Abstract
Background
Catheter ablation (CA) is an established therapy for atrial fibrillation (AF). However, the assessment of anatomical information and predictors of AF recurrence remain unclear. We investigated the relationship between anatomical information on the left atrium (LA) and pulmonary veins (PVs) from three-dimensional computed tomography images and the recurrence of AF after CA.
Methods
Sixty-seven consecutive AF patients (mean age: 62±10 years, median AF history: 42 (12; 60) months, mean LA size: 41±7 mm, paroxysmal: 56%) underwent CA and were followed for 19±10 months. The segmented surface areas (antral, posterior, septal, and lateral) and dimensions (between the anterior and posterior walls, the right inferior PV and mitral annulus [MA], the right superior PV and MA, the left superior PV and MA, and the mitral isthmus) of the LA were evaluated three dimensionally using the NavX system. The cross-sectional areas of the PVs were also evaluated.
Results
After the follow-up period, 49 patients (73%) remained free from AF. A multivariate analysis showed that the diameter of the mitral isthmus and cross-sectional area of the right upper PV were associated with AF recurrence (odds ratio: 1.070, CI: 1.02–1.12, p=0.001; odds ratio: 0.41, CI: 0.21–0.77, p=0.006).
Conclusion
Enlargement of the mitral isthmus and a smaller right superior PV cross-sectional area were associated with AF recurrence.
Abbreviations: LA, left atrium; PV, pulmonary vein; 3D-CT, three-dimensional computed tomography; AF, atrial fibrillation; MA, mitral annulus
Keywords: Computed tomography, Left atrium, Pulmonary vein, Atrial fibrillation, Catheter ablation
1. Introduction
Catheter ablation (CA) is a curative treatment option for patients with atrial fibrillation (AF) [1–3]. Reported rates of recurrence following pulmonary vein isolation (PVI) vary according to AF type, lesion concept, operator experience, technical equipment used, and the quality of follow-up [4–8]. Over the past two decades, the procedure has evolved from the ablation of focal AF triggers inside the pulmonary veins (PVs) to wide-area circumferential PV antrum isolation (PVAI) [4,5]. Data concerning the predictors of AF recurrence are limited. In particular, anatomical predictors as assessed by three-dimensional computed tomography (3D-CT) imaging are not well established [9–12]. In the present study, we sought to determine whether anatomical variables of the left atrium (LA) and PVs assessed by 3D-CT imaging were associated with the recurrence of AF after CA.
2. Materials and methods
2.1. Patient selection
This study enrolled 67 consecutive patients with highly symptomatic, medically refractory AF who had been treated with CA. Paroxysmal AF was defined as self-terminating AF of <1 week in duration. The exclusion criteria were as follows: (1) significant renal dysfunction and/or heart failure and (2) AF ablation performed without image integration.
2.2. Ablation procedure and procedural endpoint
Prior to the procedure, transesophageal echocardiography was performed to exclude thrombus formation. Patients were studied under deep propofol or dexmedetomidine sedation while breathing spontaneously. Unfractionated heparin was administered in bolus form before the transseptal puncture to maintain an activated clotting time >300 s. In case of AF, internal electrical cardioversion was performed to restore sinus rhythm. Mapping and ablation were performed under the guidance of a NavX system (St. Jude Medical, Inc.) after the integration of a three-dimensional (3D) model of LA and PV anatomy obtained from a pre-interventional CT. Prior to the ablation, the ablation catheter (IBI Therapy Cooled Path, St. Jude Medical, Inc.) and reconstructed LA posterior anatomies were aligned with the CT image [13]. A radiofrequency (RF) alternating current was delivered in the unipolar mode between the irrigated tip electrode of the ablation catheter and an external back-plate electrode. The initial RF generator settings were: an upper catheter tip temperature of 43 °C, maximal RF power of 30 W, and an irrigation flow rate of 13 ml/min. During RF applications to the posterior wall, the maximal RF power was initially set to 20 W. All patients underwent PVI. RF applications could be performed in a ‘point-by-point’ manner. The maximum time at the anterior and posterior walls was 40 and 20 s, respectively. When ablating the posterior wall, RF energy was routinely reduced by 10 W according to the esophageal temperature measured with an esophageal temperature probe (SensiTherm, St. Jude Medical). If the esophageal temperature exceeded 39 °C, the ablation was stopped immediately and the energy reduced further. Once the esophageal temperature decreased to the normal range (37 °C), RF application was resumed. If ablation could not be performed with 20 W of power, the line was placed either more antrally or closer to the PV, depending on individual anatomical findings. Catheter navigation was carried out using a steerable sheath (Agilis, St. Jude Medical) [14].
The procedural endpoint was the electrophysiologically proven bidirectional block of the PV-encircling ablation lines confirmed with a circular mapping catheter (Optima, St. Jude Medical). After proving the bidirectional block of the PVs, we executed a stimulation protocol (burst pacing from the coronary sinus at 300 ms, 250 ms, and 200 ms for 10 s each) to test inducibility. If AF was induced, the patient was cardioverted and the procedure ended. Pharmacological testing (high dose isoproterenol infusion: 20 µg/min) was performed to identify non-PV triggers. If an AF trigger from the superior vena cava (SVC) was documented, SVC isolation was performed. Ablation of the cavotricuspid isthmus or linear lesion was performed if a typical right atrial flutter or macroreentrant atrial flutter were induced by burst pacing, respectively. Proton pump inhibitors were started at admission and continued for 4 weeks, and esophagoscopy was repeated after 3–4 days if mucosal ulcerations >10 mm were observed initially [15–17].
2.3. Measurement of anatomical parameters by 3D-CT
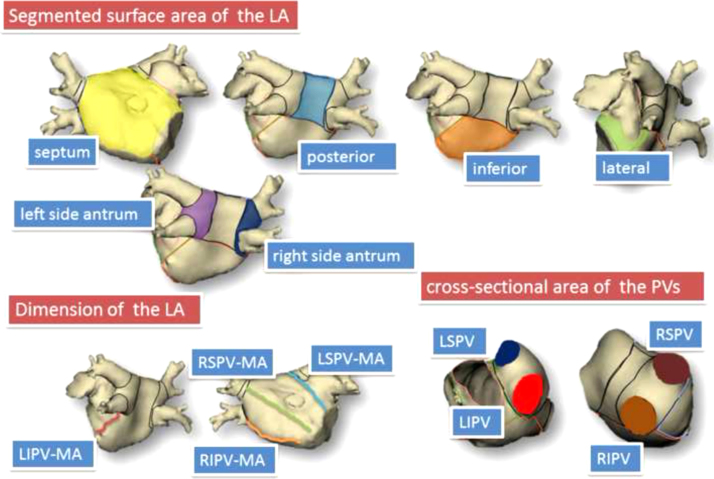
Preoperative CT data were transferred to the NavX system for analysis. The following variables were measured using the NavX Verismo software: the segmented surface area of the LA (cm2) (the antral, posterior, septal, and lateral areas, left-sided antrum, and right-sided antrum); cross-sectional PV area (cm2) (left superior PV [LSPV], left inferior PV [LIPV], right superior PV [RSPV], right inferior PV [RIPV]); LA dimensions (mm) (distance between the anterior and posterior walls, the RSPV and mitral annulus [MA], the RIPV and MA, the LSPV and MA, and the LIPV and MA) (Fig. 1). Patients with a common left PV and/or right middle PV were excluded from this NavX system measurement. The cross-sectional areas of the PVs were measured at the “ostium” level. The PV ostium was defined as the point of maximal inflection between the PV wall and the LA wall. As described in a previous report, the NavX system automatically calculates the PV cross-sectional area using a manually selected PV ostium. The distance between the PVs and MA was defined as the shortest distance between the selected point of the PV ostium and the MA.
Fig. 1.
Anatomical variables assessed by the NavX Verismo. The yellow, light blue, orange, green, purple, and blue areas indicate, respectively, the surface areas of the septal wall, posterior wall, inferior wall, lateral wall, left-sided antrum, and right-sided antrum. The red, orange, green, and light blue lines indicate, respectively, the distances between the LIPV and MA, the RIPV and MA, the RSPV and MA, and the LSPV and MA. The blue, red, brown, and light brown areas indicate, respectively, the cross-sectional areas of the LSPV, LIPV, RSPV, and RIPV. LA=left atrium, LSPV=left superior pulmonary vein, LIPV=left inferior pulmonary vein, RSPV=right superior pulmonary vein, RIPV=right inferior pulmonary vein, MA=mitral annulus.
2.4. Follow-up
Anti-arrhythmic treatment was discontinued post-interventionally and beta-blockers were administered to all patients. After treatment, patients received oral anticoagulation for 6 months (target INR: 2.0–3.0) or longer, depending on individual stroke risk as assessed using the CHADS2 score. In all patients, 24-h Holter electrocardiograms (SCM-6600; Fukuda Denshi) were obtained after 3, 6, 12, 24, and 36 months. If symptoms occurred outside the recording period, patients were asked to contact our center or the referring physician to obtain ECG documentation. AF and/or any atrial tachycardia (AT) episodes lasting longer than 30 s were considered recurrences.
2.5. Statistics
The data were evaluated using the Kolmogorov–Smirnov test and are presented as the mean±standard deviation for normally distributed variables. Medians and quartiles are given for non-normally distributed variables. Categorical variables are expressed as numbers and percentages. Cox proportional hazards regression models were used to estimate hazard ratios and 95% confidence intervals for AF/AT recurrence. Receiver operating characteristic curves were used to determine the cross-sectional RSPV area (c-RSPV) and distance between the LIPV and MA (d-LIPV–MA) that provided optimal sensitivities and specificities for the elimination of AT and AF recurrence after a single PVAI procedure. The analysis prioritized specificity rather than sensitivity. Regarding the relative cost of false positive and false negative results, a false positive result of <10%, indicating AF and AT recurrences despite a c-RSPV greater than the cutoff value, was considered an optimal cutoff for the c-RSPV. Meanwhile, a false positive result of <10%, indicating freedom from AF and AT recurrences despite a d-LIPV–MA greater than the cutoff value, was considered an optimal cutoff for the d-LIPV–MA. Freedom from AF and AT was determined using the Kaplan–Meier method. A survival analysis grouped by isolated surface area was performed using a log-rank test. The variables in the multivariable Cox proportional hazards regression model included the cross-sectional areas of the LSPV and RSPV and the d-LIPV–MA, which had a p-value <0.05 in the univariate Cox proportional hazard regression analysis. A p-value <0.05 was considered statistically significant. All statistical analyses were performed with SPSS, Release 11.0.
3. Results
3.1. Patient characteristics and rhythm outcome
Patient characteristics are displayed in Table 1. A total of 67 patients with symptomatic AF and a median AF history of 42 months (1st–3rd quartile, 12–60) were included. The mean patient age was 62±10 years, 48 patients (72%) were male, the mean LA diameter was 41±7 mm, and the mean left ventricular ejection fraction (LVEF) was 61±10%. The cohort included 32 (52%) patients with hypertension and 1 (1%) with coronary artery disease. During a follow-up period of 19±10 months, 49 (73%) patients remained free from AF. Of the 18 patients who suffered a recurrence, AF was documented in 17 patients and roof-dependent AT was documented in one patient. A univariate Cox proportional hazard regression analysis revealed no statistically significant differences in any patient characteristics except hypertension between patients with and without arrhythmia recurrences (Table 2).
Table 1.
Patient characteristics.
| All patients (n=67) | |
|---|---|
| Age (years) | 62±10 |
| Male (n – %) | 48 (72) |
| AF history (months) | 42 (12; 60) |
| Persistent AF (n – %) | 21 (31) |
| Antiarrhythmic drugs (n) | 1.2±1.0 |
| Arterial hypertension (n – %) | 35 (52) |
| Coronary artery disease (n – %) | 1 (1) |
| Diabetes mellitus (n – %) | 8 (12) |
| Lone AF (n – %) | 25 (37) |
| Left atrial diameter (mm) | 41±7 |
| LV ejection fraction (%) | 61±10 |
| Prior CTI ablation (n – %) | 2 (3) |
| Left common pulmonary vein (n – %) | 3 (4) |
| Right middle pulmonary vein (n – %) | 9 (13) |
AF=atrial fibrillation, LV=left ventricle, CTI=cavotricuspid isthmus.
Table 2.
Patient characteristics associated with AF recurrence.
|
Univariate |
|||
|---|---|---|---|
| Unadjusted OR | CI 95% | p Value | |
| Age | 1.01 | 0.97–1.06 | 0.61 |
| Male | 0.77 | 0.29–2.06 | 0.60 |
| AF history | 1.00 | 1.00–1.01 | 0.30 |
| Antiarrhythmic drugs | 0.97 | 0.60–1.56 | 0.90 |
| Arterial hypertension | 2.76 | 0.99–7.74 | 0.05 |
| Coronary artery disease | – | – | – |
| Diabetes mellitus | 0.92 | 0.21–3.99 | 0.91 |
| Lone AF | 0.87 | 0.32–2.55 | 0.79 |
| Persistent AF | 1.50 | 0.58–3.88 | 0.39 |
| Left atrial diameter | 1.03 | 0.96–1.11 | 0.37 |
| LV ejection fraction | 0.99 | 0.95–1.03 | 0.62 |
| Prior CTI ablation | – | – | – |
| Left common pulmonary vein | – | – | – |
| Right middle pulmonary vein | 0.83 | 0.19–3.60 | 0.79 |
| Additional ablation | 0.64 | 0.08–4.71 | 0.65 |
| AF inducible | 1.02 | 0.39–2.63 | 0.97 |
AF=atrial fibrillation, LV=left ventricle, CTI=cavotricuspid isthmus.
3.2. Procedural parameters and rhythm outcome
Table 3 summarizes the procedural parameters and rhythm outcomes. Ablation of the cavotricuspid isthmus was previously performed in 2 patients (3%). Ablation was performed by 6 experienced electrophysiologists. Complete PVAI was achieved in all patients. Isolation of the SVC and linear ablation were performed in 7 and 9 patients, respectively. Complete SVC isolation was achieved in all patients. Early AF recurrence was strongly associated with AF recurrence (odds ratio: 5.10, CI: 1.96–13.2, p=0.001). A univariate Cox proportional hazard regression analysis revealed no statistically significant differences in any procedural parameters except for early recurrence between patients with and without arrhythmia recurrences. A 2nd procedure was performed in 16 (89%) of the 18 patients with AF recurrence. LA–PV re-isolation and additional ablation were performed in 13 and 14 patients, respectively. The majority of patients with AF recurrence required LA modification and/or non-PV foci ablation to achieve the procedural endpoints.
Table 3.
Procedural parameters associated with AF recurrence.
|
Univariate |
|||
|---|---|---|---|
| Unadjusted OR | CI 95% | p Value | |
| Early recurrence | 5.10 | 1.96–13.2 | 0.001 |
| Arrhythmogenic PV | 0.58 | 0.22–1.47 | 0.24 |
| APC from extra PV | 1.37 | 0.52–3.67 | 0.52 |
| SVC isolation | 1.00 | 0.30–4.33 | 1.00 |
| Linear ablation | 0.63 | 0.43–5.13 | 0.53 |
AF=atrial fibrillation, PV=pulmonary vein, APC=atrial premature contraction.
3.3. Topographic variability and rhythm outcome
Regarding abnormal PV anatomy, 3 of the 67 patients (4%) had a left common pulmonary vein (LCPV) and 9 (13%) had a right middle pulmonary vein (RMPV). The presence of an LCPV or RMPV was not associated with AF recurrence (Table 2). A univariate Cox proportional hazard regression analysis identified three anatomical parameters related to AF and/or AT recurrence: the d-LIPV–MA, the distance between the RIPV and MA, and the c-RSPV. In the multivariate Cox proportional hazard regression analysis, the predictive model of AF and/or AT recurrence consisted of the d-LIPV–MA and c-RSVP (odds ratio: 1.07, CI: 1.02–1.12, p=0.001; odds ratio: 0.42, CI: 0.21–0.77, p=0.006, Table 4). Fig. 2 shows representative cases with and without AF recurrence.
Table 4.
anatomical parameters associated with AF recurrence.
|
Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Unadjusted HR | CI 95% | p Value | Adjusted HR | CI 95% | p Value | |
| Segmented surface areas of the LA | ||||||
| Left-sided antrum | 1.04 | 0.93–1.16 | 0.52 | |||
| Right-sided antrum | 0.98 | 0.82–1.16 | 0.78 | |||
| Posterior area | 0.97 | 0.79–1.18 | 0.73 | |||
| Inferior area | 1.08 | 0.99–1.17 | 0.07 | |||
| Septal area | 1.05 | 0.99–1.11 | 0.12 | |||
| Lateral area | 1.07 | 0.94–1.23 | 0.31 | |||
| Dimensions of the LA | ||||||
| AP | 1.03 | 0.96–1.12 | 0.41 | |||
| RIPV-MA | 1.11 | 1.03–1.19 | 0.006 | 1.07 | 1.00–1.15 | 0.06 |
| RSPV–MA | 1.03 | 0.98–1.09 | 0.23 | |||
| LSPV–MA | 1.02 | 0.98–1.06 | 0.42 | |||
| LIPV–MA | 1.10 | 1.04–1.16 | <0.001 | 1.07 | 1.02–1.12 | 0.01 |
| Cross-sectional areas of the PV | ||||||
| LSPV | 0.70 | 0.37–1.32 | 0.27 | |||
| LIPV | 0.64 | 0.31–1.33 | 0.23 | |||
| RSPV | 0.39 | 0.20–0.76 | 0.006 | 0.41 | 0.21–0.77 | 0.006 |
| RIPV | 0.58 | 0.30–1.11 | 1.00 | |||
AF=atrial fibrillation, LA=left atrium, AP=anterior–posterior, RIPV=right inferior pulmonary vein, RSPV=right superior pulmonary vein, LSPV=left superior pulmonary vein, LIPV=left inferior pulmonary vein, MA=mitral annulus.
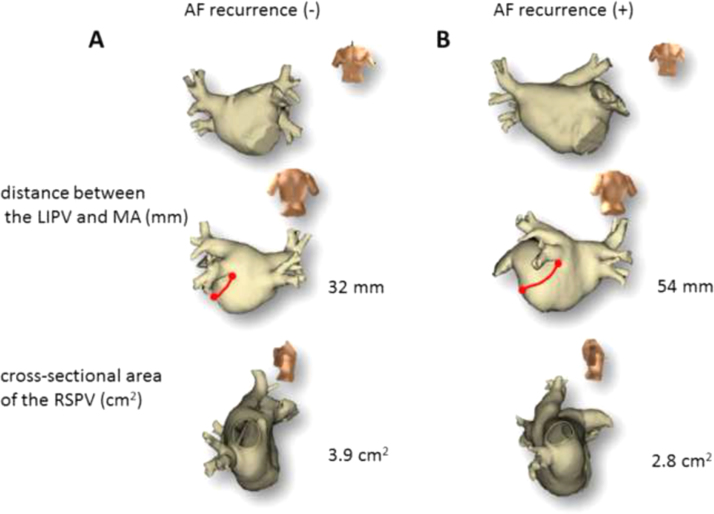
Fig. 2.
Representative cases with and without AF recurrence. (A) Representative case without AF recurrence. (B) Representative case with AF recurrence. In contrast to the patient with AF recurrence, a shorter distance between the LIPV and MA and a larger cross-sectional RSPV area were observed in the patient without AF recurrence. AF=atrial fibrillation, LIPV=left inferior pulmonary vein, RSPV=right superior pulmonary vein.
3.4. Optimal cutoff values of c-RSPV and d-LIPV–MA associated with rhythm outcomes
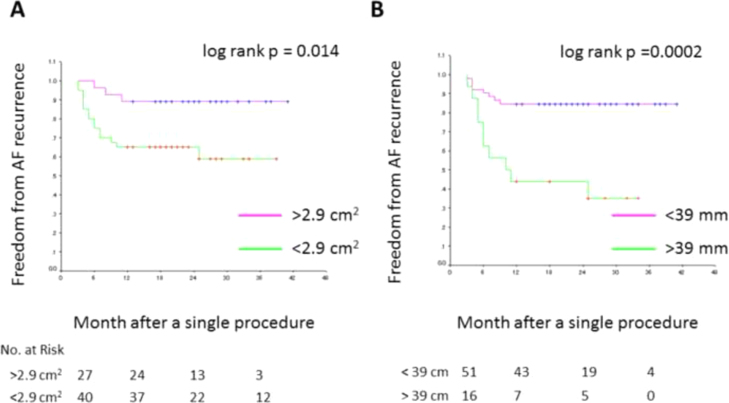
A receiver operating characteristic curve analysis yielded an optimal cutoff value of 2.9 cm2 for the c-RSPV and 39 mm for the d-LIPV–MA. The sensitivity and specificity associated with AF freedom at the c-RSPV cutoff value were 49% and 83%, respectively. The sensitivity and specificity associated with AF recurrence at the d-LIPV–MA cutoff value were 56% and 89%, respectively. A Kaplan–Meier curve revealed that the rate of freedom from AF and macroreentrant tachycardia recurrences after a single procedure was 89% in patients with a larger c-RSPV (>2.9 cm2) and 63% in patients with a smaller c-RSPV (<2.9 cm2) (log-rank test, p=0.014, Fig. 3A). The rate of freedom from AF was 38% in patients with a longer d-LIPV–MA (>39 mm) and 84% in patients with a shorter d-LIPV–MA (<39 mm) (log-rank test, p=0.0002, Fig. 3B).
Fig. 3.
A Kaplan–Meier curve of freedom from AF according to anatomical parameters. (A) A comparison of freedom from AF recurrence between patients with and without an RSPV with a cross-sectional area greater than 2.9 cm2. (B) A comparison of freedom from AF recurrence between patients with and without a distance shorter than 39 mm between the LIPV and MA.
4. Discussion
4.1. Main findings
The present study demonstrated associations of the d-LIPV–MA and c-RSPV with AF and/or AT recurrence after a single AF procedure. For every 1-mm increase, the d-LIPV–MA was associated with a 7.0% increase in the probability of AF and/or AT recurrence. For every 1 cm2 increase, the c-RSPV was associated with a 59% decrease in the probability of AF and/or AT recurrence.
4.2. Conventional parameters associated with AF recurrence
AF and/or AT recurred in 18 (27%) of the 67 patients after the first AF ablation procedure. A Cox univariate hazard regression analysis performed during the follow-up period identified no procedural parameters or patient characteristics with the exception of early recurrence and hypertension. Previous studies reported that age, gender, LVEF, LA size, LA volume, structural heart disease, AF duration, and abnormal pulmonary vein anatomy could predict recurrence [18]. In the current study, however, these parameters were not associated with AF recurrence This discrepancy may be related to the heterogeneity of AF ablation strategies and to differences in study inclusion criteria. In addition, most of the studies performed a multivariate analysis using multiple logistic regression. No survival analyses were performed [19].
4.3. The d-LIPV–MA in association with AF and/or AT recurrence
LA enlargement as assessed by echocardiography was not a reliable predictor of recurrence according to several multivariate analyses [18–21]. This may be related to inter-observer variations in LA assessment and to the absence of any defined criteria for an LA enlargement evaluation by echo studies. By contrast, our measurement of the d-LIPV–MA was performed on a 3D-CT image that could be segmented by the NavX system׳s NavX Verismo software. In a previous study, we reported excellent inter- and intra-variability for anatomical measurements of both the LA and PVs [8]. In brief, there was excellent correlation between the observers and between the initial and second calculations (inter-observer correlations, respectively: r=0.97, p<0.001; r=0.96, p<0.001; r=0.85, p<0.001; intra-observer correlations, respectively: r=0.99, p<0.001; r=0.99, p<0.001; r=0.97, p<0.001). This suggests that both the inter- and intra-observer variabilities were within an acceptable range as compared to echocardiography measurements. McGann et al. reported that atrial fibrosis assessed by delayed enhancement magnetic resonance imaging (DE-MRI) was associated with a poor response to CA therapy for AF [22–24]. They concluded that patients with a >30% enhancement on MRI showed a poor procedure outcome with a >70% failure rate. Interestingly, the localization of the atrial fibrosis was likely the LA bottom as well as the LA posterior wall. Considering the organs surrounding the LA, the LA bottom was likely to be more easily enlarged compared to the LA posterior wall. The enlargement of the LA posterior wall was limited by the PVs at the corners of the posterior wall and by the pulmonary artery at the roof of the posterior wall. Of note, posterior wall extension was likely limited by the descending aortic artery and spine, whereas the enlargement of the LA bottom was not limited by any surrounding organs. Thus, the d-LIPV–MA may reflect the degree of structural remodeling including atrial fibrosis induced by AF as compared to the conventional LA anterior–posterior diameter. Bisbal et al. reported that the LA remodeling process associated with AF leads to spherical deformation of the LA and that LA sphericity, a measure of spherical remodeling, is an independent and strong predictor of AF ablation success [25]. Notably, this deformation may induce enlargement of the LA bottom. This finding is likely consistent with our results.
4.4. The c-RSPV in association with AF and/or AT recurrence
Regarding PV anatomy, the presence of an LCPV or RMPV has been reported to be associated with increased freedom from AF ablation [26,27]. It was speculated that an LCPV could facilitate greater contact force along the left PV–left atrial appendage (LAA) ridge and would not interfere with the CA, and that the minimal risk of PV stenosis would allow for a slightly deeper ablation of the venous aspect of the left PV–LAA ridge. LCPVs may also more frequently harbor triggers responsible for AF episodes. In the current study, an LCPV and RMPV were reported in only 4% and 13% of patients, respectively. Therefore, we could not evaluate the relationship between PV anatomy and AF recurrence with statistical power. Of note, larger LSPV and RSPV cross-sectional areas were associated with AF recurrence in the univariate Cox hazard model. This indicated that the anatomical and arrhythmogenic properties of an LSPV with a large cross-sectional area were similar to those of an LCPV. Uijl et al. investigated the effects of LA dimensions, PV dimensions, and PV anatomy on AF ablation outcomes in a similar patient population (72% in paroxysmal AF, 28% in persistent AF). They concluded that an enlargement of the anteroposterior LA diameter and normal right PV anatomy were independent risk factors for AF recurrence [28]. No relationship was found between PV dimensions and the outcome of AF ablation. We speculated that a dilated PV might stretch the myocardial sleeve within the PV, which could induce arrhythmogenicity. Thus, the impact of a complete PVI is likely greater in patients with a dilated PV. The spine and pulmonary artery, with respective locations behind the inferior PVs and over the LSPV, may limit PV dilatation. By contrast, the SVC is located in the anterior part of the RSPV. No organs are located over or behind the RSPV, which may facilitate the easy dilatation of the RSPV as compared to other PVs. We considered that a different evaluation of the dilated PV may yield a contradictory outcome. The PV may be dilated three-dimensionally but not equally in the superior–inferior and anterior–posterior directions. In the present study, we evaluated the degree of PV dilatation by measuring the cross-sectional area of the PV. This three-dimensional measurement may evaluate the degree of PV dilatation more precisely as compared to the conventional two-dimensional measurement.
4.5. Clinical implications
The current study identified two novel anatomical predictors of AF recurrence after a single AF ablation: (1) a long d-LIPV–MA and (2) a small c-RSPV. The recommended cutoff values were 39 mm for the d-LIPV–MA and 2.9 cm2 for the c-RSPV. These two anatomical parameters can be easily determined using 3D-CT prior to the procedure. When considering patient selection for AF ablation or a more aggressive AF strategy, the observation of an enlarged LA bottom and/or small PVs constituted very useful anatomical information.
4.6. Study limitations
Our study had two major limitations. First, the sample size was relatively small. Second, this study was nonrandomized and not designed to clearly compare procedural outcomes with those obtained by other methods. Our findings should therefore be verified in a randomized prospective study.
5. Conclusions
A long d-LIPV–MA and small c-RSPV were associated with AF recurrence after a single AF ablation procedure. Three-dimensional assessment of both the LA and PV anatomies is useful for selecting patients suitable for AF ablation or a more aggressive AF ablation procedure.
Conflict of interest
The authors have no conflicts to disclose.
Acknowledgments
We would like to thank Mr. John Martin for his linguistic assistance.
Footnotes
Financial support: none.
References
- 1.Haissaguerre M., Jais P., Shah D.C. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Oral H., Knight B.P., Ozaydin M. Segmental ostial ablation to isolate the pulmonary veins during atrial fibrillation: feasibility and mechanistic insights. Circulation. 2002;106:1256–1262. doi: 10.1161/01.cir.0000027821.55835.00. [DOI] [PubMed] [Google Scholar]
- 3.Ouyang F., Bansch D., Ernst S. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004;110:2090–2096. doi: 10.1161/01.CIR.0000144459.37455.EE. [DOI] [PubMed] [Google Scholar]
- 4.Arya A., Piorkowski C., Sommer P. Clinical implications of various follow up strategies after catheter ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2007;30:458–462. doi: 10.1111/j.1540-8159.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 5.Arentz T., Weber R., Burkle G. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. 2007;115:3057–3063. doi: 10.1161/CIRCULATIONAHA.107.690578. [DOI] [PubMed] [Google Scholar]
- 6.Ouyang F., Tilz R., Chun J. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368–2377. doi: 10.1161/CIRCULATIONAHA.110.946806. [DOI] [PubMed] [Google Scholar]
- 7.Kottkamp H., Tanner H., Kobza R. Time courses and quantitative analysis of atrial fibrillation episode number and duration after circular plus linear left atrial lesions: trigger elimination or substrate modification: early or delayed cure? J Am Coll Cardiol. 2004;44:869–877. doi: 10.1016/j.jacc.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 8.Kiuchi K., Kircher S., Watanabe N. Quantitative analysis of isolation area and rhythm outcome in patients with paroxysmal atrial fibrillation after circumferential pulmonary vein antrum isolation using the pace-and-ablate technique. Circ Arrhythm Electrophysiol. 2012;5:667–675. doi: 10.1161/CIRCEP.111.969923. [DOI] [PubMed] [Google Scholar]
- 9.Anselmino M., Blandino A., Beninati S. Morphologic analysis of left atrial anatomy by magnetic resonance angiography in patients with atrial fibrillation: a large single center experience. J Cardiovasc Electrophysiol. 2011;22:1–7. doi: 10.1111/j.1540-8167.2010.01853.x. [DOI] [PubMed] [Google Scholar]
- 10.Kurotobi T., Iwakura K., Inoue K. The significance of the shape of the left atrial roof as a novel index for determining the electrophysiological and structural characteristics in patients with atrial fibrillation. Europace. 2011;13:803–808. doi: 10.1093/europace/eur039. [DOI] [PubMed] [Google Scholar]
- 11.Ejima K., Kato K., Arai K. Impact of atrial remodeling on the outcome of radiofrequency catheter ablation of paroxysmal atrial fibrillation. Circ J. 2014;78:872–877. doi: 10.1253/circj.cj-13-1391. [DOI] [PubMed] [Google Scholar]
- 12.Amin V., Finkel J., Halpern E. Impact of left atrial volume on outcomes of pulmonary vein isolation in patients with non-paroxysmal (persistent) and paroxysmal atrial fibrillation. Am J Cardiol. 2013;112:966–970. doi: 10.1016/j.amjcard.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Piorkowski C., Kircher S., Arya A. Computed tomography model-based treatment of atrial fibrillation and atrial macro-re-entrant tachycardia. Europace. 2008;10:939–948. doi: 10.1093/europace/eun147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piorkowski C., Kottkamp H., Gerds-Li J.H. Steerable sheath catheter navigation for ablation of atrial fibrillation: a case-control study. Pacing Clin Electrophysiol. 2008;31:863–873. doi: 10.1111/j.1540-8159.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 15.Knopp H., Halm U., Lamberts R. Incidental and ablation-induced findings during upper gastrointestinal endoscopy in patients after ablation of atrial fibrillation: a retrospective study of 425 patients. Heart Rhythm. 2014;11:574–578. doi: 10.1016/j.hrthm.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Kuwahara T., Takahashi A., Kobori A. Safe and effective ablation of atrial fibrillation: importance of esophageal temperature monitoring to avoid periesophageal nerve injury as a complication of pulmonary vein isolation. J Cardiovasc Electrophysiol. 2009;20:1–6. doi: 10.1111/j.1540-8167.2008.01280.x. [DOI] [PubMed] [Google Scholar]
- 17.Kiuchi K., Okajima K., Shimane A. Delayed-enhancement magnetic resonance imaging could detect the substrate of an unusual macroreentrant atrial tachycardia? J Cardiovasc Electrophysiol. 2014;25:1032–1033. doi: 10.1111/jce.12434. [DOI] [PubMed] [Google Scholar]
- 18.Hof I., Chilukuri K., Arbab-Zadeh A. Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J Cardiovasc Electrophysiol. 2009;20:1005–1010. doi: 10.1111/j.1540-8167.2009.01504.x. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki S., Kuwahara T., Kobori A. Preprocedural predictors of atrial fibrillation recurrence following pulmonary vein antrum isolation in patients with paroxysmal atrial fibrillation: long-term follow-up results. J Cardiovasc Electrophysiol. 2011;22:621–625. doi: 10.1111/j.1540-8167.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- 20.Montserrat S., Gabrielli L., Borras R. Left atrial size and function by three-dimensional echocardiography to predict arrhythmia recurrence after first and repeated ablation of atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2014;15:515–522. doi: 10.1093/ehjci/jet194. [DOI] [PubMed] [Google Scholar]
- 21.Sohns C., Sohns J.M., Vollmann D. Left atrial volumetry from routine diagnostic work up prior to pulmonary vein ablation is a good predictor of freedom from atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2013;14:684–691. doi: 10.1093/ehjci/jet017. [DOI] [PubMed] [Google Scholar]
- 22.Akkaya M., Higuchi K., Koopmann M. Relationship between left atrial tissue structural remodelling detected using late gadolinium enhancement MRI and left ventricular hypertrophy in patients with atrial fibrillation. Europace. 2013;15:1725–1732. doi: 10.1093/europace/eut147. [DOI] [PubMed] [Google Scholar]
- 23.McGann C., Akoum N., Patel A. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol. 2014;7:23–30. doi: 10.1161/CIRCEP.113.000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGann C.J., Kholmovski E.G., Oakes R.S. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52:1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 25.Bisbal F., Guiu E., Calvo N. Left atrial sphericity: a new method to assess atrial remodeling. Impact on the outcome of atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2013;24:752–759. doi: 10.1111/jce.12116. [DOI] [PubMed] [Google Scholar]
- 26.McLellan A.J., Ling L.H., Ruggiero D. Pulmonary vein isolation: the impact of pulmonary venous anatomy on long-term outcome of catheter ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2014;11:549–556. doi: 10.1016/j.hrthm.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Sohns C., Sohns J.M., Bergau L. Pulmonary vein anatomy predicts freedom from atrial fibrillation using remote magnetic navigation for circumferential pulmonary vein ablation. Europace. 2013;15:1136–1142. doi: 10.1093/europace/eut059. [DOI] [PubMed] [Google Scholar]
- 28.den Uijl D.W., Tops L.F., Delgado V. Effect of pulmonary vein anatomy and left atrial dimensions on outcome of circumferential radiofrequency catheter ablation for atrial fibrillation. Am J Cardiol. 2011;107:243–249. doi: 10.1016/j.amjcard.2010.08.069. [DOI] [PubMed] [Google Scholar]