Abstract
One of the key features of tumor cells is the acquisition of resistance to apoptosis. Thus, novel therapeutic strategies that circumvent apoptotic resistance and result in tumor elimination are needed. One strategy to induce apoptosis is to activate death receptor signaling pathways. In the tumor microenvironment, stimulation of Fas, Death receptor 4 (DR4) and tumor necrosis factor receptor 1 (TNFR1) can initiate multiple signaling pathways driving either tumor promotion or elimination. Nitric oxide (NO) is an important signaling molecule now understood to play a dual role in cancer biology. More and more attention is directed toward the role displayed by S-nitrosylation, the incorporation of an NO moiety to a cysteine thiol group, in promoting cell death in tumor cells. Protein post-translation modification by S-nitrosylation has decisive roles in regulating signal-transduction pathways. In this review, we summarize several examples of protein modification by S-nitrosylation that regulate signaling pathways engaged by members of the TNF superfamily (Fas ligand (FasL), Tumor-necrosis-factor-related apoptosis inducing ligand (TRAIL) and TNFalpha (TNFα)) and the way it influences cell fate decisions.
Keywords: Cancer, S-nitrosylation, Signaling, Death receptors, TNF cytokine
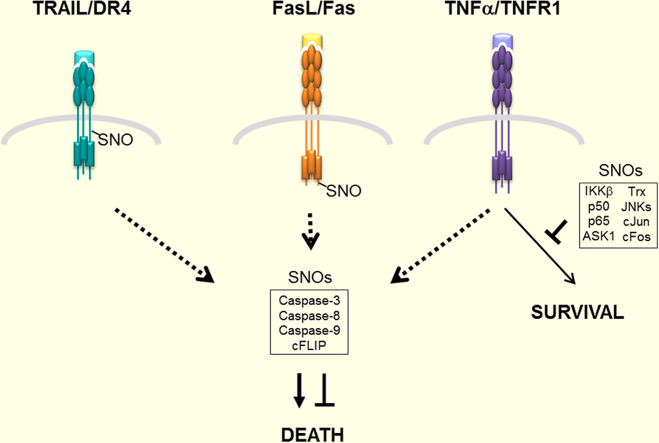
Graphical abstract
Highlights
-
•
An overview of NO in regulating signaling pathways engaged by FasL, TRAIL and TNFα.
-
•
S-nitrosylation regulates protein activity and cancer cell death.
-
•
Exploiting NO for cancer therapy.
1. Introduction
Signaling through members of tumor necrosis factor (TNF) superfamily ligands and their receptors is required for normal physiological responses including differentiation, survival, proliferation and cell death.
It has been well established over the last three decades that TNF superfamily signaling is a double-edged sword in cancer driving either tumor promotion or elimination. TNF superfamily is one of the largest family of cytokines until now including 19 different ligands and nearly 30 receptors [1]. TNF superfamily cytokines such as Fas ligand (FasL), TNF-related apoptosis inducing ligand (TRAIL) and TNFalpha (TNFα) can originate from various types of cells, immune and non-immune cells, within the tumor microenvironment. It is generally assumed that protumoral and antitumoral signaling coexist in the tumor microenvironment. The abundance of innate and adaptive immune cells in the tumor microenvironment and the expression of members of the TNF superfamily cytokines contribute to dictate whether tumor cells are more likely to proliferate or die.
Cell fate decisions leading to either protumoral or antitumoral effects are linked to transcriptional gene expression changes and/or protein post-translational modifications. Post-translational modification (PTM) is a key feature that mediates signal transduction. The modification of proteins by PTM allows the regulation of protein properties and function. TNF superfamily signal-transduction pathways rely on multiple PTM, phosphorylation and ubiquitination being among the best documented. A large number of studies have provided evidence that PTM by S-nitrosylation modulates signal transduction upon certain stimuli just like phosphorylation.
S-nitrosylation is a non-enzymatic process by which nitric oxide (NO) modulates the function of various target proteins. It consists on the covalent incorporation of an NO moiety to a reactive thiol group in specific cysteine residues. The S-nitrosylation of proteins is relatively unstable and can be reversed by denitrosylation reaction through now well established enzymatic systems such as thioredoxins (Trxs) and S-nitrosoglutathione reductase (GSNOR) [14,36]. The property of the thiol group of the cysteine also makes it a privileged site for a variety of other PTM such as S-palmitoylation, S-glutathionylation, and S-sulfhydration [17]. So a mutually competitive but also cooperative relationship between these PTM and S-nitrosylation can exist depending on the redox environment. A link between S-nitrosylation and S-glutathionylation (for review see [41,43] seems to be established supporting the notion that NO and S-nitrosylated proteins can be involved in S-glutathionylation formation. In line with this, NO has been reported to mediate c-Jun S-glutathionylation and inhibition [29].
More particularly, S-nitrosylation can affect protein structure and function in many different ways by modulating enzymatic activities, protein–protein interaction, protein subcellular location or even the level of expression of proteins in cells [15,32,46]. Thereby, S-nitrosylation has emerged as critical physiological regulators of a wide range of intracellular signaling pathways. Here we review how S-nitrosylation regulates some of the TNF-superfamily signaling pathways. We focus on the signaling pathways engaged by FasL, TRAIL and TNFα through their cognate receptors (Fas, Death receptor 4 (DR4) and tumor necrosis factor receptor 1 (TNFR1)) and the manner in which S-nitrosylation mediates cancer cell death along signaling cascades.
2. TNF-superfamily signaling pathways and apoptosis: role of DR4 and Fas
Two main signaling pathways leading to the apoptotic machinery are now well established: the intrinsic and extrinsic pathways. After FasL and TRAIL binding to their cognate receptor and receptor trimerization, the trimer recruits the adaptor protein Fas-Associated protein with Death Domain (FADD), then the initiator caspase-8 and/or -10 resulting in the formation of death-inducing signaling complex (DISC) (Fig. 1). Activation of caspase-8 and/or -10 can trigger subsequent activation of downstream caspase-3 that ultimately leads to cell death via the extrinsic pathway [66]. A cross-talk exists between extrinsic and intrinsic pathway which requires initiator caspase-dependent cleavage of the pro-apoptotic arm of the B-cell lymphoma-2 (Bcl-2) gene superfamily permitting the release of cytochrome c and ATP from mitochondria promoting the formation of the apoptosome, resulting in additional caspase activation that collectively leads to cell apoptosis. Upon ligand binding, most of the death-receptors aggregate at the plasma membrane and more particularly in raft nanodomains enriched in cholesterol and glycosphingolipids [11,16,19]. Some of these receptors may undergo a variety of post-translational modifications including S-palmitoylation and S-nitrosylation.
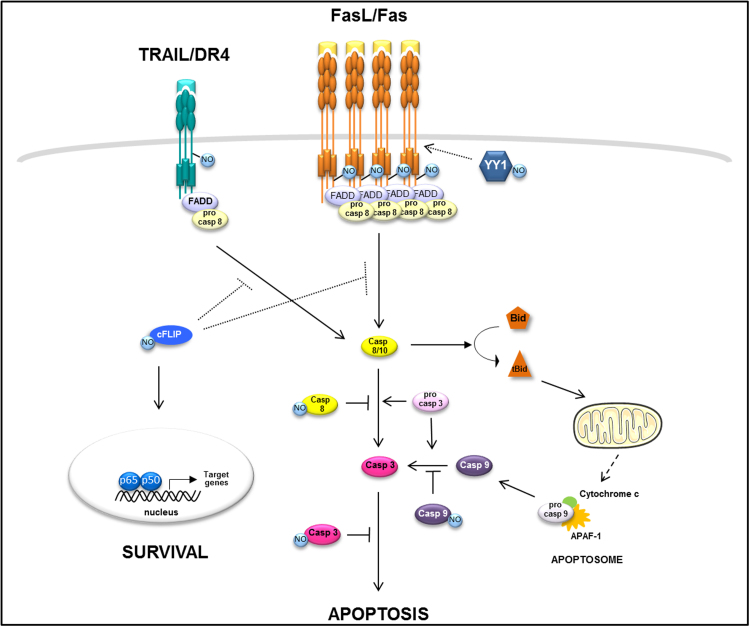
Fig. 1.
S-nitrosylation in TRAIL/DR4 and FaL/Fas signaling pathways. NO affects several proteins in death receptor-mediated apoptotic pathways. Among them, the TRAIL-receptor DR4 and Fas have been shown to be S-nitrosylated in their cytoplasmic domain thereby increasing their activity to trigger apoptosis of target cells. The Ying Yang 1, inhibitor of Fas, is also shown to be S-nitrosylated upon NO donnor treatment. This S-nitrosylation inhibits its activity toward Fas and permits dowstream apoptotic signal transduction. NO inhibits caspase 3 or 9. More recently, the S-nitrosylation of cFLIP, an inhibitor of Fas and DR4 signaling pathway has been shown to inhibit its degradation by the proteasome, thereby making it a more potent inhibitor of Fas and DR4 signaling. S-nitrosylated cFLIP will bind to RIP1 and subsequently activate the NF-KB signaling pathway leading to cell proliferation and survival.
2.1. S-nitrosylation of TRAIL receptor DR4
DR4 is considered as a tumor-suppressor protein. Thus, the increase in its expression and/or its aggregation is a major challenge in cancer research. Over the two last decades, nitric oxide has emerged as a potent antiproliferative and pro-apoptotic molecule against several cancer cell lines involving TRAIL signaling through DR4 for example. NO donor nitrosylcobalamin (NO-Cbl) (an analog of vitamin B12 that delivers NO) has demonstrated a potent antiproliferative activity in several human cell lines from different origins (melanoma, renal, and ovarian carcinomas) [61]. This effect is triggered through the ability of NO-Cbl to S-nitrosylate DR4 (but not Death receptor 5 (DR5)). Cysteine residue 336 (C336) was characterized to be targeted by NO, and cells expressing a C336A mutant form of DR4, which lacked S-nitrosylation, were resistant to NO-Cbl-mediated cell growth inhibition. Of note, DR4 did not exhibit the linear consensus motif for the S-nitrosylation of proteins [58]; however, it may exist in the three-dimensional structure of DR4 but not of DR5 that could explain the discrepancy between the two receptors. Interestingly, S-nitrosylation of DR4 has not been observed with other NO donors such as S-nitroso-N-acetylpenicillamine (SNAP) and 2, 2′-(hydroxynitrosohydrazono)bis-ethanamine (NOC-18) [61]. This discrepancy seems to be related to absorption characteristics and kinetics of NO release of different donors, as well as the microenvironment and concentration of reducing agents. S-nitrosylation is not the only PTM of DR4, it could be also S-palmitoylated, a process that targets the receptor to raft nanodomains and confers efficient TRAIL-induced cell death [54].
2.2. S-nitrosylation of Fas
We have shown that the NO donor glyceryl trinitrate or GTN sensitizes human colorectal cancer cells to Fas agonist-inducing apoptosis [45]. Bonavida and collaborators have shown that S-nitrosylation of Ying Yang 1 (YY1), a repressive transcription factor of Fas receptor, inhibits the function of this factor that results in the increase of Fas level of expression and cancer cell sensitization to Fas agonists [18]. Our team has shown that GTN S-nitrosylated Fas in colorectal cancer cells on both cysteines 199 and 304, and the pro-apoptotic functional effect of S-nitrosylation on Fas was only demonstrated for cysteine 304. We also demonstrated that S-nitrosylation of Fas targets the receptor to raft nanodomains and promotes its aggregation without affecting its plasma membrane expression [31]. Of note, cysteine residues can be a site for other post-translational modifications. S-palmitoylation of Fas on both cysteines 199 and 194 (by a palmitoyl acyltransferase) has also been reported as an essential PTM for the redistribution of this receptor into lipid rafts, a mandatory step for the death signal transmission that increases Fas expression and thereby circumvents its degradation by lysosomal proteolysis [5,55].
In conclusion, the PTM of DR4 and Fas through either S-nitrosylation or S-palmitoylation renders cancer cells more sensitive to receptor-induced cell death (Fig. 1). Although the functional relevance of the S-nitrosylation of several proteins of the TNF-superfamily signaling pathways have been assessed in response to nitrosative stress, the antitumoral properties of NO are not restricted to PTM by S-nitrosylation. An increase in intracellular levels of NO following NO donor administration or nitric oxide synthase 3 (NOS3) overexpression has been shown to increase Fas, cFLIP-L expression levels and cell death sensitization in a number of human hepatoma cell lines and mouse models. A similar enhanced expression of DR4 and TNFR1 was also observed [13].
3. S-nitrosylation of proteins involved in death receptor signaling pathways
3.1. S-nitrosylation of caspases
During apoptosis, caspase zymogens are either cleaved by auto-proteolysis or cleaved by other caspases to form active tetrameric enzymes. All caspases have a catalytic site cysteine, critical for enzymatic activity, that could be S-nitrosylated. S-nitrosylation and denitrosylation of cysteine thiol group in the active site of caspase represents a potent mechanism for NO regulation of apoptosis. S-nitrosylation of the catalytic site cysteine of caspase-1 and -3 in human endothelial cells inhibits their activity leading to TNFα-mediated cell death [10]. Furthermore, high concentrations of NO inhibit, in vitro, 7 recombinant caspases (caspases-1, 2, 3, 4, 6, 7, and 8) [33]. However, NO-mediated S-nitrosylation of cellular endogenous caspases is not confirmed for all of these enzymes. NO can prevent apoptosis in non-cancerous hepatocyte through S-nitrosylation of caspase-8 and caspase-3 [27,28]. In addition, caspase-3 was found to be S-nitrosylated in unstimulated lymphocyte cell lines. Upon Fas stimulation then caspase-3 undergoes denitrosylation and subsequent activation [37]. In addition, S-nitrosylation and inhibition of caspase 9 has been demonstrated in cholangiocarcinoma cells [63].
We have shown that NO can inhibit and activate caspases. Indeed, the NO donor glyceryl trinitrate activated caspase-1 and -10 and inhibited, at the same time, caspase-3. Together, these events lead to colorectal cell apoptosis [45]. Our results suggest that NO can target some caspases but not all. Some may be sensitive and some others more resistant to S-nitrosylation or also subjected to rapid changes of S-nitrosylation/denitrosylation processes along the signaling cascade. Along these lines, X-linked inhibitor of apoptosis protein (XIAP), a member of the Inhibitor of APoptotis (IAP) protein family which is a powerful inhibitor of caspases, has been shown to undergo S-nitrosylation but via a trans-S-nitrosylation process catalyzed by caspase 3. The authors have demonstrated, by means of in vitro experiments, that an NO moiety was transferred from S-nitrosylated caspase 3 to XIAP on cysteine residue 450 but not vice-versa [47]. S-nitrosylation of XIAP has been detected in the brain of patient suffering from neurodegenerative disorders [47,62]. It compromises its anti-apoptotic function and then contributes to the degenerative process. More recently, S-nitrosylation of XIAP cysteine 213 was found to impair the anti-apoptotic function of XIAP (anti-caspase 3) [67]. XIAP overexpression has been associated with tumorigenesis and survival of many malignant cells. However, S-nitrosylation of XIAP has never been reported in cancer cells and NO-based cancer treatment represents an interesting approach.
3.2. S-nitrosylation of cellular FLICE-inhibitory protein (cFLIP)
Death receptor signaling can also be modulated by various proteins such as the cellular FLICE-inhibitory protein (cFLIP). At least two variants of this protein are expressed, the short cFLIP (cFLIP-S) and the long cFLIP (cFLIP-L). This protein has been identified as an inhibitor of apoptosis triggered by engagement of death receptors such as Fas or DR4. However, when overexpressed, cFLIP-L, but not cFLIP-S has been reported to be pro-apoptotic [12]. cFLIP-L could associate with caspase-8 and exhibit a proteolytic activity which could account for the pro-apoptotic activity of cFLIP-L under non-physiologic conditions [44]. cFLIP can undergo S-nitrosylation at cysteine residues 254 and 259 preventing its degradation via the ubiquitin proteasome pathway [6]. Recently, the same group showed that S-nitrosylation of cFLIP mediates the receptor-interacting serine/threonine protein kinase 1 (RIPK1) binding to induce Nuclear Factor-kappa B (NF-kB) activation and confers resistance to apoptosis, since double mutation of cysteine 254 and 259 increased apoptosis when mammary cancer cells were stimulated by either TNFα or FasL [60].
4. TNF-superfamily signaling pathways and growth control: role of TNFR1
The binding of TNFα to TNFR1, the major signaling receptor for TNFα, triggers cellular responses as diverse as proliferation, differentiation and survival predominantly which suppress apoptosis. Signals transmitted through TNFR1 can trigger concomitantly the classical NF-kB signaling (Fig. 2) and certain Mitogen-Activated Protein Kinase (MAPK) pathways, such as c-Jun NH2-terminal kinase (JNK) signaling (Fig. 3), that ultimately activate the transcriptions factors NF-kB and Activator Protein-1 (AP-1) respectively [4]. Through abnormal activation of NF-kB and AP-1, TNFα contribute to tumor development [2,70].
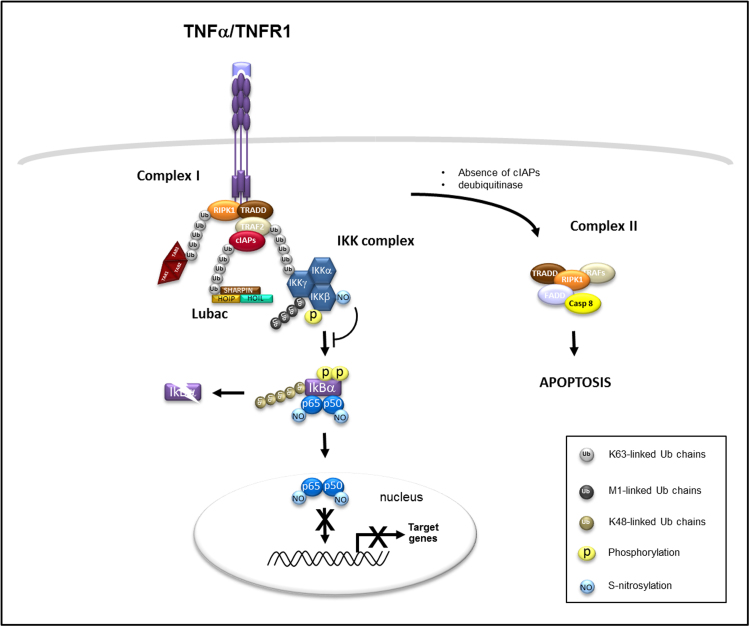
Fig. 2.
S-nitrosylation in classical TNF-NF-kB signaling. When TNFα binds its receptor TNF receptor 1 (TNFR1), it stimulates the formation of complex I that results in the recruitment of TNFR-associated via death domain (TRADD), receptor-interacting serine/threonine protein kinase 1 (RIPK1), TNFR-associated factor 2 (TRAF2), cellular inhibitor of apoptosis cIAPs (cIAP1 and cIAP2). The cIAPs, via their E3 ubiquitin ligases activities, promote the ubiquitination (through K63-linked ubiquitin chains) of RIPK1, TRAF2, and themselves which subsequently allows the recruitment of other complexes. Linear ubiquitin chain assembly complex (LUBAC) associates with ubiquitin linkages attached to cIAPs, transforming growth factor-β activated kinase (TAK1)/TAK1-binding 2 (TAB2)/TAK1 binding 3 (TAB3) associates with ubiquitin linkages attached to RIPK1 and inhibitor of kappaB alpha (IkBα) kinase (IKK) complex associates with ubiquitin linkages attached to TRAF2. LUBAC ubiquitylates (through M1-linked ubiquitin chains) IKKγ and stably recruits the IKK complex. The IKK complex (IKKβ) is then phosphorylated by TAK1/TAB2/TAB3. IKKβ in turn phosphorylates IkBα that leads to the addition of K48-linked ubiquitin chains and its proteasome-mediated degradation with the subsequent activation and nuclear translocation of the NF-kB transcription factor heterodimer. S-nitrosylation of IKKβ subunit prevents the phosphorylation of IkBα and its degradation. S-nitrosylation of either p50–p65 NF-kB subunits inhibits DNA binding and transcriptional upregulation of NF-kB target genes. In the absence of cIAPs or in presence of CYLD, which allows the removal of K63-linked ubiqtuitin chains, RIPK1 translocate to the cytoplasm to induce the formation and activation of complex II that leads to apoptosis.
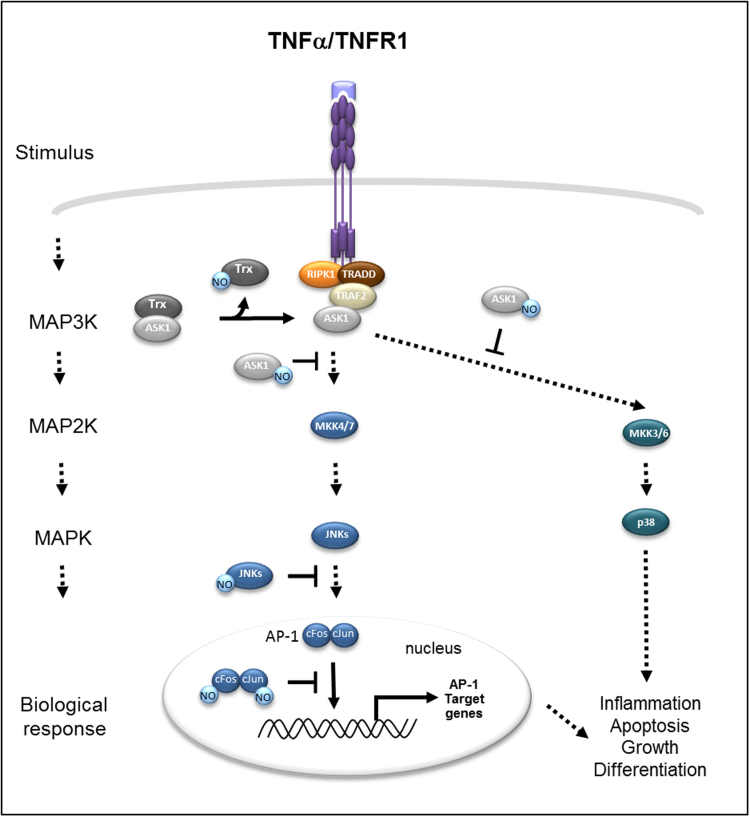
Fig. 3.
S-nitrosylation in TNF-JNK-AP-1 signaling. Upon TNFα binding, TNFR1 activates JNK-AP-1 signaling through the association of TRADD, RIPK1 and TRAFs (especially TRAF2) to the cytoplasmic death domain of TNFR1. TRAF2 recruits ASK1 (when released from Trx) an upstream MAP3K that can activate both JNKs (via the activation of MKK4 and MKK7) and p38 MAPKs (via the activation of MKK3 and MKK6). The activation of JNKs leads to the activation of the transcription factor AP-1. S-nitrosylation can stimulate (disruption of ASK1-Trx interaction) or prevent (inhibition of ASK1 and JNKs kinase activities as well as AP-1 DNA-binding ability) JNK-AP-1 signaling.
Dysregulation of the NF-kB pathway is found in many types of tumor. Constitutive activation of NF-kB in tumor cells can be either the result of oncogenic mutations in the signaling molecules or exposure to proinflammatory stimuli such as TNFα or other TNF superfamily members in the tumor microenvironment. The transcription factor NF-kB is a mediator of immune response. NF-kB overactivity contributes to tumorigenesis by promoting the expression of various genes that are involved in survival, proliferation, invasion and metastasis of tumor cells [2]. Furthermore, activation of NF-kB can prevent tumor cell death to contribute to promote tumorigenesis and confer acquired resistance following chemotherapy. Current molecular therapy strategies such as selective inhibitors of the catalytic subunit of Inhibitor of NF-kB (IkB) kinase (IKK), consist in the inhibition of this pathway [68]. Therapies targeted toward NF-kB signaling components represent an attractive approach for cancer treatment.
Two major protein complexes (complexes I and II) with different outcomes are initiated upon TNFR1 stimulation (Fig. 2). On one hand, TNFR1 recruits adaptor proteins such as TNF receptor-1-associated death domain protein (TRADD) which functions as a platform adaptator for other proteins including TNF-receptor-associated factor (TRAF2), cellular Inhibitor of apoptosis 1 (cIAP1), cIAP2 and RIPK1 to initiate the formation of the complex I. In complex I, RIPK1 modification with non-degradative Lys-63 polyubiquitin chains is critical for the activation of the NF-kB pathway. Additional molecular complexes including IKK, transforming growth factor beta-activated kinase 1 (TAK1)/TAK1 binding protein 2 (TAB2)/ TAK1 binding protein 3 (TAB3) and Linear ubiquitin chain-assembly complex (LUBAC) are then recruited to complex I while bound to the plasma membrane [9]. The complex I is a driver of NF-kB activation pathway that leads to angiogenesis, inflammation and cell survival responses. This process is initiated by the IKK complex that triggers the phosphorylation of Inhibitor of NF-kB alpha (IkBα), the NF-kB repressor, its subsequent ubiquitination and degradation by the ubiquitin–proteasome system and allows the transcription factor NF-kB, held in the cytoplasm, to translocate to the nucleus to promote gene transcription of targeted genes [21,22].
On the other hand, in the absence of proper complex I formation, a second molecular complex (complex II), is formed in the cytoplasm and initiates signals leading to cell death. Under conditions where RIPK1 is deubiquitinated (by deubiquitinase cylindromatosis protein (CYLD) or in the absence of cIAP1 and cIAP2), RIPK1 then interacts with receptor-interacting serine/threonine protein 3 (RIP3) into the complex II along with TRADD, Fas-associated protein with death domain (FADD) and procaspase-8 [9,30]. Basically, the complex II can trigger either apoptosis or necroptosis. Under certain biological contexts, when caspase-8 is deleted or inhibited (by p35 or a pan-caspase inhibitor zVAD.fmk), a RIP1-RIP3 dependent complex II-like called necroptosome is formed and leads to the activation of necroptosis. In such context, it remains to determine whether the putative S-nitrosylation of caspase-8 could contribute to induce necroptosis. But in the presence of caspase-8, RIP1 and RIP3 are constitutively cleaved thus sensitizing the cells to apoptosis [9].
4.1. S-nitrosylation in NF-kB signaling engaged by TNFα/TNFR1
NF-kB signaling pathway is sensitive to nitrosative stress and is strongly correlated with NF-kB inhibition and apoptosis [39]. NF-kB activation is tightly regulated by a variety of post-translational modifications, including phosphorylation, ubiquitylation and S-nitrosylation. S-nitrosylation is now considered as an important mechanism of inhibition of NF-kB signal transduction [38]. Several studies have demonstrated that NO exerts controls over NF-kB at multiple levels within the signaling pathway through S-nitrosylation of essential components.
S-nitrosylation of DR4 and Fas has been shown to sensitized tumor cells to their cognate ligands; however, no such modification on TNFR1 receptor by S-nitrosylation has been reported so far.
The IKK complex is a central regulator of the classical NF-kB pathway activation which is composed of three subunits IKKα, IKKβ and NF-kB essential modifier (NEMO or IKKγ) [20,23]. One major function of IKKβ kinase activity is the phosphorylation and subsequent inactivation of IkBα in response to many stimuli. Under physiological conditions, as demonstrated in lung epithelial cells and Jurkat cells, the IKKβ kinase activity is constitutively repressed by S-nitrosylation at position Cysteine 179. Following TNFα stimulation, IKKβ is denitrosylated at Cysteine 179 and allows further downstream signaling [52].
Other in vitro studies demonstrated that inhibition of NF-kB through S-nitrosylation occurred further downstream in the activation pathway at the nuclear level [38]. Within cells, NF-kB is a dimeric transcription factor composed among five different types of monomers (p50, p52, p65, c-Rel and RelB) with p50–p65 being the primary mediator of NF-kB. Epithelial lung cancer cells either treated with the NO donor S-nitrosocysteine or NO derived from NOS2 following cytokine stimulation, had a great ability to inhibit the heterodimeric NF-kB p50–p65 DNA-binding through S-nitrosylation of p50 at position Cysteine 62 [8,38]. Similarly, NF-kB p65 monomer has been found as a target of S-nitrosylation, following nitric oxide synthase 2 (NOS2) activity, at position Cysteine 38 [24]. In unstimulated lung cells, NF-kB p65 inactivation has been reported to result in constitutive S-nitrosylation. However, following stimuli exposure (such as Lipopolysaccharide (LPS)) NF-kB p65 is denitrosylated along with NF-kB activation [25]. Thioredoxin, a SNO-protein denitrosylase, is responsible for TNFα -mediated NF-kB p65 denitrosylation [26]. Both NF-kB subunits p50 and p65 are S-nitrosylated at a cysteine in the DNA-binding region of the Rel homology domain (RHD) conserved in all NF-kB proteins [24,38]. These observations suggest a common mechanism by which S-nitrosylation negatively regulates the DNA-binding activity of NF-kB and subsequent gene transcription. Endogenous NO production and NF-kB signaling pathways are reciprocally regulated. Inflammation increases the abundance of NOS2 gene transcription which is a NF-kB target gene and the other way round, NO generated by NOS2 induces S-nitrosylation of specific components of NF-kB signaling [38].
Under normal physiological conditions, NF-kB activation prevents the pro-apoptotic effects of TNFα. The NF-kB pathway represents a relevant therapeutic target in cancer for drug development to inhibit tumor growth or even promote tumor regression. Promoting IKKβ, p50 or p65 S-nitrosylation and thereby counteracting cell growth for the benefit of tumor cell death appears as a general mechanism of action for a class of NO-donating non-steroidal anti-inflammatory drugs (NSAIDs). The anticancer effect of some NO-NSAIDS including NO-aspirin (NO-ASA) and NO-naproxen in colon cancer is in part mediated by S-nitrosylation and subsequent inhibition of NF-kB p65 subunit [7,65].
The components of NF-kB signaling targeted by S-nitrosylation in cells appear to be cell type and nitrosative stress dependent.
4.2. S-nitrosylation in MAPK signaling engaged by TNFα/TNFR1
The MAPK signaling pathways are the result of stimulation by a variety of growth factors, pathogens, cellular stresses and also pro-inflammatory cytokines. MAPK signaling can be divided into four independent pathways promoted through extracellular signal-regulated kinases (ERK) 1 and 2 (ERK1/2), the Big MAP kinase-1 (BMK-1), p38 MAPK and JNK that regulate a broad range of cellular decisions including proliferation, differentiation, apoptosis and stress response. JNK activation broadly results in apoptosis induction or promotion of cellular survival, and the choice of cell fate decision appears to depend on the cell type and on the stimulating signal [56]. Activation of JNK-AP-1 signaling depends on various members of the MAPK family which are serine/threonine kinases. Activation of the JNK pathway is among the most important signaling cascade induced in response of TNFα/TNFR1 stimulation. Accumulating evidence suggest that JNK-AP-1 signaling may play a role in carcinogenesis. The TNFR1/MAPK kinase 7 (MMK7)/JNK/AP-1 signaling cascade has been shown to promote epidermal neoplasia [70]. The JNK pathway activation requires a phosphorylation signaling cascade in which MAP3Ks activates MAP2Ks that in turn activates the terminal MAPK JNK. As shown by many groups, S-nitrosylation mediated by NO from exogenous sources or endogenous NOS2 expression can affect the JNK pathway at several levels, from the upstream molecules to the transcription factor AP-1 subunits (Fig. 3). Apoptosis signal-cell fate decision results in balance control regulating kinase 1 (ASK1) functions as a MAP3K protein in both JNK and p38 MAPK signaling cascades [35]. It has been shown that endogenous NO production in murine fibrosarcoma L929 cells inhibits the kinase activity of ASK1 through a S-nitrosylation mechanism, targeting Cysteine 869 of the catalytic domain. S-nitrosylation of ASK1 also disrupts the interaction between ASK1 and downstream MAP2Ks substrates (MKK3 or MKK6) notably involved in the activation of the p38 MAPK pathway [51]. In contradiction with this result, in a model of brain ischemia-reperfusion, ASK1 S-nitrosylation mediated by endogenous NO produced by NOS1 would facilitate ASK1 activity to exert deleterious effects [34]. Hence, further studies are needed to clearly determine how NO, from endogenous sources, affects ASK1 activity in different pathological conditions. Nevertheless, another report seems to point toward an explanation. In basal conditions, thioredoxin (trx) acts to inhibit ASK1 activation and subsequent enzyme activity through a direct interaction [59]. Further studies have demonstrated that the amount of NO compared to the superoxide producing system would govern the S-nitrosylation of the complex ASK1/trx (i.e. subsequent disruption and inactivation of ASK1) [69].
JNK is a subfamily of MAPK protein composed of three isoforms (JNK1, JNK2 and JNK3) with splice variants. Upon activation by upstream kinases, JNK proteins mediate phosphorylation and activation of transcription factors such as AP-1. Like NF-kB, AP-1 functions as dimeric complexes to trigger the expression of a wide variety of genes. AP-1 is composed of Jun (c-Jun, Jun B, Jun D) and Fos (c-Fos, Fos B, Fra 1, Fra 2) family members. JNK1 and JNK2 are ubiquitously expressed in tissues while JNK3 is only expressed in brain, heart and testes. Opposing effects have been reported as regard to JNK1 and JNK2 which could act as either pro-tumoral or anti-tumoral kinases. All 3 JNK proteins are S-nitrosylated upon NO generation via interferon gamma (IFNγ)-induced NOS2 or SNAP NO-donor in murine microglial cells thus suppressing their interaction and kinase activity toward downstream substrate like c-Jun [49,50,57]. In addition, NO can negatively regulate AP-1 DNA binding. The DNA binding activity of c-Jun and c-Fos, major components of AP-1, has been shown to be dependent on the redox status of c-Jun cysteine 272 and c-Fos cysteine 154 [48]. Although the S-nitrosylation of these cysteine residues more likely explains how NO affects AP-1 function, the S-nitrosylation of c-Jun and c-Fos has not been clearly evidenced yet.
5. Concluding remarks
The classical homeostasis control system depends on a fine balance between cell death and survival signaling. Endogeneous S-nitrosylation of proteins is an important physiological process in the maintenance of cellular homeostasis. Particularly, it contributes to keep off TNF-superfamily survival pathways in the absence of ligand. Similarly, nitrosative stress-mediated by exogeneous NO donors could negatively regulate these pathways.
Protein S-nitrosylation in cancer cells is receiving increasing attention as a mechanism to regulate proteins involved in apoptosis and cell growth control. Redox-based modifications of cystein thiols by S-nitrosylation have emerged as a major signaling mechanism. S-nitrosylation appears to exert mainly a negative regulatory effect over protein activities and function which could have a significant impact, either positive or negative, on cell death. Nevertheless, several critical factors influence S-nitrosylation of target proteins. Although it is a non-enzymatic process, S-nitrosylation formation in a cell is not left to chance but is tightly dependent on NO source conditions, i.e. subcellular location (e.g. NOS proximity), concentration, time of exposure in cell, and reactivity of the cysteine residue (for review see [40,42].
Stimulation of death receptors such as Fas, DR4 and TNFR1 can initiate diverse cellular responses including apoptosis. TNF-superfamily cytokines including FasL, TRAIL and TNFα from the tumor microenvironment contribute to both tumor progression and antitumor response. A growing number of studies suggest that protein S-nitrosylation is a key mode by which NO regulates the function of target proteins involved in FasL/Fas, TRAIL/DR4 and TNFα/TNFR1 signaling pathways which can sensitize cancer cells to death. For many years, a better understanding of the regulation of these signaling pathways has resulted in intense drug development. The antitumoral efficacy of recombinant FasL, TRAIL and TNFα ligands or derivatives, has been evaluated in preclinical and clinical studies as monotherapy. The development of TNFα as a systemic treatment has not been translated to patient due to significant hepatotoxicity and lack of efficacy [53]. Similar acute toxicity toward normal cells has been reported for recombinant FasL. However, a more promising recombinant molecule (APO010) has been under investigation in a phase I clinical trial (NCT00437736). However, a great step forward in the treatment of irresectable extremity soft tissue sarcomas has been achieved in the recent years with the addition of TNFα to isolated limb perfusion with chemotherapy (melphalan) [64]. Despite potent antitumor activity of TRAIL and safety toward normal tissues, recombinant TRAIL or anti-DR4 agonistic monoclonal antibodies evaluated in clinic as single agents are currently no effective therapies.
The design of combinatorial treatment of TNF-superfamily based therapies with chemotherapies represents a more promising approach. Novel therapy involving a combination of TRAIL-based therapeutic with NO donors will be of great interest in cancer treatment. Inhibition of TNFα-induced NF-kB signaling by pharmacological agents represents also a powerful strategy to enhance the efficacy of cancer chemotherapy. Several selective IKK inhibitors and other compounds have been reported yet. Similarly, S-nitrosylation of IKK subunits represents another tool to interfere with the NF-kB pathway. Many important functional properties of key signaling proteins are similarly affected by targeted or conventional chemotherapies and NO-mediated S-nitrosylation [3]. Exploiting the redox state and bioactivity of NO, especially through S-nitrosylation, for cancer therapy seems to be a promising strategy. Nevertheless, further studies are required to determine the safety and effectiveness of NO donors or S-nitrosylating agents.
Acknowledgments
The authors aknowledge the financial support from “La Ligue Contre le Cancer”, “Fondation ARC pour la Recherche sur le Cancer” and “Région Bourgogne”.
Footnotes
This article belongs to a special issue on Nitric Oxide and Cancer, edited by Jordi Muntané and Benjamin Bonavida.
References
- 1.Aggarwal B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 3.Bettaieb A., Plenchette Sp, Paul C., Laurens Vr, Romagny S., Jeannin J.-Fo. S-nitrosylation in cancer cells: to prevent or to cause? In: B.B., editor. Nitric Oxide and Cancer: Pathogenesis and Therapy. Springer; 2015. pp. 97–105. [Google Scholar]
- 4.Cabal-Hierro L., Lazo P.S. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24:1297–1305. doi: 10.1016/j.cellsig.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabandhu K., Hérincs Z., Huault S., Dost B., Peng L., Conchonaud F., Marguet D., He H.T., Hueber A.O. Palmitoylation is required for efficient Fas cell death signaling. EMBO J. 2007;26:209–220. doi: 10.1038/sj.emboj.7601456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanvorachote P., Nimmannit U., Wang L., Stehlik C., Lu B., Azad N., Rojanasakul Y. Nitric oxide negatively regulates Fas CD95-induced apoptosis through inhibition of ubiquitin–proteasome-mediated degradation of FLICE inhibitory protein. J. Biol. Chem. 2005;280:42044–42050. doi: 10.1074/jbc.M510080200. [DOI] [PubMed] [Google Scholar]
- 7.Chattopadhyay M., Goswami S., Rodes D.B., Kodela R., Velazquez C.A., Boring D., Crowell J.A., Kashfi K. NO-releasing NSAIDs suppress NF-κB signaling in vitro and in vivo through S-nitrosylation. Cancer Lett. 2010;298:204–211. doi: 10.1016/j.canlet.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Colasanti M., Persichini T. Nitric oxide: an inhibitor of NF-kappaB/Rel system in glial cells. Brain Res. Bull. 2000;52:155–161. doi: 10.1016/s0361-9230(00)00262-8. [DOI] [PubMed] [Google Scholar]
- 9.Dickens L.S., Powley I.R., Hughes M.A., MacFarlane M. The ‘complexities’ of life and death: death receptor signalling platforms. Exp. Cell Res. 2012;318:1269–1277. doi: 10.1016/j.yexcr.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Dimmeler S., Haendeler J., Nehls M., Zeiher A.M. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J. Exp. Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajate C., Del Canto-Jañez E., Acuña A.U., Amat-Guerri F., Geijo E., Santos-Beneit A.M., Veldman R.J., Mollinedo F. Intracellular triggering of Fas aggregation and recruitment of apoptotic molecules into Fas-enriched rafts in selective tumor cell apoptosis. J. Exp. Med. 2004;200:353–365. doi: 10.1084/jem.20040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goltsev Y.V., Kovalenko A.V., Arnold E., Varfolomeev E.E., Brodianskii V.M., Wallach D. CASH, a novel caspase homologue with death effector domains. J. Biol. Chem. 1997;272:19641–19644. doi: 10.1074/jbc.272.32.19641. [DOI] [PubMed] [Google Scholar]
- 13.González R., Ferrín G., Aguilar-Melero P., Ranchal I., Linares C.I., Bello R.I., De la Mata M., Gogvadze V., Bárcena J.A., Alamo J.M., Orrenius S., Padillo F.J., Zhivotovsky B., Muntané J. Targeting hepatoma using nitric oxide donor strategies. Antioxid. Redox Signal. 2013;18:491–506. doi: 10.1089/ars.2011.4476. [DOI] [PubMed] [Google Scholar]
- 14.Haendeler J., Hoffmann J., Tischler V., Berk B.C., Zeiher A.M., Dimmeler S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat. Cell Biol. 2002;4:743–749. doi: 10.1038/ncb851. [DOI] [PubMed] [Google Scholar]
- 15.Hara M.R., Agrawal N., Kim S.F., Cascio M.B., Fujimuro M., Ozeki Y., Takahashi M., Cheah J.H., Tankou S.K., Hester L.D., Ferris C.D., Hayward S.D., Snyder S.H., Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 16.Henkler F., Behrle E., Dennehy K.M., Wicovsky A., Peters N., Warnke C., Pfizenmaier K., Wajant H. The extracellular domains of FasL and Fas are sufficient for the formation of supramolecular FasL–Fas clusters of high stability. J. Cell Biol. 2005;168:1087–1098. doi: 10.1083/jcb.200501048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess D.T., Stamler J.S. Regulation by S-nitrosylation of protein post-translational modification. J. Biol. Chem. 2012;287:4411–4418. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hongo F., Garban H., Huerta-Yepez S., Vega M., Jazirehi A.R., Mizutani Y., Miki T., Bonavida B. Inhibition of the transcription factor Yin Yang 1 activity by S-nitrosation. Biochem. Biophys. Res. Commun. 2005;336:692–701. doi: 10.1016/j.bbrc.2005.08.150. [DOI] [PubMed] [Google Scholar]
- 19.Hueber A.O. Role of membrane microdomain rafts in TNFR-mediated signal transduction. Cell Death Differ. 2003;10:7–9. doi: 10.1038/sj.cdd.4401155. [DOI] [PubMed] [Google Scholar]
- 20.Israël A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010;2:a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanarek N., London N., Schueler-Furman O., Ben-Neriah Y. Ubiquitination and degradation of the inhibitors of NF-kappaB. Cold Spring Harb. Perspect. Biol. 2010;2:a000166. doi: 10.1101/cshperspect.a000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 23.Karin M., Yamamoto Y., Wang Q.M. The IKK NF-kappa B system: a treasure trove for drug development. Nat. Rev. Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 24.Kelleher Z.T., Matsumoto A., Stamler J.S., Marshall H.E. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J. Biol. Chem. 2007;282:30667–30672. doi: 10.1074/jbc.M705929200. [DOI] [PubMed] [Google Scholar]
- 25.Kelleher Z.T., Potts E.N., Brahmajothi M.V., Foster M.W., Auten R.L., Foster W.M., Marshall H.E. NOS2 regulation of LPS-induced airway inflammation via S-nitrosylation of NF-{kappa}B p65. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;301:L327–L333. doi: 10.1152/ajplung.00463.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelleher Z.T., Sha Y., Foster M.W., Foster W.M., Forrester M.T., Marshall H.E. Thioredoxin-mediated denitrosylation regulates cytokine-induced nuclear factor κB (NF-κB) activation. J. Biol. Chem. 2014;289:3066–3072. doi: 10.1074/jbc.M113.503938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y.M., Kim T.H., Chung H.T., Talanian R.V., Yin X.M., Billiar T.R. Nitric oxide prevents tumor necrosis factor alpha-induced rat hepatocyte apoptosis by the interruption of mitochondrial apoptotic signaling through S-nitrosylation of caspase-8. Hepatology. 2000;32:770–778. doi: 10.1053/jhep.2000.18291. [DOI] [PubMed] [Google Scholar]
- 28.Kim Y.M., Talanian R.V., Billiar T.R. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J. Biol. Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 29.Klatt P., Molina E.P., Lamas S. Nitric oxide inhibits c-Jun DNA binding by specifically targeted S-glutathionylation. J. Biol. Chem. 1999;274:15857–15864. doi: 10.1074/jbc.274.22.15857. [DOI] [PubMed] [Google Scholar]
- 30.Laukens B., Jennewein C., Schenk B., Vanlangenakker N., Schier A., Cristofanon S., Zobel K., Deshayes K., Vucic D., Jeremias I., Bertrand M.J., Vandenabeele P., Fulda S. Smac mimetic bypasses apoptosis resistance in FADD- or caspase-8-deficient cells by priming for tumor necrosis factor α-induced necroptosis. Neoplasia. 2011;13:971–979. doi: 10.1593/neo.11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leon-Bollotte L., Subramaniam S., Cauvard O., Plenchette-Colas S., Paul C., Godard C., Martinez-Ruiz A., Legembre P., Jeannin J.F., Bettaieb A. S-nitrosylation of the death receptor fas promotes fas ligand-mediated apoptosis in cancer cells. Gastroenterology. 2011;140:2009–2018. doi: 10.1053/j.gastro.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 32.Li F., Sonveaux P., Rabbani Z.N., Liu S., Yan B., Huang Q., Vujaskovic Z., Dewhirst M.W., Li C.Y. Regulation of HIF-1alpha stability through S-nitrosylation. Mol. Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J., Billiar T.R., Talanian R.V., Kim Y.M. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem. Biophys. Res. Commun. 1997;240:419–424. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- 34.Liu D.H., Yuan F.G., Hu S.Q., Diao F., Wu Y.P., Zong Y.Y., Song T., Li C., Zhang G.Y. Endogenous nitric oxide induces activation of apoptosis signal-regulating kinase 1 via S-nitrosylation in rat hippocampus during cerebral ischemia-reperfusion. Neuroscience. 2013;229:36–48. doi: 10.1016/j.neuroscience.2012.10.055. [DOI] [PubMed] [Google Scholar]
- 35.Liu H., Nishitoh H., Ichijo H., Kyriakis J.M. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L., Yan Y., Zeng M., Zhang J., Hanes M.A., Ahearn G., McMahon T.J., Dickfeld T., Marshall H.E., Que L.G., Stamler J.S. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 37.Mannick J.B., Hausladen A., Liu L., Hess D.T., Zeng M., Miao Q.X., Kane L.S., Gow A.J., Stamler J.S. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 38.Marshall H.E., Stamler J.S. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40:1688–1693. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 39.Marshall H.E., Stamler J.S. Nitrosative stress-induced apoptosis through inhibition of NF-kappa B. J. Biol. Chem. 2002;277:34223–34228. doi: 10.1074/jbc.M201638200. [DOI] [PubMed] [Google Scholar]
- 40.Martínez-Ruiz A., Araújo I.M., Izquierdo-Álvarez A., Hernansanz-Agustín P., Lamas S., Serrador J.M. Specificity in S-nitrosylation: a short-range mechanism for NO signaling? Antioxid. Redox Signal. 2013;19:1220–1235. doi: 10.1089/ars.2012.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martínez-Ruiz A., Cadenas S., Lamas S. Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free Radic. Biol. Med. 2011;51:17–29. doi: 10.1016/j.freeradbiomed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Martínez-Ruiz A., Lamas S. S-nitrosylation: a potential new paradigm in signal transduction. Cardiovasc. Res. 2004;62:43–52. doi: 10.1016/j.cardiores.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Martínez-Ruiz A., Lamas S. Signalling by NO-induced protein S-nitrosylation and S-glutathionylation: convergences and divergences. Cardiovasc. Res. 2007;75:220–228. doi: 10.1016/j.cardiores.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Micheau O., Thome M., Schneider P., Holler N., Tschopp J., Nicholson D.W., Briand C., Grütter M.G. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J. Biol. Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 45.Millet A., Bettaieb A., Renaud F., Prevotat L., Hammann A., Solary E., Mignotte B., Jeannin J.F. Influence of the nitric oxide donor glyceryl trinitrate on apoptotic pathways in human colon cancer cells. Gastroenterology. 2002;123:235–246. doi: 10.1053/gast.2002.34310. [DOI] [PubMed] [Google Scholar]
- 46.Mocellin S., Bronte V., Nitti D. Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med. Res. Rev. 2007;27:317–352. doi: 10.1002/med.20092. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura T., Wang L., Wong C.C., Scott F.L., Eckelman B.P., Han X., Tzitzilonis C., Meng F., Gu Z., Holland E.A., Clemente A.T., Okamoto S., Salvesen G.S., Riek R., Yates J.R., Lipton S.A. Transnitrosylation of XIAP regulates caspase-dependent neuronal cell death. Mol. Cell. 2010;39:184–195. doi: 10.1016/j.molcel.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikitovic D., Holmgren A., Spyrou G. Inhibition of AP-1 DNA binding by nitric oxide involving conserved cysteine residues in Jun and Fos. Biochem. Biophys. Res. Commun. 1998;242:109–112. doi: 10.1006/bbrc.1997.7930. [DOI] [PubMed] [Google Scholar]
- 49.Park H.S., Huh S.H., Kim M.S., Lee S.H., Choi E.J. Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc. Natl. Acad. Sci. U.S.A. 2000;97:14382–14387. doi: 10.1073/pnas.97.26.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park H.S., Mo J.S., Choi E.J. Nitric oxide inhibits an interaction between JNK1 and c-Jun through nitrosylation. Biochem. Biophys. Res. Commun. 2006;351:281–286. doi: 10.1016/j.bbrc.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 51.Park H.S., Yu J.W., Cho J.H., Kim M.S., Huh S.H., Ryoo K., Choi E.J. Inhibition of apoptosis signal-regulating kinase 1 by nitric oxide through a thiol redox mechanism. J. Biol. Chem. 2004;279:7584–7590. doi: 10.1074/jbc.M304183200. [DOI] [PubMed] [Google Scholar]
- 52.Reynaert N.L., Ckless K., Korn S.H., Vos N., Guala A.S., Wouters E.F., van der Vliet A., Janssen-Heininger Y.M. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts N.J., Zhou S., Diaz L.A., Holdhoff M. Systemic use of tumor necrosis factor alpha as an anticancer agent. Oncotarget. 2011;2:739–751. doi: 10.18632/oncotarget.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossin A., Derouet M., Abdel-Sater F., Hueber A.O. Palmitoylation of the TRAIL receptor DR4 confers an efficient TRAIL-induced cell death signalling. Biochem. J. 2009;419(185–192):182. doi: 10.1042/BJ20081212. [DOI] [PubMed] [Google Scholar]
- 55.Rossin A., Durivault J., Chakhtoura-Feghali T., Lounnas N., Gagnoux-Palacios L., Hueber A.O. Fas palmitoylation by the palmitoyl acyltransferase DHHC7 regulates Fas stability. Cell Death Differ. 2015;22:643–653. doi: 10.1038/cdd.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabapathy K. Role of the JNK pathway in human diseases. Prog. Mol. Biol. Transl. Sci. 2012;106:145–169. doi: 10.1016/B978-0-12-396456-4.00013-4. [DOI] [PubMed] [Google Scholar]
- 57.So H.S., Park R.K., Kim M.S., Lee S.R., Jung B.H., Chung S.Y., Jun C.D., Chung H.T. Nitric oxide inhibits c-Jun N-terminal kinase 2 (JNK2) via S-nitrosylation. Biochem. Biophys. Res. Commun. 1998;247:809–813. doi: 10.1006/bbrc.1998.8788. [DOI] [PubMed] [Google Scholar]
- 58.Stamler J.S., Toone E.J., Lipton S.A., Sucher N.J. (S)NO signals: translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 59.Sumbayev V.V. S-nitrosylation of thioredoxin mediates activation of apoptosis signal-regulating kinase 1. Arch. Biochem. Biophys. 2003;415:133–136. doi: 10.1016/s0003-9861(03)00199-1. [DOI] [PubMed] [Google Scholar]
- 60.Talbott S.J., Luanpitpong S., Stehlik C., Azad N., Iyer A.K., Wang L., Rojanasakul Y. S-nitrosylation of FLICE inhibitory protein determines its interaction with RIP1 and activation of NF-κB. Cell Cycle. 2014;13:1948–1957. doi: 10.4161/cc.28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang Z., Bauer J.A., Morrison B., Lindner D.J. Nitrosylcobalamin promotes cell death via S nitrosylation of Apo2L/TRAIL receptor DR4. Mol. Cell. Biol. 2006;26:5588–5594. doi: 10.1128/MCB.00199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsang A.H., Lee Y.I., Ko H.S., Savitt J.M., Pletnikova O., Troncoso J.C., Dawson V.L., Dawson T.M., Chung K.K. S-nitrosylation of XIAP compromises neuronal survival in Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 2009;106:4900–4905. doi: 10.1073/pnas.0810595106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Török N.J., Higuchi H., Bronk S., Gores G.J. Nitric oxide inhibits apoptosis downstream of cytochrome C release by nitrosylating caspase 9. Cancer Res. 2002;62:1648–1653. [PubMed] [Google Scholar]
- 64.Verhoef C., de Wilt J.H., Grünhagen D.J., van Geel A.N., ten Hagen T.L., Eggermont A.M. Isolated limb perfusion with melphalan and TNF-alpha in the treatment of extremity sarcoma. Curr. Treat. Options Oncol. 2007;8:417–427. doi: 10.1007/s11864-007-0044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams J.L., Ji P., Ouyang N., Kopelovich L., Rigas B. Protein nitration and nitrosylation by NO-donating aspirin in colon cancer cells: Relevance to its mechanism of action. Exp. Cell Res. 2011;317:1359–1367. doi: 10.1016/j.yexcr.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson N.S., Dixit V., Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat. Immunol. 2009;10:348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 67.Wu W., Wan O.W., Chung K.K. S-nitrosylation of XIAP at Cys 213 of BIR2 domain impairs XIAP’s anti-caspase 3 activity and anti-apoptotic function. Apoptosis. 2015;20:491–499. doi: 10.1007/s10495-015-1087-3. [DOI] [PubMed] [Google Scholar]
- 68.Yang J., Amiri K.I., Burke J.R., Schmid J.A., Richmond A. BMS-345541 targets inhibitor of kappaB kinase and induces apoptosis in melanoma: involvement of nuclear factor kappaB and mitochondria pathways. Clin. Cancer Res. 2006;12:950–960. doi: 10.1158/1078-0432.CCR-05-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yasinska I.M., Kozhukhar A.V., Sumbayev V.V. S-nitrosation of thioredoxin in the nitrogen monoxide/superoxide system activates apoptosis signal-regulating kinase 1. Arch. Biochem. Biophys. 2004;428:198–203. doi: 10.1016/j.abb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J.Y., Adams A.E., Ridky T.W., Tao S., Khavari P.A. Tumor necrosis factor receptor 1/c-Jun-NH2-kinase signaling promotes human neoplasia. Cancer Res. 2007;67:3827–3834. doi: 10.1158/0008-5472.CAN-06-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]