Abstract
Background
Ventricular fibrillation (VF) in Brugada syndrome (BrS) is known to occur more frequently during nighttime and from spring to early summer. In this study, we investigated whether early repolarization syndrome (ERS) has the same seasonal, weekly, and circadian distribution of VF events as BrS using data from the “J-wave associated with prior cardiac event” (J-PREVENT) registry.
Methods
The study included 90 consecutive patients with BrS and 31 patients with ERS during a mean follow-up of 49±37 months. Follow-up data from implantable cardioverter-defibrillators were evaluated in all cases.
Results
In patients with ERS, the circadian distribution of VF episodes differed among the four 6-h periods, with a significant peak from midnight to 6:00 am (p<0.01) similar to that observed in BrS patients. However, VF occurred more frequently on weekends in patients with ERS, whereas on weekdays in patients with BrS (p<0.01). The months of peak VF occurrence also differed between the groups, with the frequency of VF episodes at peak between December and March in ERS patients and between March and June in BrS patients. In ERS patients, VF events had an inverse correlation with air temperature (r=−0.726, p<0.01).
Conclusions
ERS and BrS patients show similar nighttime increases in the occurrence of VF, but different seasonal and weekly distributions, suggesting a pathophysiological difference between the two syndromes.
Keywords: Electrocardiography, Ventricular fibrillation, Sudden death, Early-repolarization, Brugada syndrome
1. Introduction
Early repolarization (ER), a term classically used to refer to ST segment elevation on an electrocardiogram (ECG), is predominantly found in healthy young males and has traditionally been viewed as a completely benign entity [1,2]. However, it was recently reported that an ER ECG pattern in the inferior or lateral precordial leads is associated with ventricular fibrillation (VF), leading to the recognition of a pathological entity termed early repolarization syndrome (ERS) [3–8]. The Brugada syndrome (BrS) differs from ERS with respect to the magnitude and lead location of the abnormal J-wave; the two entities are thought to belong to a continuous spectrum of phenotypic expression known as “J-wave syndromes” [9]. Although ERS and BrS share similar clinical features including gender distribution and arrhythmia triggers, Kawata et al. hypothesized that different pathophysiological mechanisms were responsible for each condition based on their pharmacological responses [10].
It is known that in BrS, VF occurs more frequently during nighttime and from spring to early summer [11]. Our study aimed to investigate whether ERS has the same seasonal, weekly, and circadian distribution of VF events as BrS using data from the “J-wave associated with PRior cardiac EVENT” (J-PREVENT) registry [12].
2. Material and methods
2.1. Patient population
Patients were eligible for enrollment if they met the following criteria: an unexplained cardiac arrest with documented cardiovascular collapse during ventricular tachycardia (VT) or VF without identifiable structural heart disease on echocardiography as assessed based on biventricular dimensions and function; no detectable coronary artery disease on coronary angiography or exercise testing; and no known repolarization abnormalities [6,16]. A drug-induced coronary spasm provocation test was carried out using intracoronary acetylcholine administration to induce coronary spasm. After resuscitation, all patients underwent an electrophysiological study and received an implantable cardioverter defibrillator (ICD).
Patients were excluded if they had long or short QT syndrome [17,18] or catecholaminergic polymorphic VT. Patients with a transient long QT interval after resuscitation were included. All patients gave their written informed consent for the study protocol, which was approved by our institutional review board (approval number: 11-06, approval date: 12/9/2011). ICD follow-up data were obtained for all cases. In studying the temporal patterns of events, we evaluated all cardiac arrests and documented ventricular arrhythmias that occurred before ICD implantation, as well as appropriate ICD shock therapies. VF storms, defined as three or more VF episodes daily, were counted as a single event. No patient was taking antiarrhythmic drugs (AADs) at the time of study enrollment. However, in patients who had multiple episodes of appropriate ICD therapy, an AAD was administered at the discretion of each center.
2.2. Implanted cardioverter defibrillator settings
The ICD was programmed according to the documented arrhythmia with at least two detection zones. The lowest VT detection zone was set at a cycle length (CL) of 360 ms. In the VT zone, anti-tachycardia pacing included more than one burst pacing and/or one ramp pacing therapy followed by cardioversion. The maximum shocks were programmed in the VF zone (CL <320 ms) for rapid VT or VF.
2.3. ECG analysis for ER ECG pattern and Brugada-type ECG pattern
All ICD electrograms and serial 12-lead ECGs recorded at admission were reviewed by two cardiologists who were blinded to patient diagnoses, according to the HRS/EHRA/APHRS expert consensus statement [15]. The ECG pattern was classified as ER if the J-point elevation (≥0.1 mV) was notched (a positive J deflection inscribed on the S-wave) or slurred (a smooth transition from the QRS to the ST segment) in at least two inferior or lateral leads excluding the anterior precordial leads [3,5,13–15]. BrS was diagnosed in patients showing ST-segment elevation with type 1 morphology ≥2 mm or a type 2 or 3 ST-segment elevation in at least one right precordial lead (V1–V2) positioned in the 2nd, 3rd, or 4th intercostal space and occurring either spontaneously or after provocative drug testing using an intravenously administered class I AAD [15].
2.4. Climate parameters
All appropriate shock episodes with accurate time and date by the ICD recordings were evaluated as VF episodes. Data on the mean temperature and atmospheric air pressure in Tokyo were obtained from Japan Meteorological Agency releases.
2.5. Statistical analysis
Continuous variables are presented as the mean±SD. Continuous variables were compared using Student t-test. Categorical variables were compared using a χ2 analysis or Fisher exact test. Cosinor curve fitting was applied to VF frequency over the months of the year. Differences in the frequency of VF and syncope were analyzed by the goodness-of-fit test for a multinomial distribution. Pearson r coefficient was calculated for the relationship between arrhythmic events and climate parameters (temperature and atmospheric pressure). A p-value <0.05 was considered significant. R, Version 2.15.2. (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis.
3. Results
3.1. Patient characteristics
This study included 90 patients with BrS (age: 45±13 years, 85 men) and 31 patients with ERS (age: 42±14 years, 27 men) who were followed for 49±37 months (Table 1). Of the 90 patients diagnosed with BrS, 25 also exhibited ER in the inferolateral leads. The two patient groups did not differ with respect to the proportion of males, age, left ventricular ejection fraction, electrocardiographic PR interval, QRS width, QTc interval, number of follow-up months, and AAD use. A VF storm occurred in 9 BrS patients and 2 ERS patients. An acetylcholine provocation test was performed in 25 ERS patients, and coronary spasm was induced in 4 patients (16%).
Table 1.
Patient characteristics.
| BrS (n=90) | ERS (n=31) | p-Value | |
|---|---|---|---|
| Male (%) | 94 | 87 | 0.18 |
| Age (years) | 45±13 | 42±14 | 0.36 |
| Ejection fraction (%) | 67±8 | 64±15 | 0.16 |
| PR interval (ms) | 139±73 | 152±57 | 0.36 |
| QRS width (ms) | 90±49 | 89±33 | 0.89 |
| QTc interval (ms) | 399±34 | 408±32 | 0.22 |
| Follow-up (months) | 48±40 | 50±40 | 0.76 |
| AAD at study end | 19 | 7 | 0.86 |
| Quinidine | 12 | 2 | 0.30 |
| Disopyramide | 2 | 1 | 0.76 |
| Bepridil | 3 | – | – |
| Amiodarone | – | 4 | – |
| Cilostazol | 2 | – | – |
AAD, anti-arrhythmic drug; BrS, Brugada syndrome; ERS, early repolarization syndrome; QTc, corrected QT interval. A p-value <0.05 was considered significant.
3.2. Seasonal distribution
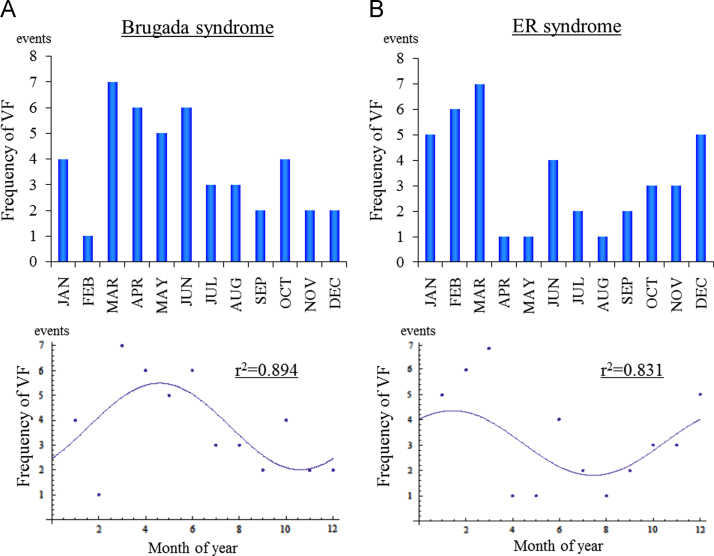
In this part of the study, we evaluated the number of confirmed VF episodes occurring per season. In BrS, 24 of the total 45 VF events occurred during the 4-month period of March through June. The cosine fit indicated a peak between April and May (Fig. 1A, r2=0.894). By contrast, 20 of the total 37 VF events recorded in ERS patients occurred during the 4-month period of December through March, and the cosine fit indicated a peak between January and February (Fig. 1B, r2=0.831). We interpreted this as a difference in the seasonal distribution of VF between ERS patients and BrS patients.
Fig. 1.
Seasonal distribution of VF episodes. (A) Brugada syndrome. (B) Early repolarization syndrome. The upper graph shows the number of VF episodes. The lower graph shows the cosinor fitting. BrS: y=3.75+1.73833 cos[0.523599 (t−4.59482)], ERS: y=3.08333+1.27707 cos[0.523599 (t−1.43548)]. The fit was r2=0.894 for BrS and r2=0.831 for ERS.
3.3. Relationship between arrhythmic events and climate parameters
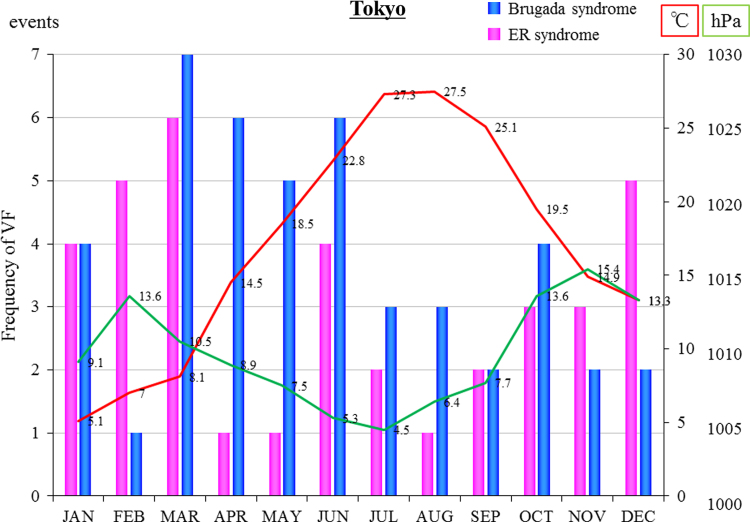
Most of the study patients were from the greater Tokyo metropolitan area. Fig. 2 shows the relationship between the number of VF events and Tokyo׳s temperature and atmospheric air pressure in patients with ERS. The blue bar graph (BrS) and the pink bar graph (ERS) show the monthly distribution of events. The red line shows the mean monthly temperature in Tokyo and the green line shows the mean atmospheric air pressure in Tokyo. In ERS patients, events were modestly positively correlated with the atmospheric air pressure (r=0.446, p=0.15) and significantly inversely correlated with the temperature (r=−0.726, p<0.01). However, no significant correlations between arrhythmic events and climate parameters were detected in patients with BrS.
Fig. 2.
VF events related to Tokyo׳s temperature and atmospheric air pressure in patients with ERS. The blue bar graph (Brugada syndrome) and the pink bar graph (early repolarization syndrome) show the monthly distribution of events. The red line shows the temperature in Tokyo and the green line shows the atmospheric air pressure in Tokyo.
3.4. Weekly distribution
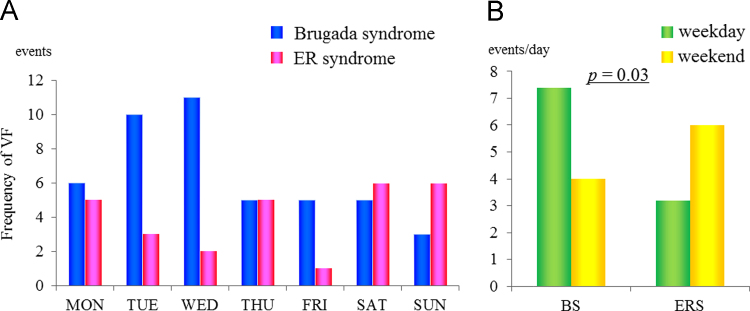
Fig. 3A shows the distribution of VF events in BrS and ERS patients over the course of the week. When normalized for the number of days, the weekday/weekend distribution of VF events differed between the two patient groups (Fig. 3B, p=0.03). VF events occurred more frequently on weekdays in patients with BrS whereas on the weekend in patients with ERS.
Fig. 3.
Distribution of VF episodes. (A) The weekly distribution of VF episodes. (B) A comparison of the number of events per day occurring on weekdays and weekends.
3.5. Circadian distribution
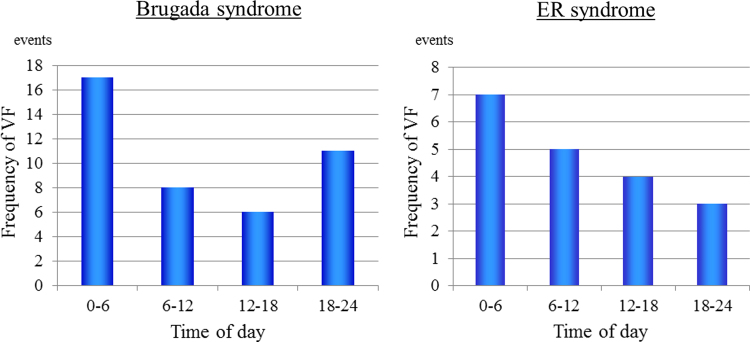
Fig. 4 shows the circadian distribution of VF episodes. For 7 events, the time of cardiac arrest was not available as it was missing from the medical chart. The circadian distribution of VF differed statistically among the four 6-h periods, and there was a significant peak from midnight to 6:00 AM (p<0.01).
Fig. 4.
The circadian distribution of VF episodes among four 6-h periods.
4. Discussion
4.1. Main findings
We investigated the seasonal, weekly, and circadian distribution of VF in patients with BrS and ERS. In patients with ERS, episodes of VF mainly occurred over the winter months of December through March, in contrast with BrS patients whose episodes were most frequent between March and June. In patients with ERS, the frequencies of VF events by month negatively correlated with the monthly mean external air temperatures. In addition, VF occurred more frequently on weekends in patients with ERS and on weekdays in patients with BrS. Both ERS and BrS patients displayed a peak of VF events in the between midnight to 6 AM block.
4.2. Seasonal distribution
Several studies reported that in patients with structural heart disease, coronary death and cardiac arrest are more frequent in winter than in other seasons [19–22]. Similarly, we found that VF episodes were concentrated in the winter months of December through March in patients with ERS. This contrasted with the seasonal peak occurring between spring and early summer we observed in our BrS patients, a finding consistent with a previous study [11]. However, a prior study of a South Korean cohort of ERS patients likewise found a seasonal peak in appropriate shocks between spring and summer, in contrast to the results in our ERS patients [23]. We can only speculate on the reasons for this summer peak. There are certainly differences in climate between the two regions such as the timing of the rainy season, which takes place in June in Japan and in July and August in South Korea. The rainy season may be related to the incidence of VF. The mechanisms underlying these seasonal distributions remain unclear. Moreover, we know of no other studies of the seasonal variability of VF episodes in ERS patients from other countries.
While the existence of seasonal variability in cardiac events is well established, the manner in which seasons affect cardiac pathophysiology is still largely unknown. Physiological factors such as autonomic nerve system activity and hormonal activity are thought to be associated with the occurrence of VF. For example, it has been reported that in winter, sympathetic nerve activity tends to be higher and vagal nerve activity tends to be lower [24]. Kruse et al. reported that plasma catecholamine levels were highest during the winter and lowest during the summer [25].
At the cardiomyocyte level, increased sympathetic tone and catecholamine levels create a spatial dispersion of the action potential duration due to alterations in the expression of L-type Ca2+ channels and K+ channels. Action potential prolongation and augmented Ca2+ influx through L-type Ca2+ channels combine to increase susceptibility to early after-depolarization and/or delayed after-depolarization triggered activity, resulting in arrhythmogenicity [26].
4.3. The influence of atmospheric air pressure and temperature
The influence of atmospheric air pressure and temperature on coronary events and sudden cardiac death has been described previously [20,27,28]. It was reported that the number of coronary events was inversely correlated with temperature, and that a change in air pressure was associated with an increase in coronary events [29]. Seasonal and temperature-related variations in blood pressure and sympathetic nerve activity have also been described [24,29]. In our study, VF events in ERS patients had a modestly positive correlation with the absolute value of atmospheric air pressure and a significantly inverse correlation with temperature, similar to coronary event patterns. These environmental factors may be recognized as triggers of the incidence of VF. However, the mechanism is still unknown.
4.4. Weekly distribution
It has been reported that there is a peak in the onset of acute myocardial infarction on Mondays in the working population [29]. In that paper, the beginning of the week was one of the risk factors for sudden cardiac death. In our cases, VF events occurred more frequently on weekdays in patients with BrS, similar to the myocardial infarction event distribution, while episodes of VF occurred more frequently on the weekend in patients with ERS.
4.5. Circadian distribution
In BrS, it is well known that episodes of ventricular tachyarrhythmia occur most frequently during mealtimes [30,31], between postprandial periods [32], and in the early morning [11,24,33]. It is also well known that the incidence of sudden death is highest in the daytime in patients with structural heart disease including patients with a history of myocardial infarction [19,22]. Our results were similar to findings previously reported for patients with BrS and ERS [23]. Importantly, the previous study indicated that VF occurrence was affected only by vagal nerve activity. In addition, Mizumaki et al. [34] reported that vagal activity modulates spontaneous augmentation of the J-wave elevation in patients with idiopathic VF. In their paper, the J-wave elevation was more strongly augmented during bradycardia and was associated with an increase in vagal activity. This could also be related to the incidence of VF, which occurred predominantly at night in patients with ERS.
We found that VF events in ERS patients had a significant inverse correlation with temperature, which was similar to coronary event patterns, suggesting that sympathetic nerve activity is related to the seasonal distribution of VF in ERS patients. However, we also found that the peak of VF events occurred between midnight and 6 AM in both BrS and ERS, which suggests that vagal nerve activity also plays an important role in the genesis of VF in both syndromes. It is difficult to clarify which is predominant in the genesis of VF in ERS. We speculated that higher sympathetic nerve activity might be related to a higher incidence of VF throughout the year, but that vagal nerve activity also plays an important role in the genesis of VF on the daily basis. A dynamic change of autonomic nervous activity may influence the incidence of VF. In this study, we did not assess patients’ autonomic nervous activity. An analysis of heart rate variability may be instrumental in evaluating the relationship between the dynamic changes in autonomic tone and the occurrence of VF.
4.6. Clinical implications
The present study may improve the recognition and treatment of individuals. For one, it shows that VF occurs more frequently during nighttime and in winter in patients with ERS, and during nighttime and in spring to early summer in patients with BrS. Haïssaguerre et al. reported the effects of oral AADs on VF recurrence during long-term follow-up [35]. They reported that β-blockers, verapamil, mexiletine, amiodarone, and class 1C AADs are not effective in preventing recurrent VF, and that only quinidine is effective in correcting the ECG pattern and suppressing the recurrence of arrhythmia in patients with ERS. In this paper, complete success was achieved in 9 out of 9 patients using quinidine (in 3) or hydroquinidine (in 6), with the number of VF recurrences reduced from a mean of 33±35/median of 25 (IQR 8–62) episodes to nil with a current follow-up of 25±18 months on therapy. In addition, these authors reported that rapid atrial pacing at 90 beats/min was used effectively in 1 patient. Therefore, perhaps quinidine should be taken during the high frequency seasons. Furthermore, in patients with ICD implantation, high rate pacing may exert a beneficial effect by preventing VF during nighttime sleep [35].
4.7. Study limitations
The study has several limitations. Although this was a multicenter study, the study population was small, especially regarding patients with ERS. A larger number of participants will be necessary to evaluate the incidence and mechanism of VF. Second, the seasonal distribution of VF differed between our cohort and a South Korean cohort of ERS patients. We speculate that the impact of physiological factors and environmental factors besides climate may be more strongly related to the incidence of VF than climate factors. A further study is needed to clarify the mechanism. Third, the weekly distribution of VF may depend on patients’ work lifestyles, which we did not assess. Fourth, the percentages of patients taking AADs were quite small in both groups. This may influence the circadian or weekly pattern of VF events. Fifth, it was reported that the J-wave was augmented by myocardial ischemia during coronary spasms, and that the presence and augmentation of J-waves were associated with VF during spasms [36]. In our study, 16% of ERS patients had a coronary spasm during acetylcholine provocation testing. Because most VF episodes occurred out of hospital, we could not investigate the association between the ER ECG pattern and the occurrence of coronary spasms. Finally, in our study, VF events in ERS patients had a modestly positive correlation with the absolute value of atmospheric air pressure and a significantly inverse correlation with temperature. However, the mechanism is still unknown.
5. Conclusions
The present study showed that in patients with ERS, VF occurred more frequently during nighttime similarly to BrS, while the seasonal and weekly distributions of VF episodes differed from those of BrS. Although ERS and BrS share many clinical features, these findings suggest a difference in the pathophysiology of ERS and BrS.
Conflict of interest
The authors have no conflicts of interest to disclose.
Acknowledgments
The authors thank M. Sato for assistance with statistical analysis.
References
- 1.Wasserburger R.H., Alt W.J. The normal RS-T segment elevation variant. Am J Cardiol. 1961;8:184–192. doi: 10.1016/0002-9149(61)90204-1. [DOI] [PubMed] [Google Scholar]
- 2.Mehta M.C., Jain A.C. Early repolarization on scalar electrocardiogram. Am J Med Sci. 1995;309:305–311. doi: 10.1097/00000441-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Haïssaguerre M., Derval N., Sacher F. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 4.Nam G.B. Brugada syndrome and early repolarization syndrome: cellular basis and clinical features. J Arrhythm. 2013;29:126–133. [Google Scholar]
- 5.Tikkanen J.T., Anttonen O., Junttila M.J. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki S., Shah A.J., Haïssaguerre M. Early repolarization syndrome—a new electrical disorder associated with sudden cardiac death. Circ J. 2010;74:2039–2044. doi: 10.1253/circj.cj-10-0753. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe H., Minamino T. Similarities and differences of clinical characteristics between Brugada syndrome and early repolarization syndrome. J Arrhythm. 2013;29:134–137. [Google Scholar]
- 8.Murakoshi N., Aonuma K. Epidemiology of arrhythmias and sudden cardiac death in Asia. Circ J. 2013;77:2419–2431. doi: 10.1253/circj.cj-13-1129. [DOI] [PubMed] [Google Scholar]
- 9.Antzelevitch C., Yan G.X. J wave syndromes. Heart Rhythm. 2010;7:549–558. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawata H., Noda T., Yamada Y. Effect of sodium-channel blockade on early repolarization in inferior/lateral leads in patients with idiopathic ventricular fibrillation and Brugada syndrome. Heart Rhythm. 2012;9:77–83. doi: 10.1016/j.hrthm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Takigawa M., Noda T., Shimizu W. Seasonal and circadian distributions of ventricular fibrillation in patients with Brugada syndrome. Heart Rhythm. 2008;5:1523–1527. doi: 10.1016/j.hrthm.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi Y., Osaka Y., Nogami A. Inducibility of ventricular arrhythmias in early repolarization syndrome and Brugada syndrome: from the J-wave associated with prior cardiac event (J-PREVENT) registry. J Arrhythm. 2014;30:300–304. [Google Scholar]
- 13.Klatsky A.L., Oehm R., Cooper R.A. The early repolarization normal variant electrocardiogram: correlates and consequences. Am J Med. 2003;115:171–177. doi: 10.1016/s0002-9343(03)00355-3. [DOI] [PubMed] [Google Scholar]
- 14.Gussak I., Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000;33:299–309. doi: 10.1054/jelc.2000.18106. [DOI] [PubMed] [Google Scholar]
- 15.Priori S.G., Wilde A.A., Horie M. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Zipes D.P., Wellens H.J. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 17.Moss A.J., Schwartz P.J., Crampton R.S. The long QT syndrome: a prospective international study. Circulation. 1985;71:17–21. doi: 10.1161/01.cir.71.1.17. [DOI] [PubMed] [Google Scholar]
- 18.Gaita F., Giustetto C., Bianchi F. Short QT Syndrome: a familial cause of sudden death. Circulation. 2003;108:965–970. doi: 10.1161/01.CIR.0000085071.28695.C4. [DOI] [PubMed] [Google Scholar]
- 19.Lampert R., Rosenfeld L., Batsford W. Circadian variation of sustained ventricular tachycardia in patients with coronary artery disease and implantable cardioverter-defibrillators. Circulation. 1994;90:241–247. doi: 10.1161/01.cir.90.1.241. [DOI] [PubMed] [Google Scholar]
- 20.Arntz H.R., Willich S.N., Schreiber C. Diurnal, weekly and seasonal variation of sudden death. Population-based analysis of 24,061 consecutive cases. Eur Heart J. 2000;21:315–320. doi: 10.1053/euhj.1999.1739. [DOI] [PubMed] [Google Scholar]
- 21.Müller D., Lampe F., Wegscheider K. Annual distribution of ventricular tachycardias and ventricular fibrillation. Am Heart J. 2003;146:1061–1065. doi: 10.1016/S0002-8703(03)00426-5. [DOI] [PubMed] [Google Scholar]
- 22.Anand K., Aryana A., Cloutier D. Circadian, daily, and seasonal distributions of ventricular tachyarrhythmias in patients with implantable cardioverter-defibrillators. Am J Cardiol. 2007;100:1134–1138. doi: 10.1016/j.amjcard.2007.04.063. [DOI] [PubMed] [Google Scholar]
- 23.Kim S.H., Nam G.B., Baek S. Circadian and seasonal variations of ventricular tachyarrhythmias in patients with early repolarization syndrome and Brugada syndrome: analysis of patients with implantable cardioverter defibrillator. J Cardiovasc Electrophysiol. 2012;23:757–763. doi: 10.1111/j.1540-8167.2011.02287.x. [DOI] [PubMed] [Google Scholar]
- 24.Kristal-Boneh E., Froom P., Harari G. Summer–winter differences in 24 h variability of heart rate. J Cardiovasc Risk. 2000;7:141–146. doi: 10.1177/204748730000700209. [DOI] [PubMed] [Google Scholar]
- 25.Kruse H.J., Wieczorek I., Hecker H. Seasonal variation of endothelin-1, angiotensin II, and plasma catecholamines and their relation to outside temperature. J Lab Clin Med. 2002;140:236–241. doi: 10.1067/mlc.2002.127169. [DOI] [PubMed] [Google Scholar]
- 26.Rubart M., Zipes D.P. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115:2305–2315. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spencer F.A., Goldberg R.J., Becker R.C. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol. 1998;31:1226–1233. doi: 10.1016/s0735-1097(98)00098-9. [DOI] [PubMed] [Google Scholar]
- 28.Danet S., Richard F., Montaye M. Unhealthy effects of atmospheric temperature and pressure on the occurrence of myocardial infarction and coronary deaths. A 10-year survey: the Lille-World Health Organization MONICA project (Monitoring trends and determinants in cardiovascular disease) Circulation. 1999;100:E1–E7. doi: 10.1161/01.cir.100.1.e1. [DOI] [PubMed] [Google Scholar]
- 29.Willich S.N., Löwel H., Lewis M. Weekly variation of acute myocardial infarction. Increased Monday risk in the working population. Circulation. 1994;90:87–93. doi: 10.1161/01.cir.90.1.87. [DOI] [PubMed] [Google Scholar]
- 30.Nishizaki M., Sakurada H., Ashikaga T. Effects of glucose-induced insulin secretion on ST segment elevation in the Brugada syndrome. J Cardiovasc Electrophysiol. 2003;14:243–249. doi: 10.1046/j.1540-8167.2003.02389.x. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda T., Abe A., Yusu S. The full stomach test as a novel diagnostic technique for identifying patients at risk of Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17:602–607. doi: 10.1111/j.1540-8167.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 32.Mizumaki K., Fujiki A., Nishida K. Postprandial augmentation of bradycardia-dependent ST elevation in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2007;18:839–844. doi: 10.1111/j.1540-8167.2007.00872.x. [DOI] [PubMed] [Google Scholar]
- 33.Matsuo K., Kurita T., Inagaki M. The circadian pattern of the development of ventricular fibrillation in patients with Brugada syndrome. Eur Heart J. 1999;20:465–470. doi: 10.1053/euhj.1998.1332. [DOI] [PubMed] [Google Scholar]
- 34.Mizumaki K., Nishida K., Iwamoto J. Vagal activity modulates spontaneous augmentation of J-wave elevation in patients with idiopathic ventricular fibrillation. Heart Rhythm. 2012;9:249–255. doi: 10.1016/j.hrthm.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 35.Haïssaguerre M., Sacher F., Nogami A. Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy. J Am Coll Cardiol. 2009;53:612–619. doi: 10.1016/j.jacc.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 36.Sato A., Tanabe Y., Chinushi M. Analysis of J waves during myocardial ischemia. Europace. 2011;14:715–723. doi: 10.1093/europace/eur323. [DOI] [PubMed] [Google Scholar]