Abstract
Background
Congenital long QT syndrome (LQTS) is a genetically transmitted cardiac channelopathy that can lead to lethal arrhythmia and sudden cardiac death in healthy young people. The clinical characteristics of LQTS are variable and depend on the subtype of long QT syndrome, which differ among populations. This single hospital-based case review study examined the clinical presentation of long QT syndrome and the outcomes of its treatment in 20 Thai children at King Chulalongkorn Memorial Hospital in Bangkok, Thailand.
Methods
Inpatient and outpatient records of children (aged 0–14 years) diagnosed with long QT syndrome from January 1, 1998, to September 30, 2013, were retrospectively reviewed. Presentation at diagnosis, treatments, and clinical courses were collected and analyzed. In the 20 subjects, total Schwartz scores totaled 5.2±0.9 points, and mean age at diagnosis was 7.6±4.4 years (range, 1 day–13.8 years). The patients were assigned to one of 3 groups based on trigger events: 50% of patients had events at rest (sleep or at rest), 35% experienced adrenergic-mediated events (e.g., stress, exercise, startle), and 15% were asymptomatic. Excluding the 3 patients who died at first presentation, 100% of patients received a beta blocker, and 47.1% were treated with an automatic implantable cardioverter-defibrillator (AICD).
Results
At follow-up (median=959 days; range, 1–4170 days), 4 patients (20%) were known to have died, 3 of whom died shortly after the diagnosis. Among patients who survived the initial event, 52.9% (9 of 17) experienced cardiac events (appropriate AICD shock, death, and/or syncope) during the follow-up period. The mean duration from diagnosis to cardiac event was 1420±759 days (range, 497–2499 days).
Conclusions
All 20 patients with LQTS were mostly symptomatic at presentation. Owing to the geographical region and ethnicity of the Thai population, we conclude that the ratio of patients who develop cardiac symptoms at rest or during sleep might be higher than in other Asian countries.
Keywords: Cardiac arrest, Cardiac arrhythmia, Cardiac conditions of childhood, Congenital long QT syndrome, Syncope, Thai children
1. Introduction
Congenital long QT syndrome (LQTS) is an inheritable cardiac condition that may lead to fatal cardiac arrhythmia (e.g., torsades de pointes, ventricular fibrillation). Typical presentation consists of syncope, seizure, or even sudden death in otherwise healthy young people. A large neonatal screening study from Italy reported the prevalence of long QT syndrome to be at least 1 in 2500 [1], but the true prevalence of LQTS in other populations is still unknown. Some studies suggest that LQTS might be an underrecognized rather than a rare disease [2]. This underestimation might be the result of a large number of silent-mutation carriers in the total population.
LQTS is classified into subtypes LQT1 to LQT13 based on the site of genetic mutation. Each type possesses distinct clinical characteristics and a different prognosis. Treatment choice depends partly on individual phenotype and partly on genotype. Among Asian countries, several reports have been published on the clinical characteristics and course of LQTS patients from Japan, Korea, and China, but reports from other regions are rare [3–6].
Our study examined the clinical presentation and outcome of 20 LQTS patients who presented to the Pediatric Department at King Chulalongkorn Memorial Hospital (KCMH) in Bangkok, Thailand, between 1998 and 2013.
2. Materials and methods
2.1. Data collection
This study is a single hospital-based case review. Our study population derived from patients who presented to the Pediatric Department at KCMH with the diagnosis of congenital LQTS from January 1, 1998, to September 30, 2013. Demographic data, family history, clinical presentation, echocardiogram findings, and others special investigations, treatments, and outcomes were collected. Data were reviewed from outpatient and inpatient records at KCMH. The ethics committee of the Faculty of Medicine at Chulalongkorn University approved this study. Informed consent was obtained from all surviving patients at the time of this study.
2.2. Diagnosis
The diagnosis of LQTS was made based on clinical background, using the 2013 HRS/EHRS/APHRS consensus guidelines [7]. Only patients who were diagnosed as having the congenital form of LQTS were included. Those with cardiac arrest and/or a prolonged QT interval from an identifiable cause, such as myocardial ischemia, trauma, infection, electrolyte abnormalities, known history of exposure to QT-prolonging medications, or known congenital heart disease, were excluded. QT interval was measured in lead II or V5 and corrected for differences in heart rate using Bazett׳s formula [8].
2.3. Patients׳ clinical characteristics grouped by trigger events
The patients were divided into 3 groups based on trigger events: those who experienced events occurring at rest (group 1), those with adrenergic-mediated events (e.g., stress, exercise, and startle) (group 2), and those who were asymptomatic (group 3).
2.4. Statistical analysis
Continuous data were expressed as mean±SD, and nominal data were expressed as percentages. Among the 3 groups, one-way ANOVA was used to compare continuous data, and Chi-square analysis was used to compare nominal data. Treatment outcomes were presented using Kaplan–Meier survival curves, which showed the cumulative event rates for death, cardiac arrest/ventricular arrhythmia, and/or syncope. A log-rank test was used to compare the groups׳ cumulative-event-free survival curves.
3. Results
3.1. Clinical characteristics
The clinical characteristics of the study subjects are summarized in Table 1.
Table 1.
Clinical characteristics of the study subjects (N=20).
| Study subjects (N=20) | n | % of total |
|---|---|---|
| Male | 9 | 45.0 |
| Age at diagnosis (years) | Mean (±SD) | 7.6 (±4.4) |
| range | 0–13.8 | |
| Comorbid conditions | 2 | 10.0 |
| Mental retardation | 2 | 10.0 |
| Congenital anomalies | 4 | 20.0 |
| Double aortic arch | 1 | 5.0 |
| Left ventricular aneurysm | 1 | 5.0 |
| SNHL BE | 2 | 10.0 |
| Family history | ||
| Definite LQTS | 4 | 20.0 |
| SCD | 4 | 20.0 |
| SNHL | 2 | 10.0 |
| Presenting symptoms | ||
| Asymptomatic | 3 | 15.0 |
| Stress-related | 7 | 35.0 |
| Sleep/rest-related | 10 | 50.0 |
| Total Schwartz score (points) | Mean (±SD) | 5.2 (± 0.9) |
| range | 4–7 | |
| Electrocardiographic characteristics | ||
| Torsades de pointes | 3 | 15.0 |
| T-wave alternans | 1 | 5.0 |
| Notched T wave | 5 | 25.0 |
| Giant negative T wave | 3 | 15.0 |
| Bradycardia | 6 | 30.0 |
| QTc (ms) | Mean (±SD) | 548.1 (±61.5) |
| range | 465–707 | |
| HR (bpm) | Mean (±SD) | 79.9 (±21.4) |
| Range | 46–120 | |
| Treatments (n=17; excluding patients who died shortly after diagnosis) | ||
| Beta blocker | 17 | 100.0 |
| Atenolol | 8 | 47.1 |
| Propanolol | 9 | 52.9 |
| AICD | 8 | 47.1 |
| Current status (n=20) | ||
| Alive | 15 | 75.0 |
| Died | 4 | 20.0 |
| Lost to follow-up | 1 | 5.0 |
BE=both ears; SNHL, sensorineural hearing loss; SCD, sudden cardiac death; QTc, corrected QT interval; bpm, beat per minute; HR, heart rate; AICD, automatic implantable cardioverter-defibrillator.
A total of 20 patients were identified, most of whom had no comorbid conditions. Nine patients (45%) were male. The mean age at diagnosis was 7.6±4.4 years (range, 1 day–13.8 years). The subjects׳ total Schwartz score at presentation was 5.2±0.9 points (range, 4–7 points). All patients were categorized as having a high probability of LQTS based on their Schwartz score (≥4 points).
Congenital anomalies were noted in 4 subjects (20%). Two subjects had congenital sensorineural hearing loss, and after echocardiogram was performed in all patients to exclude structural heart disease, 2 were found to have a left ventricular aneurysm (1 patient) and a double aortic arch (1 patient). Coronary angiography was performed in the patient with left ventricular aneurysm and the result was normal. Two patients had comorbid mental retardation. The number of subjects with a family history of definite LQTS was 4 (20%), and an additional 4 patients (20%) had a family history of unexplained sudden cardiac death.
3.2. Electrocardiographic characteristics
The mean QTc interval at the first presentation was 548.1±61.5 ms (range 465–707), and the mean heart rate was 79.9±21.4 bpm (range 46–120 bpm). Six patients (30%) had bradycardia compared with age-matched normal individuals. Torsades de pointes was documented in 3 patients (15%). Notched T wave in at least 3 leads (Fig. 1A) was found in 5 patients (25%). One patient (5%) had T-wave alternans (Fig. 1B). Giant negative T-wave with prolonged QT intervals was observed in 3 patients (15%) (Fig. 1C).
Fig. 1.
Example of T-wave abnormalities in the study subjects. (A) Notched T-wave; QTc interval=623 ms (group 2: catecholamine mediated). (B) T-wave alternans; QTc interval=764 ms (group 1: events at rest/sleep). (C) Giant negative T-wave; QTc interval=723 ms (group 1: events at rest/sleep).
3.3. Triggers for cardiac events
Among the 20 patients, cardiac symptoms developed at rest or sleep in 10 (50%) and during stress in 7 (35%). The remaining 3 patients (15%) were asymptomatic at presentation: 1 patient was diagnosed based on a family screening (a sibling with congenital LQTS with sensorineural hearing loss), 1 patient was diagnosed because of fetal bradycardia, and 1 patient presented with bradycardia compared with age-matched normal individuals.
3.4. Treatment and outcome
Excluding the 3 patients who died shortly after presentation, all 17 patients (100%) received a beta blocker; 8 patients received atenolol, and 9 patients received propanolol. One patient was prescribed phenytoin (Dilantin) after a standard dose of propranolol (5 mg/kg/day) failed to control ventricular arrhythmia. Mexiletine is not available in Thailand; therefore, no patient was administered this medication. Eight patients (47.1%) were treated with automated implantable cardioverter-defibrillators (AICDs); 7 of these 8 patients presented with ventricular arrhythmia during sleep. In this retrospective study, 1 patient (5%) was lost to follow-up after the initial clinic visit, but the remaining 19 patients׳ follow-up data were available.
Four patients (20%) had died at the time of this report. Three died shortly after the diagnosis (duration from diagnosis to death was <1 day for 2 patients and 48 days for 1 patient). One patient treated with an AICD died 2499 days after the diagnosis and had been lost to follow-up for 4.3 years before death. Nine of 20 patients (45%) experienced cardiac events during the follow-up period. Excluding the 3 patients who died shortly after diagnosis, the mean time to cardiac event was 1420±759 days (range, 497–2499 days).
3.5. Patients׳ clinical characteristics grouped by trigger events
The patients were divided into 3 groups: 10 patients (50%) who had cardiac events occurring at rest (group 1), 7 patients (35%) with adrenergic-mediated events (group 2), and 3 patients (15%) who were asymptomatic (group 3). The clinical characteristics of each group are shown in Table 2.
Table 2.
Clinical characteristics of subjects based on trigger events (N=20).
| Subjects (N=20) | Group 1 events at rest or sleep | % | Group 2 catecholamine mediated | % | Group 3 asymptomatic | % | P-value |
|---|---|---|---|---|---|---|---|
| n | 10 | 7 | 3 | ||||
| Male | 7 | 70.0 | 1 | 14.3 | 1 | 33.3 | 0.069 |
| Age at diagnosis (years) | Mean (±SD) | Range | Mean (±SD) | Range | Mean (±SD) | Range | |
| 9.1 (±4.2) | 0.8–13.9 | 7.3 (±4.2) | 0.1–12.3 | 3.5 (±3.3) | 0–6.5 | 0.149 | |
| Comorbid conditions | 2 | 20.0 | 0 | 0.0 | 0 | 0.0 | 0.329 |
| Mental retardation | 2 | 20.0 | 0 | 0.0 | 0 | 0.0 | |
| Congenital anomalies | 1 | 10.0 | 1 | 14.3 | 2 | 66.7 | 0.088 |
| Double aortic arch | 1 | 10.0 | 0 | 0.0 | 0 | 0.0 | |
| Left ventricular aneurysm | 0 | 0.0 | 1 | 14.3 | 0 | 0.0 | |
| SNHL BE | 0 | 0.0 | 0 | 0.0 | 2 | 66.7 | |
| Family history | |||||||
| Definite LQTS | 2 | 20.0 | 0 | 0.0 | 2 | 66.7 | 0.054 |
| SCD | 4 | 40.0 | 0 | 0.0 | 0 | 0.0 | 0.082 |
| SNHL | 0 | 0.0 | 0 | 0.0 | 2 | 66.7 | 0.002 |
| Total Schwartz score (points) | Mean (±SD) | Range | Mean (±SD) | Range | Mean (±SD) | Range | |
| 5.1 (±0.88) | 4–6.5 | 5.6 (±0.85) | 5–7 | 4.5 (±0.0) | 4.5 | 0.139 | |
| Electrocardiographic characteristics | |||||||
| Torsades de pointes | 1 | 10 | 2 | 28.6 | 0 | 0.0 | 0.420 |
| T-wave alternans | 1 | 10 | 0 | 0.0 | 0 | 0.0 | 0.591 |
| Notched T wave | 3 | 30 | 1 | 14.3 | 1 | 33.3 | 0.714 |
| Giant negative T wave | 2 | 20 | 0 | 0.0 | 1 | 33.3 | 0.329 |
| Bradycardia for age | 4 | 40 | 1 | 14.3 | 1 | 33.3 | 0.518 |
| Mean (±SD) | Range | Mean (±SD) | Range | Mean (±SD) | Range | ||
| QTc (ms) | 550.3 (±65.3) | 465–707 | 535.9 (±69.1) | 467–650 | 527.3 (±40.9) | 494–573 | 0.879 |
| HR (bpm) | 72.4 (±17.9) | 46–120 | 84.3 (±25.4) | 56–120 | 94.7 (±17.6) | 75–109 | 0.239 |
| Treatments (N=17) (excluding 3 patients who died shortly after diagnosis) | |||||||
| Beta blocker | 10 | 100.0 | 4 | 100.0 | 3 | 100.0 | 0.871 |
| Atenolol | 5 | 50.0 | 2 | 50.0 | 1 | 33.3 | |
| Propanolol | 5 | 50.0 | 2 | 50.0 | 2 | 66.7 | |
| AICD | 7 | 70.0 | 1 | 25.0 | 0 | 0 | 0.062 |
| Current status | 0.056 | ||||||
| Alive | 9 | 90.0 | 4 | 57.1 | 2 | 66.7 | |
| Died | 1 | 10.0 | 3 | 42.9 | 0 | 0.0 | |
| Lost to follow-up | 0 | 0.0 | 0 | 0.0 | 1 | 33.3 | |
| Events | |||||||
| Available data | 10 | 100.0 | 7 | 100.0 | 2 | 66.7 | |
| Died shortly after diagnosis | 0 | 0 | 3 | 42.9 | 0 | 0 | |
| Death, syncope, AICD shock | 7.0 | 70.0 | 0 | 0.0 | 2 | 66.7 | 0.052 |
| (excluding patients who died shortly after diagnosis) | Mean (±SD) | Range | Mean (±SD) | Range | Mean (±SD) | Range | |
| Time to event (days) | 1109.1 (±693.3) | 267–2499 | 1820.0 (±1784.5) | 9–4170 | 2195.0 (±1276.0) | 0–2225 | 0.861 |
| Duration until last follow-up or death (days) | 1277.7 (±836.0) | 267–2768 | 1820.0 (±1784.5) | 9–4170 | 1876.7 (±1625.3) | 0–2830 | 0.653 |
BE=Both ears; SNHL, sensorineural hearing loss; SCD, sudden cardiac death; QTc, corrected QT interval; bpm, beats per minute; HR, heart rate; AICD, automatic implantable cardioverter-defibrillator.
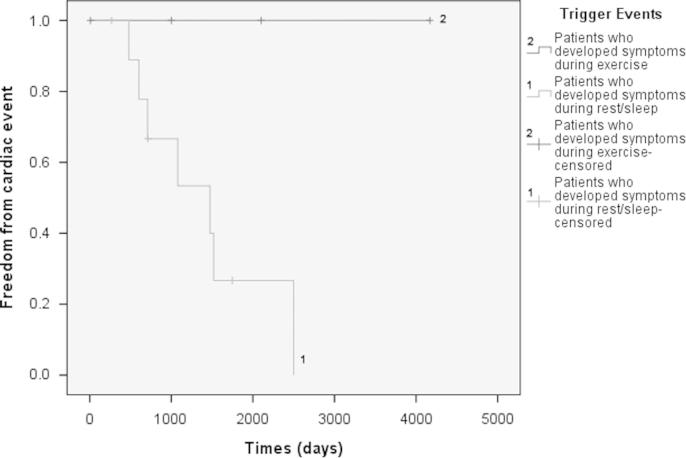
Patients who experienced events at rest (group 1) were older at presentation (P=0.149) and were more likely to be male (P=0.069), though these differences were not statistically significant. The corrected QT interval, incidence of T-wave abnormalities, and Schwartz score did not significantly differ among the 3 groups. Patients who developed symptoms during rest/sleep (group 1) underwent more AICD implantations than the other groups (P=0.062). Event-free survival times after treatment in symptomatic patients are shown in Fig. 2. Note that none of the group 2 patients developed symptoms of cardiac event after treatment with a beta blocker, whereas in group 1 patients, the incidence of recurrent cardiac events was much higher; however, the difference was not statistically significant (P=0.057).
Fig. 2.
Kaplan–Meier curve demonstrating freedom from cardiac events (death, appropriate AICD discharge, and/or syncope) from the time of diagnosis in symptomatic patients (excluding patients who died shortly after diagnosis) divided into groups according to triggered event. Curve 1=patients who developed symptoms during rest/during sleep (group 1). Curve 2=patients who developed symptoms during exercise/adrenergic-mediated events (group 2).
4. Discussion
Cardiac ion channels, including potassium, calcium, and sodium, are crucial for transporting ions across cell membranes, resulting in cardiac depolarization and repolarization. Defective ion channels can lead to abnormal repolarization, prolonged QT interval, and ventricular tachyarrhythmia.
Multiple genes encoding these cardiac ion channels have been identified as causes of congenital LQTS, which has been historically divided into two main subtypes: Romano–Ward syndrome (autosomal dominant) and Jervell and Lange–Nielsen syndrome (autosomal recessive with neurosensory deafness) [7,9]. Both subtypes are now further subclassified based on genotype. This classification system provides an important guide for selecting proper treatment and preventive method for specific patients. Unfortunately, clinical presentation, heart rate, QTc interval, and Schwartz score cannot be used with certainty to differentiate among subtypes of LQTS [10]. Instead, ECG phenotypes have been used to predict the genotypes of LQTS based on differentiate T-wave morphology and ST-T segment. However, the application of this method is not specific to all types of LQTS [11].
4.1. Genotype–phenotype correlation of LQTS
Romano–Ward syndrome is the most common form of LQTS. It is classified numerically as LQT1 to LQT13 according to various sites of mutation in the gene loci. LQT1, LQT2, and LQT3 account for 92% of cases in which gene mutations are found [7]. LQT1 features a heterogeneous mutation in the KCNQ1 gene, which encodes the alpha subunit of the cardiac slow delayed rectifier potassium channel. Most arrhythmia events are triggered by strong emotional stimuli or exercise. LQT2 is characterized by a defect in the KCNH2 gene, which encodes the alpha subunit of the rapid delayed rectifier potassium channel. Ventricular arrhythmia in patients with LQT2 is often triggered by strong emotional or auditory stimuli. LQT3 features a defect in the SCN5A gene, which causes a gain-of-function mutation in the cardiac sodium channel. Patients with LQT3 often develop cardiac arrhythmia during rest or sleep.
Genetic screening by sequencing the protein-coding region of the genes responsible for LQTS is the best method of differentiating among genotypes. Genetic testing has shown to be beneficial in the prognostication, diagnosis, and treatment of patients with LQTS [7,12]. However, genetic testing is not always available in Thailand and many other developing countries. Therefore, data on the phenotype (clinical presentation, ECG findings, etc.) may be used as an alternative in order to elicit differences in LQTS subtype among Thai patients and those in other developing countries; these data were not previously available for Thailand.
Studies from Caucasian populations [1] as well as Northern Asian countries [3,6,9] have demonstrated that the LQT1 and LQT2 genotypes of LQTS are more common than LQT3. For example, a study of 54 patients from a Chinese registry using ECG (morphology of ST-T wave) criteria suggested that the LQT1 and LQT2 phenotypes are more common than the LQT3 phenotype [5]. In this study, cardiac events were more frequently triggered by exercise (50.0%) or emotional stress (41.7%) than by rest or sleep (14.8%). These results contradict with our finding that the proportion of Thai children with LQTS who develop symptoms during rest/sleep (50%) is higher than that of patients who present with stress-induced events (35%). This discrepancy might be because of the ethnic differences between our population and those in other Asian countries.
According to the phenotype and genotype correlation, most patients with LQT1 experienced cardiac events after catecholamine-mediated triggers, such as exercise or emotional stress. Patients with LQT2 developed cardiac events after startle, auditory stimuli, or even at rest. Those with LQT3 were more likely to develop symptoms during sleep. We hypothesize that the subtypes of LQTS in our population might differ from those in other Asian countries. Further studies to investigate the genotypes of our LQTS patients are needed to answer these questions.
4.2. Study limitations
Among its limitations, first, our study was based on single-hospital data; therefore, generalizing its results to the entire Thai population may not seem appropriate. However, our hospital is one of the major referral centers for children with cardiac arrhythmia in Thailand, and our patients came from all parts of the country. Thus, our data are representative of the Thai population.
Second, the number of patients was small, as only those who presented to the hospital were included. However, this population may not include those patients who did not receive medical attention and/or those who were asymptomatic or minimally symptomatic.
Third, classification of LQTS patients based on phenotype has important limitations for some subtypes, such as LQT2. In these patients, either rest or exercise (including strong emotional or auditory stimuli) can trigger arrhythmia. Thus, further genetic testing would be beneficial in our cohort.
5. Conclusion
Our study is the first to describe the clinical characteristics, treatment, and outcomes of congenital LQTS patients in Thailand. Most patients in our study developed cardiac symptoms at rest or during sleep, which was higher than that reported in other countries. Further studies are needed to identify the genotypes of people with LQTS in the Thai population.
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- 1.Schwartz P.J., Stramba-Badiale M., Crotti L. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elena V.Z., Hugues A. Prevalence of significant genetic variants in congenital long QT syndrome is largely underestimated. Front Pharmacol. 2012;3:1–3. doi: 10.3389/fphar.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiraoka M. Inherited arrhythmic disorders in Japan. J Cardiovasc Electrophysiol. 2003;14:431–434. doi: 10.1046/j.1540-8167.2003.02435.x. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi K., Fujino N., Uchiyama K. Long QT syndrome and associated gene mutation carriers in Japanese children: results from ECG screening examinations. Clin Sci. 2009;117:415–424. doi: 10.1042/CS20080528. [DOI] [PubMed] [Google Scholar]
- 5.Li C., Hu D., Qin X. Clinical features and management of congenital long QT syndrome: a report on 54 patients from a national registry. Heart Vessels. 2004;19:38–42. doi: 10.1007/s00380-003-0733-9. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y.S., Kwon B.S., Kim G.B. Long QT syndrome: a Korean single center study. J Korean Med Sci. 2013;28:1454–1460. doi: 10.3346/jkms.2013.28.10.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Priori S.G., Wilde A.A., Horie M. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Qiu H., Bird G.L., Qu L. Evaluation of QT interval correction methods in normal pediatric resting ECGs. Comput Cardiol. 2007;34:431–434. [Google Scholar]
- 9.Horigome H., Nagashima M., Sumitomo N. Clinical characteristics and genetic background of congenital long-QT syndrome diagnosed in fetal, neonatal, and infantile life: a nationwide questionnaire survey in Japan. Circ Arrhythm Electrophysiol. 2010;3:10–17. doi: 10.1161/CIRCEP.109.882159. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi T., Shimizu W., Itoh H. Age- and genotype-specific triggers for life-threatening arrhythmia in genotyped long QT syndrome. J Cardiovasc Electrophysiol. 2008;19:794–799. doi: 10.1111/j.1540-8167.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 11.Zareba W. Genotype-specific ECG patterns in long QT syndrome. J Electrocardiol. 2006;39:S101–S106. doi: 10.1016/j.jelectrocard.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Zareba W., Moss A.J., Schwartz P.J. Influence of genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N Engl J Med. 1998;339:960–965. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]