Abstract
Oxidative stress plays a central role in the pathogenesis of diverse chronic inflammatory disorders including diabetic complications, cardiovascular disease, aging, and chronic kidney disease (CKD). Patients with moderate to advanced CKD have markedly increased levels of oxidative stress and inflammation that likely contribute to the unacceptable high rates of morbidity and mortality in this patient population. Oxidative stress is defined as an imbalance of the generation of reactive oxygen species (ROS) in excess of the capacity of cells/tissues to detoxify or scavenge them. Such a state of oxidative stress may alter the structure/function of cellular macromolecules and tissues that eventually leads to organ dysfunction. The harmful effects of ROS have been largely attributed to its indiscriminate, stochastic effects on the oxidation of protein, lipids, or DNA but in many instances the oxidants target particular amino acid residues or lipid moieties. Oxidant mechanisms are intimately involved in cell signaling and are linked to several key redox-sensitive signaling pathways in fibrogenesis. Dysregulation of antioxidant mechanisms and overproduction of ROS not only promotes a fibrotic milieu but leads to mitochondrial dysfunction and further exacerbates kidney injury. Our studies support the hypothesis that unique reactive intermediates generated in localized microenvironments of vulnerable tissues such as the kidney activate fibrogenic pathways and promote end-organ damage. The ability to quantify these changes and assess response to therapies will be pivotal in understanding disease mechanisms and monitoring efficacy of therapy.
Abbreviations: AGEs, advanced glycation end-products; ALE, advanced lipoxidation end-products; BH4, tetrahydrobiopterin; CML, Nε-(carboxymethyl)lysine; eNOS, endothelial nitric oxide synthase; ESRD, end stage renal disease; MS, mass spectrometry; HO•, hydroxyl radical; HODE, hydroxyoctadecadienoic acid; H2O2, hydrogen peroxide; HOCl, hypochlorous acid; LDL, low density lipoprotein; MPO, myeloperoxidase; NEFA, non esterified fatty acid; NOX, NAD(P)H oxidase; NO•, nitric oxide; NO2•, nitrogen dioxide; ONOO−, peroxynitrite; PUFA, polyunsaturated fatty acid; RAGE, Receptor for AGE; RNase, ribonuclease; O2•−, superoxide anion
Keywords: Oxidative stress, Oxidized amino acids, Mass spectrometry, Chronic kidney disease, Fibrosis
1. Background
Oxygen forms the basis of aerobic life but, it is well-recognized that it can be modified by cellular metabolism to form highly reactive free radicals termed as “reactive oxygen species” (ROS) which in turn can form a variety of intermediates including “reactive nitrogen species” (RNS). Oxidative stress may be viewed as an essential consequence related to the fundamental biological need for utilization of molecular oxygen (O2) for energy production from simple aerobic eukaryotes to more complex mammalian species [1]. While physiological levels of such oxidants have a beneficial role in energy production, cellular signaling and host defense, excess oxidants can lead to pathological consequences.
Acute kidney injury (AKI) is characterized by the sudden deterioration of kidney function and is associated with a high incidence of morbidity and mortality [2]. Chronic kidney disease (CKD) affects approximately 26 million people in the US and premature death from cardiovascular disease and from all causes is higher in adults with CKD compared to adults without CKD [3]. During the past decade these two syndromes were conceptually distinct, however, evidence from experimental models and recent epidemiological studies suggest that AKI and CKD are closely interconnected: AKI can result in end-stage kidney disease (ESKD), AKI is a risk factor for CKD, CKD is a risk factor for AKI, and both are risk factors for cardiovascular disease [2,4]. The exact mechanisms by which AKI initiates or accelerates CKD in humans are unknown and as a result there are currently no therapies to halt or reverse AKI or to address the relentless progression of CKD.
Fibrosis is characterized by maladaptive wound repair following tissue injury that leads to the progressive accumulation of interstitial matrix proteins with gradual destruction of renal tubules and functional nephrons. Increased oxidative stress is a consistent characteristic of both AKI and CKD. Although once conceptualized as a random process, it is now recognized that oxidant-mediated injury occurs along predictable pathways and through specific cell types. One of the ways that oxidant and antioxidants may affect disease pathogenesis is through the modulation of reversible oxidation-reduction (redox) processes. Redox is a dynamic process that involves the ROS/RNS to oxidize a critical protein, such as a kinase, phosphatase or enzyme, and an antioxidant, typically a thiol containing protein such as glutathione (GSH) to reduce the protein back to its original state [5,6]. Redox signaling is advantageous because ROS/RNS are diffusible, permitting action at a distance. In this review, we will discuss the major oxidant and redox sensitive pathways in kidney injury and fibrosis.
2. Oxidative regulation of the fibrotic response
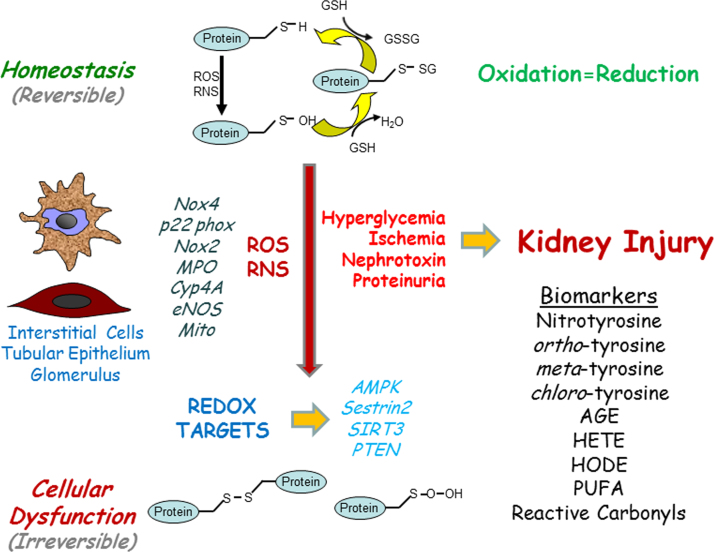
Oxidative stress is commonly viewed as a disturbance in the balance between oxidant production and antioxidant defense mechanisms within the tissue. Both acute and chronic kidney injury are characterized by an over-production of oxidants in the presence of a diminished antioxidant reserve. This imbalance of pro-oxidants or free radicals can oxidize macromolecules such as proteins, lipids, and nucleic acids altering redox sensitive pathways resulting in subsequent cell and tissue injury (Fig. 1). Reactive oxygen species (ROS) and reactive nitrogen (RNS) are collective terms that include not only highly reactive oxygen and nitrogen radicals (O2•−, OH•, and NO• derived NO2•) but also non-radical derivatives (ONOO−). Several enzyme complexes generate ROS and RNS such as NADPH oxidase, myeloperoxidase, nitric oxide synthase, and superoxide dismutase. Although many of these oxidant complexes are important in kidney injury [7], we will focus on oxidant pathways most thoroughly investigated in regards to redox regulation in kidney disease.
Fig. 1.
Redox Pathways and Kidney Injury. Under normal physiological conditions, redox reactions are balanced and cellular homeostasis is preserved. With pathological stimuli, renal cells produce oxidants in an uncontrolled manner which can lead to irreversible cellular damage due to oxidation of proteins and lipids resulting in kidney injury. Thiol oxidation in proteins is depicted as an illustrative example in the figure. AMPK, 5′ adenosine monophosphate-activated protein kinase; AGEs, advanced glycosylation end-products; eNOS, endothelial nitric oxide synthase; HETE, Hydroxyeicosatetraenoic acid; HODE, hydroxyoctadecadienoic acid; Mito, mitochondrial electron transport chain; MPO, myeloperoxidase; NOX, NADPH oxidase; PTEN, Phosphatase and tensin homolog; PUFA, polyunsaturated fatty acids; ROS, reactive oxygen Species; RNS, reactive nitrogen species.
2.1. NADPH oxidase
Superoxide anion (O2•−) is the major free radical generated in-vivo through the action of the enzyme complex, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. The phagocyte NADPH oxidase enzyme complex is composed of the membrane bound flavocytochrome subunits Nox (formerly known as gp91phox) and p22phox and cytosolic regulatory proteins p47phox, p67phox, p40phox, and the GTPase Rac1/2. All Nox proteins contain a heme binding site on a membrane spanning region of the N-terminal half and NADPH- and FAD-binding domains in the C-terminal half. Membrane bound Nox along with p22phox form the catalytic core that can transfer electrons across biological membranes using NADPH as the electron donor to molecular O2 to generate superoxide anion (O2•−). At neutral pH, O2•− is a reducing agent rather than an oxidant. However, O2•− dismutates enzymatically or nonenzymatically into hydrogen peroxide (H2O2), which can then oxidize thiol residues, a mechanism for cellular signaling via the inactivation of cysteine-containing phosphatases [8]. It can also function as an oxidizing substrate for heme proteins such as myeloperoxidase (MPO). O2•− also reacts at a diffusion-controlled rate with nitric oxide (NO) to form peroxynitrite (ONOO−), a powerful reactive nitrogen species that nitrates tyrosine residues on proteins and may induce oxidative damage to other macromolecular substrates.
Nox and p22phox are activated after recruitment and phosphorylation of the three regulatory proteins (p47phox, p67phox, p40phox) and the GTPase Rac1/2, which assemble with the membrane-bound proteins to form a functional NADPH oxidase [9,10]. This regulatory pathway allows Nox to remain inactive in resting cells and rapidly activated to provide the respiratory burst in leukocytes or ROS in nonphagocytic cells.
There are currently seven Nox isoforms (Nox1-5, Duox1, Duox2). Several Nox isoforms (Nox1-4) share a number of critical structural and functional domains with Nox2. Excessive production of ROS by the Nox complex is commonly thought to be responsible for tissue injury associated with a range of chronic inflammatory diseases and has long been considered a unique property of phagocytic cells. Deficiency of Nox2 in humans results in chronic granulomatous disease characterized by multiple abscess formation due to inability to remove bacterial pathogens. However, in animal studies of chronic injury, Nox2 deficiency was associated with enhanced inflammation with subsequent tissue damage [11–15], implying that Nox2 also has beneficial functions in immune responses and cell signaling. Recent studies have broadened our understanding of Nox’s function to include cellular processes as diverse as cell proliferation, migration, differentiation, signal transduction, and oxygen sensing [9,16,17].
2.2. Nox-dependent pathways in kidney injury
Nox proteins are increasingly recognized as important mediators and modulators of specific intracellular signal transduction pathways by activating redox-sensitive kinases. Potential mediators include angiotensin II, endothelin-1, hypercholesterolemia, shear stress, non-esterified fatty acids, hyperglycemia, and growth factors that can also augment Nox activity. Angiotensin II may represent a pathophysiologically relevant pathway for stimulating the production of reactive intermediates in the kidney because inhibitors of this pathway lower the risk for renal progression [18]. Nox4 appears to produce a higher H2O2 to superoxide ratio compared to Nox1, Nox2, and Nox5. Studies strongly suggest that H2O2 formation occurs via the third extracytosolic loop (E-loop) of Nox4 that is 28 amino acids longer than that of Nox1 or Nox2. The E-loop of Nox4, in contrast to Nox1 and Nox2, contains highly conserved histidine that could serve as a source of protons to accelerate spontaneous dismutation of superoxide to form H2O2 [19]. Nox4 is the predominant form in the kidney due to its expression in proximal tubules and is up-regulated in renal tubular epithelial cell injury and diabetic nephropathy. Nox4 is also present in podocytes, mesangial cells, and microvascular endothelial cells making it a key oxidation complex in the kidney.
Mesangial cell hypertrophy and podocyte injury are two important disease promoting pathways in the progression of diabetic nephropathy and other glomerular diseases, the leading causes of chronic kidney disease and end-stage kidney disease. In cultured mesangial cells, glucose elicits a rapid upregulation in Nox4 protein levels, including in the mitochondrial fraction and is associated with an increase in cellular and mitochondrial ROS production [20–22]. In diabetic nephropathy and other kidney diseases, Angiotensin II (Ang II) activates glomerular mesangial cells and leads to hypertrophy and extracellular matrix accumulation. Ang II induces an increase in 3-phosphoinositide-dependent protein kinase-1 (PDK-1) kinase activity that requires phosphorylation on tyrosine 9 and 373/376. Mutation of these tyrosine sites blocks AngII-dependent mesangial cell hypertrophy and fibronectin accumulation. The tyrosine kinase Src is upstream of PDK-1 and is the site of redox regulation. Small interfering RNA for Nox4 inhibits Ang II-induced activation of Src and PDK-1 tyrosine phosphorylation [23].
Nox4 localizes to mitochondria in mesangial cells and the cortex of the kidney and silencing of Nox4 blocks glucose-induced mitochondrial superoxide generation [20]. In cardiac myocytes, overexpression of Nox4 leads to an increase in ROS oxidation of cysteine residues in mitochondrial proteins [24]. In endothelial cells, Nox4 depletion induced alterations in mitochondria morphology, stabilized mitochondrial membrane potential, and decreased production of H2O2 and that Nox4-derived ROS decreased mitochondrial function via disruption of the electron transport chain I. These findings suggest that mitochondrial electron transport chain may be a downstream effector of Nox4. In addition, two recent studies demonstrated that another important downstream target of Nox4-derived ROS is the uncoupling of endothelial nitric oxide synthase (eNOS) and a decrease in nitric oxide (NO) bioavailability [21,25]. Lee and colleagues demonstrated that mesangial cell fibronectin production induced by Ang II involved the reaction of Nox4-derived superoxide with eNOS-derived NO resulting in ONOO− that subsequently uncouples eNOS further promoting superoxide generation [25]. Eid and colleagues demonstrated in a rat model of diabetes that Nox4-dependent eNOS uncoupling induced by hyperglycemia not only eliminates the protective effect of eNOS-derived NO but enhances ROS generation and the mesangial cell fibrotic response. They further identified two novel upstream regulators of Nox4, Sestrin 2 and AMP-activated protein kinase (AMPK) [21]. Sestrin 2 and AMPK are important for the maintenance of metabolic homeostasis and also function as stress-inducible proteins that are critical for the suppression of ROS production and protection from oxidative stress [26–29]. Sestrin 2 counteracts Nox4-derived ROS production by blocking the rapid upregulation of Nox4 protein elicited by hyperglycemia. AMPK mediates the inhibitory effects of Sestrin 2 on hyperglycemia-stimulated and Nox4-dependent eNOS dysfunction and extracellular matrix protein accumulation in mesangial cells. In summary, hyperglycemia promotes AMPK inactivation via downregulation of Sestrin 2, which leads to increased Nox4 and Nox4-derived ROS production followed by eNOS uncoupling and a decrease in NO bioavailability and enhancement of the mesangial cell fibrotic response.
Podocytes demand a high energy supply that is primarily derived from the respiratory chain of mitochondria to maintain cellular functions like the organization of cytoskeletal and other extracellular matrix proteins. Dysregulation in the metabolic homeostasis of the podocytes may result in podocyte injury and glomerular disease. In diabetic nephropathy and other chronic kidney diseases characterized by albuminuria there is an increase in Nox4 and ROS production especially in podocytes [30–32]. In human podocytes both Nox4 and Nox5 are expressed and upon exposure to hyperglycemia Nox4 mRNA expression significantly increases but has no effect on Nox5 mRNA expression [33]. Stimulation of human podocytes with Ang II upregulated Nox5 expression and increased ROS generation, while silencing of Nox5 blocked ROS and altered podocyte cytoskeleton and lead to a more motile phenotype [34]. As mice do not express Nox5, transgenic mice expressing human Nox5 in a podocyte-specific manner led to early onset albuminuria, podocyte foot process effacement, and elevated systolic blood pressure. Subjecting the mice to streptozotocin-induced diabetes further exacerbated these changes [34]. Even in nondiabetic kidney injury, ROS plays a major role in progressive glomerular injury. The cytosolic p47phox subunit is a key regulator of the assembly of Nox1 and Nox2 and its expression and phosphorylation are upregulated during kidney injury. Deletion of p47phox protected mice from albuminuria and glomerulosclerosis in experimental models of focal segmental glomerulosclerosis (FSGS) [35].
Hyperglycemia, transforming growth factor beta (TGFβ), and Ang II promote podocyte apoptosis. Hyperglycemia results in ROS production and podocyte apoptosis via sequential regulation of Nox oxidases by cytochrome P450 of the 4A family (CYP4A). Upregulation of CYP4A leads to increased generation of 20-hydroxyeicosatetraenoic acid (20-HETE) and subsequent increase in Nox1 and Nox4, and blocking 20-HETE production by inhibiting CYP4A not only inhibited podocyte apoptosis in vitro but decreased oxidative stress, podocyte apoptosis, and proteinuria in a diabetic model [36]. During hyperglycemia, Nox4 promotes podocyte cell death via activation of p53- and PUMA-dependent apoptotic pathways [32]. Furthermore, the oxidative stress triggered by hyperglycemia appears to be exacerbated by Nox4-derived ROS that affects the balance between oxidants and antioxidants through decreasing activity of key antioxidant enzymes such as glutathione peroxidase and catalase [37].
Myofibroblasts are the major matrix producing cells in fibrosis and are hallmarked by their expression of α-smooth muscle actin (αSMA) and their contractile phenotype. The origins of myofibroblasts are strongly debated at present [38], and the role of oxidative stress in fibrotic cell transformation has been reported in all cells of origin [39–47]. Both Nox4 and Nox2 are expressed in kidney fibroblasts, and both homologs are upregulated by TGFβ, implicating their importance and the role of ROS in activation of the profibrotic phenotype. Upon TGFβ activation, Nox4 expression levels greatly exceed Nox2 and siRNA inhibition of Nox4 substantially inhibits αSMA and extracellular matrix production [48,49]. TGFβ binds to a receptor on the cell surface and forms a complex of subunits called TGFR1 and TGFR2. Both TGFR1 and TGFR2 activate serine/threonine kinases that subsequently signal through the Smad family of transcription factors [50,51]. However, unlike cardiac and lung myofibroblasts where Nox4 acts upstream of Smad2/3 [52,53], Nox4 is positioned downstream of Smad3 and proximal to ERK in kidney myofibroblast activation and extracellular matrix production [49]. A recent study by Manickam and colleagues demonstrated that in kidney myofibroblasts inhibition of RhoA and Rho A kinase (ROCK) reduced TGFβ stimulation of Nox4 protein, NADPH oxidase activity, and myofibroblast activation suggesting that RhoA/ROCK is upstream of Nox4 myofibroblast signaling. Interestingly, RhoA/ROCK seems to act via increasing the expression of polymerase (DNA-directed) δ-interacting protein 2 (Polydip2), a newly discovered Nox4 enhancer protein [43]. Furthermore, a recent study by Hecker and colleagues suggests that the persistent fibrosis in aging may be related to a redox imbalance between Nox4 and the antioxidant response protein nuclear factor (erythroid derived-2)-related factor 2 (Nrf2). Lung fibroblasts from aged mice showed sustained expression levels of Nox4 and an impaired capacity to induce Nrf2. In fibroblasts from human subjects with idiopathic pulmonary fibrosis (IPF), Nox4 mediated a senescent and apoptotic resistant phenotype and genetic and pharmacologic targeting of Nox4 in aged mice showed a reversal of established tissue fibrosis [53]. You et al. recently showed that NOX4 is a major mediator of diabetes-associated glomerular dysfunction through targeting of renal fumarate hydrolase, which increases fumarate levels. These authors propose that fumarate is a key link connecting metabolic pathways to diabetic kidney disease pathogenesis, and measuring urinary fumarate levels may have application for monitoring renal NOX4 [54]. Thus, Nox4 may serve as viable redox-sensitive target for modulation of fibrosis and diabetic kidney disease [55,56].
The role of Nox4 in renal tubular cells, however, has been more controversial. Several studies have demonstrated that tubular toxins such as glycated albumin and the uremic toxins indoxyl sulfate and p-cresyl sulfate, trigger Nox-dependent fibrogenic pathways [57–61]. During the progressive loss of glomerular filtration, there is increasing inability of the kidney to remove uremic toxins from the bloodstream. Particular attention has been paid to protein bound uremic toxins in the prototype group of phenols and indoles. Both indoxyl sulfate and p-cresyl sulfate have been shown to accumulate in patients with progressive chronic kidney disease and have been linked with increasing cardiovascular mortality [62–65]. A recent study by Watanabe and colleagues [60] demonstrated that Nox4- and p22phox-based NADPH oxidase derived ROS is a significant mediator of p-cresyl sulfate-dependent human tubular cell damage and increased expression of inflammatory cytokines and profibrotic factors. The study also showed that accumulation of p-cresyl sulfate contributes to renal tubular injury and extracellular matrix deposition in the 5/6 nephrectomized rat model of CKD. However, studies by Babelova and collegues [66] and Nlandu Khodo and colleagues [67] demonstrated that global or cell-specific transgenic deletion of Nox4 in several experimental models of chronic kidney injury did not reduce fibrosis and in some cases resulted in increased tubular apoptosis and oxidative stress and suggested that Nox4 in certain instances of tubular injury was protective. Possible explanations to these conflicting results from the studies discussed above could be, at least in part, attributed to: (1) the importance of Nox4-dependent ROS in homeostasis and early response to injury; (2) different models of diabetes [33]; (3) the genetic manipulation used to generate the global or cell specific knockouts;and (4) another example of where genetic mouse models do not accurately represent the human condition. Therefore, use of pharmacologic inhibitors of Nox may be a preferred approach than genetic manipulation to study its utility in clinical studies.
3. Neutralizing the oxidant response
The ability to reduce or remove ROS and RNS by endogenous antioxidant enzymes is a key step in limiting tissue injury and modulating redox sensitive pathways. During both acute and chronic injury, many intracellular and extracellular antioxidant systems become depleted resulting in increased oxidative stress within the tissue. Several antioxidant defense systems have evolved to detoxify specific free radicals.
3.1. Protein thiols
A thiol is a compound that contains the functional group – SH (reduced) and can be oxidized to sulfenic acid resulting in disulfide formation (oxidized). Thiol groups react with almost all physiologic oxidants and serves as a key antioxidant buffer for both intracellular and tissue reduction/oxidation (redox) state [68]. Redox reactions of thiol proteins are thought to be a major mechanism by which reactive oxidants integrate into the cellular signal transduction pathways [69–73]. Thiol proteins are well suited as targets because cysteine residues are sensitive to oxidation, and changes in enzymatic activity or binding characteristics due to oxidation provide a mechanism for transmission of the signal. Reactive thiols (–SH) in proteins are subject to a wide array of modifications in oxidation state. Cysteine residues (HO2CCH(NH2)CH2–SH) in proteins and enzymes serve important roles in cell signaling, protein-protein interactions, substrate and metal binding, and catalysis. [5,68]. It has been proposed that approximately 0.5% of all cysteine residues in the proteome are subject to continuous and reversible oxidation–reduction cycles and that this redox cycling controls many aspects of structure and function in cells [74,75]. As our understanding of signaling networks has become more sophisticated, it has become evident that key regulators play essential roles in multiple outcomes that are context dependent. For example, a recent study by Numajiri and colleagues demonstrated that phosphatase with sequence homology to tensin (PTEN) is highly sensitive to relative low concentrations of NO and that its enzymatic activity is inhibited by the resulting S-nitrosylation (SNO) of Cys-83. The authors further demonstrated that at low (physiologic) concentrations of NO, SNO-PTEN is formed and enhanced Akt phosphorylation, whereas high (pathologic) concentrations of NO lead to SNO-Akt and inhibited its function [76].
The reversible oxidation of cysteine residues on cell signaling enzymes such as protein-tyrosine phosphatase illustrate how the glutathione/thioredoxin system modulates oxidant-mediated cell signaling pathways [77,78]. The three major biological thiol/disulfide couples include glutathione (GSH/GSSG), thioredoxin, and cysteine containing proteins (R-Cys/R-CysSS). The micromolar concentrations of thioredoxin compared to millimolar GSH concentrations could suggest that thioredoxin may be used for specificity of signaling or other highly critical functions while GSH is used more globally for detoxification and redox buffering [6]. Extracellular thiols also constitute an important component of antioxidant defense and when depleted could result in tipping the redox equilibrium toward increased levels of systemic oxidative stress. The plasma protein reduced thiols (located primarily on the albumin molecule) are depleted in patients with AKI and are thus not able to participate in antioxidant defense [79]. Furthermore, although protective reduced thiols are depleted in kidney injury, oxidized thiols that include homocysteine and cysteine accumulate and may have toxic effects on the endothelium [80]. Studies in our lab have demonstrated that small molecule thiols such as cysteamine bitartrate have significant antifibrotic properties. Administration of cysteamine bitartrate blocked fibrosis progression through reducing ROS production, limiting myofibroblast activation, and myofibroblast proliferation in vitro and in vivo and may serve as a novel adjunctive antifibrotic therapeutic agent [40].
3.2. Reduction of hydrogen peroxide
Peroxiredoxins, glutathione peroxidase, thioredoxin reductase, and catalase represent an important group of enzymes that reduce hydrogen peroxide. Thiol proteins likely represent an important partner in the ability of these enzymes to limit the oxidative damage of H2O2. For example, intracellular recycling of oxidized glutathione peroxidase by GSH and oxidized peroxiredoxin by thioredoxin enable these enzymes to inactivate greater amounts of H2O2. The thioredoxin system (thioredoxin, thioredoxin reductase, and peroxiredoxin) are ubiquitous and abundant proteins, with different family members distributed through the cytoplasm, mitochondria, and other cell compartments [81]. These thioredoxin proteins are highly abundant in the renal tubules [82], but it's role in CKD is not known. Catalase is also a key enzyme in antioxidant defense in the kidney during injury. Studies of progressive kidney disease in catalase-deficient mice demonstrated that loss of catalase buffering capacity leads to an increase in oxidative products and more severe renal fibrosis [47,83].
3.3. Redox regulation of mitochondrial ROS
After an ischemic or toxic insult, dysfunction of tubular epithelial cells plays a key role in the evolution of AKI. Central to tubular injury is mitochondrial dysregulation manifesting as a reduction in cell respiration and ATP production [84–86]. Mitochondrial function rests on a complex molecular machinery of finely tuned and balanced by regulatory proteins of two opposing forces fission and fusion [87,88]. In experimental AKI, fission predominates, resulting in mitochondrial fragmentation, outer membrane permeabilization, and release of apoptogenic factors which together with ROS overproduction determine tubular cell injury and apoptosis [85,89].
Homologs of the Saccharomyces cerevisiae, sirtuins, promote longevity in many organisms and are an evolutionarily conserved family comprising 7 proteins with NAD+-dependent deacetylase activity in mammals, 3 of which (SIRT3–SIRT5) are mainly localized in the mitochondrion [90]. SIRT3 is the major mitochondrial deacetylase that maintains basal ATP levels by direct physical association with complex I and the activation of FoF1ATPase, as well as ROS homeostasis through the regulation of detoxifying enzymes [90–93]. SIRT3 overexpression protects cells against Bax-mediated apoptosis, acting on NF-κB downstream target genes such as manganese SOD (MnSOD) and Bcl2, thus making cells resistant to H2O2–mediated cell injury [94]. Elevated expression levels of SIRT3 in the kidney reduces ROS and ameliorates mitochondria dynamics that translate into the longevity phenotype in mice deficient for angiotensin II type 1 receptor [95]. In cisplatin tubular injury models, increased oxidative stress and mitochondrial fragmentation are associated with reduced levels of SIRT3. Treatment with an AMPK agonist or the antioxidant agent acetyl-l-carnitine (ALCAR) restored SIRT3 levels, improved renal function, and decreased tubular injury in wild-type animals but had no effect on SIRT3-deficient mice. SIRT3-deficient mice had more severe AKI than wild-type animals and died. In proximal tubular cells, SIRT3 is critical in preserving mitochondrial integrity by preventing dynamin-related protein-dependent (DRP1-dependent) fission, loss of membrane potential, and PTEN-induced putative kinase 1-related (PINK1-related) mitophagy [96]. Therapeutic agents to augment SIRT3 to stabilize and preserve mitochondrial function hold great promise not only for AKI but for CKD as well.
4. Biomarkers of pathologic oxidant pathways
During disease progression, there are several key oxidant pathways that lead to the excessive generation of oxidative intermediates. Amino acids and lipids serve as natural targets of these pathways and can serve as biomarkers of organ injury in addition to serving as a ROS/RNS intermediate.
4.1. Myeloperoxidase
Myeloperoxidase (MPO) which is present in phagocytes can be used to generate several oxidative intermediates: H2O2 can be used by MPO to convert chloride ion to hypochlorous acid (HOCl) [97,98]; and oxidation of NO• with oxygen yields nitrite (NO2−) which MPO converts to nitrogen dioxide radical (NO2•), a potent nitrating intermediate [99,100]. Lipoproteins oxidized by MPO have been detected in human atherosclerotic lesions [101–104]. In clinical studies, MPO levels correlate with prospective mortality risk in subjects on maintenance hemodialysis [105], and MPO-oxidized LDL was noted to be elevated in the dialysis patients in a different study [106]. While traditionally associated with autoimmune vasculitic renal disease, more recent evidence point out that MPO-mediated oxidation is a key element in diabetic kidney disease and CKD progression [107–109]. It may also in part mediate increased cardiovascular morbidity in CKD patients [110,111].
4.2. Mitochondrial electron transport
Mitochondrial overproduction of O2•− and the subsequent dismutation to H2O2 in diabetic tissues can lead to oxidant injury. Both hyperglycemia and excess free fatty acids can induce mitochondrial dysfunction and lead to excess O2•− production [112]. Moreover, O2•− inhibits glyceraldehyde phosphate dehydrogenase, a key glycolytic enzyme whose inactivity could make upstream metabolites accumulate. Such inhibition of glycolysis might promote end-organ damage by diverting metabolites into the hexosamine pathway or stimulating the polyol and diacylglycerol-protein kinase C pathways. Exposing endothelial cells to exogenous oxidants leads to mitochondrial damage and can augment O2•− production, a mechanism whereby oxidative stress perpetuates oxidative stress in a pathologic feedforward pathway [113]. Benfotiamine, a lipid-soluble thiamine analog that inhibits these pathways by activating transketolase, an enzyme in the pentose pathway shunt, can prevent complications from experimental diabetes in animal models [114]. Recent evidence has cast some doubts on this hypothesis, but attribute Nox4 activity to diabetic kidney disease progression [115].
4.3. Uncoupled eNOS
NO synthesized by eNOS in endothelial cells and its uncoupling plays a major role in diabetes, hypertension, and CKD. Oxidation of its cofactor tetrahydrobiopterin (BH4) [116] uncouples eNOS which then transfers electrons to molecular oxygen, generating O2•−[117] and subsequent ONOO−. Peroxynitrite is a potent oxidant that converts tyrosine residues to 3-nitrotyrosine, which serves as a biomarker for the activation of the RNS. Indeed, elevated levels of nitrotyrosine have been identified in mouse models of diabetic kidney disease and neuropathy [118,119]. An alternative mechanism for uncoupling eNOS involves overproduction of angiotensin II, which can induce dihydrofolate reductase deficiency. Dihydrofolate reductase maintains BH4 in its reduced form, and therefore its deficiency uncouples eNOS. Administering BH4 improves endothelium-dependent vasodilation in experimental animals and humans with those conditions [120]. Studies of eNOS deficient mice reported from two separate groups show dramatic histopathology and decline in glomerular filtration rate in settings of diabetic hyperglycemia attesting to the importance of this pathway in diabetic nephropathy [121,122].
The uncoupled eNOS has also been suggested as a key mediator of both vascular and renal injury in Fabry's disease, a lysosomal storage disorder secondary to a deficiency in alpha-galactosidase A [123], and 3-nitrotyrosine, might be a novel biomarker of Fabry vasculopathy [124].
4.4. Glycoxidation
The glycoxidation pathway is particularly relevant in high-glucose states such as diabetes mellitus, a well-established cause of CKD. In its open-chain form, glucose has a carbonyl group that is a target of oxidative chemistry. Glucose auto-oxidization has been reported to mediate covalent linkage of glucose to amino groups on proteins by a hydroxyl radical-dependent mechanism [125]. Glucose also reacts nonenzymatically with proteins to form the reversible Schiff base adduct, which subsequently can rearrange itself into the stable Amadori product and advanced glycosylation end products (AGE). In vitro, free metal ions catalyze steps in a nonenzymatic glycoxidation pathway that generates AGE products. Metal-catalyzed hydroxyl radical formation can peroxidize lipids and convert phenylalanine residues of proteins into isomers of tyrosine such as ortho-tyrosine and meta-tyrosine [126–128]. Reduced, redox-active metal ions (Mn+) such as Fe2+ and Cu1+ generate hydroxyl radical (HO•) when they react with H2O2. AGEs can damage tissues through a number of mechanisms, including generation of oxidizing intermediates, formation of immune complexes, interaction with a cellular receptor called RAGE (Receptor for AGE), and induction of cytokine release [129,130]. Although RAGE binds to AGE-modified proteins in vitro with high affinity, its ligands in vivo are unclear. High levels of AGEs accumulate in renal failure, even in nondiabetic patients, and this process reverses after renal transplantation, implicating the kidneys in AGE production and/or clearance [131–134]. Many studies have shown that age-adjusted levels of pentosidine and Nε-carboxymethyllysine, two known AGE products, correlate with the development of diabetic complications [127,135–138].
4.5. Glucose-polyunsaturated fatty acids
Glucose can also generate reactive intermediates by interacting with polyunsaturated fatty acids (PUFA). When incubated with LDL or a control protein such as ribonuclease (RNAse), pathophysiologically relevant concentrations of glucose induce formation of oxidatively-modified amino acids in LDL even in the absence of free metal ions; in contrast, glucose exposure did not increase levels of oxidized amino acids in RNAse [139]. This study indicates that glucose is capable of generating a species resembling the hydroxyl radical by a carbonyl/PUFA pathway, a potential mechanism for localized oxidative stress in tissues vulnerable to diabetic damage [139].
4.6. Detection of oxidized biomolecules in vivo
ROS and RNS intermediates are difficult to detect in vivo because they are extremely short-lived due to their high reactivity with substrates; however, these oxidized substrates may serve as biomarkers for the activation of relevant oxidative pathways. Immunohistochemistry and dihydroethidium fluorescence have been extensively used to study oxidation-specific epitopes and oxidant production. These techniques are highly sensitive, and their ability to provide epitope-specific structural data can localize oxidative events to cell types or to subcellular locations. However, they are nonspecific as antibodies can bind to structurally similar compounds and, at best, only semiquantitative. Mass spectrometry (MS) offers a highly sensitive and specific approach to quantify oxidative biomarkers. When combined with isotope dilution, in which a labeled internal standard which is identical to the target analyte except for the heavy isotope is added to the mixture, accurate quantitation can be achieved.
4.7. Oxidized amino acid content correlates with degree of oxidative stress in vivo.
To understand the molecular mechanisms that promote oxidative stress in vivo, we first identified the patterns of oxidation products that are formed by well-characterized oxidant-generating model systems. The phenylalanine residues in proteins when subjected to glycoxidation or hydroxyl radical damage form ortho-tyrosine and meta-tyrosine. Oxidation reactions of tyrosine residues include cross-linking (to form oo′-dityrosine; mediated by tyrosine radicals, ROS and RNS), chlorination (to form 3-chlorotyrosine; catalyzed by myeloperoxidase) and nitration (to from 3-nitrotyrosine; mediated by RNS). Quantifying these unnatural amino acids characterizes the underlying oxidant pathway operative in target tissue. Using a combination of free radical generating systems in vitro and studying biospecimens from animal models of disease and humans, we and others defined patterns of these oxidative markers that accurately indicate pathways of oxidation that are activated [101,104,118,119,123,124,139–157]. Similar techniques can be used to accurately quantify oxidatively modified bioactive lipids [118,119,149,152,158,159].
5. Conclusions
Using a wide variety of different biomarkers of increased oxidative stress status, numerous laboratories around the world have now unequivocally demonstrated that CKD and ESKD is a state of increased oxidative stress due to an overproduction of ROS and a diminished antioxidant reserve. This leads to a dysregulation in redox signaling pathways that augment kidney injury and promote fibrogenesis. Disappointingly, traditional approaches with broad antioxidants to reduce systemic oxidant stress have not resulted in significant alterations in limiting or reversing disorders in which oxidative stress has been implicated by model system studies. Such observations highlight the importance of documenting that a proposed antioxidant intervention actually inhibits oxidative reactions in vivo [160–162]. The ability to accurately quantify amino acid and lipid oxidation markers in tissue samples, plasma, and urine can provide a means of monitoring the efficacy of therapeutic interventions, in addition to providing mechanistic insights into disease pathogenesis. Potential therapies interrupting only reactive pathways that are activated in target tissues are likely to be beneficial in modulating kidney injury and hold the promise of attenuating the relentless progression of CKD.
Acknowledgements
This work is supported in part by grants from the National Institutes of Health: HL094230 (SP); DK094292 (SP), DK089503 (SP), DK082841 (SP), DK081943 (SP), and DK097153 (SP): and from the Cystinosis Research Foundation (DO) (Date of award is from 9/1/13-8/31/15).
References
- 1.Thannickal V.J. Oxygen in the evolution of complex life and the price we pay. Am. J. Respir. Cell Mol. Biol. 2008:507–510. doi: 10.1165/rcmb.2008-0360PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawla L.S., Eggers P.W., Star R.A., Kimmel P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. New Engl. J. Med. 2014;371(1):58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCullough P.A., Li S., Jurkovitz C.T., Stevens L., Collins A.J., Chen S.C., Norris K.C., McFarlane S., Johnson B., Shlipak M.G. Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am. Heart J. 2008;156(2):277–283. doi: 10.1016/j.ahj.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Chawla L.S., Kimmel P.L. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82(5):516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 5.Forman H.J., Fukuto J.M., Torres M. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am. J. Physiol. Cell Physiol. 2004;287(2):C246–C256. doi: 10.1152/ajpcell.00516.2003. [DOI] [PubMed] [Google Scholar]
- 6.Kemp M., Go Y.M., Jones D.P. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic. Biol. Med. 2008;44(6):921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamura D.M., Himmelfarb J. Tipping the redox balance of oxidative stress in fibrogenic pathways in chronic kidney disease. Pediatr. Nephrol. 2009;24(12):2309–2319. doi: 10.1007/s00467-009-1199-5. [DOI] [PubMed] [Google Scholar]
- 8.Rhee S.G., Bae Y.S., Lee S.R., Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE 2000. 2000;53:PE1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 9.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4(3):181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 10.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 11.Segal B.H., Davidson B.A., Hutson A.D., Russo T.A., Holm B.A., Mullan B., Habitzruther M., Holland S.M., Knight P.R., 3rd Acid aspiration-induced lung inflammation and injury are exacerbated in NADPH oxidase-deficient mice. Am. J. Physiol. Lung.: Cell Mol. Physiol. 2007;292(3):L760–L768. doi: 10.1152/ajplung.00281.2006. [DOI] [PubMed] [Google Scholar]
- 12.Gao X.P., Standiford T.J., Rahman A., Newstead M., Holland S.M., Dinauer M.C., Liu Q.H., Malik A.B. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox-/- and gp91phox-/- mice. J. Immunol. 2002;168(8):3974–3982. doi: 10.4049/jimmunol.168.8.3974. [DOI] [PubMed] [Google Scholar]
- 13.Morgenstern D.E., Gifford M.A., Li L.L., Doerschuk C.M., Dinauer M.C. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J. Exp. Med. 1997;185(2):207–218. doi: 10.1084/jem.185.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kassim S.Y., Fu X., Liles W.C., Shapiro S.D., Parks W.C., Heinecke J.W. NADPH oxidase restrains the matrix metalloproteinase activity of macrophages. J. Biol. Chem. 2005;280(34):30201–30205. doi: 10.1074/jbc.M503292200. [DOI] [PubMed] [Google Scholar]
- 15.Snelgrove R.J., Edwards L., Williams A.E., Rae A.J., Hussell T. In the absence of reactive oxygen species, T cells default to a Th1 phenotype and mediate protection against pulmonary Cryptococcus neoformans infection. J. Immunol. 2006;177(8):5509–5516. doi: 10.4049/jimmunol.177.8.5509. [DOI] [PubMed] [Google Scholar]
- 16.Geiszt M., Leto T.L. The Nox family of NAD(P)H oxidases: host defense and beyond. J. Biol. Chem. 2004;279(50):51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- 17.Terada L.S. Specificity in reactive oxidant signaling: think globally, act locally. J. Cell Biol. 2006;174(5):615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy, Heart Outcomes Prevention Evaluation Study Investigators, Lancet, 2000; 355,9200, pp. 253–259. [PubMed]
- 19.Takac I., Schroder K., Zhang L., Lardy B., Anilkumar N., Lambeth J.D., Shah A.M., Morel F., Brandes R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011;286(15):13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block K., Gorin Y., Abboud H.E. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. USA. 2009;106(34):14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eid A.A., Lee D.Y., Roman L.J., Khazim K., Gorin Y. Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression. Mol. Cell Biol. 2013;33(17):3439–3460. doi: 10.1128/MCB.00217-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah A., Xia L., Goldberg H., Lee K.W., Quaggin S.E., Fantus I.G. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J. Biol. Chem. 2013;288(10):6835–6848. doi: 10.1074/jbc.M112.419101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Block K., Eid A., Griendling K.K., Lee D.Y., Wittrant Y., Gorin Y. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J. Biol. Chem. 2008;283(35):24061–24076. doi: 10.1074/jbc.M803964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ago T., Kuroda J., Pain J., Fu C., Li H., Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010;106(7):1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee D.Y., Wauquier F., Eid A.A., Roman L.J., Ghosh-Choudhury G., Khazim K., Block K., Gorin Y. Nox4 NADPH oxidase mediates peroxynitrite-dependent uncoupling of endothelial nitric-oxide synthase and fibronectin expression in response to angiotensin II: role of mitochondrial reactive oxygen species. J. Biol. Chem. 2013;288(40):28668–28686. doi: 10.1074/jbc.M113.470971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budanov A.V. Stress-responsive sestrins link p53 with redox regulation and mammalian target of rapamycin signaling. Antioxid. Redox Signal. 2011;15(6):1679–1690. doi: 10.1089/ars.2010.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budanov A.V., Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134(3):451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J.H., Budanov A.V., Talukdar S., Park E.J., Park H.L., Park H.W., Bandyopadhyay G., Li N., Aghajan M., Jang I. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metab. 2012;16(3):311–321. doi: 10.1016/j.cmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanli T., Linher-Melville K., Tsakiridis T., Singh G. Sestrin2 modulates AMPK subunit expression and its response to ionizing radiation in breast cancer cells. PLoS One. 2012;7(2):e32035. doi: 10.1371/journal.pone.0032035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashraf S., Gee H.Y., Woerner S., Xie L.X., Vega-Warner V., Lovric S., Fang H., Song X., Cattran D.C., Avila-Casado C. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J. Clin. Investig. 2013;123(12):5179–5189. doi: 10.1172/JCI69000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eid A.A., Ford B.M., Bhandary B., de Cassia Cavaglieri R., Block K., Barnes J.L., Gorin Y., Choudhury G.G., Abboud H.E. Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes. 2013;62(8):2935–2947. doi: 10.2337/db12-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eid A.A., Ford B.M., Block K., Kasinath B.S., Gorin Y., Ghosh-Choudhury G., Barnes J.L., Abboud H.E. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J. Biol. Chem. 2010;285(48):37503–37512. doi: 10.1074/jbc.M110.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jha J.C., Gray S.P., Barit D., Okabe J., El-Osta A., Namikoshi T., Thallas-Bonke V., Wingler K., Szyndralewiez C., Heitz F. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J. Am. Soc. Nephrol. 2014;25(6):1237–1254. doi: 10.1681/ASN.2013070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holterman C.E., Thibodeau J.F., Towaij C., Gutsol A., Montezano A.C., Parks R.J., Cooper M.E., Touyz R.M., Kennedy C.R. Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J. Am. Soc. Nephrol. 2014;25(4):784–797. doi: 10.1681/ASN.2013040371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H., Chen X., Su Y., Paueksakon P., Hu W., Zhang M.Z., Harris R.C., Blackwell T.S., Zent R., Pozzi A. p47(phox) contributes to albuminuria and kidney fibrosis in mice. Kidney Int. 2015;87(5):948–962. doi: 10.1038/ki.2014.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eid A.A., Gorin Y., Fagg B.M., Maalouf R., Barnes J.L., Block K., Abboud H.E. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58(5):1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piwkowska A., Rogacka D., Audzeyenka I., Jankowski M., Angielski S. High glucose concentration affects the oxidant-antioxidant balance in cultured mouse podocytes. J. Cell Biochem. 2011;112(6):1661–1672. doi: 10.1002/jcb.23088. [DOI] [PubMed] [Google Scholar]
- 38.Falke L.L., Gholizadeh S., Goldschmeding R., Kok R.J., Nguyen T.Q. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat. Rev. Nephrol. 2015;11(4):233–244. doi: 10.1038/nrneph.2014.246. [DOI] [PubMed] [Google Scholar]
- 39.Jain M., Rivera S., Monclus E.A., Synenki L., Zirk A., Eisenbart J., Feghali-Bostwick C., Mutlu G.M., Budinger G.R., Chandel N.S. Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J. Biol. Chem. 2013;288(2):770–777. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamura D.M., Bahrami N.M., Ren S., Pasichnyk K., Williams J.M., Gangoiti J.A., Lopez-Guisa J.M., Yamaguchi I., Barshop B.A., Duffield J.S. Cysteamine modulates oxidative stress and blocks myofibroblast activity in CKD. J. Am. Soc. Nephrol. 2014;25(1):43–54. doi: 10.1681/ASN.2012090962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.L. Alili, M. Sack, K. Puschmann, P. Brenneisen, Fibroblast-to-myofibroblast switch is mediated by NAD(P)H oxidase generated reactive oxygen species, Biosci. Rep, 2013. [DOI] [PMC free article] [PubMed]
- 42.Chen Y.T., Chang Y.T., Pan S.Y., Chou Y.H., Chang F.C., Yeh P.Y., Liu Y.H., Chiang W.C., Chen Y.M., Wu K.D. Lineage tracing reveals distinctive fates for mesothelial cells and submesothelial fibroblasts during peritoneal injury. J. Am. Soc. Nephrol. 2014;25(12):2847–2858. doi: 10.1681/ASN.2013101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manickam N., Patel M., Griendling K.K., Gorin Y., Barnes J.L. RhoA/Rho kinase mediates TGF-beta1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species. Am. J. Physiol. Renal. Physiol. 2014;307(2):F159–F171. doi: 10.1152/ajprenal.00546.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.A. Andersson-Sjoland, J.C. Karlsson, K. Rydell-Tormanen, ROS-induced endothelial stress contributes to pulmonary fibrosis through pericytes and Wnt signaling, Lab. Investig., 2015. [DOI] [PubMed]
- 45.Montorfano I., Becerra A., Cerro R., Echeverria C., Saez E., Morales M.G., Fernandez R., Cabello-Verrugio C., Simon F. Oxidative stress mediates the conversion of endothelial cells into myofibroblasts via a TGF-beta1 and TGF-beta2-dependent pathway. Lab. Investig. 2014;94(10):1068–1082. doi: 10.1038/labinvest.2014.100. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X., Liang D., Guo L., Liang W., Jiang Y., Li H., Zhao Y., Lu S., Chi Z.H. Curcumin protects renal tubular epithelial cells from high glucose-induced epithelial-to-mesenchymal transition through Nrf2-mediated upregulation of heme oxygenase-1. Mol. Med. Rep. 2015;12(1):1347–1355. doi: 10.3892/mmr.2015.3556. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi M., Sugiyama H., Wang D.H., Toda N., Maeshima Y., Yamasaki Y., Masuoka N., Yamada M., Kira S., Makino H. Catalase deficiency renders remnant kidneys more susceptible to oxidant tissue injury and renal fibrosis in mice. Kidney Int. 2005;68(3):1018–1031. doi: 10.1111/j.1523-1755.2005.00494.x. [DOI] [PubMed] [Google Scholar]
- 48.Barnes J.L., Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 2011;79(9):944–956. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bondi C.D., Manickam N., Lee D.Y., Block K., Gorin Y., Abboud H.E., Barnes J.L. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J. Am. Soc. Nephrol. 2010;21(1):93–102. doi: 10.1681/ASN.2009020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Attisano L., Wrana J.L. Signal transduction by the TGF-beta superfamily. Science. 2002;296(5573):1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 51.Schnaper H.W., Hayashida T., Poncelet A.C. It’s a Smad world: regulation of TGF-beta signaling in the kidney. J. Am. Soc. Nephrol. 2002;13(4):1126–1128. doi: 10.1681/ASN.V1341126. [DOI] [PubMed] [Google Scholar]
- 52.Cucoranu I., Clempus R., Dikalova A., Phelan P.J., Ariyan S., Dikalov S., Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005;97(9):900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 53.Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T.R., Horowitz J.C., Pennathur S., Martinez F.J., Thannickal V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15(9):1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.YouY.H., QuachT., SaitoR., PhamJ., and SharmaK.Metabolomics Reveals a Key Role for Fumarate in Mediating the Effects of NADPH Oxidase 4 in Diabetic Kidney Disease. Journal of the American Society of Nephrology: JASN.2015. [DOI] [PMC free article] [PubMed]
- 55.Sturrock A., Cahill B., Norman K., Huecksteadt T.P., Hill K., Sanders K., Karwande S.V., Stringham J.C., Bull D.A., Gleich M. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290(4):L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 56.Waghray M., Cui Z., Horowitz J.C., Subramanian I.M., Martinez F.J., Toews G.B., Thannickal V.J. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. Faseb J. 2005;19(7):854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 57.Qi W., Niu J., Qin Q., Qiao Z., Gu Y. Glycated albumin triggers fibrosis and apoptosis via an NADPH oxidase/Nox4-MAPK pathway-dependent mechanism in renal proximal tubular cells. Mol. Cell Endocrinol. 2015;405:74–83. doi: 10.1016/j.mce.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Gorin Y. Nox4 as a potential therapeutic target for treatment of uremic toxicity associated to chronic kidney disease. Kidney Int. 2013;83(4):541–543. doi: 10.1038/ki.2012.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimizu H., Saito S., Higashiyama Y., Nishijima F., CREB Niwa T. NF-kappaB, and NADPH oxidase coordinately upregulate indoxyl sulfate-induced angiotensinogen expression in proximal tubular cells. Am. J. Physiol. Cell Physiol. 2013;304(7):C685–C692. doi: 10.1152/ajpcell.00236.2012. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe H., Miyamoto Y., Honda D., Tanaka H., Wu Q., Endo M., Noguchi T., Kadowaki D., Ishima Y., Kotani S. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013;83(4):582–592. doi: 10.1038/ki.2012.448. [DOI] [PubMed] [Google Scholar]
- 61.Kim S.M., Kim Y.G., Jeong K.H., Lee S.H., Lee T.W., Ihm C.G., Moon J.Y. Angiotensin II-induced mitochondrial Nox4 is a major endogenous source of oxidative stress in kidney tubular cells. PLoS One. 2012;7(7):e39739. doi: 10.1371/journal.pone.0039739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin C.J., Wu V., Wu P.C., Wu C.J. Meta-analysis of the associations of p-cresyl sulfate (pcs) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One. 2015;10(7):e0132589. doi: 10.1371/journal.pone.0132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han H., Zhu J., Zhu Z., Ni J., Du R., Dai Y., Chen Y., Wu Z., Lu L., Zhang R. p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes. J. Am. Heart Assoc. 2015;4(6):e001852. doi: 10.1161/JAHA.115.001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barreto F.C., Barreto D.V., Liabeuf S., Meert N., Glorieux G., Temmar M., Choukroun G., Vanholder R., Massy Z.A. and European Uremic Toxin Work G. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J. Am. Soc. Nephrol. 2009;4(10):1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liabeuf S., Barreto D.V., Barreto F.C., Meert N., Glorieux G., Schepers E., Temmar M., Choukroun G., Vanholder R., Massy Z.A. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transplant. 2010;25(4):1183–1191. doi: 10.1093/ndt/gfp592. [DOI] [PubMed] [Google Scholar]
- 66.Babelova A., Avaniadi D., Jung O., Fork C., Beckmann J., Kosowski J., Weissmann N., Anilkumar N., Shah A.M., Schaefer L. Role of Nox4 in murine models of kidney disease. Free Radic. Biol. Med. 2012;53(4):842–853. doi: 10.1016/j.freeradbiomed.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 67.Nlandu Khodo S., Dizin E., Sossauer G., Szanto I., Martin P.Y., Feraille E., Krause K.H., de Seigneux S. NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J. Am. Soc. Nephrol. 2012;23(12):1967–1976. doi: 10.1681/ASN.2012040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghezzi P., Bonetto V., Fratelli M. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid. Redox Signal. 2005;7(7–8):964–972. doi: 10.1089/ars.2005.7.964. [DOI] [PubMed] [Google Scholar]
- 69.Hamdane D., Kiger L., Dewilde S., Green B.N., Pesce A., Uzan J., Burmester T., Hankeln T., Bolognesi M., Moens L. The redox state of the cell regulates the ligand binding affinity of human neuroglobin and cytoglobin. J. Biol. Chem. 2003;278(51):51713–51721. doi: 10.1074/jbc.M309396200. [DOI] [PubMed] [Google Scholar]
- 70.Jordan P.A., Gibbins J.M. Extracellular disulfide exchange and the regulation of cellular function. Antioxid. Redox Signal. 2006;8(3–4):312–324. doi: 10.1089/ars.2006.8.312. [DOI] [PubMed] [Google Scholar]
- 71.Kerblat I., Drouet C., Chesne S., Marche P.N. Importance of thioredoxin in the proteolysis of an immunoglobulin G as antigen by lysosomal Cys-proteases. Immunology. 1999;97(1):62–68. doi: 10.1046/j.1365-2567.1999.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reeves J.P., Bailey C.A., Hale C.C. Redox modification of sodium-calcium exchange activity in cardiac sarcolemmal vesicles. J. Biol. Chem. 1986;261(11):4948–4955. [PubMed] [Google Scholar]
- 73.Yang J., Chen H., Vlahov I.R., Cheng J.X., Low P.S. Evaluation of disulfide reduction during receptor-mediated endocytosis by using FRET imaging. Proc. Natl. Acad. Sci. USA. 2006;103(37):13872–13877. doi: 10.1073/pnas.0601455103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Go Y.M., Duong D.M., Peng J., Jones D.P. Protein Cysteines Map to Functional Networks According to Steady-state Level of Oxidation. J. Proteom. Bioinform. 2011;4(10):196–209. doi: 10.4172/jpb.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iyer S.S., Ramirez A.M., Ritzenthaler J.D., Torres-Gonzalez E., Roser-Page S., Mora A.L., Brigham K.L., Jones D.P., Roman J., Rojas M. Oxidation of extracellular cysteine/cystine redox state in bleomycin-induced lung fibrosis. Am. J. Physiol. : Lung. Cell Mol. Physiol. 2009;296(1):L37–L45. doi: 10.1152/ajplung.90401.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Numajiri N., Takasawa K., Nishiya T., Tanaka H., Ohno K., Hayakawa W., Asada M., Matsuda H., Azumi K., Kamata H. On-off system for PI3-kinase-Akt signaling through S-nitrosylation of phosphatase with sequence homology to tensin (PTEN) Proc. Natl. Acad. Sci. USA. 2011;108(25):10349–10354. doi: 10.1073/pnas.1103503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.den Hertog J., Groen A., van der Wijk T. Redox regulation of protein-tyrosine phosphatases. Arch. Biochem. Biophys. 2005;434(1):11–15. doi: 10.1016/j.abb.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 78.Groen A., Lemeer S., van der Wijk T., Overvoorde J., Heck A.J., Ostman A., Barford D., Slijper M., den Hertog J. Differential oxidation of protein-tyrosine phosphatases. J. Biol. Chem. 2005;280(11):10298–10304. doi: 10.1074/jbc.M412424200. [DOI] [PubMed] [Google Scholar]
- 79.Himmelfarb J., McMonagle E., Freedman S., Klenzak J., McMenamin E., Le P., Pupim L.B., Ikizler T.A., The P.G. Oxidative stress is increased in critically ill patients with acute renal failure. J. Am. Soc. Nephrol. 2004;15(9):2449–2456. doi: 10.1097/01.ASN.0000138232.68452.3B. [DOI] [PubMed] [Google Scholar]
- 80.Scholze A., Rinder C., Beige J., Riezler R., Zidek W., Tepel M. Acetylcysteine reduces plasma homocysteine concentration and improves pulse pressure and endothelial function in patients with end-stage renal failure. Circulation. 2004;109(3):369–374. doi: 10.1161/01.CIR.0000109492.65802.AD. [DOI] [PubMed] [Google Scholar]
- 81.Wood Z.A., Schroder E., Robin Harris J., Poole L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003;28(1):32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 82.Oberley T.D., Verwiebe E., Zhong W., Kang S.W., Rhee S.G. Localization of the thioredoxin system in normal rat kidney. Free Radic. Biol. Med. 2001;30(4):412–424. doi: 10.1016/s0891-5849(00)00486-x. [DOI] [PubMed] [Google Scholar]
- 83.Sunami R., Sugiyama H., Wang D.H., Kobayashi M., Maeshima Y., Yamasaki Y., Masuoka N., Ogawa N., Kira S., Makino H. Acatalasemia sensitizes renal tubular epithelial cells to apoptosis and exacerbates renal fibrosis after unilateral ureteral obstruction. Am. J. Physiol. Renal Physiol. 2004;286(6):F1030–F1038. doi: 10.1152/ajprenal.00266.2003. [DOI] [PubMed] [Google Scholar]
- 84.Bonventre J.V., Yang L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011;121(11):4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pabla N., Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 86.Zhan M., Brooks C., Liu F., Sun L., Dong Z. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int. 2013;83(4):568–581. doi: 10.1038/ki.2012.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Friedman J.R., Nunnari J. Mitochondrial form and function. Nature. 2014;505(7483):335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palmer C.S., Osellame L.D., Stojanovski D., Ryan M.T. The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell. Signal. 2011;23(10):1534–1545. doi: 10.1016/j.cellsig.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 89.Brooks C., Wei Q., Cho S.G., Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Investig. 2009;119(5):1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lombard D.B., Alt F.W., Cheng H.L., Bunkenborg J., Streeper R.S., Mostoslavsky R., Kim J., Yancopoulos G., Valenzuela D., Murphy A. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell Biol. 2007;27(24):8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahn B.H., Kim H.S., Song S., Lee I.H., Liu J., Vassilopoulos A., Deng C.X., Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA. 2008;105(38):14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kong X., Wang R., Xue Y., Liu X., Zhang H., Chen Y., Fang F., Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5(7):e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu Y.T., Lee H.C., Liao C.C., Wei Y.H. Regulation of mitochondrial F(o)F(1)ATPase activity by Sirt3-catalyzed deacetylation and its deficiency in human cells harboring 4977bp deletion of mitochondrial DNA. Biochim. Biophys. Acta. 2013;1832(1):216–227. doi: 10.1016/j.bbadis.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 94.Chen C.J., Fu Y.C., Yu W., Wang W. SIRT3 protects cardiomyocytes from oxidative stress-mediated cell death by activating NF-kappaB. Biochem. Biophys. Res. Commun. 2013;430(2):798–803. doi: 10.1016/j.bbrc.2012.11.066. [DOI] [PubMed] [Google Scholar]
- 95.Benigni A., Corna D., Zoja C., Sonzogni A., Latini R., Salio M., Conti S., Rottoli D., Longaretti L. Cassis P, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J. Clin. Invest. 2009;119(3):524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morigi M., Perico L., Rota C., Longaretti L., Conti S., Rottoli D., Novelli R., Remuzzi G., Benigni A. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Invest. 2015;125(2):715–726. doi: 10.1172/JCI77632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klebanoff S.J. Oxygen metabolism and the toxic properties of phagocytes. Ann. Intern. Med. 1980;93:480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 98.S.J. Klebanoff, R.A., Clark Amsterdam: North Holland Biochemical Press, 1978, pp. 447–451.
- 99.Eiserich J.P., Hristova M., Cross C.E., Jones A.D., Freeman B.A., Halliwell B., van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391(6665):393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 100.Gaut J.P., Byun J., Tran H.D., Lauber W.M., Carroll J.A., Hotchkiss R.S., Belaaouaj A., Heinecke J.W. Myeloperoxidase produces nitrating oxidants in vivo. J. Clin. Investig. 2002;109(10):1311–1319. doi: 10.1172/JCI15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bergt C., Pennathur S., Fu X., Byun J., O’Brien K., McDonald T.O., Singh P., Anantharamaiah G.M., Chait A., Brunzell J. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA. 2004;101(35):13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pennathur S., Bergt C., Shao B., Byun J., Kassim S.Y., Singh P., Green P.S., McDonald T.O., Brunzell J., Chait A. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J. Biol. Chem. 2004;279(41):42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 103.Hazen S.L., Heinecke J.W. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest. 1997;99(9):2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leeuwenburgh C., Hardy M.M., Hazen S.L., Wagner P., Oh-ishi S., Steinbrecher U.P., Heinecke J.W. Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J. Biol. Chem. 1997;272(3):1433–1436. doi: 10.1074/jbc.272.3.1433. [DOI] [PubMed] [Google Scholar]
- 105.Kalantar-Zadeh K., Brennan M.L., Hazen S.L. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2006;48(1):59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 106.Himmelfarb J., McMenamin M.E., Loseto G., Heinecke J.W. Myeloperoxidase-catalyzed 3-chlorotyrosine formation in dialysis patients. Free Radic. Biol. Med. 2001;31(10):1163–1169. doi: 10.1016/s0891-5849(01)00697-9. [DOI] [PubMed] [Google Scholar]
- 107.Malle E., Buch T., Grone H.J. Myeloperoxidase in kidney disease. Kidney Int. 2003;64(6):1956–1967. doi: 10.1046/j.1523-1755.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- 108.Malle E., Woenckhaus C., Waeg G., Esterbauer H., Grone E.F., Grone H.J. Immunological evidence for hypochlorite-modified proteins in human kidney. Am. J. Pathol. 1997;150(2):603–615. [PMC free article] [PubMed] [Google Scholar]
- 109.Porubsky S., Schmid H., Bonrouhi M., Kretzler M., Malle E., Nelson P.J., Grone H.J. Influence of native and hypochlorite-modified low-density lipoprotein on gene expression in human proximal tubular epithelium. Am. J. Pathol. 2004;164(6):2175–2187. doi: 10.1016/S0002-9440(10)63775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hazen S.L. Myeloperoxidase and plaque vulnerability. Arterioscler. Thromb. Vasc. Biol. 2004;24(7):1143–1146. doi: 10.1161/01.ATV.0000135267.82813.52. [DOI] [PubMed] [Google Scholar]
- 111.Wang Z., Nicholls S.J., Rodriguez E.R., Kummu O., Horkko S., Barnard J., Reynolds W.F., Topol E.J., DiDonato J.A., Hazen S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007;13(10):1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 112.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 113.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ. Res. 2005;96(8):818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 114.Hammes H.P., Du X., Edelstein D., Taguchi T., Matsumura T., Ju Q., Lin J., Bierhaus A., Nawroth P., Hannak D. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat. Med. 2003;9(3):294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 115.Dugan L.L., You Y.H., Ali S.S., Diamond-Stanic M., Miyamoto S., DeCleves A.E., Andreyev A., Quach T., Ly S., Shekhtman G. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J. Clin. Investig. 2013;123(11):4888–4899. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Landmesser U., Dikalov S., Price S.R., McCann L., Fukai T., Holland S.M., Mitch W.E., Harrison D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003;111(8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vasquez-Vivar J., Kalyanaraman B., Martasek P., Hogg N., Masters B.S., Karoui H., Tordo P., Pritchard K.A., Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl. Acad. Sci. USA. 1998;95(16):9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wiggin T.D., Kretzler M., Pennathur S., Sullivan K.A., Brosius F.C., Feldman E.L. Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology. 2008;149(10):4928–4937. doi: 10.1210/en.2008-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang H., Saha J., Byun J., Schin M., Lorenz M., Kennedy R.T., Kretzler M., Feldman E.L., Pennathur S., Brosius F.C., 3rd Rosiglitazone reduces renal and plasma markers of oxidative injury and reverses urinary metabolite abnormalities in the amelioration of diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2008;295(4):F1071–F1081. doi: 10.1152/ajprenal.90208.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guzik T.J., Harrison D.G. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov. Today. 2006;11(11–12):524–533. doi: 10.1016/j.drudis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 121.Nakagawa T., Sato W., Glushakova O., Heinig M., Clarke T., Campbell-Thompson M., Yuzawa Y., Atkinson M.A., Johnson R.J., Croker B. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J. Am. Soc. Nephrol. 2007;18(2):539–550. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- 122.Zhao H.J., Wang S., Cheng H., Zhang M.Z., Takahashi T., Fogo A.B., Breyer M.D., Harris R.C. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J. Am. Soc. Nephrol. 2006;17(10):2664–2669. doi: 10.1681/ASN.2006070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shu L., Park J.L., Byun J., Pennathur S., Kollmeyer J., Shayman J.A. Decreased nitric oxide bioavailability in a mouse model of Fabry disease. J. Am. Soc. Nephrol. 2009;20(9):1975–1985. doi: 10.1681/ASN.2008111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shu L., Vivekanandan-Giri A., Pennathur S., Smid B.E., Aerts J.M., Hollak C.E., Shayman J.A. Establishing 3-nitrotyrosine as a biomarker for the vasculopathy of Fabry disease. Kidney Int. 2014;86(1):58–66. doi: 10.1038/ki.2013.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hunt J.V., Dean R.T., Wolff S.P. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem. J. 1988;256(1):205–212. doi: 10.1042/bj2560205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huggins T.G., Wells-Knecht M.C., Detorie N.A., Baynes J.W., Thorpe S.R. Formation of o-tyrosine and dityrosine in proteins during radiolytic and metal-catalyzed oxidation. J. Biol. Chem. 1993;268(17):12341–12347. [PubMed] [Google Scholar]
- 127.Huggins T.G., Staton M.W., Dyer D.G., Detorie N.J., Walla M.D., Baynes J.W., Thorpe S.R. o-Tyrosine and dityrosine concentrations in oxidized proteins and lens proteins with age. Ann. NY Acad. Sci. 1992;663:436–437. doi: 10.1111/j.1749-6632.1992.tb38692.x. [DOI] [PubMed] [Google Scholar]
- 128.Kaur H., Halliwell B. Detection of hydroxyl radicals by aromatic hydroxylation. Methods Enzymol. 1994;233:67–82. doi: 10.1016/s0076-6879(94)33009-3. [DOI] [PubMed] [Google Scholar]
- 129.Stern D.M., Yan S.D., Yan S.F., Schmidt A.M. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res. Rev. 2002;1(1):1–15. doi: 10.1016/s0047-6374(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 130.Baynes J.W., Thorpe S.R. Glycoxidation and lipoxidation in atherogenesis. Free Radic. Biol. Med. 2000;28(12):1708–1716. doi: 10.1016/s0891-5849(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 131.Baynes J.W., Thorpe S.R. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48(1):1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 132.Miyata T., van Ypersele de Strihou C., Kurokawa K., Baynes J.W. Alterations in nonenzymatic biochemistry in uremia: origin and significance of “carbonyl stress” in long-term uremic complications. Kidney Int. 1999;55(2):389–399. doi: 10.1046/j.1523-1755.1999.00302.x. [DOI] [PubMed] [Google Scholar]
- 133.Miyata T., Ueda Y., Yoshida A., Sugiyama S., Iida Y., Jadoul M., Maeda K., Kurokawa K., van Ypersele de Strihou C. Clearance of pentosidine, an advanced glycation end product, by different modalities of renal replacement therapy. Kidney Int. 1997;51(3):880–887. doi: 10.1038/ki.1997.124. [DOI] [PubMed] [Google Scholar]
- 134.Miyata T., Fu M.X., Kurokawa K., van Ypersele de Strihou C., Thorpe S.R., Baynes J.W. Autoxidation products of both carbohydrates and lipids are increased in uremic plasma: is there oxidative stress in uremia? Kidney Int. 1998;54(4):1290–1295. doi: 10.1046/j.1523-1755.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- 135.Dyer D.G., Blackledge J.A., Thorpe S.R., Baynes J.W. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J. Biol. Chem. 1991;266(18):11654–11660. [PubMed] [Google Scholar]
- 136.McCance D.R., Dyer D.G., Dunn J.A., Bailie K.E., Thorpe S.R., Baynes J.W., Lyons T.J. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J. Clin. Investig. 1993;91(6):2470–2478. doi: 10.1172/JCI116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Monnier V.M. Transition metals redox: reviving an old plot for diabetic vascular disease. J. Clin. Investig. 2001;107(7):799–801. doi: 10.1172/JCI12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sell D.R., Lapolla A., Odetti P., Fogarty J., Monnier V.M. Pentosidine formation in skin correlates with severity of complications in individuals with long-standing IDDM. Diabetes. 1992;41(10):1286–1292. doi: 10.2337/diab.41.10.1286. [DOI] [PubMed] [Google Scholar]
- 139.Pennathur S., Ido Y., Heller J.I., Byun J., Danda R., Pergola P., Williamson J.R., Heinecke J.W. Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: a potential pathway for oxidative damage of retinal proteins in diabetes. J. Biol. Chem. 2005;280(24):22706–22714. doi: 10.1074/jbc.M500839200. [DOI] [PubMed] [Google Scholar]
- 140.Brennan M.L., Hazen S.L. Amino acid and protein oxidation in cardiovascular disease. Amino Acids. 2003;25(3–4):365–374. doi: 10.1007/s00726-003-0023-y. [DOI] [PubMed] [Google Scholar]
- 141.Hazen S.L., Crowley J.R., Mueller D.M., Heinecke J.W. Mass spectrometric quantification of 3-chlorotyrosine in human tissues with attomole sensitivity: a sensitive and specific marker for myeloperoxidase-catalyzed chlorination at sites of inflammation. Free Radic. Biol. Med. 1997;23(6):909–916. doi: 10.1016/s0891-5849(97)00084-1. [DOI] [PubMed] [Google Scholar]
- 142.Leeuwenburgh C., Rasmussen J.E., Hsu F.F., Mueller D.M., Pennathur S., Heinecke J.W. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J. Biol. Chem. 1997;272(6):3520–3526. doi: 10.1074/jbc.272.6.3520. [DOI] [PubMed] [Google Scholar]
- 143.Pennathur S., Jackson-Lewis V., Przedborski S., Heinecke J.W. Mass spectrometric quantification of 3-nitrotyrosine, ortho-tyrosine, and o,o’-dityrosine in brain tissue of 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson’s disease. J. Biol. Chem. 1999;274(49):34621–34628. doi: 10.1074/jbc.274.49.34621. [DOI] [PubMed] [Google Scholar]
- 144.Pennathur S., Wagner J.D., Leeuwenburgh C., Litwak K.N., Heinecke J.W. A hydroxyl radical-like species oxidizes cynomolgus monkey artery wall proteins in early diabetic vascular disease. J. Clin. Investig. 2001;107(7):853–860. doi: 10.1172/JCI11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shishehbor M.H., Brennan M.L., Aviles R.J., Fu X., Penn M.S., Sprecher D.L., Hazen S.L. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108(4):426–431. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- 146.Zheng L., Nukuna B., Brennan M.L., Sun M., Goormastic M., Settle M., Schmitt D., Fu X., Thomson L., Fox P.L. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Investig. 2004;114(4):529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Back S.H., Scheuner D., Han J., Song B., Ribick M., Wang J., Gildersleeve R.D., Pennathur S., Kaufman R.J. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10(1):13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Malhotra J.D., Miao H., Zhang K., Wolfson A., Pennathur S., Pipe S.W., Kaufman R.J. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl. Acad. Sci. USA. 2008;105(47):18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Okamura D.M., Pennathur S., Pasichnyk K., Lopez-Guisa J.M., Collins S., Febbraio M., Heinecke J., Eddy A.A. CD36 regulates oxidative stress and inflammation in hypercholesterolemic CKD. J. Am. Soc. Nephrol. 2009;20(3):495–505. doi: 10.1681/ASN.2008010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pop-Busui R., Oral E., Raffel D., Byun J., Bajirovic V., Vivekanandan-Giri A., Kellogg A., Pennathur S., Stevens M.J. Impact of rosiglitazone and glyburide on nitrosative stress and myocardial blood flow regulation in type 2 diabetes mellitus. Metabolism. 2009;58(7):989–994. doi: 10.1016/j.metabol.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 151.Song B., Scheuner D., Ron D., Pennathur S., Kaufman R.J. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Investig. 2008;118(10):3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vincent A.M., Hayes J.M., McLean L.L., Vivekanandan-Giri A., Pennathur S., Feldman E.L. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58(10):2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Veiga-Lopez A., Pennathur S., Kannan K., Patisaul H.B., Dolinoy D.C., Zeng L., Padmanabhan V. Impact of gestational bisphenol a on oxidative stress and free fatty acids: human association and interspecies animal testing studies. Endocrinology. 2015;156(3):911–922. doi: 10.1210/en.2014-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith C.K., Vivekanandan-Giri A., Tang C., Knight J.S., Mathew A., Padilla R.L., Gillespie B.W., Carmona-Rivera C., Liu X., Subramanian V. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66(9):2532–2544. doi: 10.1002/art.38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vivekanandan-Giri A., Slocum J.L., Byun J., Tang C., Sands R.L., Gillespie B.W., Heinecke J.W., Saran R., Kaplan M.J., Pennathur S. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Ann. Rheum. Dis. 2013;72(10):1725–1731. doi: 10.1136/annrheumdis-2012-202033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vivekanandan-Giri A., Byun J., Pennathur S. Quantitative analysis of amino Acid oxidation markers by tandem mass spectrometry. Methods Enzymol. 2011;491:73–89. doi: 10.1016/B978-0-12-385928-0.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sparks M.A., Parsons K.K., Stegbauer J., Gurley S.B., Vivekanandan-Giri A., Fortner C.N., Snouwaert J., Raasch E.W., Griffiths R.C., Haystead T.A. Angiotensin II type 1A receptors in vascular smooth muscle cells do not influence aortic remodeling in hypertension. Hypertension. 2011;57(3):577–585. doi: 10.1161/HYPERTENSIONAHA.110.165274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meagher E.A., Barry O.P., Lawson J.A., Rokach J., FitzGerald G.A. Effects of vitamin E on lipid peroxidation in healthy persons. J. Am. Med. Assoc. 2001;285(9):1178–1182. doi: 10.1001/jama.285.9.1178. [DOI] [PubMed] [Google Scholar]
- 159.Pennathur S., Pasichnyk K., Bahrami N.M., Zeng L., Febbraio M., Yamaguchi I., Okamura D.M. The macrophage phagocytic receptor cd36 promotes fibrogenic pathways on removal of apoptotic cells during chronic kidney injury. Am. J. Pathol. 2015;185(8):2232–2245. doi: 10.1016/j.ajpath.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Heinecke J.W. Is the emperor wearing clothes? Clinical trials of vitamin E and the LDL oxidation hypothesis. Arterioscler. Thromb. Vasc. Biol. 2001;21(8):1261–1264. doi: 10.1161/hq0801.095084. [DOI] [PubMed] [Google Scholar]
- 161.Heinecke J.W. Oxidative stress: new approaches to diagnosis and prognosis in atherosclerosis. Am. J. Cardiol. 2003;91(3A):12A–16AA. doi: 10.1016/s0002-9149(02)03145-4. [DOI] [PubMed] [Google Scholar]
- 162.Mashima R., Witting P.K., Stocker R. Oxidants and antioxidants in atherosclerosis. Curr. Opin. Lipidol. 2001;12(4):411–418. doi: 10.1097/00041433-200108000-00007. [DOI] [PubMed] [Google Scholar]