Highlights
-
•
We surveyed common myeloid leukemia cell lines undergoing differentiation therapy.
-
•
Lineage progression in induced leukemia cell lines differs from nonmalignant cells.
-
•
Aberrant transcription factor expression occurs in acute myeloid leukemia cells.
-
•
A given differentiation therapy elicits different effects in specific cell lines.
-
•
Although potent tools, cell lines must be employed with circumspection.
Abbreviations: FAB, French–American–British [myeloid leukemia classification]; RA, retinoic acid; D3, 1,25-dihydroxyvitamin D3; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; RARα, retinoic acid receptor α; VDR, vitamin D receptor; Gfi-1, growth factor independent protein 1; EGR1, early growth response protein 1; C/EBPα, CCAAT-enhancer binding protein α; PU.1, binds PU-box, also called Spi-1; IRF-1, interferon regulatory factor 1; AhR, aryl hydrocarbon receptor; Oct4, octamer-binding transcription factor 4; CD, cluster of differentiation [marker]
Keywords: Retinoic acid, Vitamin D3, Myeloid leukemia, Differentiation, Lineage selection
Abstract
Transcription factors that drive non-neoplastic myelomonocytic differentiation are well characterized but have not been systematically analyzed in the leukemic context. We investigated widely used, patient-derived myeloid leukemia cell lines with proclivity for differentiation into granulocytes by retinoic acid (RA) and/or monocytes by 1,25-dihyrdroxyvitamin D3 (D3). Using K562 (FAB M1), HL60 (FAB M2), RA-resistant HL60 sublines, NB4 (FAB M3), and U937 (FAB M5), we correlated nuclear transcription factor expression to immunophenotype, G1/G0 cell cycle arrest and functional inducible oxidative metabolism. We found that myelomonocytic transcription factors are aberrantly expressed in these cell lines. Monocytic-lineage factor EGR1 was not induced by D3 (the monocytic inducer) but instead by RA (the granulocytic inducer) in lineage bipotent myeloblastic HL60. In promyelocytic NB4 cells, EGR1 levels were increased by D3, while Gfi-1 expression (which promotes the granulocytic lineage) was upregulated during D3-induced monocytic differentiation in HL60, and by RA treatment in monocytic U937 cells. Furthermore, RARα and VDR expression were not strongly correlated to differentiation. In response to different differentiation inducers, U937 exhibited the most distinct transcription factor expression profile, while similarly mature NB4 and HL60 were better coupled. Overall, the differentiation induction agents RA and D3 elicited cell-specific responses across these common FAB M1-M5 cell lines.
1. Introduction
Differentiation induction therapy agents like all-trans retinoic acid (RA) and 1,25-dihyrodxyvitamin D3 (D3) show promise in many cancer cells types [1–3]. Although acute myeloid leukemias (AML) are extremely heterogeneous diseases, with over 200 known AML-related cytogenic aberrations [4], RA and D3 evoke comparable responses in human myeloid leukemia cell lines, i.e. RA induces granulocytic events while D3 induces monocytic events. Whether RA and D3 can act additively, synergistically or antagonistically is an outstanding question, since each behavior has been observed in different contexts. Although lineage-determining myeloid transcription factors are well characterized for the nonmalignant case [5–7], systematic analysis of their expression during differentiation induction therapy in leukemia is lacking. In this study we used sequentially more mature, human myeloid leukemia cell lines K562 (FAB M1), HL60 (FAB M2), NB4 (FAB M3) and U937 (FAB M5) and compared treatment-induced expression of an ensemble of well-known transcription factors that govern myelomonocytic lineage selection.
K562 is a chronic myelogenous leukemia (CML) cell line (FAB M1) that harbors the Bcr-Abl fusion protein [8,9]. K562 cells exhibit inducible erythroleukemic and megakaryocytic characteristics [10,11], but are not responsive to either RA [12,13] or D3 treatment [14], and thus serve as a negative control for RA- or D3-induced differentiation. HL60 leukemia cells are FAB M2 lineage-bipotent myeloblasts [15,16] that can differentiate along either the granulocytic lineage (using RA) or monocytic lineage (using D3). HL60 cells are t(15;17)-negative, so RA-induced therapy must act through a mechanism independent of PML-RARα. We previously isolated and described two sequentially emergent RA-resistant HL60 cell lines that differ in their RA-inducible CD38 expression, termed R38+ and R38− [17,18]. These lines, which do not growth arrest or exhibit other RA-induced markers when treated with RA, demonstrate that as RA resistance becomes more profound, progressive resistance to D3 also develops. NB4 is an acute promyelocytic leukemia (APL) cell line (FAB M3) that does contain the t(15;17) translocation pathognomonic for APL [19–21]. NB4 cells are highly RA-responsive, but are less responsive to D3 than wild-type HL60 cells are, and require combination treatment to achieve any degree of monocytic differentiation [22,23]. U937 monocytic leukemia cells (FAB M5), the most mature cells in this study, are highly responsive to D3-induced monocytic/macrophage differentiation. RA exerts ambiguous differentiative effects in U937, which at times have been considered either monocytic or granulocytic [24–26]. U937 cells harbor a t(10;11) translocation, a recurrent event found in AML cells and T cell acute lymphoblastic leukemia [4,27].
During non-neoplastic myelomonopoiesis, the transcription factors PU.1 (a myeloid lineage master regulator) and C/EBPα have positive effects on both granulocytic and monocytic maturation, but the ratio of PU.1 to C/EBPα determines granulocytic versus monocytic lineage selection [28]. This is due to a bistable switch described by Laslo et al. (2006) [29] that involves mutually antagonistic repressors Gfi-1 and EGR1 which lie downstream of PU.1 and C/EBPα. Gfi-1 represses monocytic differentiation and promotes granulocytic lineage selection, while EGR1 acts conversely. In addition to retinoid acid receptor α (RARα) and vitamin D receptor (VDR), other transcription factors found to be significant, specifically to RA-induced differentiation, are IRF-1, AhR and Oct4 [30,31]. Aryl hydrocarbon receptor (AhR) expression increases during myeloid differentiation of HL60 [30] as well as during monocytic differentiation of HL60 and U937 [32], and promotes Oct4 downregulation, putatively relieving stemness. IRF-1 expression is induced by RA in HL60, NB4 and U937 cells [31,33], but not K562 cells [34], and this expression appears to be Stat1-independent [35].
In this study we treated K562, wild-type and RA-resistant HL60, NB4 and U937 cells with RA, D3, or combination RA + D3 and assessed differentiation using immunophenotypic markers CD38 and CD11b (myelomonocytic markers) and CD14 (a monocytic-specific marker). Additionally we assessed G1/G0 cell cycle arrest and inducible oxidative metabolism, a functional differentiation marker of mature myelomonocytic cells. We surveyed nuclear expression of the nine aforementioned transcription factors and analyzed their coupling to cellular phenotype. Our intentions were to: (1) provide ourselves and others with a comparative index of responses by these cell lines, (2) identify departures from the norm of myelomonocytic transcription factor expression in the leukemic differentiation context, (3) determine how the transcription factor expression and phenotypic marker signatures couple to the individual treatments (RA, D3 or RA + D3) and to myeloid cell maturity, and (4) determine if combined RA + D3 treatment promotes one lineage over the other.
2. Materials and methods
2.1. Cell lines and treatments
Original HL60 patient isolates were a gift of Dr. Robert Gallagher and maintained in this laboratory. Two retinoic acid (RA)-resistant HL60 sublines (R38+ and R38−) were isolated as described previously [17]. NB4 cells were provided by Ethan Dmitrovsky (Dartmouth University). American Type Cell Culture (ATCC)-obtained U937 cells were provided by Tracy Stokol (Cornell University) and ATCC-obtained K562 cells were provided by Hening Lin (Cornell University). No human primary tissues were obtained or used in this study—all parent cell lines were established approximately 30 years ago [8,15,19,25] and are purchasable from ATCC. Cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 5% (HL60) or 10% (K562, NB4, U937) heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT) and 1% antibiotic–antimycotic (Invitrogen) and maintained in a 5% CO2 humidified environment at 37 °C. Cells were seeded at either 0.1 × 106 cells/ml (HL60), 0.2 × 106 cells/ml (HL60, NB4, U937) or 0.3 × 106 cells/ml (K562). Cell viability consistently exceeded 95%. All-trans retinoic acid (RA; Sigma, St. Louis MO) was added from a 5 mM stock solution in 100% ethanol to a final concentration of 1 μM in culture. 1,25-Dihydroxyvitamin D3 (D3; Cayman Chemicals, Ann Arbor, MI) was added from a 1 mM stock solution in 100% ethanol to a final concentration of 0.5 μM in culture.
2.2. Flow cytometry
CD surface markers were detected using anti-CD38 (PE), anti-CD11b (APC) and anti-CD14 (PE) antibodies (BD Biosciences, San Jose, CA). 0.5 × 106 cells were harvested and analyzed by flow cytometry on a BD LSRII flow cytometer (BD Biosciences) as described previously [18]. For cell cycle analysis, cells were harvested and stained with propidium iodide-containing solution and analyzed as described previously [18]. For reactive oxygen species quantification, cells were treated with 0.2 μg/ml 12-o-tetradecanoylphorbol-13-acetate (TPA, Sigma, St. Louis, MO) or dimethyl sulfoxide (DMSO) carrier and stained with 5 μM 5-(and-6)-chloromethyl-2′,7′-dichlorodihydro–fluorescein diacetate acetyl ester (H2-DCF, Molecular Probes, Eugene, OR), then analyzed by flow cytometry as described previously [18].
2.3. Western blotting
Cell nuclear lysates were isolated using the NE-PER extraction kit (Thermo Scientific, Rockford, IL). Equal amounts of protein lysate (15 μg) were resolved by SDS–PAGE and transferred onto PVDF membrane (Millipore, Billerica, MA). Blots were blocked and incubated with primary and secondary antibody as described previously [18]. Primary antibodies were specific against C/EBPα, RARα, VDR, EGR1, PU.1, Oct4 (Cell Signaling, Danvers, MA), AhR, Gfi-1 (Santa Cruz Biotechnology, Santa Cruz, CA) and IRF-1 (BD Biosciences). Anti-Histone 3 or anti-TATA-binding protein (Cell Signaling) were used to ensure even loading.
2.4. Statistical analysis
p-Values between treatment group means were calculated using ANOVA within GraphPad software. Repeat Western blot data were quantified using ImageJ. Pearson correlation coefficient calculation and hierarchical clustering analysis (average linking method) were performed in MATLAB or Cluster 3.0.
3. Results
3.1. Induced phenotypic changes in K562, HL60, NB4 and U937 cells
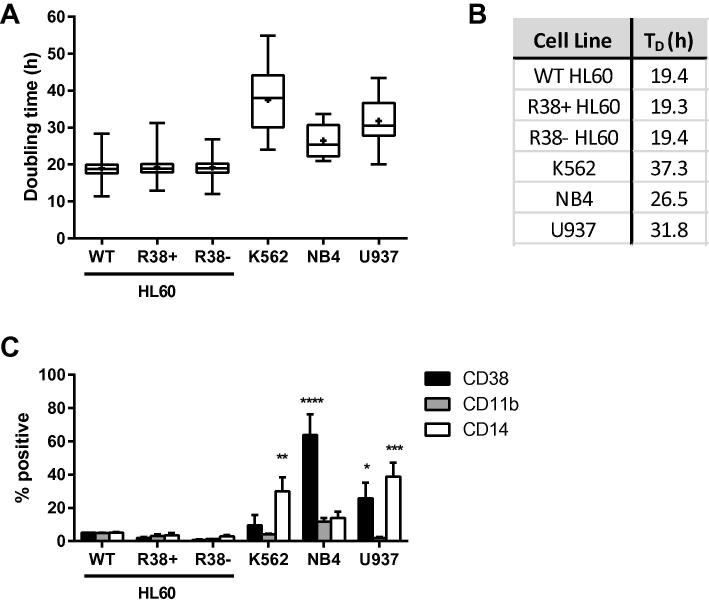
Wild-type and RA-resistant HL60 maintained in our laboratory have a doubling time of approximately 20 h (Fig. 1A, B) and are grown in RPMI 1640 medium with 5% FBS. We obtained K562, NB4 and U937 cell lines which have slower doubling times and are maintained in RPMI medium with 10% FBS (Fig. 1A, B). Compared to untreated wild-type HL60, untreated NB4 and U937 are more mature, having higher basal expression levels of CD38 and CD14 (Fig. 1C). K562 also have significantly higher basal CD14 expression than the HL60 cell lines.
Fig. 1.
Comparison of cell line characteristics. (A) Box-and-whisker plots of cell line doubling times when grown in RPMI 1640 with serum. (B) Mean doubling times of cell lines. (C) CD38, CD11b and CD14 expression were analyzed by flow cytometry and shown as percent positive cells for each myeloid cell line compared to wild-type (WT) HL60, the bipotent system. Percent positive shift was determined by setting the WT HL60 control to exclude 95% of the population peak. NB4 (p < 0.0001) and U937 (p < 0.05) had significantly higher CD38 expression than WT HL60. All cell lines had similar CD11b expression levels when untreated, but K562 (p < 0.01) and U937 (p < 0.001) had higher CD14 expression compared to WT HL60.
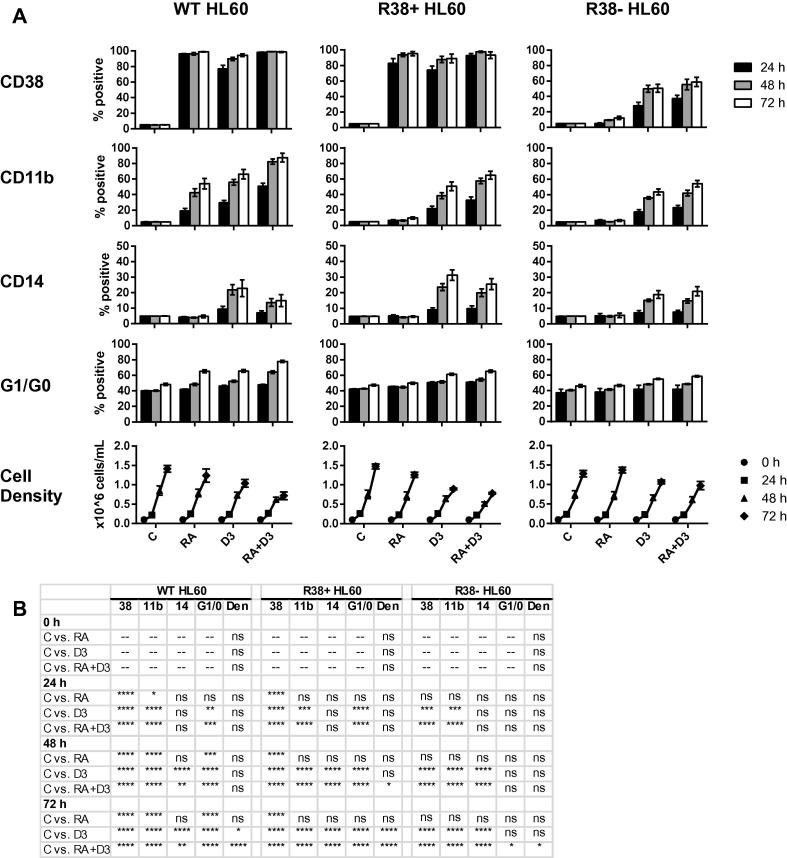
We investigated the phenotypic changes that result from differentiation induction therapy in the myeloid leukemia cell line panel. CD38, CD11b and CD14 expression, G1/G0 cell cycle arrest, and cell densities were assessed in wild-type and the two RA-resistant HL60 cell lines after 24, 48, and 72 h of treatment with either retinoic acid (RA), 1,25-dihydroxyvitamin D3 (D3), or combination (RA + D3). Wild-type HL60 cells display a strong response to both RA and D3 (Fig. 2A, B), with D3 treatment resulting in greater CD11b expression as well as CD14 expression, as reported previously [18]. Combined RA + D3 treatment further enhances G1/G0 arrest and CD11b expression compared to either individual treatment. However, lower CD14 expression was observed with combined RA + D3 compared to D3 alone. This may suggest that RA has an antagonistic effect on this monocytic-specific marker. Meanwhile RA-resistant cells lines R38+ and R38− do not respond to RA, with the exception of RA-inducible CD38 expression in R38+ HL60, as previously reported for 48 h [17]. Both R38+ and R38− are responsive to D3 treatment, displaying increased CD11b, CD14 and G1/G0 arrest, with the sequentially emergent line R38− being less responsive. Interestingly, unlike wild-type HL60, RA fails to reduce the D3-induced CD14 expression in R38+ and R38− HL60.
Fig. 2.
Induced phenotype in wild-type, R38+ and R38− HL60 cells. (A) CD38, CD11b, CD14, G1/G0 arrest and cell densities for wild-type (WT), R38+ and R38− HL60 cells at 24, 48 and 72 h of culture for indicated control, RA, D3 or RA + D3 treatment. (B) p-Values for data in (A). Each treated sample group was compared to its respective timepoint control.
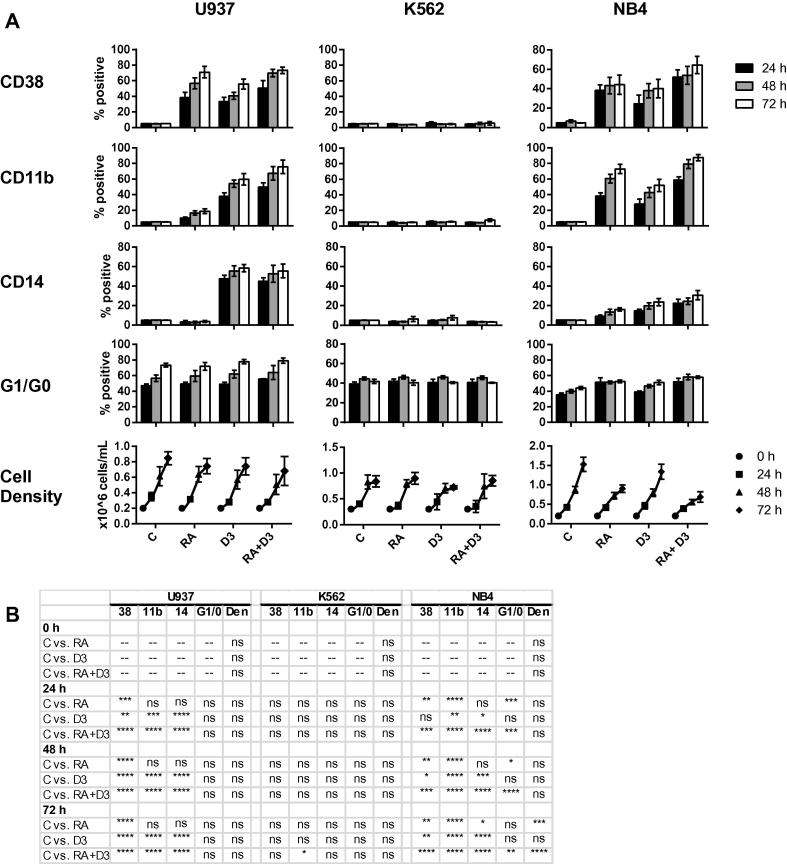
In K562 cells, our negative phenotypic control, RA, D3 or combined RA + D3 treatment do not induce any change in CD38, CD11b or CD14 expression, G1/G0 arrest, or cell density after 24, 48 and 72 h (Fig. 3A,B). In contrast, NB4 cells are highly responsive to RA treatment, which induced CD38 and CD11b expression, G1/G0 arrest, and greatly reduced cell density over time. Interestingly, minor CD14 expression is RA-inducible in NB4 cells. This effect has been reported previously [36], and may be due to aberrant effects of the PML-RARα fusion protein (see Section 4). D3 treatment of NB4 cells does not significantly reduce cell density, but does induce comparable levels of CD38, CD11b and CD14 surface markers. In NB4, combined RA + D3 treatment enhanced the effect of either individual inducer. U937 cells appeared to arrest in culture over time, even without treatment (Fig. 3A). This may reflect the greater maturity of this cell line. U937 cells display the strongest response to D3 treatment compared to the other cell lines, with over 50% CD14 expression occurring by 72 h in the D3 and RA + D3 treated cases (Fig. 3A,B). Unlike wild-type HL60, combined RA treatment fails to reduce the D3-induced CD14 expression in U937 cells. However, RA enhances D3-induced CD38 and CD11b expression, and RA alone can induce significant CD38 expression and very minor CD11b expression in U937.
Fig. 3.
Induced phenotype in U937, K562 and NB4 cells. (A) CD38, CD11b, CD14, G1/G0 arrest and cell densities for U937, K562 and NB4 cells at 24, 48 and 72 h of culture for indicated control, RA, D3 or RA + D3 treatment. (B) p-Values for data in (A). Each treated sample group was compared to its respective timepoint control.
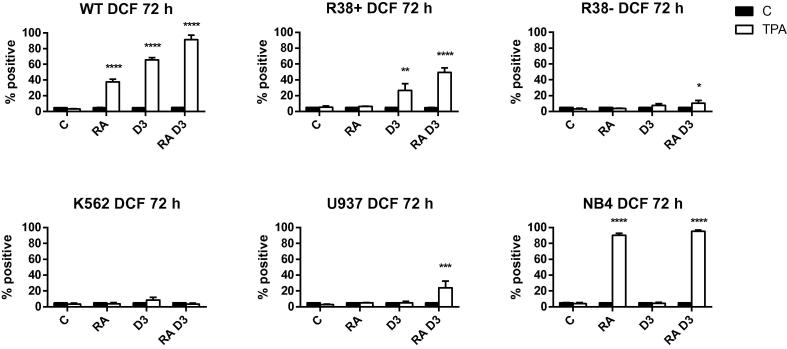
We also assessed reactive oxygen species (ROS) production, or inducible oxidative metabolism in response to 12-o-tetradecanoylphorbol-13-acetate (TPA), which is a marker of functional differentiation in mature myelomonocytic leukemia cells. RA and D3 significantly increase ROS, with RA + D3 causing greater ROS production compared to either inducer alone, in wild-type HL60 (Fig. 4). R38+ HL60 only exhibit TPA-induced ROS in the presence of D3, and R38− only minimally during RA + D3 treatment. K562 are functionally unresponsive to RA or D3 induction, while NB4 display striking TPA-induced oxidative metabolism with RA treatment. However, U937 had surprisingly low ROS production, with a significant increase occurring only after the combined treatment.
Fig. 4.
Inducible ROS production in wild-type HL60, R38+ HL60, R38− HL60, U937, K562 and NB4 cells at 72 h. Cells were stimulated with TPA to induce respiratory burst activity. Percent positive cells were determined by flow cytometric detection of DCF fluorescence.
For response to combined RA + D3 treatment, generally U937 and the RA-resistant HL60 lines R38+ and R38− were comparable in that D3 induces monocytic changes that are only minimally enhanced or unchanged by additional RA treatment. In wild-type HL60, RA enhances D3 effects with the exception of CD14 expression, which is reduced. NB4 cells display a complex response, with both RA and D3 inducing expression of CD38, CD11b and CD14 to some extent. However in NB4 cells, cell density is greatly reduced and functional oxidative metabolism is greatly enhanced only when RA is present. These characterizations of phenotypic responses based on cell line identity and maturity can be compared to the intracellular transcription factors expression profiles determined below.
3.2. Transcription factor expression in K562, HL60, NB4 and U937 cells
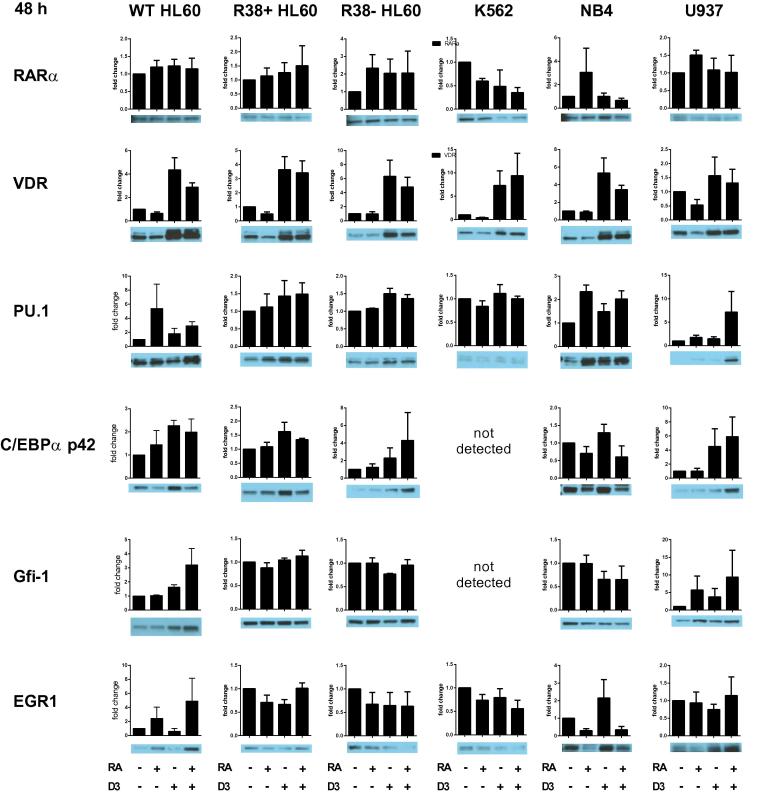
We surveyed nine transcription factors for their nuclear expression levels in the myeloid cell line ensemble treated with RA, D3 or RA + D3 for 48 h. Nuclear RARα protein levels are unchanged in treated HL60 (Fig. 5), which has been shown previously [18], but in R38+ and R38− RA-resistant HL60 cells, RARα expression often increased with treatments, whereas in the less mature K562 cells, treatment tends to decrease RARα. The more mature NB4 and to a lesser extent U937 appear to exhibit RA-inducible nuclear RARα expression which is blunted by combined RA + D3 treatment. In contrast nuclear VDR expression is greatly enhanced whenever D3 is present in every cell line, including phenotypically-unresponsive K562 cells.
Fig. 5.
RARα, VDR, PU.1, C/EBPα, Gfi-1 and EGR1 nuclear transcription factor expression in wild-type HL60, R38+ HL60, R38− HL60, U937, K562 and NB4 cells at 48 h. Fold change of quantified Western blot data (from at least three biological repeat nuclear lysates) using ImageJ are shown with a representative blot. Note the scale on the y-axis may differ. Error bars represent standard error. T-test analysis is not possible for quantified blot data as luminescent signal may be in the linear or nonlinear range.
Nuclear PU.1 levels tend to be higher with RA treatment in wild-type HL60 (Fig. 5). PU.1 expression is higher in U937 with combined RA + D3 treatment, while expression is higher when D3 is present in RA-resistant HL60. In NB4 cells, both RA- and combined RA + D3-treated cells tend to have increased nuclear PU.1 expression, although D3 induced PU.1 expression as well. The C/EBPα transcription factor could not be detected in K562. Nuclear C/EBPα expression is highest with D3 treatment alone in wild-type HL60, R38+ HL60 and NB4. R38− and U937 are similar in their expression of C/EBPα across treatments, which is highest for combined RA + D3.
Nuclear Gfi-1 (which promotes granulocytic differentiation) paradoxically increases during D3 treatment in wild-type HL60, and is also higher in RA-treated U937 (Fig. 5). NB4 cells do not exhibit RA-induced Gfi-1 upregulation, although Gfi-1 expression decreases slightly with D3 treatment. Gfi-1 expression is more or less unchanged in treated RA-resistant HL60 and was undetected in K562. Strangely, nuclear EGR1 (which promotes monocytic differentiation) expression is increased with D3 treatment alone in NB4 cells, although combined RA + D3 suppresses EGR1 expression (Fig. 5). Meanwhile wild-type HL60 cells display increased EGR1 expression whenever exposed to RA. EGR1 expression is unchanged in U937 and RA-resistant HL60, and may be slightly decreased by all treatments in K562.
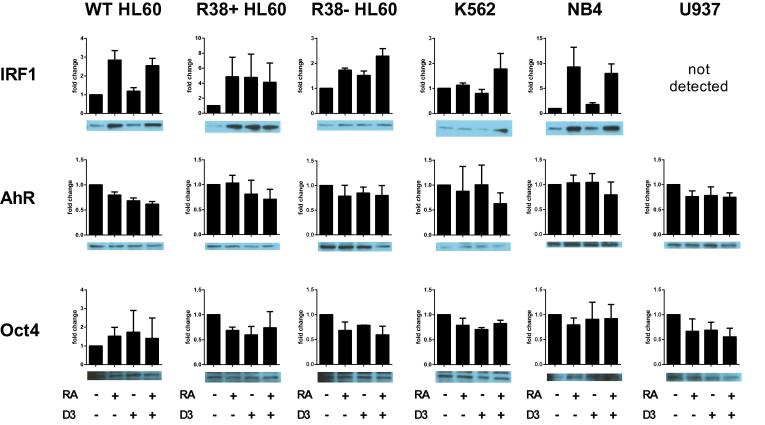
Nuclear IRF-1 is clearly induced by RA in the most RA-responsive lines, wild-type HL60 and NB4 (Fig. 6). Interestingly, IRF-1 expression is higher across all treatments in the RA-resistant HL60, and not detectable in U937. Combined RA + D3 treatment could increase IRF-1 expression in K562. Nuclear AhR levels decrease with treatments in wild-type HL60, but remain more or less the same in all other cell lines, therefore nuclear AhR expression alone did not reveal significant dependencies on RA- versus D3-induced differentiation (Fig. 6). Nuclear Oct4 expression levels, as a marker for stemness, were generally reduced with treatments (Fig. 6), but quantified fold change results were sensitive to small differences due to the faintness of the band. Below, we address the importance of myeloid leukemia cell line maturity versus differentiation-inducing agents by performing clustering analyses on transcription factor expression and phenotype data.
Fig. 6.
IRF-1, AhR and Oct4 nuclear transcription factor expression in wild-type HL60, R38+ HL60, R38− HL60, U937, K562 and NB4 cells at 48 h. Fold change of quantified Western blot data (from at least three biological repeat nuclear lysates) using ImageJ are shown with a representative blot. Note the scale on the y-axis may differ. Error bars represent standard error. T-test analysis is not possible for quantified blot data as luminescent signal may be in the linear or nonlinear range.
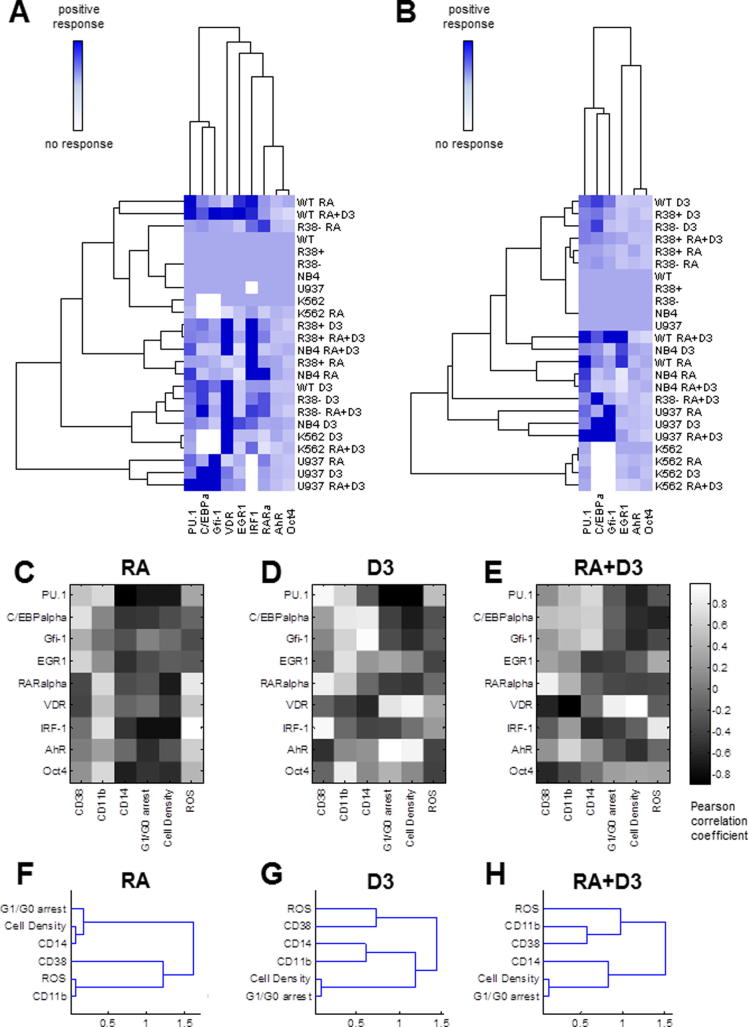
3.3. Clustering of treated cell lines based on transcription factor expression
Quantified 48 h nuclear transcription factor expression data were subject to hierarchical clustering analysis. The simple anticipation was to observe clusters grouped by treatment rather than by cell line, to indicate that the same treatment evokes a similar response across all myeloid types. But contrary to this anticipation, we found that the induced transcription factor response in U937 cells is highly divergent from all other cases, indicating a cell line-specific expression pattern (Fig. 7A). However, all other myeloid leukemia cell lines treated with D3 (with the exception of R38+) are grouped within a single subcluster. This could suggest that for less mature cell lines, the identity of the inducer, rather individual cell lines with distinct karyotypes, can take precedence in determining transcription factor expression. Also included in this cluster are the least RA-responsive lines, K562 and R38-, when treated with combination RA + D3. But this cluster seemed to be grouped based on VDR expression, which could be uncorrelated from phenotypic maturation (as exemplified by K562 cells). R38+ HL60 and NB4 cells, respectively poor and good RA-responders, are closely coupled based on similar treatments (RA alone or combined RA + D3). However these also seemed to be grouped based on a single transcription factor, IRF-1, rather than on a collectively similar expression profile of several transcription factors. Finally, RA-treated and combined RA + D3-treated wild-type HL60 are grouped closely together but distal from the other strong RA responder, NB4.
Fig. 7.
Clustering of cell line transcription factor profile and correlation of transcription factor to observed phenotype. (A) Average transcription factor expression matrix and clustering of treated cell lines based on 48 h nuclear transcription factor expression data. Mean quantified Western blot data for each cell line treatment case was subject to hierarchical clustering analysis using the Pearson correlation coefficient as a distance metric and using an average linking method. (B) Correlation and clustering without VDR, IRF-1 or RARα. (C–E) Pearson correlation coefficient matrix between average transcription factor expression and phenotypic results at 48 h across all cell lines for (C) RA treatments, (D) D3 treatments and (E) RA + D3 treatments. Hierarchical clustering of phenotypic results using average linkage for all cell lines across (F) RA treatments, (G) D3 treatments and (H) RA + D3 treatments.
We repeated hierarchical clustering analysis after eliminating VDR and IRF-1, which had very dominant (high) nuclear expression levels and were poorly coupled to the transcription factor ensemble. We also removed RARα from the analysis since RARα expression was found above to correspond poorly to RA response. Without these transcription factors, the highly non-responsive K562 cells (treated and untreated) are then tightly grouped, while the treated U937 cells remain in a highly coupled cluster (Fig. 7B). This illustrates that differentiation induction agents result in very cell line-specific transcription factor expression patterns in very mature (U937) or very immature (K562) cells. Meanwhile cell lines of closer maturity, NB4 (M3) and HL60 (M2), including the emergent RA-resistant HL60 cell lines, are better clustered based on treatment. RA-treated wild-type HL60 and NB4 cells are highly coupled, as are D3-treated NB4 with RA + D3-treated wild-type HL60. D3-treated wild-type and likewise D3-treated R38+ and R38− HL60 cells are highly correlated. RA-treated R38+ and R38− HL60 are clustered with the untreated cases.
3.4. Correlations between treatments, cell lines and transcription factor expression
To identify couplings between nuclear transcription factor expression and differentiation state (induced phenotypic shift), we calculated correlation coefficient matrices between quantified transcription factor expression and 48 h phenotypic marker data across all six myeloid leukemia cell lines for RA treatment (Fig. 7C), D3 treatment (Fig. 7D) and RA + D3 treatment (Fig. 7E). We also assessed hierarchical clustering of phenotypic response markers across all cell lines for RA treatment, D3 treatment and RA + D3 treatment (Figs. 7F–H). Results did not indicate that RA or D3 necessarily took precedence over the other agent during combined treatment (see Section 4). For RA differentiation induction, CD38 expression, CD11b expression and ROS production are more positively correlated with the transcription factor ensemble compared to CD14 expression, G1/G0 arrest and cell culture density (Fig. 7C). For D3 differentiation induction, individual transcription factors have very strong correlations (close to 1) with various phenotypic responses, e.g. AhR or VDR with cell density and G1/G0 arrest, Gfi-1 or C/EBPα with CD14 expression, and PU.1, RARα or IRF-1 with CD38 expression (Fig. 7D). Combined RA + D3 treatment appears to result in a mixed phenotype, preserving some couplings observed for both the RA and D3 treatment cases (Fig. 7E). For example, VDR expression remains highly correlated to G1/G0 arrest while AhR expression is positively correlated to both CD38 and CD11b expression.
CD38, CD11b and inducible ROS production are strongly correlated in RA-treated cases (Fig. 7F). But G1/G0 groups with CD14 and cell density in a cluster distinct from CD38, CD11b, and ROS. During D3 treatments, CD11b and CD14 are tightly coupled, and better correlated to G1/G0 arrest and cell density (which were also tightly coupled) than to CD38 or ROS production (Fig. 7G). Combined RA + D3 treatment clustering is generally similar to RA (versus to D3) clustering, with CD14, G1/G0 arrest and cell density grouped into the same cluster while CD11b is more highly correlated with the cluster containing CD38 and ROS production (Fig. 7H).
4. Discussion
4.1. Impetus for cell line use and differentiation therapies
The limitations of monoculture and the divergence of continuously passaged ex vivo cells from their original isolates [37] remain valid concerns. However one study showed that several myeloid cell lines, including K562, HL60, NB4, are faithful models of leukemia [9]. Another group showed that compared to single cell lines, panels of cell lines can at times better recapitulate features of heterogeneous tissue populations [38]. Established cell lines provide a cornerstone for basic research before progressing to primary cells or animal models, thus clarification of the characteristics of broadly-employed cell lines like NB4 and U937 is of significant value. Here we sought to examine the transcription factor expression profiles and their coupling to phenotypic state of a panel of progressively mature myeloid leukemia cell lines.
Retinoic acid (RA) therapy induces remission in 80–90% of t(15;17)-positive acute promyelocytic leukemia (APL) patients [39], but has been limited by naïve/emergent RA resistance, cell-type specific effects and limited understanding of the mechanism of RA action. RA can exert its pro-differentiative and anti-proliferative properties in breast, lung, kidney and prostate cancer [1,2]. As a combination therapy, RA can be used to lower the dosage of traditional chemotherapeutics or kinase inhibitors, or when combined with other differentiation induction agents, mitigate the negative and augment the positive effects of the individual inducers. For example, differentiation therapy using 1,25-dihydroxyvitamin D3 (D3) has been less widespread since it can induce hypercalcemia and hyperphosphatemia, but combination with RA or use of D3 analogs [40,41] could reduce these side effects.
4.2. Effects of RA and D3 treatment on their receptors and on maturation in myeloid leukemia cell lines
RA and D3 act as ligand agonists for their cognate receptor/transcription factors, retinoic acid receptors (RARs) and vitamin D receptor (VDR), each of which can heterodimerize with retinoid X receptor (RXR, whose ligand is 9-cis RA). Treatment with D3 increases the expression of VDR protein in HL60 [42], while RA treatment results in a downregulation of VDR mRNA in HL60 [4] and protein levels in K562, NB4, U937 and R38+ HL60 (this study). However in KG1 cells, which are RA-responsive but not D3-repsonsive, RA treatment actually induces upregulation of VDR mRNA [4]. Exposure of KG1 cells to both RA and D3 can thus rescue expression of the monocytic marker CD14. Another FAB M2 cell line, Kasumi-1, undergoes monocytic differentiation in response to either D3 or RA [43]. Thus RA and D3 have differing effects on expression of their cognate receptors in different myeloid lines.
There are also various crosstalk effects. Bastie et al. (2004) [44] showed that in HL60 cells, D3 treatment (i.e. VDR) transrepresses RA transcriptional activity, while RA does not transrepress D3. However, we saw that combined RA + D3 treatment decreased the D3-induced CD14 expression in HL60, which suggests the opposite (i.e. RA inhibits D3 action). Meanwhile in NB4 cells the opposite is true: D3 is not a transrepressor of RA transcriptional activity, and RA treatment releases VDR and increases RARα binding to promoter regions [45]. Combined RA treatment can rescue D3-induced differentiation in NB4 cells [23], but here we found that D3 alone can induce several CD markers to some extent in NB4. In fact, RA-induced CD14 expression in NB4 cells is known [36], and several CD markers not expressed in RA-induced HL60 are expressed in RA-induced NB4, and vice versa [46]. This may be directly related to the aberrant function of the PML-RARα fusion protein which HL60 cells lack. Additionally, induced differentiation of K562 (with compounds other than RA or D3) along the erythroid and megakaryocytic lineages is accompanied by ambiguous expression of CD markers for one or more lineages [11]. Since lineage infidelity is a recurrent theme for induced leukemia, it is no surprise to find that nuclear transcription factor expression deviates from what is established for the nonmalignant case.
4.3. Aberrant transcription factor expression and cell-type versus treatment-specific responses
The roles of transcription factors, especially PU.1 and C/EBPα, in myelomonocytic lineage selection have been well characterized via knockout, antisense and overexpression studies [5-7]. In this study, the most salient result after surveying the nuclear expression of several transcription factors was deviation from the mutually antagonistic Gfi-1/EGR1 circuit [29]. HL60, NB4 and U937 leukemia cells exhibit paradoxical effects compared to what has been reported for Gfi-1 and EGR1, which promote granulocytic and monocytic differentiation, respectively, in nonmalignant myelomonocytic fate selection. In the most mature cell lines, NB4 and U937, the treatment respective to each lines’ maturity (RA for NB4 and D3 for U937) does not result in an increase in the transcription factor that promotes that lineage (Gfi-1 is unchanged with RA treatment in NB4, EGR1 is unchanged with D3 treatment in U937). Oddly, treatment increases its corresponding transcription factor in the cell line of the opposite lineage (RA increases Gfi-1 expression in U937 and D3 increases EGR1 expression in NB4). We could speculate that lack of activation is prompting an increase in expression: Gfi-1 is not getting activated during RA treatment in U937 cells, and EGR1 is not getting activated during D3 treatment in NB4 cells, hence a compensatory expression mechanism is being driven. Meanwhile in the bipotent HL60, which are less mature, there is a different effect altogether. Treatment instead drives expression of the transcription factor not corresponding to that lineage, i.e. D3 induces Gfi-1 expression and RA induces EGR1 expression. This could again indicate that lack of activation is prompting an increase in expression.
We compared the cell line transcription factor expression signature presented here to primary patient data curated from published literature. While Gfi-1 overexpression is a known characteristic in acute lymphoid leukemia, increased Gfi-1 expression can sometimes be detected in patients with chronic and acute myeloid leukemias [47]. Additionally, Gfi-1 expression is low in mature, end-stage granulocytes [47], which suggests that the lack of increase in Gfi-1 expression after RA-induced differentiation in NB4 and HL60 cells is not atypical. Also, Gfi-1 may actively repress granulocytic genes in CD14+ monocytes [47], accounting for its upregulation either by D3, or by RA in the monocytic cell line U937. In the context of leukemia, EGR1 is generally known to have a tumor suppressing function [48], which could explain its upregulation by D3 in NB4 cells, although not its lack of upregulation in U937 cells. EGR1 expression is increased by RA in HL60 cells but, in fresh AML patient samples, RA is known to reduce EGR1 expression [49]. However, EGR1 acts as a tumor suppressor in cells with deregulated c-Myc [48], which could account for its RA-induced expression in the HL60 cell line. Freshly isolated cells from AML patients have even higher constitutive VDR transcript expression compared to established AML cell lines—VDR protein becomes protected from degradation when ligand is bound, and resistance mechanisms typically occur downstream of the VDR protein [50]. This supports the consistent D3-induced VDR expression seen across all cell lines in this study regardless of D3 responsiveness. Meanwhile RARα expression is inducible by RA, but only in primary cells from AML M3 patients and perhaps not in other types of AML [51]. This parallels our data where only the NB4 (FAB M3) cell line tended to exhibit RA-inducible RARα expression. One group found a correlation between increasing AML maturity (M0-M5) and PU.1 expression levels with the exception of M3 (APL), which had very low PU.1 expression [52], and it is known that RA treatment rescues PU.1 expression in primary APL cells [53]. However in a study that examined 109 non-APL AML patient samples, C/EBPα and PU.1 expression were both highly heterogeneous with a broad distribution across samples—expression could be either much higher, similar to or much lower than that of nonmalignant granulocytes or monocytes [54]. Overall this highlights that leukemias can exhibit extremely variable characteristics, with individual cell lines being either capable or incapable of representing specific leukemias in patients. Cell lines can be used to represent a defined leukemia subset, or as stated previously, cell line panels may be the mode d’emploi when comparing to patient tissues.
We assessed whether a given agent evokes a similar response across cell lines tested by investigating whether transcription factor expression profiles couple by treatment. We found results both in favor and against this idea. We showed that there is a high correlation between all D3-treated cell lines and RA + D3-treated K562 and R38- (the least RA-responsive cell lines). This suggests that the identity of the inducer, rather individual cell lines with distinct karyotypes, can determine transcription factor expression. Cell lines like NB4 and R38+ (respectively a good RA responder and poor RA responder) could also be highly correlated based on the same treatment pattern like RA alone or RA + D3 treatment. However, the importance of cell identity and maturity can still hold sway as evidenced by U937 cells, which when treated with any inducer were the least correlated from the remaining cases. Also, clustering of treated cell lines appeared to associate strongly with the expression of an individual transcription factor—rather than an expression profile of several transcription factors—and these factors (i.e. VDR and IRF-1) were not significantly coupled to the remaining factors. However, removal of VDR, IRF-1 and RARα from the clustering analysis did not significantly skew the clustering toward same-treatment groups.
We questioned whether induced myelomonocytic lineage selection, during combined treatment with RA + D3, has a hierarchical differentiation relationship or solely a bifurcation structure. We previously found that progressive resistance to RA also resulted in progressive resistance to D3, i.e. lineage induction occurred at the expense of the other lineage [18]. Comparing correlations between transcription factor expression and phenotypic response across all cell lines for each treatment, RA + D3 treatment resulted in a mixed response with aspects from both RA- and D3-induced differentiation (Fig. 7C). Figs. 7D–F suggest that the RA + D3 phenotypic changes are more RA-like, and RA + D3 treatment enhances CD marker expression (including CD14) in NB4 cells. However, in wild-type HL60, RA + D3 treatment decreases CD14 expression compared to D3 alone, suggesting that D3-induced signaling can be inhibited by RA-induced signaling. Without nuclear VDR expression overpowering the clustering analysis (Fig. 7B), D3-treated cases are separated across different clusters. Reports in U937 cells have not truly clarified whether RA enhances monocytic differentiation or promotes granulocytic changes in these cells. While we found that RA can induce certain changes interpretable as either granulocytic or monocytic (CD38 expression, oxidative metabolism), RA fails to upregulate the monocytic specific marker CD14 in U937 cells. Despite the distinctions between RA-induced granulocytic or D3-induced monocytic lineage selection, the two pathways exhibit a degree of crosstalk.
5. Conclusions
Although potent tools, cell lines must be employed with circumspection. We showed that transcription factor expression—which is not a measure for activity—cannot be assumed similar between nonmalignant lineage selection and corresponding induced differentiation in leukemia cell lines. Additionally, a given agent can elicit variable transcription factor expression across different myeloid cell lines. The most mature cell line U937 exhibited transcription factor expression responses the most distinct from the other cells, while more similarly mature cells like NB4 and HL60 could be better coupled based on the same treatments. Generally combined RA + D3 treatment results in a mixed phenotype in the responsive cell lines; however, cell line-specific effects remain, e.g. RA potentiated D3-induced phenotypic changes in NB4 cells but not in wild-type or RA-resistant HL60 cells.
Authors contributions
Conceived and designed the experiments: HAJ. Performed the experiments: HAJ, HBY. Analyzed the data: HAJ, HBY, RPB, AY. Contributed reagents/materials/analysis tools: JDV, AY. Wrote the paper: HAJ, RPB, JDV, AY.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
NB4 cells were generously provided by David Sekula of Ethan Dmitrovsky’s laboratory (Dartmouth University). U937 cells were provided by Wee Ming of Tracy Stokol’s laboratory (Cornell University), who obtained them from the ATCC. K562 cells were provided by Jonathan Shrimp of Hening Lin’s laboratory (Cornell University), who obtained them from the ATCC. This work was supported by grants R01 CA033505, R01 CA152870 from NIH (A.Y.) and NIH/PS-OC (Shuler/A.Y./R.P.B.), NYSTEM NY Dept. Health (A.Y.), Cornell Vertebrate Genomics (VERGE) (R.P.B.), and CBET-0846876 from NSF (J.D.V./H.A.J.).
References
- 1.Bushue N., Wan Y.Y. Retinoid pathway and cancer therapeutics. Adv. Drug. Deliv. Rev. 2010;62:1285–1296. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang X., Gudas L.J. Retinoids, retinoic acid and cancer. Annu. Rev. Pathol. Mech. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 3.Cheung F.S., Lovicu F.J., Reichardt J.K. Current progress in using vitamin D and its analogs for cancer prevention and treatment. Expert Rev. Anticancer Ther. 2012;12(6):811–837. doi: 10.1586/era.12.53. [DOI] [PubMed] [Google Scholar]
- 4.Gocek E., Marchwicka A., Baurska H., Chrobak A., Marcinkowska E. Opposite regulation of vitamin D receptor by ATRA in AML cells susceptible and resistant to vitamin D-induced differentiation. J. Steroid Biochem Mol. Biol. 2012;132(3–5):220–226. doi: 10.1016/j.jsbmb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Tenen D.G., Hromas R., Licht J.D., Zhang D.E. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90(2):489–519. [PubMed] [Google Scholar]
- 6.Rosenbauer F., Tenen D.G. Transcription factors in myeloid development: balancing differentiation with transformation. Nat. Rev. Immunol. 2007;7(2):105–117. doi: 10.1038/nri2024. [DOI] [PubMed] [Google Scholar]
- 7.Friedman A.D. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007;26(47):6816–6828. doi: 10.1038/sj.onc.1210764. [DOI] [PubMed] [Google Scholar]
- 8.Lozzio C.B., Lozzio B.B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45(3):321–334. [PubMed] [Google Scholar]
- 9.Rücker F.G., Sander S., Döhner K., Döhner H., Pollack J.R., Bullinger L. Molecular profiling reveals myeloid leukemia cell lines to be faithful model systems characterized by distinct genomic aberrations. Leukemia. 2006;20(6):994–1001. doi: 10.1038/sj.leu.2404235. [DOI] [PubMed] [Google Scholar]
- 10.Andersson L.C., Nilsson K., Gahmberg C.G. K562–a human erythroleukemic cell line. Int. J. Cancer. 1979;23(2):143–147. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland J.A., Turner A.R., Mannoni P., McGann L.E., Turc J.M. Differentiation of K562 leukemia cells along erythroid, macrophage, and megakaryocyte lineages. J. Biol. Response Mod. 1986;5(3):250–262. [PubMed] [Google Scholar]
- 12.Koiso Y., Nakajima O., Matsumura D., Fujimoto Y., Hashimoto Y. Chemical control of cell differentiation of human myeloleukemia K562 cell line. Yakugaku Zasshi. 2000;120(1):104–112. doi: 10.1248/yakushi1947.120.1_104. [DOI] [PubMed] [Google Scholar]
- 13.Robertson K.A., Mueller L., Collins S.J. Retinoic acid receptors in myeloid leukemia: characterization of receptors in retinoic acid-resistant K-562 cells. Blood. 1991;77(2):340–347. [PubMed] [Google Scholar]
- 14.Munker R., Norman A., Koeffler H. Vitamin D compounds. Effect on clonal proliferation and differentiation of human myeloid cells. J. Clin. Invest. 1986;78:424–430. doi: 10.1172/JCI112593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontana J.A., Colbert D.A., Deisseroth A.B. Identification of a population of bipotent stem cells in the HL-60 human promyelocytic leukemia cell line. Proc. Natl. Acad. Sci. U.S.A. 1981;78(6):3863–3866. doi: 10.1073/pnas.78.6.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalton W.T., Ahearn M.J., McCredie K.B., Freireich E.J., Stass S.A., Trujillo J.M. HL-60 cell line was derived from a patient with FAB-M2 and not FAB-M3. Blood. 1988;71:242–247. [PubMed] [Google Scholar]
- 17.Jensen H.A., Styskal L.E., Tasseff R., Bunaciu R.P., Congleton J., Varner J.D. The Src-family kinase inhibitor PP2 rescues inducible differentiation events in emergent retinoic acid-resistant myeloblastic leukemia cells. PLoS One. 2013;8(3):e58621. doi: 10.1371/journal.pone.0058621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen H.A., Bunaciu R.P., Ibabao C.N., Myers R., Varner J.D., Yen A. Retinoic acid therapy resistance progresses from unilineage to bilineage in HL-60 leukemic blasts. PLoS One. 2014;9(6):e98929. doi: 10.1371/journal.pone.0098929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanotte M., Martin-Thouvenin V., Najman S., Balerini P., Valensi F., Berger R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3) Blood. 1991;77:1080–1086. [PubMed] [Google Scholar]
- 20.Drexler H.G., Quentmeier H., MacLeod R.A., Uphoff C.C., Hu Z.B. Leukemia cell lines: in vitro models for the study of acute promyelocytic leukemia. Leuk. Res. 1995;19(10):681–691. doi: 10.1016/0145-2126(95)00036-n. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher R.E. Retinoic acid resistance in acute promyelocytic leukemia. Leukemia. 2002;16:1940–1958. doi: 10.1038/sj.leu.2402719. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia M., Kirkland J.B., Meckling-Gill K.A. M-CSF and 1,25 Dihydroxyvitamin D3 synergize with 12-O-tetradecanoylphorbol-13-acetate to induce macrophage differentiation in acute promyelocytic leukemia NB4 cells. Leukemia. 1994;8:1744–1749. [PubMed] [Google Scholar]
- 23.Testa U., Grignani F., Barberi T., Fagioli M., Masciulli R., Ferrucci P.F. PML/RAR alpha+ U937 mutant and NB4 cell lines: retinoic acid restores the monocytic differentiation response to vitamin D3. Cancer Res. 1994;54(16):4508–4515. [PubMed] [Google Scholar]
- 24.Nakajima H., Kizaki M., Ueno H., Muto A., Takayama N., Matsushita H. All-trans and 9-cis retinoic acid enhance 1,25-dihydroxyvitamin D3-induced monocytic differentiation of U937 cells. Leuk. Res. 1996;20(8):665–676. doi: 10.1016/0145-2126(96)00020-3. [DOI] [PubMed] [Google Scholar]
- 25.Olsson I.L., Breitman T.R. Induction of differentiation of the human histiocytic lymphoma cell line U-937 by retinoic acid and cyclic adenosine 3’:5’-monophosphate-inducing agents. Cancer Res. 1982;42(10):3924–3927. [PubMed] [Google Scholar]
- 26.Defacque H., Commes T., Contet V., Sevilla C., Marti J. Differentiation of U937 myelomonocytic cell line by all-trans retinoic acid and 1,25-dihydroxyvitamin D3: synergistic effects on tissue transglutaminase. Leukemia. 1995;9(10):1762–1767. [PubMed] [Google Scholar]
- 27.Caudell D., Aplan P.D. The role of CALM-AF10 gene fusion in acute leukemia. Leukemia. 2008;22(4):678–685. doi: 10.1038/sj.leu.2405074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahl R., Walsh J.C., Lancki D., Laslo P., Iyer S.R., Singh H. Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPalpha ratio and granulocyte colony-stimulating factor. Nat. Immunol. 2003;4(10):1029–1036. doi: 10.1038/ni973. [DOI] [PubMed] [Google Scholar]
- 29.Laslo P., Spooner C.J., Warmflash A., Lancki D.W., Lee H.J., Sciammas R. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126:755–766. doi: 10.1016/j.cell.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 30.Bunaciu R.P., Yen A. Activation of the aryl hydrocarbon receptor AhR promotes retinoic acid-induced differentiation of myeloblastic leukemia cells by restricting expression of the stem cell transcription factor Oct4. Cancer Res. 2011;71:2371–2380. doi: 10.1158/0008-5472.CAN-10-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen M., Bunaciu R.P., Congleton J., Jensen H.A., Sayam L.G., Varner J.D. Interferon regulatory factor-1 binds c-Cbl, enhances mitogen activated protein kinase signaling and promotes retinoic acid-induced differentiation of HL-60 human myelo-monoblastic leukemia cells. Leuk. Lymphoma. 2011;52:2372–2379. doi: 10.3109/10428194.2011.603449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi S., Okabe-Kado J., Honma Y., Kawajiri K. Expression of Ah receptor (TCDD receptor) during human monocytic differentiation. Carcinogenesis. 1995;16(6):1403–1409. doi: 10.1093/carcin/16.6.1403. [DOI] [PubMed] [Google Scholar]
- 33.Matikainen S., Ronni T., Hurme M., Pine R., Julkunen I. Retinoic acid activates interferon regulatory factor-1 gene expression in myeloid cells. Blood. 1996;88(1):114–123. [PubMed] [Google Scholar]
- 34.Grande A., Montanari M., Manfredini R., Tagliafico E., Zanocco-Marani T., Trevisan F. A functionally active RARα nuclear receptor is expressed in retinoic acid non responsive early myeloblastic cell lines. Cell Death Differ. 2001;8(1):70–82. doi: 10.1038/sj.cdd.4400771. [DOI] [PubMed] [Google Scholar]
- 35.Luo X.M., Ross A.C. Retinoic acid exerts dual regulatory actions on the expression and nuclear localization of interferon regulatory factor-1. Exp. Biol. Med (Maywood) 2006;231(5):619–631. doi: 10.1177/153537020623100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D.Y., Choi S.J., Kim S.H., Chung H.Y., Yi S., Kim D.W. Upregulated hoxC4 induces CD14 expression during the differentiation of acute promyelocytic leukemia cells. Leuk. Lymphoma. 2005;46(7):1061–1066. doi: 10.1080/10428190500102589. [DOI] [PubMed] [Google Scholar]
- 37.Leupin N., Kuhn A., Hügli B., Grob T.J., Jaggi R., Tobler A. Gene expression profiling reveals consistent differences between clinical samples of human leukaemias and their model cell lines. Br. J. Haematol. 2006;135(4):520–523. doi: 10.1111/j.1365-2141.2006.06342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domcke S., Sinha R., Levine D.A., Sander C., Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tallman M.S., Altman J.K. How I treat acute promyelocytic leukemia. Blood. 2009;114(25):5126–5135. doi: 10.1182/blood-2009-07-216457. [DOI] [PubMed] [Google Scholar]
- 40.Zhou J.Y., Norman A.W., Akashi M., Chen D.L., Uskokovic M.R., Aurrecoechea J.M. Development of a novel 1,25(OH)2-vitamin D3 analog with potent ability to induce HL-60 cell differentiation without modulating calcium metabolism. Blood. 1991;78:75–82. [PubMed] [Google Scholar]
- 41.Rebel V.I., Ossenkoppele G.J., van de Loosdrecht A.A., Wijermans P.W., Beelen R.H., Langenhuijsen M.M. Monocytic differentiation induction of HL-60 cells by MC 903, a novel vitamin D analogue. Leuk. Res. 1992;16:443–451. doi: 10.1016/0145-2126(92)90169-8. [DOI] [PubMed] [Google Scholar]
- 42.Gocek E., Kiełbiński M., Marcinkowska E. Activation of intracellular signaling pathways is necessary for an increase in VDR expression and its nuclear translocation. FEBS Lett. 2007;581:1751–1757. doi: 10.1016/j.febslet.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 43.Manfredini R., Trevisan F., Grande A., Tagliafico E., Montanari M., Lemoli R. Induction of a functional vitamin D receptor in all-trans-retinoic acid-induced monocytic differentiation of M2-type leukemic blast cells. Cancer Res. 1999;59(15):3803–3811. [PubMed] [Google Scholar]
- 44.Bastie J.N., Balitrand N., Guidez F., Guillemot I., Larghero J., Calabresse C. 1 alpha,25-dihydroxyvitamin D3 transrepresses retinoic acid transcriptional activity via vitamin D receptor in myeloid cells. Mol. Endocrinol. 2004;18(11):2685–2699. doi: 10.1210/me.2003-0412. [DOI] [PubMed] [Google Scholar]
- 45.Bastie J.N., Balitrand N., Guillemot I., Chomienne C., Delva L. Cooperative action of 1alpha, 25-dihydroxyvitamin D3 and retinoic acid in NB4 acute promyelocytic leukemia cell differentiation is transcriptionally controlled. Exp. Cell Res. 2005;310(2):319–330. doi: 10.1016/j.yexcr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Barber N., Belov L., Christopherson R.I. All-trans retinoic acid induces different immunophenotypic changes on human HL60 and NB4 myeloid leukaemias. Leuk. Res. 2008;32(2):315–322. doi: 10.1016/j.leukres.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 47.van der Meer L.T., Jansen J.H., van der Reijden B.A. Gfi1 and Gfi1b: key regulators of hematopoiesis. Leukemia. 2010;24(11):1834–1843. doi: 10.1038/leu.2010.195. [DOI] [PubMed] [Google Scholar]
- 48.Gibbs J.D., Liebermann D.A., Hoffman B. Leukemia suppressor function of Egr-1 is dependent on transforming oncogene. Leukemia. 2008;22(10):1909–1916. doi: 10.1038/leu.2008.189. [DOI] [PubMed] [Google Scholar]
- 49.Tokura Y., Shikami M., Miwa H., Watarai M., Sugamura K., Wakabayashi M. Augmented expression of P-gp/multi-drug resistance gene by all-trans retinoic acid in monocytic leukemic cells. Leuk Res. 2002;26(1):29–36. doi: 10.1016/s0145-2126(01)00094-7. [DOI] [PubMed] [Google Scholar]
- 50.Gocek E., Baurska H., Marchwicka A., Marcinkowska E. Regulation of leukemic cell differentiation through the vitamin D receptor at the levels of intracellular signal transduction, gene transcription, and protein trafficking and stability. Leuk. Res. Treat. 2012;2012:713243. doi: 10.1155/2012/713243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chomienne C., Balitrand N., Ballerini P., Castaigne S., de The H., Degos L. All-trans retinoic acid modulates the retinoic acid receptor-alpha in promyelocytic cells. J. Clin. Invest. 1991;88:2150. doi: 10.1172/JCI115547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu X., Zhang H., Qian M., Zhao X., Yang W., Wang P. The significance of low PU.1 expression in patients with acute promyelocytic leukemia. J. Hematol. Oncol. 2012;5:22. doi: 10.1186/1756-8722-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mueller B.U., Pabst T., Fos J., Petkovic V., Fey M.F., Asou N. ATRA resolves the differentiation block in t(15;17) acute myeloid leukemia by restoring PU.1 expression. Blood. 2006;107(8):3330–3338. doi: 10.1182/blood-2005-07-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Alò F., Di Ruscio A., Guidi F., Fabiani E., Greco M., Rumi C. PU.1 and CEBPA expression in acute myeloid leukemia. Leuk. Res. 2008;32(9):1448–1453. doi: 10.1016/j.leukres.2008.01.007. [DOI] [PubMed] [Google Scholar]