organs inducing secondary damage [1]. WD is caused by mutations in the ATP7B gene on chromosome 13, which encodes ATPase 7B involved in copper transport. In WD, copper excretory mechanisms fail to develop and copper accumulation begins at birth and continues throughout life, gradually producing clinical symptoms such as neurologic and hepatic complications [2]. WD has been found worldwide, with an estimated prevalence of one case per 30,000 live births in most populations [3]. The prevalences of WD are various, showing higher prevalences in Sardinia, China, Japan, and other Asian populations [4,5,6]. The prevalence of pediatric WD was recently reported to be one in 37,000 children in Korea [7]. Most patients present with WD between the first and fourth decade of life, although age at presentation can vary from 2 to 70 years of age [8,9,10].
Despite its low incidence, early diagnosis and treatment are important to prevent permanent damage to the liver and to avoid disease progression in the brain. In addition, WD can progress to severe hemolytic anemia or fulminant hepatic failure, which can lead to death if diagnosis is delayed. For the initial screening of WD, measurement of serum ceruloplasmin levels is the first-line diagnostic test due to its rapidity and low cost. Conventionally, the reference range of serum ceruloplasmin concentration is between 20 and 40 mg/dL in healthy adults [11]. Serum ceruloplasmin concentration is age-dependent; lower levels are seen in normal neonates and increases by about 6 months of age, with the maximum concentration seen between 2 and 3 years of age, at which point the levels decrease gradually until the teenage period. In addition, various health conditions such as hepatitis can alter serum ceruloplasmin levels.
The main role of serum ceruloplasmin is a screening test, of which the nature is to maintain high sensitivity in identifying WD. However, it could be confusing in non-WD children with hepatitis and low ceruloplasmin, unless the urine copper and ophthalmologic examination show abnormal findings. Contrarily, in fulminant WD, normal level of serum ceruloplasmin may delay appropriate diagnostic approach. Hence, in this retrospective pediatric study, we evaluated the diagnostic value of ceruloplasmin levels for the diagnosis of WD among children with hepatitis.
MATERIALS AND METHODS
Subject
We conducted a retrospective review of patient records of serum ceruloplasmin tests performed at Asan Medical Center (Seoul, Korea) between January 2004 and November 2013. We selected patients under 20 years of age who had a diagnosis of hepatitis defined as elevated serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) at the time of ceruloplasmin measurement. Medical information extracted from digital records included age, gender, weight, height, and diagnosis. The study was approved by the institutional review board of Asan Medical Center (IRB No. 2015-0571).
Measurement of serum ceruloplasmin levels
Serum ceruloplasmin concentration was measured by the IMMAGE Immunochemistry System (Beckman Coulter, Fullerton, CA, USA). The analyzers employed a nephelometric assay calibrated against the same primary standard CRM470 (RPPHS 91/0619) for measurement of ceruloplasmin levels.
Analysis of the ATP7B gene
Genomic DNA was isolated from peripheral blood using the Puregene DNA isolation kit (Gentra, Minneapolis, MN, USA). Twenty-one exons of the ATP7B gene and their intronic flanking sequences were amplified by polymerase chain reaction (PCR) using 25 sets of primers. After verifying that a single specific PCR product was amplified, DNA sequencing was performed using the same primers used in PCR and the Big Dye Terminatore V3.0 Cycle Sequencing Ready reaction kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. Electrophoresis and analysis of the reaction mixtures were carried out using the ABI 3100 Genetic analyzer (Applied Biosystems).
Statistics
The paired t-test was used to compare the means of ceruloplasmin levels between WD and non-WD groups and other continuous variables. Receiver operation characteristic (ROC) curve and the area under curve (AUC) were used to determine the optimal cut-off value for predicting WD [12]. All statistical calculations were performed using IBM SPSS Statistics ver. 19.0 for Windows (IBM Co., Armonk, NY, USA) and R package (ver. 3.0.2; R Foundation for Statistical Computing, Vienna, Austria). A p-value <0.05 was considered statistically significant.
RESULTS
Basic characteristics
Measurement of ceruloplasmin levels was performed in 2,834 children who had serologically confirmed hepatitis. Among these, 181 (6.4%) children were diagnosed as having WD. The mean age of the WD group at the time of ceruloplasmin testing was 10.96±5.01 years. There were 106 male WD patients and 75 female patients. The mean height and weight of the WD group were 143.6±26.7 cm and 35±21.24 kg, respectively (Table 1). The mean age of the WD and non-WD groups at the time of ceruloplasmin testing was not significantly different (Table 2). In addition, no differences were seen in the basic characteristics between the two groups.
Table 1. Basic Characteristics of the Population.
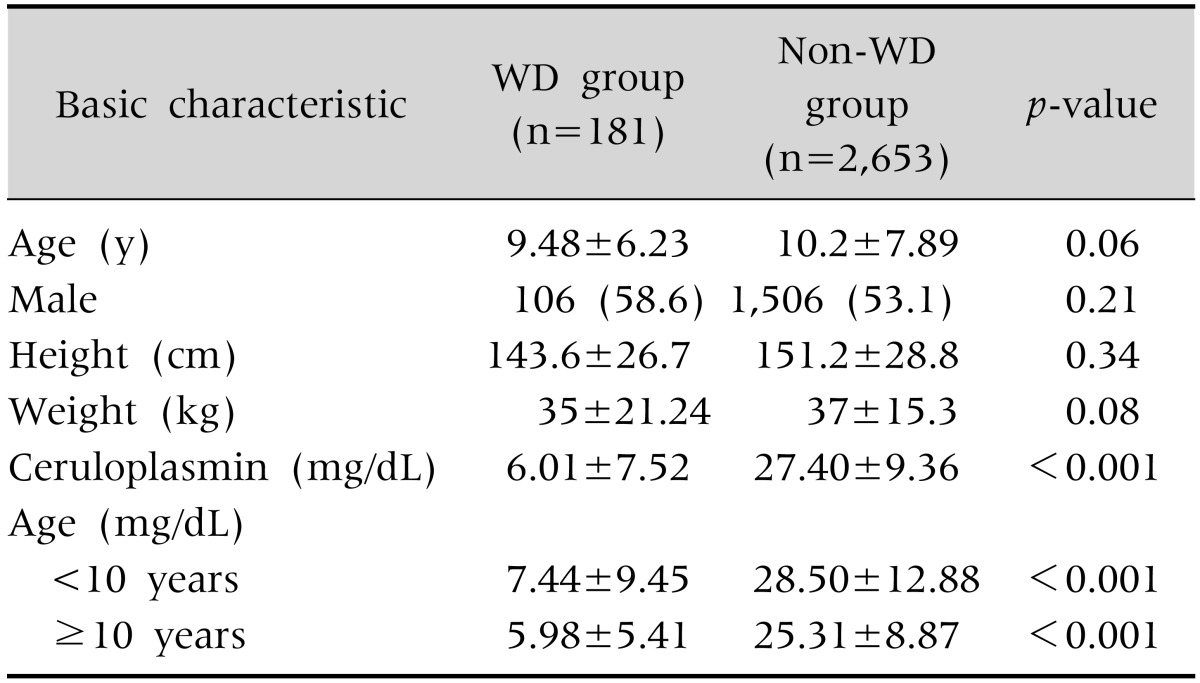
Values are presented as mean±standard deviation or number (%). WD: Wilson's disease.
Table 2. Diagnostic Value of Ceruloplasmin Testing.
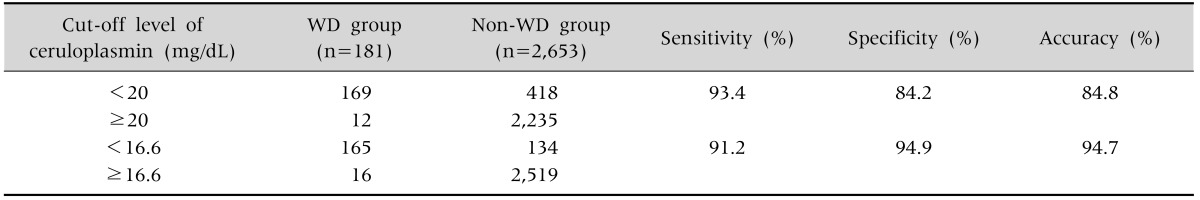
WD: Wilson's disease.
Diagnostic value of conventional cut-off ceruloplasmin level
The mean serum ceruloplasmin level in the overall 2,834 children was 26.04±10.63 mg/dL. The WD group had a lower mean ceruloplasmin level (6.01±7.52 mg/dL) than the non-WD group (27.40±9.36 mg/dL, p<0.001). The mean levels of serum ceruloplasmin in children under 10 years of age and older children ≥10 years of age were 7.44±9.45 mg/dL and 5.98±5.41 mg/dL, respectively. In non-WD group, the corresponding findings are 28.5±12.88 mg/dL and 25.31±8.87 mg/dL, respectively. The WD group had a lower mean ceruloplasmin level (6.01±7.52 mg/dL) than the non-WD group (27.40±9.36 mg/dL, p<0.001). Using the conventional ceruloplasmin cut-off level of <20 mg/dL, WD was discriminated in the 181 patients with a sensitivity of 93.4%, a specificity of 84.2%, and an accuracy of 84.8%, resulting in a positive predictive rate of 27.7%.
False positivity and false negativity of ceruloplasmin testing
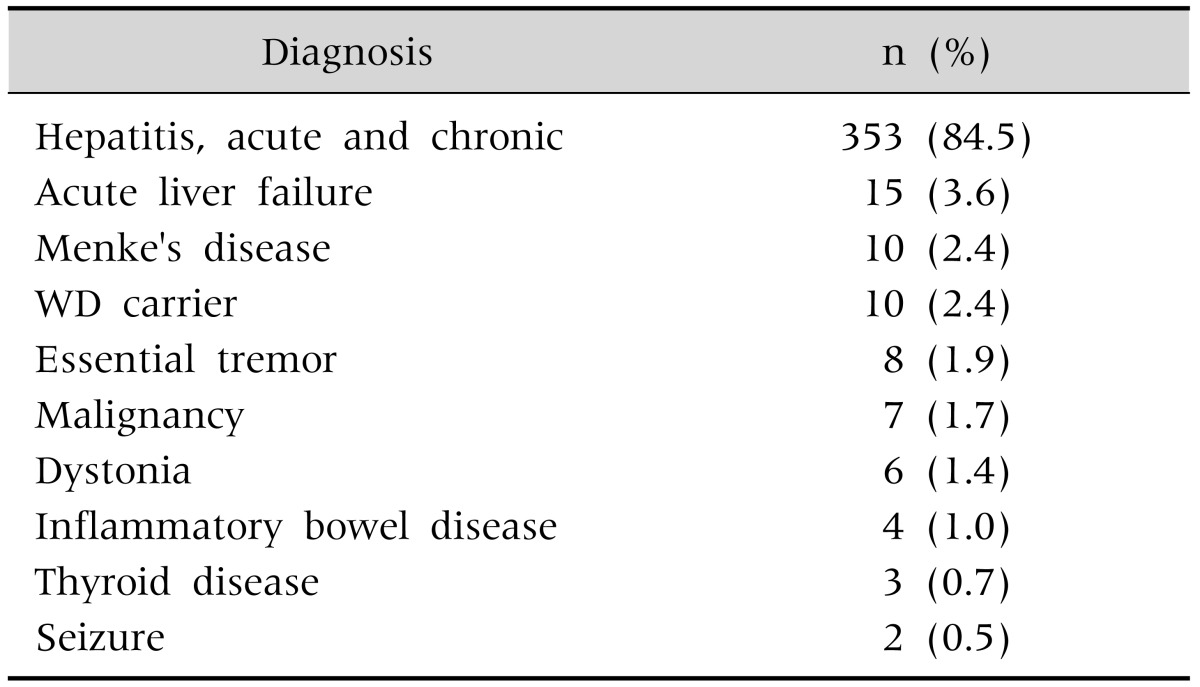
Using the conventional ceruloplasmin cut-off level of <20 mg/dL, 418 (14.7%) false-positive cases (Table 3) and 12 (0.4%) false-negative cases were noted in this study, resulting in a 15.8% false-positive rate. The false-positive cases were mostly non-WD hepatitis, acute liver failure (ALF), Menke's disease, and WD carriers. Twelve patients showed initial ceruloplasmin levels of >20 mg/dL, but all were later diagnosed as having WD by analysis of the ATP7B gene. These false-negative patients were asymptomatic with the exception of two fulminant cases.
Table 3. False-Positive Cases in the Diagnosis of Wilson's Disease (WD) Using a Serum Ceruloplasmin Cut-Off of <20 mg/dL (n=418).
ROC analysis
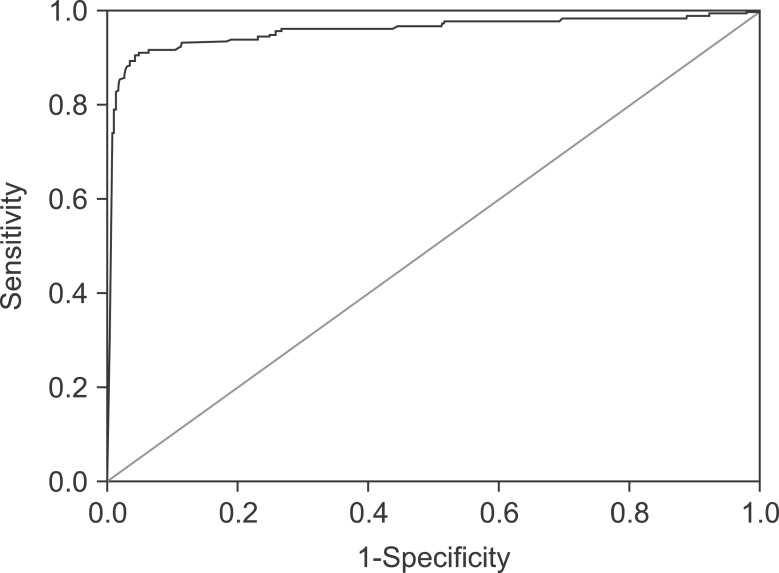
ROC curve analysis indicated that the most useful cut-off value for serum ceruloplasmin concentration was 16.6 mg/dL, for which the AUC was 0.956 (Fig. 1). A ceruloplasmin level of ≤16.6 mg/dL showed the highest AUC value of 0.956 (95% confidence interval, 0.935 to 0.978) with a sensitivity of 91.2%, a specificity of 94.9%, and an accuracy of 94.7%, providing both higher specificity and accuracy than the conventional cut-off level of 20 mg/dL. There were differences of optimal ceruloplasmin levels based on the 10-year-old. A ceruloplasmin level of 10.35 mg/dL has the maximal AUC in a younger group of <10 years, while 16.8 mg/dL has one in the older group of ≥10 years.
Fig. 1. A cut-off value of serum ceruloplasmin of <16.6 mg/dL showed maximal area under curve of 0.956 on the receiver operation characteristic analysis.
DISCUSSION
A ceruloplasmin concentration <20 mg/dL is conventionally considered as one of the major diagnostic criteria for WD. Although the diagnosis of WD must be based on both clinical symptoms and laboratory findings, measurement of ceruloplasmin is a rapid and cost-effective biomarker. However, pediatric data of serum ceruloplasmin is limited. To the best of our knowledge, two studies reported pediatric data of serum ceruloplasmin in children with WD [2,7]. Both studies were not exclusively based on pediatric control groups. The present study provides the first pediatric data of ceruloplasmin in children with WD using a pediatric control group.
This retrospective pediatric study provided three main findings. First, in this study, the measurement of serum ceruloplasmin level was frequently used for the diagnosis of WD in children with elevated liver function values, despite its low positive predictive value (28.7%). A recent adult study also reported the overuse of ceruloplasmin measurements, for which the positive predictive value was only 8.4% [13]. In terms of health care resource burden, this low predictive value may suggest restriction of the test to certain cases. Nonetheless, measurement of ceruloplasmin appears to be the fastest and most important test, especially in an urgent life-threatening situation such as fulminant hepatitis and hemolytic anemia. WD is the most common metabolic condition associated with pediatric ALF in children over 5 years of age [14]. Korman et al. [15] suggested that the combination of an alkaline phosphatase to total bilirubin ratio of less than 4 and an AST to ALT ratio of more than 2.2 provide a rapid and accurate method for the diagnosis of WD presenting as ALF in adults. However, these findings have not yet been confirmed in a pediatric population.
Second, using the conventional cut-off ceruloplasmin level, a substantial portion (14.7%) of the study population showed false-positivity (Table 2). Non-WD Hepatitis was the most common etiology of false positivity in the present study. This finding was similarly shown in a previous adult study [16]. Therefore, additional effort to exclude WD and occasional early mis-treatment could be performed in the non-WD children with low ceruloplamins. In addition, even in children with WD, the level of ceruloplasmin is easily affected by chronic WD-stage and WD treatment. Indeed, with the exception of a genetic test, there is no single diagnostic modality to diagnose WD [8,9,10]. For example, Kayser-Fleischer rings are seen in most WD patients with neurologic disease, whereas less than 50% of WD patients with liver disease have such manifestations. Urinary copper levels are normally elevated in symptomatic patients, while asymptomatic or early WD-stage patients do not show high urine copper excretion. Herein, a scoring system has been developed to predict the development of hepatic WD [17]. However, the accuracy of the score system remains under debate for WD patients without symptoms [11]. Actually, application of WD scoring system requires a full clinical work up for clinical parameters.
Third, a cut-off level of <16.6 mg/dL showed maximal discrimination in diagnosing WD in Korean children. A ceruloplasmin level of <16.6 mg/dL gave the highest AUC of ROC curve, indicating better diagnostic accuracy in this study. Aside from a conventional cut-off level of 20 mg/dL, several cut-off levels of ceruloplasmin have been suggested. A cut-off of 14 mg/dL showed higher diagnostic accuracy in adult groups [11]. However, it is controversial to adopt a new cut-off value with higher accuracy, because the screening test should maintain a high sensitivity in the statistic aspect. With regards to adapting a new cut-off value in the measurement of serum ceruloplasmin, no study has been done to evaluate cost and the clinical and economic benefits.
In conclusion, the measurement of serum ceruloplasmin levels was frequently used as a screening test for children with hepatitis. Non-WD hepatitis largely contributed false-positivity in the present study. The study suggested the diagnostic value of ceruloplasmin may be strengthened by adopting a new cut-off level to avoid false positivity. However, to adopt a lower cut-off value of serum ceruloplasmin as the first-line screening test, a cost-benefit analysis to overcome decreased sensitivity is needed.
References
- 1.Litwin T, Członkowska A. Wilson disease-factors affecting clinical presentation. Neurol Neurochir Pol. 2013;47:161–169. doi: 10.5114/ninp.2013.34397. [DOI] [PubMed] [Google Scholar]
- 2.Manolaki N, Nikolopoulou G, Daikos GL, Panagiotakaki E, Tzetis M, Roma E, et al. Wilson disease in children: analysis of 57 cases. J Pediatr Gastroenterol Nutr. 2009;48:72–77. doi: 10.1097/MPG.0b013e31817d80b8. [DOI] [PubMed] [Google Scholar]
- 3.Huster D. Wilson disease. Best Pract Res Clin Gastroenterol. 2010;24:531–539. doi: 10.1016/j.bpg.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Endo F, Taketa K, Nakamura K, Awata H, Tanoue A, Eda Y, et al. Measurement of blood holoceruloplasmin by EIA using a mouse monoclonal antibody directed to holoceruloplasmin. Implication for mass screening of Wilson disease. J Inherit Metab Dis. 1994;17:616–620. doi: 10.1007/BF00711601. [DOI] [PubMed] [Google Scholar]
- 5.Ohura T, Abukawa D, Shiraishi H, Yamaguchi A, Arashima S, Hiyamuta S, et al. Pilot study of screening for Wilson disease using dried blood spots obtained from children seen at outpatient clinics. J Inherit Metab Dis. 1999;22:74–80. doi: 10.1023/a:1005455401076. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi Y, Aoki T, Arashima S, Ooura T, Takada G, Kitagawa T, et al. Mass screening for Wilson's disease: results and recommendations. Pediatr Int. 1999;41:405–408. doi: 10.1046/j.1442-200x.1999.01096.x. [DOI] [PubMed] [Google Scholar]
- 7.Seo JK. Diagnosis of Wilson disease in young children: molecular genetic testing and a paradigm shift from the laboratory diagnosis. Pediatr Gastroenterol Hepatol Nutr. 2012;15:197–209. doi: 10.5223/pghn.2012.15.4.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ala A, Borjigin J, Rochwarger A, Schilsky M. Wilson disease in septuagenarian siblings: raising the bar for diagnosis. Hepatology. 2005;41:668–670. doi: 10.1002/hep.20601. [DOI] [PubMed] [Google Scholar]
- 9.Beyersdorff A, Findeisen A. Morbus Wilson: case report of a two-year-old child as first manifestation. Scand J Gastroenterol. 2006;41:496–497. doi: 10.1080/00365520500389453. [DOI] [PubMed] [Google Scholar]
- 10.Schoen RE, Sternlieb I. Clinical aspects of Wilson's disease. Am J Gastroenterol. 1990;85:1453–1457. [PubMed] [Google Scholar]
- 11.Mak CM, Lam CW, Tam S. Diagnostic accuracy of serum ceruloplasmin in Wilson disease: determination of sensitivity and specificity by ROC curve analysis among ATP7B-genotyped subjects. Clin Chem. 2008;54:1356–1362. doi: 10.1373/clinchem.2008.103432. [DOI] [PubMed] [Google Scholar]
- 12.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn SH. Population screening for Wilson's disease. Ann N Y Acad Sci. 2014;1315:64–69. doi: 10.1111/nyas.12423. [DOI] [PubMed] [Google Scholar]
- 14.Squires RH, Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korman JD, Volenberg I, Balko J, Webster J, Schiodt FV, Squires RH, Jr, et al. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology. 2008;48:1167–1174. doi: 10.1002/hep.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126:926.e1–926.e5. doi: 10.1016/j.amjmed.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Ferenci P, Caca K, Loudianos G, Mieli-Vergani G, Tanner S, Sternlieb I, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 2003;23:139–142. doi: 10.1034/j.1600-0676.2003.00824.x. [DOI] [PubMed] [Google Scholar]