Abstract
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare condition with a mortality rate of up to 10%. Herein, we describe a case of DRESS syndrome secondary to allopurinol and which may have been precipitated by amoxicillin, the diagnostic challenge it represented and the successful treatment of the condition with corticosteroids.
Background
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare condition affecting between 1 in 1000 and 1 in 10 000 patients after exposure to associated medications.1–3 DRESS syndrome is reported to have a mortality rate of up to 10%,4–6 and therefore early recognition and treatment initiation is crucial. It is characterised by fever, lymphadenopathy, maculopapular rash, haematological abnormalities (including eosinophilia, leucocytosis, thrombocytopenia and anaemia) and multiorgan involvement, with the most commonly involved systems being the hepatic and renal systems.1 5 7 8 DRESS may mimic more common conditions, and the delay between a medication being started and the symptoms of DRESS beginning (often 2–6 weeks later) confounds diagnosis further.1 5 7 8 Herein, we describe a case of DRESS syndrome secondary to allopurinol and the diagnostic challenge it presented.
Case presentation
A 73-year-old woman presented to our accident and emergency department with a 2-week history of a neck lump and a 4-day history of fever, vomiting and diarrhoea. Her medical history revealed hypothyroidism and spinal stenosis. Medications were aspirin, levothyroxine, dosulepin, simvastatin, losartan, allopurinol and indapamide. There was no history of drug hypersensitivity reactions. Prior to presenting to hospital, she had taken two doses of clarithromycin followed by 5 days of phenoxymethylpenicillin.
The patient lived with her son and his partner. There was no history of exposure to tuberculosis or recent travel. She smoked 20 cigarettes per day. The initial recorded temperature was 38.5°C and examination revealed a smooth, tender 5 cm×5 cm lump in the submandibular region. Several small lumps were noted in the upper outer quadrant of the left breast. Admission blood tests revealed a white cell count of 6×109/L with normal differential, a C reactive protein of 29.5 mg/L and an estimated-glomerular filtration rate of 36 mL/min from a baseline of >60 mL/min. She was started on oral coamoxiclav.
An ultrasound scan of the neck lump revealed multiple reactive nodes, which were thought likely due to infection. Fevers continued and a non-pruritic maculopapular rash appeared first on the lower limbs (figure 1) and then involving the trunk and upper limbs. Antibiotics were converted to a 5-day course of intravenous vancomycin 750 mg two times a day and intravenous metronidazole 500 mg three times a day. Differential diagnoses considered at this time were infection, allergic response to penicillin and paraneoplastic phenomenon in association with the breast lumps. On day 5, the patient was given oral chlorpheniramine 4 mg and dexamethasone 4 mg intravenously with the thought that the rash could represent a drug reaction. By day 7, the eosinophil count had risen to 1.2×109/L. The patient was transferred to our infectious diseases unit and dermatological opinion was sought.
Figure 1.
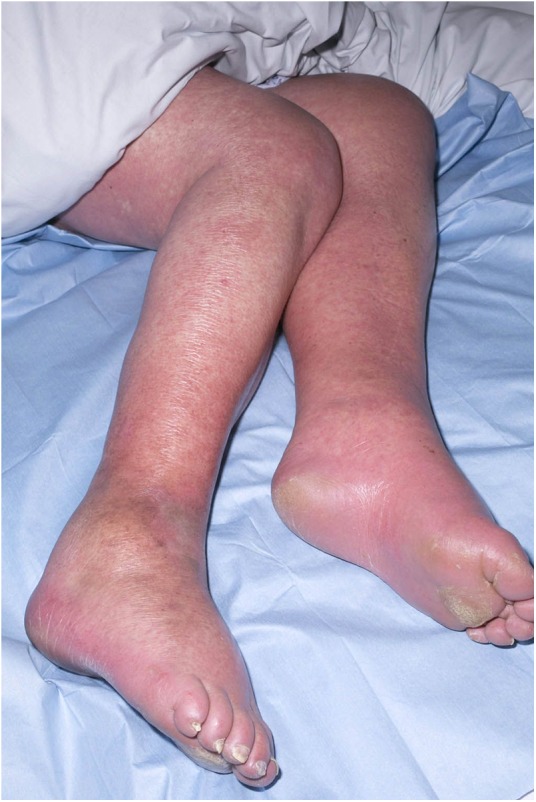
Maculopapular rash covering the lower limbs of our patient.
Core biopsy of the enlarged lymph node revealed no pus cells and no organisms were seen on the Gram stain. Culture of the excised node, including mycobacterial culture, yielded no growth. Blood cultures grew no organisms, urinalysis was unremarkable and microscopy noted an absence of pyuria, and multiplex RT-PCR of a viral throat swab was negative. Further serological and PCR testing was performed as detailed in tables 1 and 2. A CT chest, abdomen and pelvis revealed only cervical and left axillary adenopathy measuring up to 1.3 cm in diameter and a few small nodules in the left breast. An appointment at the breast centre was arranged.
Table 1.
Serological and PCR tests for herpesviruses performed during our patient's admission, all of which were negative
| Day of admission | Serological/PCR test |
|---|---|
| 6 | CMV IgM EBV IgM |
| 9 | EBV VCA IgM |
| 16 | HHV-6 DNA HHV-7 DNA HHV-8 DNA |
| 25 | CMV IgM EBV VCA IgM |
| 31 | CMV DNA HHV-6 DNA HHV-7 DNA HHV-8 DNA EBV DNA |
CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, human herpes virus; VCA, viral capsid antigen.
Table 2.
The results of other PCR and serology tests carried out on our patient
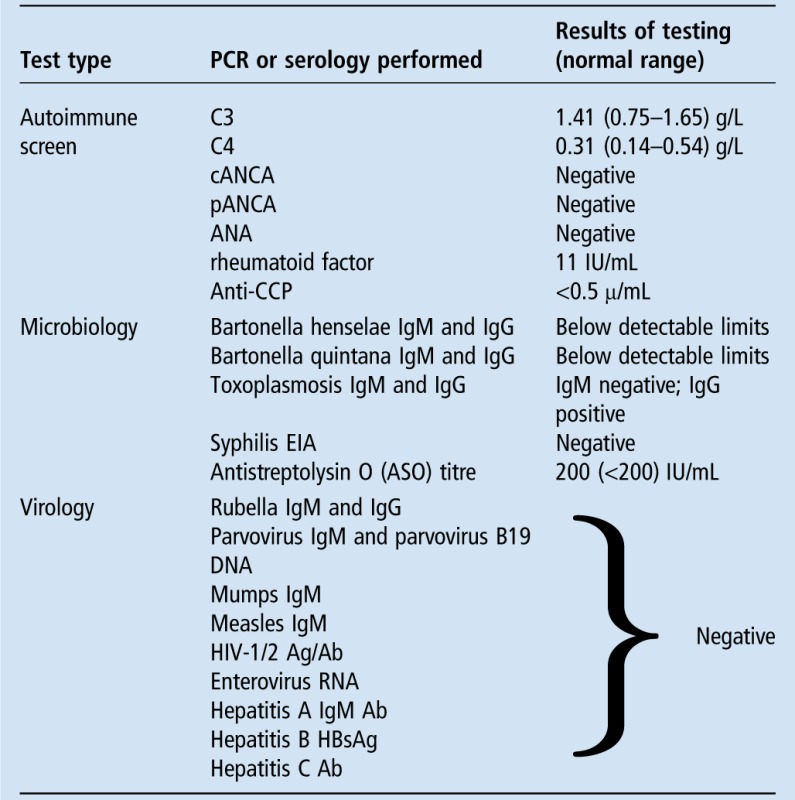 |
ANA, antinuclear antibody; cANCA, cytoplasmic antineutrophil cytoplasmic antibody; CCP, cyclic citrullinated peptides; EIA, enzyme immunoassay; pANCA, perinuclear antineutrophil cytoplasmic antibody.
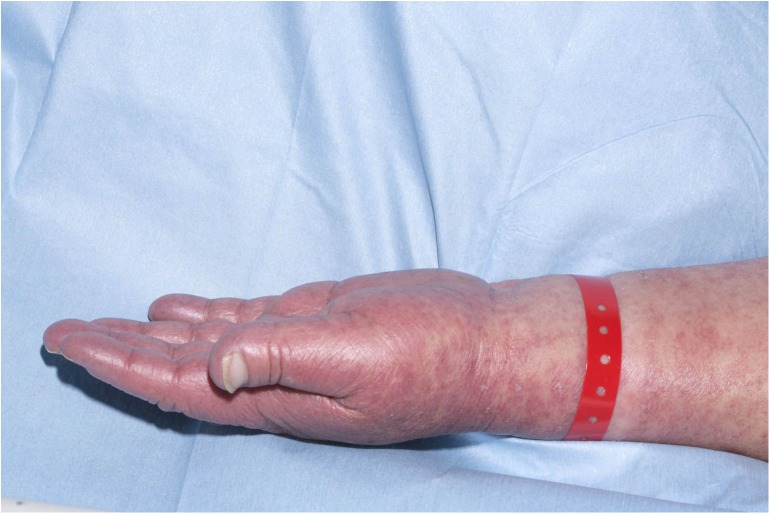
Over the following week, the patient exhibited intermittent fevers over 38°C and began having rigors. She developed dyspnoea and diffuse wheeze, receiving oxygen therapy as her SaO2 dropped below 94%. Her eosinophils rose to 2.3×109/L. Her upper and lower limbs became oedematous, she developed periorbital oedema and the rash became more erythematous and confluent in areas (figures 2 and 3).
Figure 2.
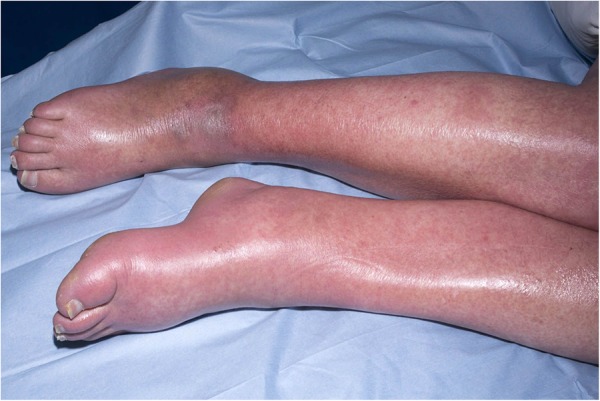
Oedema of the lower limbs and a maculopapular rash.
Figure 3.
Oedema of the upper limbs and a maculopapular rash.
On the 12th day of admission, blisters developed on the patient's lips (figure 4) without oral or genital mucosal ulceration. Lip swabs were taken and PCR was negative for herpes simplex virus one and two and varicella-zoster virus. Further dermatology advice was sought and clinical opinion favoured a drug reaction. A full medication review was conducted. Allopurinol was found to have been started 1 month prior to presentation and symptoms had begun 2–3 weeks after this, while antimicrobial therapy had been started after symptom onset. Allopurinol was immediately stopped, and on day 17, once it was clear that the diagnosis was likely to be DRESS syndrome, the patient was started on 3 days of once daily pulsed intravenous methylprednisolone 400 mg followed by oral prednisolone 40 mg once a day.
Figure 4.
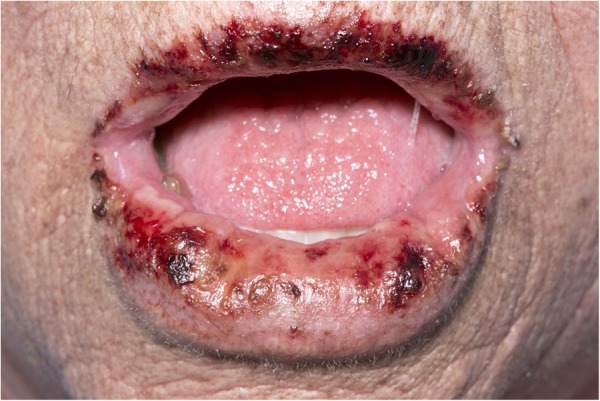
Blistering of the lips occurring in our patient.
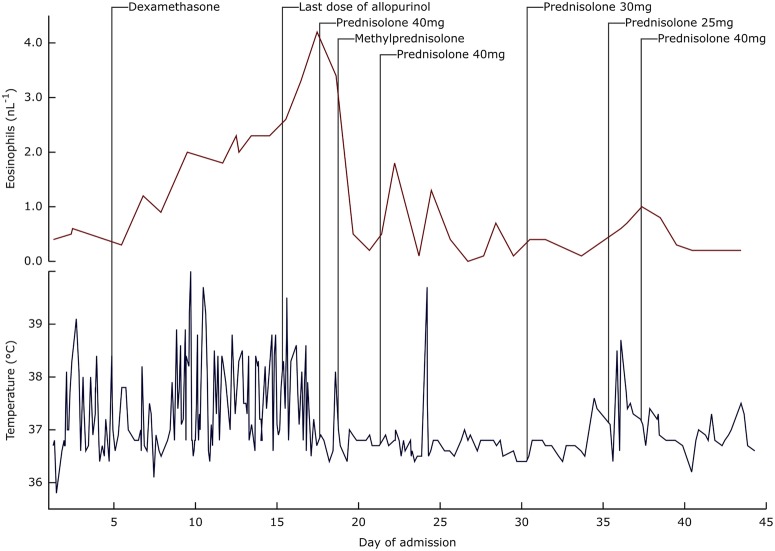
Eosinophil count peaked at 4.2×109/L 3 days after stopping allopurinol, and then resolved within 24 h of introduction of steroid therapy, as did the high fevers (figure 5). The patient reported reduced visual acuity and developed marked periorbital oedema and was subsequently found to have a bilateral conjunctivitis, which was treated with chloramphenicol 0.5% and dexamethasone 0.1% eye drops. The maculopapular rash improved and limbs became less oedematous over the following week.
Figure 5.
Eosinophil counts (red line) and temperature recordings (blue line) of our patient during her hospital admission. Also marked are the points at which dexamethasone 4 mg intravenously was administered (a second dose was administered 12 h later), the last dose of allopurinol was administered, a single dose of prednisolone 40 mg orally was administered, the first of three doses of methylprednisolone 400 mg intravenously once daily was administered, prednisolone 40 mg orally once daily was started, and prednisolone doses were switched to 30 mg orally once daily, 25 mg orally once daily and back to 40 mg orally once daily. Note that the frequency of temperature recordings was greater earlier in the admission when our patient was reporting symptoms of fever.
Liver function tests were normal on admission, but at the time of lip blistering, the International Normalised Ratio rose to 3.3; this normalised following a single 10 mg dose of intravenous phytomenadione (vitamin K). On day 23, the patient's alanine transaminase (ALT) began to rise. An ultrasound scan of the liver showed changes in keeping with hepatitis. The ALT peaked at 738 iu/L and then normalised.
Serial quantitative DNA PCR assays were undertaken for Epstein-Barr virus (EBV), cytomegalovirus (CMV), human herpes virus (HHV)-6 and HHV-7 owing to the association between reactivation of these viruses and DRESS syndrome.9–16 All were negative (table 1). The patient continued to improve and after 7 days of 40 mg prednisolone once daily, therapy was reduced first to 30 mg once daily, then after a further 5 days to 25 mg once daily. However, after two doses of 25 mg prednisolone, the patient experienced further fevers, the maculopapular rash reappeared on her limbs and her ALT rose to 533 iu/L. This was thought to reflect a relapse of DRESS syndrome, and prednisolone was increased back to 40 mg.
Mammography was performed and was unremarkable, and further clinical history and examination was consistent with a diagnosis of hidradenitis suppurativa. The patient remained on 40 mg prednisolone once daily for 1 month following discharge, after which the dose was reduced by 5 mg/week to 10 mg once daily. She remained on this until 3 months after discharge, at which point a complete tapering of steroids without symptom relapse still proved difficult and she was considered for steroid-sparing therapy. Our patient's symptoms improved, but 3 months later she was admitted with community-acquired pneumonia and acute kidney injury, for which she was treated with intravenous levofloxacin. Though she made a good recovery, renal insufficiency persisted, the cause of which is unclear.
Discussion
DRESS syndrome, also known as drug-induced hypersensitivity syndrome (DIHS), was originally observed in patients treated with anticonvulsants in the 1930s.1 It is most commonly associated with antiepileptics, allopurinol and sulfonamides.1 5 6 17 18 DRESS syndrome typically presents with fever, followed by a maculopapular rash covering the limbs, trunk and face, lymphadenopathy, haematological abnormalities and multiorgan involvement, with liver involvement being the most common.1 5–7 15 19 20 Table 3 depicts the range of involvement of different body systems. Our patient had many of the most common features of DRESS syndrome, including fever, lymphadenopathy, a maculopapular rash, mucosal blistering, limb and periorbital oedema, fevers, eosinophilia, a drop in haemoglobin, pneumonitis, hepatitis and evidence of kidney injury. However, our case is unusual in that the lymphadenopathy preceded the maculopapular rash, and this made diagnosis difficult, with initial presentation making an infective cause seem most likely.7 DRESS presenting with oropharyngeal symptoms prior to a rash has been reported previously.16 Our patient also developed conjunctivitis, a recognised but less widely reported form of mucosal involvement.6
Table 3.
| Body system | Clinical presentation |
|
|---|---|---|
| Skin |
|
|
| Mucosa |
|
|
| Lymphatic |
|
|
| Haematological |
|
|
| Hepatic |
|
|
| Renal |
|
|
| Pulmonary |
|
|
| Cardiac |
|
|
| Neurological |
|
|
| Endocrine |
|
|
| Gastrointestinal |
|
|
Diagnosis of DRESS syndrome is clinical, with several criteria for diagnosis being suggested. These include The European Registry of Severe Cutaneous Adverse Reactions to Drugs and Collection of Biological Samples (RegiSCAR) criteria, the Japanese DIHS criteria and Bocquet's criteria. A recent comparative study by Kim and Koh4 suggested that criteria set out by Bocquet (box 1) were the most appropriate in clinical practice. Patch testing or lymphocyte transformation tests can be used if there is uncertainty regarding the offending drug.21 Our patient meets all of Bocquet's criteria.
Box 1. Bocquet's criteria for the diagnosis of DRESS syndrome4.
All three of the below criteria must be met:
1. Skin eruption
2. Blood eosinophilia (>1.5×109/L) or the presence of atypical lymphocytes
3. Internal organ involvement including lymphadenopathies (>2 cm in diameter), hepatitis (liver transaminase values over twice the upper normal limit), interstitial nephritis and interstitial pneumonia or carditis.
Possible differential diagnoses for DRESS syndrome include Stevens-Johnson Syndrome (SJS), Toxic Epidermal Necrolysis (TEN), Acute Generalised Exanthematous Pustulosis (AGEP) and erythroderma (exfoliative dermatitis). Compared to these other conditions, DRESS syndrome typically occurs after a longer period from exposure to the medication and takes longer to subside. Although SJS and DRESS syndrome are both on the same spectrum of severe cutaneous adverse reactions to drugs (SCARs) and overlap between the two conditions does exist, the presence of eosinophilia and involvement of multiple viscera in our patient favour DRESS syndrome.22 23 Other differential diagnoses for DRESS syndrome include acute viral infections, haematological and lymphocytic conditions and vasculitides.1 7
DRESS syndrome is classified as a type IV T-cell mediated delayed hypersensitivity reaction,20 though pathogenesis is only partially understood. It is thought that DRESS syndrome may be caused by genetic defects in detoxification of the offending drug, leading to immunological reactions to accumulating reactive metabolites.5 24 In the case of allopurinol, it has been proposed that there is significant lymphocyte proliferation in response to both allopurinol and its metabolite, oxypurinol, with immunological cell-mediated reactions to both of these products.25 Furthermore, certain human leucocyte antigen variants may increase susceptibility to certain drugs.9 The reactivation of latent herpesviridae infections, typically HHV-6,5 7 11 16 26 CMV, EBV and HHV-7,9 10 12–15 has also been implicated, with some studies suggesting sequential reactivation of the herpes viruses, the order of which varies.13–15 However, the direction of causality is unclear. It may be that DRESS begins as an allergic reaction to a certain drug, causing stimulation of T cells leading to herpes virus reactivation, or that a primary herpes virus infection causes stimulation of T cells which then cross-react with certain drugs.15 In one reported study, 62 of 100 patients had a rise in HHV-6 IgG titre 2–4 weeks after the onset of symptoms, with significant amounts of HHV-6 DNA found in 18 of these patients. This study also demonstrated that a worsening of symptoms correlated to HHV-6 reactivation, and that it was a poor prognostic indicator.26 It is thought that the reactivation of the herpesviridae may explain why the symptoms of DRESS syndrome may persist on withdrawal of the offending drug, as well as the viral-like symptoms.10 Although herpesvirus reactivation was not detected in our patient, it is possible that we did not test soon enough or frequently enough; moreover, we assayed by RT-PCR rather than serology while one case series reported serology as more sensitive.26 It has been suggested that the herpesviridae should be tested for weekly, in order to assess the sequence of virus reactivation and how it correlates clinically,13 and clinicians should be aware of this suggestion.
It has been found that amoxicillin may induce a worsening of DRESS syndrome in patients already taking a DRESS-inducing drug; it is also hypothesised that this may be due to reactivation of herpesviruses.27 In several cases, the worsening of symptoms came only a few days after the addition of amoxicillin to the drug regime.16 27 28 Mardivirin et al27 showed that amoxicillin administration was associated with a worsening of mild symptoms of DRESS syndrome, and induced HHV-6 replication in vitro. It may be that amoxicillin is initially given in patients presenting with early symptoms of DRESS syndrome, as in our patient, with the thought of treating infection, and that this then precipitates or accentuates development of DRESS. It seems our patient developed a rash 1 day after starting coamoxiclav and 6 days after starting penicillin V, and whether either of these β-lactams could have precipitated the rash and worsening of symptoms is unclear. Ben Fredj et al29 reported a patient without previous allergy developing an allergic reaction to amoxicillin after an episode of DRESS syndrome secondary to allopurinol, suggesting that cosensitisation may occur and caution is required when considering the future use of amoxicillin in our patient. Clinicans should be aware of the relationship between amoxicillin and DRESS syndrome, as the occurrence of DRESS following amoxicillin exposure may cause them to misidentify amoxicillin as the culprit drug and not conduct a thorough medication review.
DRESS is often prolonged with several episodes of remission and relapse.17 20 In our patient, relapse occurred when steroid therapy was tapered too quickly. Kim and Koh4 analysed 37 patients and found that clinical manifestations of DRESS persisted for 8–108 days, reflecting the disease's unpredictable nature. Our patient's symptoms persisted for at least 2 months. Kim and Koh4 also suggested that lymphocytes, eosinophil and creatinine and ferritin levels taken at the onset of DRESS syndrome could be useful prognostic markers. Moreover, liver involvement and allopurinol-induced disease are both associated with a higher mortality rate.5
Currently, there are few guidelines for the management of DRESS syndrome. Most importantly, the management of DRESS syndrome is withdrawal of the offending medication, but it very much depends on organ involvement and should involve input from multiple specialties. Descamps et al30 proposed a decision tree for treatment of DRESS syndrome, based on the severity of visceral manifestations. In the absence of severe disease, it has been suggested that patients be treated with topical steroids in addition to supportive therapy such as emollients and antihistamines. For more severe cases of DRESS syndrome, high-potency topical steroids and systemic corticosteroids are the mainstay of treatment,6 20 21 30 with early administration shown to improve significantly clinical symptoms and laboratory results.17 21 Expert opinion suggests that the dose of corticosteroid should be 1.0–1.5 mg/kg/day prednisone or equivalent,7 15 21 with gradual dose tapering over 6–8 weeks once the patient is stable.7 15 31 Further, intravenous methylprednisolone at 30 mg/kg for 3 days can be used if corticosteroids are ineffective or there is significant visceral involvement.15 21 30 31 Our patient received an initial dose of 4 mg dexamethasone intravenously on day 5 (owing to diagnostic uncertainty) and received 40 mg prednisolone on day 17, followed by 3 days of 400 mg intravenous methylprednisolone and subsequently 40 mg prednisolone daily, plus topical steroids and emollients, and the symptoms resolved rapidly. Our patient weighed 84.2 kg and the doses of corticosteroid therapy given were effective initially. In the absence of specific guidance on the initial rate of steroid tapering, we decided to taper at a rate that was, in hindsight, too fast and led to symptom relapse. Our experience emphasises the lesson that clinicians should taper corticosteroids with the possibility of symptom relapse in mind. In cases where DRESS syndrome is accompanied and complicated by virus reactivation, antiviral therapy may be considered,20 but reports of such usage are rare. In a handful of cases, intravenous immunoglobulin (IVIG) has been used in addition to corticosteroid therapy, though there are mixed reports of its effectiveness.32–36 Plasmapheresis and immunosuppressive therapy may also be potential therapies in DRESS syndrome.21 37 38 There are several case reports suggesting that, following DRESS syndrome, a patient may have a predisposition to developing an autoimmune disease such as systemic lupus erythematosus, autoimmune thyroiditis, rheumatoid arthritis or type 1 diabetes mellitus, and it is thus important to consider this during follow-up.10 20 39–43 Our patient developed pneumonia 3 months after discharge from hospital and chronic renal insufficiency, and although an association cannot be confirmed, both are recognised sequelae of DRESS syndrome.6 40
In this report, we have highlighted the presentations of DRESS syndrome and how easily it can mimic severe systemic infection. Our case was typical of allopurinol-induced DRESS syndrome, but diagnosis was confounded by early lymphadenopathy. Our case supports the theory that amoxicillin may precipitate a flare of DRESS syndrome without being the instigating medication. With the high mortality rate of the condition, especially in patients taking allopurinol, DRESS syndrome is a diagnosis that should be entertained and managed as early as possible.
Learning points.
Always consider an iatrogenic cause for presenting symptoms.
A fever is not always indicative of infection.
Always take a thorough drug history.
Consider drug reactions even if onset is weeks after introduction of a new medication.
An unexplained eosinophilia can often provide an important clue to aetiology.
Footnotes
Contributors: RT gathered the information to draft the initial case report and participated in researching, drafting and revising the case discussion. RT gained access to photography, drafted figures, and was involved in communication with the patient. JPS helped to draft the case report and discussion, participated in literature searches and gaining article access, gained patient consent for publication plotted graphs and tables and analysed data. JD and DA helped to edit the initial draft and critically analysed it and drafts thereafter. They also helped with data analysis and advised on the format. All authors have approved the final version of the case report, as has the patient. All authors agree to be accountable for all aspects of the work.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part I. Clinical perspectives. J Am Acad Dermatol 2013;68:693.e1–14. 10.1016/j.jaad.2013.01.033 [DOI] [PubMed] [Google Scholar]
- 2.Roujeau JC, Stern R. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331:1272–85. 10.1056/NEJM199411103311906 [DOI] [PubMed] [Google Scholar]
- 3.Gennis MA, Vemuri R, Burns EA et al. Familial occurrence of hypersensitivity to phenytoin. Am J Med 1991;91:631–4. 10.1016/0002-9343(91)90216-K [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Koh YI. Comparison of diagnostic criteria and determination of prognostic factors for drug reaction with eosinophilia and systemic symptoms syndrome. Allergy Asthma Immunol Res 2014;6:216–21. 10.4168/aair.2014.6.3.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cacoub P, Musette P, Descamps V et al. The DRESS syndrome: a literature review. Am J Med 2011;124:588–97. 10.1016/j.amjmed.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 6.Chiou C-C, Yang L-C, Hung S-I et al. Clinicopathological features and prognosis of drug rash with eosinophilia and systemic symptoms: a study of 30 cases in Taiwan. J Eur Acad Dermatol Venereol 2008;22:1044–9. 10.1111/j.1468-3083.2008.02585.x [DOI] [PubMed] [Google Scholar]
- 7.Chen Y-C, Cho Y-T, Chang C-Y et al. Drug reaction with eosinophilia and systemic symptoms: a drug-induced hypersensitivity syndrome with variable clinical features. Dermatol Sin 2013;31:196–204. 10.1016/j.dsi.2013.09.006 [DOI] [Google Scholar]
- 8.Hamm RL. Drug-hypersensitivity syndrome: diagnosis and treatment. J Am Coll Clin Wound Spec 2011;3:77–81. 10.1016/j.jcws.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camous X, Calbo S, Picard D et al. Drug reaction with eosinophilia and systemic symptoms: an update on pathogenesis. Curr Opin Immunol 2012;24:730–5. 10.1016/j.coi.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 10.Shiohara T. The role of viral infection in the development of severe drug eruptions. Dermatol Sin 2013;31:205–10. 10.1016/j.dsi.2013.09.003 [DOI] [Google Scholar]
- 11.Descamps V, Bouscarat F, Laglenne S et al. Human herpesvirus 6 infection associated with anticonvulsant hypersensitivity syndrome and reactive haemophagocytic syndrome. Br J Dermatol 1997;137:605–8. 10.1111/j.1365-2133.1997.tb03795.x [DOI] [PubMed] [Google Scholar]
- 12.Shiohara T, Kano Y. A complex interaction between drug allergy and viral infection. Clin Rev Allergy Immunol 2007;33:124–33. 10.1007/s12016-007-8010-9 [DOI] [PubMed] [Google Scholar]
- 13.Kano Y, Hiraharas K, Sakuma K et al. Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br J Dermatol 2006;155:301–6. 10.1111/j.1365-2133.2006.07238.x [DOI] [PubMed] [Google Scholar]
- 14.Seishima M, Yamanaka S, Fujisawa T et al. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br J Dermatol 2006;155:344–9. 10.1111/j.1365-2133.2006.07332.x [DOI] [PubMed] [Google Scholar]
- 15.Criado PR, Avancini J, Santi CG et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a complex interaction of drugs, viruses and the immune system. Isr Med Assoc J 2012;14:577–82. [PubMed] [Google Scholar]
- 16.Descamps V, Ranger-Rogez S. DRESS syndrome. Joint Bone Spine 2014;81:15–21. 10.1016/j.jbspin.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 17.Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg 1996;15:250–7. 10.1016/S1085-5629(96)80038-1 [DOI] [PubMed] [Google Scholar]
- 18.Ben m'rad M, Leclerc-Mercier S, Blanche P et al. Drug-induced hypersensitivity syndrome: clinical and biologic disease patterns in 24 patients. Medicine 2009;88:131–40. 10.1097/MD.0b013e3181a4d1a1 [DOI] [PubMed] [Google Scholar]
- 19.Chi M-H, Hui RC-Y, Yang C-H et al. Histopathological analysis and clinical correlation of drug reaction with eosinophilia and systemic symptoms (DRESS). Br J Dermatol 2014;170:866–73. 10.1111/bjd.12783 [DOI] [PubMed] [Google Scholar]
- 20.Roujeau J-C, Haddad C, Paulmann M et al. Management of nonimmediate hypersensitivity reactions to drugs. Immunuol Allergy Clin North Am 2014;34:473–87. 10.1016/j.iac.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 21.Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. Management and therapeutics. J Am Acad Dermatol 2013;68:709.e1–9. 10.1016/j.jaad.2013.01.032 [DOI] [PubMed] [Google Scholar]
- 22.Roujeau J-C. Clinical heterogeneity of drug hypersensitivity. Toxicology 2005;209:123–9. 10.1016/j.tox.2004.12.022 [DOI] [PubMed] [Google Scholar]
- 23.Bouvresse S, Valeyrie-Allanore L, Ortonne N et al. Toxic epidermal necrolysis, DRESS, AGEP: do overlap cases exist? Orphanet J Rare Dis 2012;7:72–7. 10.1186/1750-1172-7-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shear NH, Spielberg SP. Anticonvulsant hypersensitivity syndrome: in vitro assessment of risk. J Clin Invest 1988;82:1826–32. 10.1172/JCI113798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braden GL, Warzynski MJ, Golightly M et al. Cell-mediated immunity in allopurinol-induced hypersensitivity. Clin Immunol Immunopathol 1994;70:145–51. 10.1006/clin.1994.1022 [DOI] [PubMed] [Google Scholar]
- 26.Tohyama M, Hashimoto K, Yasukawa M et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol 2007;157:934–40. 10.1111/j.1365-2133.2007.08167.x [DOI] [PubMed] [Google Scholar]
- 27.Mardivirin L, Valeyrie-Allanore L, Branlant-Redon E et al. Amoxicillin-induced flare in patients with DRESS (Drug Reaction with Eosinophilia and Systemic Symptoms): report of seven cases and demonstration of a direct effect of amoxicillin on Human Herpesvirus 6 replication in vitro. Eur J Dermatol 2010;20:68–73. 10.1684/ejd.2010.0821 [DOI] [PubMed] [Google Scholar]
- 28.Girelli F, Bernardi S, Gardelli L et al. A new case of DRESS syndrome induced by sulfasalazine and triggered by amoxicillin. Case Rep Rheumatol 2013;2013:1–3. 10.1155/2013/409152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben Fredj N, Aouam K, Chaabane A et al. Hypersensitivity to amoxicillin after drug rash with eosinophilia and systemic symptoms (DRESS) to carbamazepine and allopurinol: a possible co-sensitization. Br J Clin Pharmacol 2010;70:273–6. 10.1111/j.1365-2125.2010.03685.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Descamps V, Ben Saïd B, Sassolas B et al. Management of drug reaction with eosinophilia and systemic symptoms (DRESS). Ann Dermatol Venereol 2010;137:703–8. 10.1016/j.annder.2010.04.024 [DOI] [PubMed] [Google Scholar]
- 31.Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int 2006;55:1–8. 10.2332/allergolint.55.1 [DOI] [PubMed] [Google Scholar]
- 32.Scheuerman O, Nofech-Moses Y, Rachmel A et al. Successful treatment of antiepileptic drug hypersensitivity syndrome with intravenous immune globulin. Pediatrics 2001;107:e14 10.1542/peds.107.1.e14 [DOI] [PubMed] [Google Scholar]
- 33.Kito Y, Ito T, Tokura Y et al. High-dose intravenous immunoglobulin monotherapy for drug-induced hypersensitivity syndrome. Acta Derm Venereol 2012;92:100–1. 10.2340/00015555-1168 [DOI] [PubMed] [Google Scholar]
- 34.Santhamoorthy P, Alexander KJ, Alshubaili A. Intravenous immunoglobulin in the treatment of drug rash eosinophilia and systemic symptoms caused by phenytoin. Ann Indian Acad Neurol 2012;15:320–2. 10.4103/0972-2327.104348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Park H-K, Heo J et al. Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci 2008;23:521–5. 10.3346/jkms.2008.23.3.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joly P, Janela B, Tetart F et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol 2012;148:543–4. 10.1001/archderm.148.4.dlt120002-c [DOI] [PubMed] [Google Scholar]
- 37.Alexander T, Iglesia E, Park Y et al. Severe DRESS syndrome managed with therapeutic plasma exchange. Pediatrics 2013;131:e945–9. 10.1542/peds.2012-2117 [DOI] [PubMed] [Google Scholar]
- 38.Laban E, Hainaut-Wierzbicka E, Pourreau F et al. Cyclophosphamide therapy for corticoresistant drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome in a patient with severe kidney and eye involvement and Epstein-Barr virus reactivation. Am J Kidney Dis 2010;55:e11–14. 10.1053/j.ajkd.2009.10.054 [DOI] [PubMed] [Google Scholar]
- 39.Kano Y, Shiohara T. Long-term outcome of patients with severe cutaneous adverse reactions. Dermatologica Sin Issue Cutan Advers Drug React 2013;31:211–16. [Google Scholar]
- 40.Ushigome Y, Kano Y, Ishida T et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol 2013;68:721–8. 10.1016/j.jaad.2012.10.017 [DOI] [PubMed] [Google Scholar]
- 41.Aota N, Shiohara T. Viral connection between drug rashes and autoimmune diseases: how autoimmune responses are generated after resolution of drug rashes. Autoimmun Rev 2009;8:488–94. 10.1016/j.autrev.2009.02.029 [DOI] [PubMed] [Google Scholar]
- 42.Chiou C-C, Chung W-H, Hung S-I et al. Fulminant type 1 diabetes mellitus caused by drug hypersensitivity syndrome with human herpesvirus 6 infection. J Am Acad Dermatol 2006;54:S14–17. 10.1016/j.jaad.2005.03.057 [DOI] [PubMed] [Google Scholar]
- 43.Brown R, Rother K, Artman H et al. Minocycline-induced drug hypersensitivity syndrome followed by multiple autoimmune sequelae. Arch Dermatol 2009;145:63–6. 10.1001/archdermatol.2008.521 [DOI] [PMC free article] [PubMed] [Google Scholar]