Abstract
Limb salvage surgery in bone tumors has evolved in recent years and includes all of the surgical procedures designed to accomplish removal of a malignant tumor and reconstruction of the limb with an acceptable oncologic, functional, and cosmetic result. This dramatic change came about as the result of three important developments, i.e. effective chemotherapy, improved precision imaging techniques and advances in reconstructive surgery. Reconstruction with a modular custom-made oncological endoprosthesis (megaprosthesis) has become a common procedure nowadays. These large foreign bodies make infection a common and feared complication. However, the occurrence of complications may be multifactorial, including a poor nutritional and compromised immune status due to chemotherapy and/or radiotherapy, a lengthy operation, extensive dissection and resection of soft tissues, inadequate soft-tissue coverage, a longer exposure of the wound resulting in infection, etc. Management of postoperative infection in these cases remains a challenge. This article analyses the current literature available for these cases and summarizes the cause and different available methods of treatment.
Keywords: Bone tumors, Limb salvage surgery, Megaprosthesis, Infection
1. Introduction
Over the last 30 years, limb salvage surgery has evolved and the technique has been proven safe and effective in most cases.1 Limb salvage surgery includes all of the surgical procedures designed to accomplish removal of a malignant tumor and reconstruction of the limb with an acceptable oncologic, functional, and cosmetic result. Today, up to 85% of sarcomas in the extremities are treated with limb salvage surgery. This dramatic change came about as the result of three important developments, i.e. effective chemotherapy, improved precision imaging techniques, and advances in reconstructive surgery.2 Options for skeletal reconstruction include modular endoprostheses, osteoarticular or bulk allografts, allograft-prosthetic composites, vascularized bone grafts, arthrodesis, expandable prostheses, rotationplasty, and limb-lengthening techniques. Reconstruction with a modular custom-made oncological endoprosthesis (Megaprosthesis) has become a common procedure nowadays.3–7 This procedure provides a durable and functional limb immediately after the operation, achieving immediate rigid fixation, and allowing early initiation of a postoperative rehabilitation program. Therefore, increasing numbers of patients are undergoing megaprosthesis reconstruction after resection of a malignant bone tumor and some other nonmalignant conditions. Complications common to all types of limb salvage surgeries are neurovascular injuries, local tumor recurrence, deep wound infections, and soft-tissue healing problems.8–11 The occurrence of complications may be multifactorial,12 including a poor nutritional and compromised immune status due to chemotherapy and/or radiotherapy, a lengthy operation, extensive dissection and resection of soft tissues, inadequate soft-tissue coverage, a longer exposure of the wound resulting in infection, etc. Early general complications include wound necrosis, infection, joint contracture, joint stiffness, joint instability, neuropraxia, vascular injury, etc. Infection is one of the most-common complications of limb salvage operations, and the management of postoperative infection remains a challenge.
2. Significance of chemotherapy
In the early 1970s, new anti-neoplastic drugs such as adriamycin and methotrexate were introduced, and remarkable improvements in the prognosis for some sarcomas were seen.13,14 Neo-Adjuvant or “assisted” chemotherapy is now used for most bone sarcomas prior to surgery.15–19 Chemotherapy causes tumor necrosis, which allows for safer removal. In addition, chemotherapy causes the tumor to develop a rind or margin and in some cases shrink, helping the surgeon to completely resect the tumor and minimize the removal of normal tissue. However neo-adjuvant chemotherapy can compromise the immune status of the patient, which may result in complications such as postsurgical infection.20,70 Response to chemotherapy and patient fitness are important criteria in determining patient eligibility for surgery.21 Certain clinical parameters can be adopted to prevent complications for patient undergoing reconstructive surgeries after neo-adjuvant chemotherapy. Some clinical markers of recovery are:
-
•
Absolute neutrophil count (ANC) > = 1200 mm–3
-
•
Platelet count > = 100,000 mm–3
-
•
Hemoglobin > = 9.0 mg/dL
-
•
Total bilirubin must be = <the upper limit of normal (ULN)
2.1. Significance of radiotherapy
Besides chemotherapy, some bone tumors such as Ewing's sarcoma can be treated with radiation therapy prior to surgery to downsize the tumor grade.22–29 But radiation therapy also carries risk of skin necrosis, decrease in immune status, secondary malignancy, etc. Complication rates for preoperative radiotherapy reported by various authors range from 10% to 41%.26,30–33 In patients with megaprosthesis replacement, the reported risk of infection with preoperative radiation therapy is 20.7% and 35.3% in those receiving postoperative radiation therapy.34 Cutaneous radiation injury can be caused by radiations as low as 2 Gy or 200rads and risk increases with increasing doses.33 Therefore it is important to follow proper guidelines for maximum radiation dose for specific tumors.35
The reported risk of infection is significantly higher with radiotherapy alone, and interestingly, combination of chemotherapy with radiotherapy is associated with decreased rate of infection (Table 1).36
Table 1.
Infection and adjuvant therapy.
| Adjuvant | Infection rate | Relative risk |
|---|---|---|
| Chemotherapy alone | 0.20 | 1.51 |
| Radiation alone | 0.50 | 3.85 |
| Chemotherapy & radiation | 0.09 | 0.66 |
| No adjuvant therapy | 0.13 | 1.00 |
Emerging radiation delivery methods may reduce the toxicity to local normal tissue without, increasing the risk of disease recurrence. Newer techniques have been introduced for reducing the radiotherapy field in order to maximize radiation treatment and to decrease incidental morbidity. In recent years, precise field sculpting with intensity-modulated radiotherapy (IMRT) has become popular. IMRT is an advanced form of external beam irradiation and a type of three-dimensional conformal radiotherapy (3D-CRT). Intensity-modulated radiotherapy involves the use of computerized optimization techniques to deliver nonuniform radiation-beam intensities to a field that is planned with use of three-dimensional computerized tomographic scanning techniques.92 This precise delivery of radiotherapy avoids incidental treatment of surrounding tissues and therefore reduces radiation-associated complications.93
2.2. Custom-made megaprosthesis
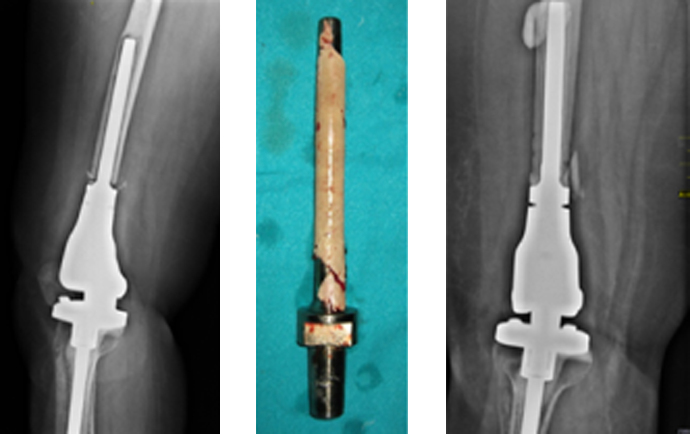
Megaprosthesis is a large metallic device designed to replace the excised length of bone and the adjacent joint (Fig. 1). Modular designs are available for the most common uses in the femur, tibia, and humerus that allow the surgeon to assemble the prosthesis intraoperatively to accommodate the needs of a particular patient. Custom prostheses are available for special applications. The prosthetic joint has a modified hinge design to substitute for the stability normally provided by the capsular and ligamentous structures that were sacrificed by the resection. An analysis of the literature shows that besides the occurrence of a local recurrence, periprosthetic infection remains the most serious complication following limb salvage procedure with megaprosthesis.37,6,38 Infection of a megaprosthesis (Fig. 2) implanted following tumor resection leads to major morbidity and sometimes amputation.
Fig. 1.
Megaprosthesis.
Fig. 2.
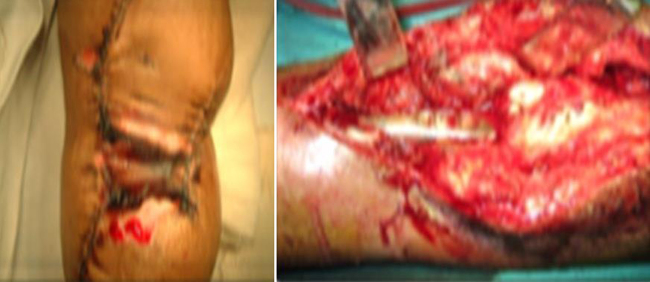
Periprosthetic Infection.
Periprosthetic infections are prevalent despite the use of systemic antibiotic treatment, operating rooms with laminar airflow, and routine screening for methicillin-resistant Staphylococcus aureus (MRSA).39 In the literature, infection rates of 3–31% have been reported (average approx. 15%), often in conjunction with risk factors, e.g. the anatomic region (pelvis implants in particular), implant alloy, and underlying reason for implantation of a megaprosthesis.37 The rate of infections varies according to the site of replacement with lower rates in upper extremities and higher rates in lower extremities.39–44 However postsurgical infection is more common in pelvic tumor surgeries. Several authors have reported infection rates ranging from 12% to 47%.6,38 The infection can be superficial to deep wound infection. The high rate of infectious complication can be attributed to the creation of a huge dead space after sarcoma resection that cannot be filled by the implant, and leading to formation of local hematoma. Other factors include prolonged operating time, wide surgical exposure, use of chemotherapy/radiotherapy, proximity of the rectum and genitourinary tract, size of the implant, and blood supply to the skin flap.37,6,39,45–48
Regarding infection rates with regard to specific diagnosis, sarcomas demonstrated the highest infection rate of 21.7% when compared to metastatic or non-metastatic disease (Table 2).36 Usually high infection rates are seen after chondrosarcome tumor resection when compared to other tumors in the pelvis. The reason for this could be the high volume of the tumor and frequent involvement of the acetabulum, which leads to increased operating time for resection.49
Table 2.
Overall infection rate by disease.
| Disease | Infection rate (%) |
|---|---|
| Sarcoma | 21.7 |
| Metastatic disease | 7.4 |
| Benign bone tumor | 15.3 |
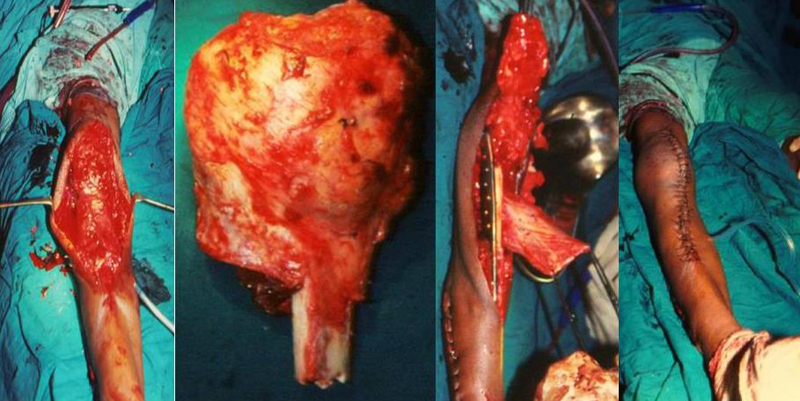
Endoprosthetic reconstruction following resection of a bone tumor of the proximal tibia creates a particular set of problems. The procedure necessarily sacrifices all the knee ligaments with extensive soft-tissue resection50–52 (Fig. 3). The problem of infection following prosthetic replacement after tumor resection is related to the difficulties in achieving adequate soft-tissue cover.51,52 Patients with proximal tibia replacements in particular are at high risk for periprosthetic infection and have a reported infection rate of 31% before the introduction of the gastrocnemius flap procedure.50 Proximal femur replacements have reported infection rates of 8%.53 High rates of infection have, in part, been improved by abandoning the use of synthetic grafts and using gastrocnemius flaps, which have decreased the rate of infection to about 14% in proximal tibia tumor resection.50
Fig. 3.
Extensive soft tissue resection in surgery around knee.
Diagnosis of periprosthesis infection is challenging because of highly variable clinical symptoms. Various preoperative, intraoperative, and postoperative diagnostic laboratory tests are nonspecific (Table 3).54,55 A combination of clinical, histological, or microbiological criteria56 is required for diagnosis of periprosthetic infection. Diagnosis of deep infection can be considered on the basis of clinical evidence of infection with a positive microbiological culture or periprosthetic pus and histology compatible with infection at operation.57
Table 3.
Diagnostic clinical and laboratory evaluation for prosthetic joint infections.
| Evaluation | Details |
|---|---|
| Clinical history | History of wound healing problems and prolonged antibiotics administration at initial surgery, previous surgery, radiation therapy, chemotherapy, malnutrition, anemia, advanced age, diabetes mellitus, previous native joint infection, obesity, skin disease, and pre-existing joint disease |
| Surgical history | Revision surgeries such as bushing exchange, patellar resurfacing, invasive lengthening of expandable tumor prostheses in children, and cemented fixation |
| Clinical signs | Persistent joint pain, fever, erythema, calor, effusion, and sinus |
| Blood investigations | White blood cell count, C-reactive protein, and erythrocyte sedimentation rate |
| Radiographs and computed tomography scans | Bone loss and loosening (late) |
| Ultrasonography | Joint effusion and synovial hypertrophy |
| Radionuclide bone scanning | Combined labeled leukocyte imaging and complementary bone marrow imaging with technetium 99-m, gallium citrate 67, or indium 111 |
| Synovial fluid aspiration | Microbiological examination |
| Biopsy | Periprosthetic tissue and synovium; histological and microbiological examination |
| Revised prostheses | Microbiological examination |
| Nucleic amplification techniques | Detection of bacterial 16S ribosomal DNA |
Infection of the megaprosthesis exposes the patient to the risks of repeated surgical procedures, long rehabilitation, pain, a possibly poor functional outcome, and amputation. The clinical impact of periprosthetic joint infection remains severe, with infection noted to be the leading cause of morbidity following joint replacement.58.59 Periprosthetic infection has been shown to carry a 2.7–18% mortality rate.58 Interestingly there are reports, which suggest increased survival in patients with osteosarcoma and developing periprosthetic infection after reconstructive surgery, suggesting a potential immunologic antineoplastic advantage induced by infection.57,59
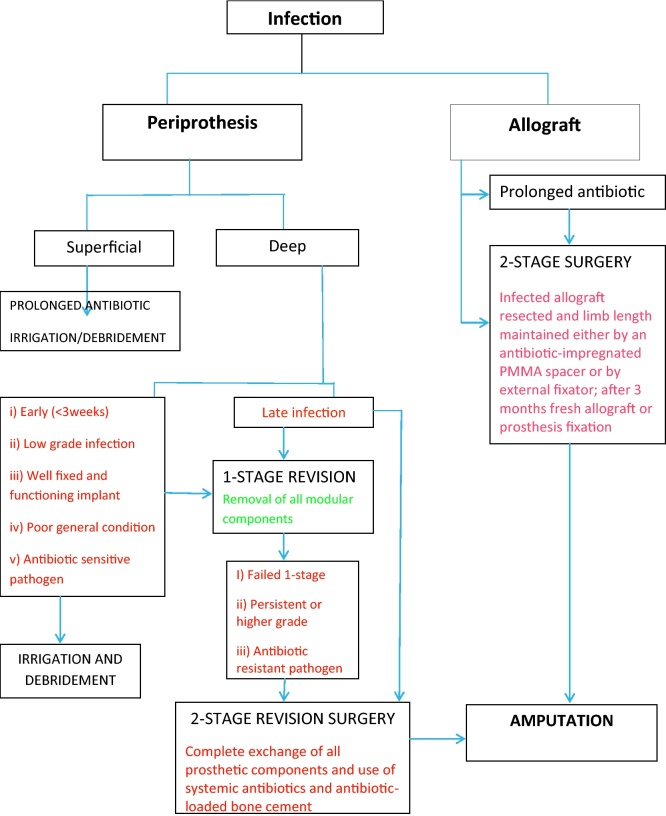
Treatment is similar to treatment of infected total knee or hip prosthesis, except there is a dramatically larger dead or infected tissue, and immune deficiency due to oncologic treatments, requiring more aggressive treatment.60 Deep infections in patients with megaprostheses require treatment by surgical methods because the long-term administration of antibiotics alone is not sufficient.60 The methods, which have been described, include irrigation without revision of the prosthesis,61 wound debridement without revision of the prosthesis,62–64 revision of prosthesis,62 two-stage revision with implantation of a cement spacer,64,65 arthrodesis with a vascularized fibular graft,62 and ultimately in some cases amputation.62,66 There are few data on the outcome of these different procedures. Late prosthetic infections are associated with poor results when treated by lavage, debridement, and prolonged antibiotics administration67; therefore, removal of infected prosthesis either as 1- or 2-stage procedure or an amputation becomes necessary.42,68–71 The reported success rate of eradicating infection with amputation ranges from 98% to 100%, 72% to 91% with 2-stage revision, and 42% with 1-stage revision.70 However Allison et al.36 reported success rate of 42% with irrigation and debridement procedures alone (without any component exchange), 70% with single-stage irrigation and debridement procedure with the addition of modular component exchange and varying degrees of suppressive antibiotics, and in contrast to other studies formal two-staged implant removal, antibiotic spacer placement with subsequent reimplantation was associated with 62%. The risk of amputation due to infected tumor prostheses for oncological reconstructions has been reported to be between 23.5% and 87%.70,72–76
Management with two-stage revision remains the gold standard,64,65,77,78 but a single-stage exchange of the prosthesis without removing the stems is possible in selected cases.60 In two-stage procedures, the first stage includes debridement, insertion of culture-specific antibiotic-loaded bone cement in the form of beads and/or rods, temporary fixation with a custom-made IM nail or self-designed, mobile hinged-joint prosthesis covered with antibiotic-loaded PMMA. Following parenteral antibiotherapy, the second staging is performed after a median of 6 weeks (5–11). The reconstruction stage includes reimplantation of a cemented prosthesis.
However some authors advocate one-stage revision without exchange of the anchorage components. The advantages are the avoidance of large bone defects, the need for only one operation putting a smaller burden on the patients, a shorter period of hospitalization, and the potential for lower costs.60
2.3. Allograft
Another treatment modality for replacement in the management of bone tumors is the use of allograft bone graft (Fig. 4). Frozen allografts have been used longer than any other tumor reconstruction option. Allografts are favored by some, for their potential for longevity, because they function as a biologic reconstruction material. Incorporation of the allograft by the host is a slow and incomplete process. The results for allograft arthrodeses were considerably poorer than osteoarticular, intercalary, and allograft plus prosthesis.79 Although numerous problems continue to limit the success of allograft reconstructions, they remain a viable choice for selected uses, especially in the upper extremity, for intercalary resections, and for patients who will not need chemotherapy. Infection remains one of the most common complications of allograft bone graft.80 Most of the allograft-associated infections80 occur within 4 months and despite antibiotic prophylaxis the reported incidence remains at 4–12%.80,81 High infection rates ranging from 15% to 50% have been reported after pelvic tumor resection surgeries.47,82–84 Factors responsible for the high incidence of infection in allogenic bone graft pointed out by Witso et al.85 include highly porous, non-cellular, and avascular foreign bodies that are prone to bacterial adhesion. Once bacteria attach, they secrete a thick glycocalyx matrix rendering them inaccessible to immune surveillance and local cellular defense mechanism.86 Majority of complications and unsatisfactory results with allografts have been reported in patients receiving chemotherapy, radiation therapy, and high doses of steroid.87,88
Fig. 4.
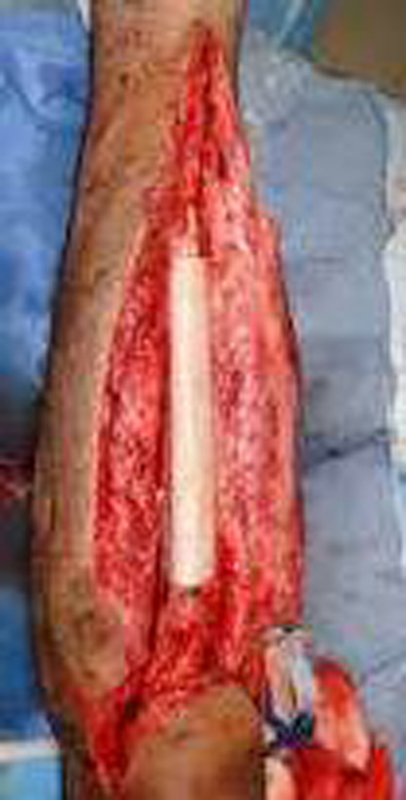
Allograft.
Allograft-associated infection has been reduced by 1) improved processing, 2) Strict donor screening, and 3) terminal sterilization.
Treatment of low grade, single microorganism allograft infection is usually successful with prolonged antibiotics alone or in combination with drainage; however, if the infection persists or in cases of multiple virulent pathogens, then a 2-stage surgery may be required.80 In the first case the infected allograft is resected, and length of the limb is maintained either with antibiotic-loaded PMMA spacer or external fixator. Pathogen sensitive antibiotics are usually given for 3 months after which a fresh allograft or prosthesis is implanted. The recommended treatment includes administration of antibiotics for a prolonged period intravenously for 2–14 days and orally for up to 16 weeks.89,90 Empirically, antibiotic impregnated bone allografts85,90 with antibiotics before implantation, achieving high local concentrations of antibiotic during the initial elution, help in prevention and treatment of infection. Use of intramedullary antibiotic bone cement91 also helps in infection prevention and outcome.
In conclusion, limb salvage surgery has become the mainstay for treatment of malignant bone tumors in recent times. One of the main contributing factors in the success of limb salvage surgery has been the introduction of neo-adjuvant chemotherapy and/or radiotherapy. However it predisposes the patient to certain complications such as wound infections postoperatively. Periprosthetic infection can lead to increased morbidity and mortality, and functional outcome. Management of infections after reconstructive surgery for bone tumors is a challenge, which requires careful planning and aggressive and multiprong approach. The treatment protocol for infection after reconstruction surgery for bone tumors is summarized in the flow chart.
Conflicts of interest
The authors have none to declare.
References
- 1.Eilber F.R., Eckhardt J., Morton D.L. Advances in the treatment of sarcomas of the extremity. Current status of limb salvage. Cancer. 1984;54(11 Suppl):2695–26701. doi: 10.1002/1097-0142(19841201)54:2+<2695::aid-cncr2820541415>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Kropej D., Schiller C., Ritschl P., Salzer-Kunt M., Rainer K. The management of IIB osteosarcoma: experience from 1976 to 1985. Clin Orthop. 1991;270:40–44. [PubMed] [Google Scholar]
- 3.Chao E.Y., Sim F.H. Modular prosthetic system for segmental bone and joint replacement after tumor resection. Orthopedics. 1985;8:641–651. doi: 10.3928/0147-7447-19850501-17. [DOI] [PubMed] [Google Scholar]
- 4.Kotz R., Ritschl P., Trachtenbrodt J. A modular femur tibia reconstruction system. Orthopaedics. 1986;9:1639–1652. doi: 10.3928/0147-7447-19861201-07. [DOI] [PubMed] [Google Scholar]
- 5.Kuo K.N., Gitelis S., Sim F.H. Segmental replacement of long bones using titaniium fiber metal composite following tumor resection. Clin Orthop. 1983;176:108–114. [PubMed] [Google Scholar]
- 6.Roberts P., Chan D., Grimer R.J., Sneath R.S., Scales J.T. Prosthetic replacement of the distal femur for primary bone tumors. J Bone Joint Surg [Br] 1991;73-B:762–769. doi: 10.1302/0301-620X.73B5.1894662. [DOI] [PubMed] [Google Scholar]
- 7.Shih L.Y., Sim F.H., Pritchard D.J., Rock M.G., Chao E.Y. Segmental total knee arthroplasty after distal femoral resection for tumor. Clin Orthop. 1993;292:269–281. [PubMed] [Google Scholar]
- 8.Delepine N., Delepine G., Desbois J.C., Cornille H., Mathe G. Results of multidisciplinary limb salvage in 240 consecutive bone sarcomas. Biomed Pharmacother. 1990;44:217–224. doi: 10.1016/0753-3322(90)90027-7. [DOI] [PubMed] [Google Scholar]
- 9.Eckardt J.J., Yang R.S., Ward W.G., Kelly C., Eilber F.R. Endoprosthetic reconstruction for malignant bone tumors and nontumorous conditions of bone. In: Stauffer R.N., editor. vol. 3. 1995. pp. 61–83. (Advances in Operative Orthopaedics). [Google Scholar]
- 10.Yang R.S. Endoprosthetic reconstruction for limb salvage surgery. Biomed Eng Appl Basis Commun. 1998;10:23–34. [Google Scholar]
- 11.Yang R.S. Endoprosthesis-related complications after limb-salvage operation of malignant bone tumors around the knee. Biomed Eng Appl Basis Commun. 2004;16:141–147. [Google Scholar]
- 12.Marcove R.C. The treatment of malignant bone tumors by conservative surgery. Recent Results Cancer Res. 1976;54:218–220. doi: 10.1007/978-3-642-80997-2_18. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe N., Patel S.R., Benjamin R.S. Chemotherapy in osteosarcoma. Basis for application and antagonism to implementation: early controversies surrounding its implementation [Review] Hemotol Oncol. 1995;9:825–840. [PubMed] [Google Scholar]
- 14.Bacci G., Picci 2, Pignatti G. Neoadjuvant chemotherapy for nonmetastatic osteosarcoma of the extremities. Clin Orthop. 1991;270:87–98. [PubMed] [Google Scholar]
- 15.Yang R.S. Limb salvage operations for patients with malignant bone tumors in the extremities. Tzu Chi Med J. 2005;17:389–396. [Google Scholar]
- 16.Goorin A.M., Schwartzentruber D.J., Devidas M. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;20:1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 17.Bielack S.S., Kempf-Bielack B., Delling G. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 18.Link M.P., Goorin A.M., Miser A.W. The effect of adjuvant chemotherapy on relapse free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;134:1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 19.Bramwell V.H., Burgers M., Sneath R. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults. J Clin Orthop. 1992;10:1579–1591. doi: 10.1200/JCO.1992.10.10.1579. [DOI] [PubMed] [Google Scholar]
- 20.McDonald D.J., Capanna R., Gherlinzoni F. Influence of chemotherapy on perioperative complications in limb salvage surgery for bone tumors. Cancer. 1990;65:1509–1516. doi: 10.1002/1097-0142(19900401)65:7<1509::aid-cncr2820650710>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Buchholz T.A., Hunt K.K., Whitman G.J. Neoadjuvant chemotherapy for breast carcinoma: multidisciplinary considerations of benefits and risks. Cancer. 2003;98:1150–1160. doi: 10.1002/cncr.11603. [DOI] [PubMed] [Google Scholar]
- 22.Bray Peter W., Robert S., Bell C., Bowen Vaughan A., Aileen Davis, Brian O'Sullivan Limb salvage surgery and adjuvant radiotherapy for soft tissue sarcomas of the forearm and hand. J Hand Surgery. 1997;22(3):495–503. doi: 10.1016/S0363-5023(97)80019-6. [DOI] [PubMed] [Google Scholar]
- 23.Fuller Brian G. The role of radiotherapy in the treatment of bone and soft-tissue sarcomas musculoskeletal cancer surgery. Malawer Chapter. 2001;05:86–133. [Google Scholar]
- 24.Marcus R.B., Jr., Cantor A., Heare T.C. Local control and function after twice-a-day radiotherapy for Ewing's sarcoma of bone. Int J Radiat Oncol Biol Phys. 1991;21:1509–1515. doi: 10.1016/0360-3016(91)90326-y. [DOI] [PubMed] [Google Scholar]
- 25.Bolek T.W., Marcus R.B., Mendenhall N.P. Local control and functional results after twice-daily radiotherapy for Ewing's sarcoma of the extremities. Int J Radiat Oncol Biol Phys. 1996;35:687–692. doi: 10.1016/0360-3016(96)00145-9. [DOI] [PubMed] [Google Scholar]
- 26.Perez C.A., Tefft M., Nesbit M.E. Radiation therapy in the mulimodal management of Ewing's sarcoma of bone. Report of the intergroup Ewing's sarcoma study. Natl Cancer Inst Monogr. 1981;56:263–271. [PubMed] [Google Scholar]
- 27.Perez C.A., Tefft M., Nesbit M.E. The role of radiation therapy in the management of non-metastatic Ewing's sarcoma of bone. Report of the intergroup Ewing's sarcoma study. Int J Radiat Oncol Biol Phys. 1981;7:141–149. doi: 10.1016/0360-3016(81)90429-6. [DOI] [PubMed] [Google Scholar]
- 28.Barbieri E., Emiliani E., Zini G. Combined therapy of localized Ewing's sarcoma of bone: analysis of results in 100 patients. Int J Radiat Oncol Biol Phys. 1990;19:1165–1170. doi: 10.1016/0360-3016(90)90223-7. [DOI] [PubMed] [Google Scholar]
- 29.Donaldson S.S., Torrey M., Link M.P. A multidisciplinary study investigating radiotherapy in Ewing's sarcoma: end results of POG #8346. Int J Radiat Oncol Biol Phys. 1998;42:125–135. doi: 10.1016/s0360-3016(98)00191-6. [DOI] [PubMed] [Google Scholar]
- 30.Mansson E., Willems J., Aparisi T., Jakobsson, Nilsonne U., Ringborg U. Preoperative radiation therapy of high malignancy grade soft tissue sarcoma: a preliminary investigation. Acta Radiol Oncol. 1983;22:461–464. doi: 10.3109/02841868309135971. [DOI] [PubMed] [Google Scholar]
- 31.Tanabe K.K., Pollock R.E., Ellis L.M., Murphy A., Sherman N., Romsdahl M.M. Influence of surgical margins on outcome in patients with preoperatively irradiated extremity soft tissue sarcomas. Cancer. 1994;73:1652–1659. doi: 10.1002/1097-0142(19940315)73:6<1652::aid-cncr2820730617>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 32.Wanebo H.J., Temple W.J., Popp M.B., Constable W., Aron B., Cunningham S.L. Preoperative regional therapy for extremity sarcoma. A tricenter update. Cancer. 1995;75:2299–2306. doi: 10.1002/1097-0142(19950501)75:9<2299::aid-cncr2820750919>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 33.Emami B. Tolerance of normal tissues to therapeutic radiation. Reports Radiother Oncol. 2013;1:35–48. [Google Scholar]
- 34.Jeys L.M., Luscombe J.S., Grimer R.J., Abudu A., Tillman R.M., Carter S.R. The risks and benefits of radiotherapy with massive endoprosthetic replacement. J Bone Joint Surg Br. 2007;89(10):1352–1355. doi: 10.1302/0301-620X.89B10.19233. [DOI] [PubMed] [Google Scholar]
- 35.Jimm Grimma, Tamara LaCouture, Raymond Croce, Inhwan Yeo, Yunping Zhu, Jinyu Xue Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys. 2011;12(2) doi: 10.1120/jacmp.v12i2.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allison D.C., Huang E., Ahlmann E.R., Carney S., Wang L., Menendez L.R. Peri-Prosthetic Infection in the Orthopedic Tumor Patient. JISRF Reconstructive Rev. 2014 Sep;4(3):13–17. [Google Scholar]
- 37.Pilge H., Gradl G., von Eisenhart-Rothe R., Gollwitzer H. Incidence and outcome after infection of megaprostheses. Hip Int. 2012;22(July-August (Suppl 8)):S83–S90. doi: 10.5301/HIP.2012.9576. [DOI] [PubMed] [Google Scholar]
- 38.Gradinger R., Rechl H., Ascherl R., Hipp E. Complications and their management following limb-salvage with total knee replacement. In: Brown K.L.B., editor. Complications of limb salvage: prevention, management and outcome. 6th International, ISOLS Symposium Montreal 1991. ISOLS; Montreal: 1991. pp. 151–153. [Google Scholar]
- 39.Hardes J. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101:389–395. doi: 10.1002/jso.21498. [DOI] [PubMed] [Google Scholar]
- 40.Gosheger G., Gebert C., Ahrens H. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164–171. doi: 10.1097/01.blo.0000223978.36831.39. [DOI] [PubMed] [Google Scholar]
- 41.Guo W., Ji T., Yang R. Endoprosthetic replacement for primary tumours around the knee: experience from Peking University. J Bone Joint Sur Br. 2008;90:1084–1089. doi: 10.1302/0301-620X.90B8.20240. [DOI] [PubMed] [Google Scholar]
- 42.Jeys L.M., Kulkarni A., Grimer R.J. Endoprosthetic replacement for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am. 2008;90:1265–1271. doi: 10.2106/JBJS.F.01324. [DOI] [PubMed] [Google Scholar]
- 43.Kumar D., Grimer R.J., Abudu A. Endoprosthetic replacement of the proximal humerus. Long-term results. J Bone Joint Surg Br. 2003;85:717–722. [PubMed] [Google Scholar]
- 44.Sim I.W., Tse L.F., Ek E.T. Salvaging the limb salvage: management of complications following endoprosthetic reconstruction for tumours around the knee. Eur J Surg Oncol. 2007;33:796–802. doi: 10.1016/j.ejso.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Mavrogenis F. Infected tumor prostheses. Orthopaedics. 2011;34(December (12)):991–998. doi: 10.3928/01477447-20111021-24. [DOI] [PubMed] [Google Scholar]
- 46.Guo W., Li D., Tang X., Yang Y., Ji T. Reconstruction with modular hemipelvic prostheses for periacetabular tumor. Clin Orthop Relat Res. 2007;461:180–188. doi: 10.1097/BLO.0b013e31806165d5. [DOI] [PubMed] [Google Scholar]
- 47.Senchenkov A., Moran S.L., Petty P.M. Predictors of complications and outcomes of external hemipelvectomy wounds: account of 160 consecutive cases. Ann Surg Oncol. 2008;15:355–363. doi: 10.1245/s10434-007-9672-5. [DOI] [PubMed] [Google Scholar]
- 48.Capanna R., van Horn J.R., Guernelli N. Complications of pelvic resections. Arch Orthop Trauma Surg. 1987;106:71–77. doi: 10.1007/BF00435417. [DOI] [PubMed] [Google Scholar]
- 49.Ozaki T., Hillmannl A., Dieter Bettin D., Wuisman P., Winkelmann W. High complication rates with pelvic allografts. Experience of 22 sarcoma resections. Acta Orthop Scand. 1996;67(4):333–338. doi: 10.3109/17453679609002326. [DOI] [PubMed] [Google Scholar]
- 50.Myers G.J., Abudu A.T., Carter S.R. The long-term results of endoprosthetic replacement of the proximal tibia for bone. Bone Joint Surg [Br] 2007;89-B:1632–1637. doi: 10.1302/0301-620X.89B12.19481. [DOI] [PubMed] [Google Scholar]
- 51.Abboud J.A., Patel R.V., Donthineni-Rao R., Lackman R.D. Proximal tibial segmental prosthetic replacement without the use of muscle flaps. Clin Orthop. 2003;414:189–196. doi: 10.1097/01.blo.0000079264.91782.83. [DOI] [PubMed] [Google Scholar]
- 52.Biau D., Faure F., Katashian S. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg [Am] 2006;88-A:1285–1293. doi: 10.2106/JBJS.E.00553. [DOI] [PubMed] [Google Scholar]
- 53.Finstein J.L., King J.J., Fox E.J. Bipolar proximal femoral replacement prostheses for musculoskeletal neoplasms. Clin Orthop Relat Res. 2007;459:66–75. doi: 10.1097/BLO.0b013e31804f5474. [DOI] [PubMed] [Google Scholar]
- 54.Zimmerli W., Trampuz A., Ochsner P. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 55.Trampuz A., Steckelberg J.M., Osmon D.R., Cockerill F.R., III, Hanssen A.D., Patel R. Advances in the laboratory diagnosis of prosthetic joint infection. Rev Med Microbiol. 2003;14(1):1–14. [Google Scholar]
- 56.Moran E., Byren I., Atkins B.L. The diagnosis and management of prosthetic joint infections. J Antimicrob Chemother. 2010;65(Suppl 3):iii45–iii54. doi: 10.1093/jac/dkq305. [DOI] [PubMed] [Google Scholar]
- 57.Jeys L.M., Grimer R.J., Carter S.R., Tillman R.M., Abudu A. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol. 2007;14(10):2887–2895. doi: 10.1245/s10434-007-9483-8. [DOI] [PubMed] [Google Scholar]
- 58.Matar W.Y., Jafari S.M., Restrepo C., Austin M., Purtill J.J., Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(December (Suppl 2)):36–46. doi: 10.2106/JBJS.J.01046. [DOI] [PubMed] [Google Scholar]
- 59.Lee J.A., Kim M.S., Kim D.H. Postoperative infection and survival in osteosarcoma patients. Ann Surg Oncol. 2008;16(January (1)):147–151. doi: 10.1245/s10434-008-0184-8. [DOI] [PubMed] [Google Scholar]
- 60.Holzer G., Windhager R., Kotz R. One-stage revision surgery for Infected megaprostheses. J Bone Joint Surg (Br) 1997;79-b(January (1)):31–35. doi: 10.1302/0301-620x.79b1.7139. [DOI] [PubMed] [Google Scholar]
- 61.Enneking W.F., Mindell E.R. Observations on massive retrieved human allografts. J Bone Joint Surg [Am] 1991;73-A:1123–1142. [PubMed] [Google Scholar]
- 62.Capanna R., Morris H.G., Campanacci D., Del Ben M., Campanacci M. Modular uncemented prosthetic reconstruction after resection of tumours of the distal femur. J Bone Joint Surg [Br] 1994;76-B:178–186. [PubMed] [Google Scholar]
- 63.Sim F.H., Beauchamp C.P., Chao E.Y.S. Reconstruction of musculoskeletal defects about the knee for tumour. Clin Orthop. 1987;221:188–201. [PubMed] [Google Scholar]
- 64.McDonald D.J., Fitzerald R.H., Ilstrup D.M. Two-stage reconstruction of a total hip arthroplasty because of infection. J Bone Joint Surg [Am] 1989;71-A:828–834. [PubMed] [Google Scholar]
- 65.Grimer R.J., Carter S.R., Sneath R.S. Two stage revision for infected endoprostheses. Procs 8th ISOLS; Florence; 1995. p. 54. [Google Scholar]
- 66.Eckhardt J.J., Eilber F.R., Rosen G. Endoprosthetic replacement for stage IIB osteosarcoma. Clin Orthop. 1991;270:202–213. [PubMed] [Google Scholar]
- 67.Flint M.N., Griffin A.M., Bell R.S., Wunder J.S., Ferguson P.C. Two-stage revision of infected uncemented lower extremity tumor endoprostheses. J Arthroplasty. 2007;22(6):859–865. doi: 10.1016/j.arth.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Gaur A.H., Liu T., Knapp K.M. Infections in children and young adults with bone malignancies undergoing limb-sparing surgery. Cancer. 2005;104(3):602–610. doi: 10.1002/cncr.21212. [DOI] [PubMed] [Google Scholar]
- 69.Malawer M.M., Chou L.B. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high bone sarcomas. J Bone Joint Surg Am. 1995;77(8):1154–1165. doi: 10.2106/00004623-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Jeys L.M., Grimer R.J., Carter S.R., Tillman R.M. Periprosthetic infection in patients treated for an orthopaedic oncological condition. J Bone Joint Surg Am. 2005;87(4):842–849. doi: 10.2106/JBJS.C.01222. [DOI] [PubMed] [Google Scholar]
- 71.Hardes J., Gebert C., Schwappach A. Characteristics and outcome of infections associated with tumor endoprostheses. Arch Orthop Trauma Surg. 2006;126(5):289–296. doi: 10.1007/s00402-005-0009-1. [DOI] [PubMed] [Google Scholar]
- 72.Shehadeh A., Noveau J., Malawer M., Henshaw R. Late complications and survival of endoprosthetic reconstruction after resection of bone tumors. Clin Orthop Relat Res. 2010;468(11):2885–2895. doi: 10.1007/s11999-010-1454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wirganowicz P.C., Eckardt J.J., Dorey F.J., Eilber F.R., Kabo J.M. Etiology and results of tumor endoprosthesis revision surgery in 64 patients. Clin Orthop Relat Res. 1999;(358):64–74. [PubMed] [Google Scholar]
- 74.Brigman B., Hornicek F.J., Gebhardt M.C., Mankin H.J. Allografts about the knee in young patients with high-grade sarcoma. Clin Orthop Relat Res. 2004;(421) doi: 10.1097/01.blo.0000127132.12576.05. 232-239.20. [DOI] [PubMed] [Google Scholar]
- 75.Jeys L., Grimer R., Carter S., Tillman R.M. Risk of amputation following limb salvage surgery with endoprosthetic replacement, in a consecutive series of 1261 patients [published online ahead of print February 8, 2003] Int Orthop. 2003;27(3):160–163. doi: 10.1007/s00264-003-0429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grimer R.J., Belthur M., Chandrasekar C., Carter S.R., Tillman R.M. Two-stage revision for infected endoprostheses used in tumor surgery. Clin Orthop Relat Res. 2002;(395):193–203. doi: 10.1097/00003086-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 77.Hardes J. Management of complications in megaprostheses. Unfallchirurg. 2014;117(July (7)):607–613. doi: 10.1007/s00113-013-2477-z. [DOI] [PubMed] [Google Scholar]
- 78.Eralp L., Ozger H., Kocaoglu M. Treatment strategies for infected megaprosthesis JBJS Br 2009. J Bone Joint Surg Br. 2009;91-B(Supp. II):301. [Google Scholar]
- 79.Mankin H.J., Gebhardt M.C., Jennings L., Candace Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;March (324):86–97. doi: 10.1097/00003086-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 80.Lord C.F., Gebhardt M.C., Tomford W.W., Mankin H.J. Infection in bone allografts. Incidence, nature, and treatment. J Bone Joint Surg Am. 1988;70:369–376. [PubMed] [Google Scholar]
- 81.Tomford W.W., Thongphasuk J., Mankin H.J., Ferraro M.J. Frozen musculoskeletal allografts. A study of the clinical incidence and causes of infection associated with their use. J Bone Joint Surg Am. 1990;72:1137–1143. [PubMed] [Google Scholar]
- 82.Loty B., Courpied J.P., Tomeno B., Postel M., Forest M., Ahelanet R. Bone allografts sterilised by irradiation. Int Orthop (SICOT) 1990;14:237–242. [PubMed] [Google Scholar]
- 83.Mnaymneh W., Lane Y., Malinin T.I., Glasser D. Pelvic allograft in surgery of pelvic bone tumors. Chir Organi Mov. 1990;75(Suppl 1):255–257. [PubMed] [Google Scholar]
- 84.Joyce M.J., Makley J.1’. Complications in hemipelvic resection/allograft reconstruction for bone sarcomas: are they prohibitive to limb salvage? In: Langlais F., Tomeno B., editors. Limb Salvage – Major Reconstructions in Oncology and Nontumoral Conditions. Springer-Verlag; Berlin: 1991. pp. 125–138. [Google Scholar]
- 85.Witso E., Persen L., Benum P., Bergh K. Cortical allograft as a vehicle for antibiotic delivery. Acta Orthop. 2005;76:481–486. doi: 10.1080/17453670510041457. [DOI] [PubMed] [Google Scholar]
- 86.Ketonis C. Bacterial colonization of bone allograft. Establishment and effect of antibiotics. Clin Orthop Relat Res. 2010;468(August (8)):2113–2121. doi: 10.1007/s11999-010-1322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mankin H., Doppelt S., Tomford W. Clinical experience with allograft implantation. The first ten years. Clin Orthop. 1983;174:69–86. [PubMed] [Google Scholar]
- 88.Fricdlaender G.E., Toss R.E., Doganis A.C., Kirkwood J.M., Barron R. Effects of cheinothcrapculic agents on bone. J Bone Joint Surg (Am) 1984;66(4):602–607. [PubMed] [Google Scholar]
- 89.Dick H.M., Strauch R.J. Infection of massive bone allografts. Clin Orthop Relat Res. 1994;306(September):46–53. [PubMed] [Google Scholar]
- 90.Degroot H., Donati D., Di Liddo M., Gozzi E., Mercuri M. The use of cement in osteoarticular allografts for proximal humerus bone tumors. Clin Orthop Relat Res. 2004;427:190–197. doi: 10.1097/01.blo.0000138959.50057.2c. [DOI] [PubMed] [Google Scholar]
- 91.Ortiz-Cruz E., Gebhardt M.C., Jennings L.C., Springfield D.S., Mankin H.J. The results of transplantation of intercalary allografts after resection of tumors. Long term follow up study. J Bone Joint Surg Am. 1997;79-A:97–106. doi: 10.2106/00004623-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 92.Intensity Modulated Radiation Therapy Collaborative Working Group Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001;51:880–914. doi: 10.1016/s0360-3016(01)01749-7. [DOI] [PubMed] [Google Scholar]
- 93.Holt G.E., Griffin A.M., Pintilie M. Fractures following radiotherapy and limb-salvage surgery for lower extremity soft-tissue sarcomas. A comparison of high-dose and low-dose radiotherapy. J Bone Joint Surg Am. 2005;87(February (2)):315–319. doi: 10.2106/JBJS.C.01714. [DOI] [PubMed] [Google Scholar]