Abstract
The mouse is commonly used for studying retinal processing, primarily because it is amenable to genetic manipulation. To accurately study photoreceptor driven signals in the healthy and diseased retina, it is of great importance to isolate the responses of single photoreceptor types. This is not easily achieved in mice because of the strong overlap of rod and M-cone absorption spectra (i.e., maxima at 498 and 508 nm, respectively). With a newly developed mouse model (Opn1lwLIAIS) expressing a variant of the human L-cone pigment (561 nm) instead of the mouse M-opsin, the absorption spectra are substantially separated, allowing retinal physiology to be studied using silent substitution stimuli. Unlike conventional chromatic isolation methods, this spectral compensation approach can isolate single photoreceptor subtypes without changing the retinal adaptation. We measured flicker electroretinograms in these mutants under ketamine-xylazine sedation with double silent substitution (silent S-cone and either rod or M/L-cones) and obtained robust responses for both rods and (L-)cones. Small signals were yielded in wild-type mice, whereas heterozygotes exhibited responses that were generally intermediate to both. Fundamental response amplitudes and phase behaviors (as a function of temporal frequency) in all genotypes were largely similar. Surprisingly, isolated (L-)cone and rod response properties in the mutant strain were alike. Thus the LIAIS mouse warrants a more comprehensive in vivo assessment of photoreceptor subtype-specific physiology, because it overcomes the hindrance of overlapping spectral sensitivities present in the normal mouse.
Keywords: keywords electrophysiology, mouse, photoreceptors, silent substitution
in recent years, the mouse has been the mainstay model for studying the physiology of the retina and retinal diseases. In vivo studies of retinal electrophysiology can be achieved by electroretinography (ERG). This method is noninvasive and enables repeated measurements from the same animal. Recent developments in ERG recordings in humans have shown that ERGs not have only a clinical value but also may give information on information processing in major retinogeniculate pathways with relevance for basic visual neuroscience (Kremers and Link 2008; Kremers et al. 2010; Parry et al. 2012). This relevance was recently confirmed for the mouse (Allen et al. 2014). To be able to study retinal signal processing and its disease-related changes, it is of great importance to isolate the responses of single photoreceptor types and record the signals that each elicits downstream. Also, in other studies of the retinal physiology, isolation of photoreceptor responses may lead to a better understanding of the information processing in the retina and for associated visual functions (Brown et al. 2010, 2012; Lall et al. 2010).
The isolation of ERG responses of single photoreceptor types can be achieved with different methods. One is to saturate one or more photoreceptor types using chromatic adaptation and to measure the responses of the nonadapted photoreceptors by flashing with a light of another chromaticity. Isolating different photoreceptor responses in this manner, however, has the disadvantage of generating results with different adaptation levels of the retina. The state of adaptation influences not only the response characteristics of the isolated photoreceptors but also the mode of operation of postreceptoral pathways (Cameron and Lucas 2009; Eisner and Macleod 1981; Kremers et al. 2003; Lee et al. 1999; Padmos and van Norren 1971; Swanson 1993). Therefore, responses obtained from differently adapted retinae do not allow direct comparisons.
An alternative method for the isolation of rod and cone responses is the silent substitution method, which was first mentioned and used by Donner and Rushton (1959) and further developed by Estévez and Spekreijse (1974, 1982). Today, the method is commonly used in human electrophysiology and psychophysics (Bessler et al. 2010; Kremers et al. 1999, 2003; Shapiro et al. 1996; Usui et al. 1998a, 1998b; Zele et al. 2006). This method has several advantages relative to the selective adaptation method, because the isolation of different photoreceptor types can be achieved without changing the state of adaptation. In addition, both transient (flash) and steady-state stimuli (sine waves, square waves, sawtooth) can be used, and their strength (expressed in terms of cone or rod contrast) can be varied without changing the mean luminance or chromaticity. Finally, instead of isolating the responses of one photoreceptor, a combination of photoreceptor outputs, each well defined, can be chosen. However, the success of the silent substitution method strongly depends on the spectral separation of the fundamentals of each photoreceptor type. The larger the spectral separation, the larger the contrast can be in the isolated photoreceptor. If the spectral separation is small, then the dynamic range of stimulus strength is limited. In the extreme case, when two photoreceptors have identical fundamentals, then a silent substitution for one photoreceptor type will, by definition, also be a silent substitution condition of the other photoreceptor type, preventing isolation of either type.
In the mouse, the M-cones and the rods have spectral sensitivities that are only about 10 nm apart (Lyubarsky et al. 1999; Nikonov et al. 2006). As a result, the dynamic range for separating rod- and M-cone-driven responses is limited with silent substitution. Hence, to date, rod and M-cone signals are still separated on the basis of measurements at different retinal illuminances (Allen et al. 2014; Cia et al. 2005; Szmajda et al. 2006).
Recently, mice were created in which the native M-cone pigment was replaced by the human L-cone pigment (Greenwald et al. 2014; Smallwood et al. 2003). In both strains, the spectral separation of the rod and the human L-cone pigments is much larger (∼60 nm) than between rods and native M-cone pigments in the wild-type mouse, providing the possibility to separate rod- and cone-driven responses using the silent substitution method. The available data suggest that the cones are functional (Greenwald et al. 2014; Smallwood et al. 2003) and can be used for some sort of spectral opponency [color vision (Jacobs et al. 2007)]. In addition, the total cone numbers, their distribution, their outer segment lengths, and the coexpression with S-cone pigment do not differ from those in the native mouse (Greenwald et al. 2014). It is not known yet, however, whether functional processing in their retinal circuitry is also similar.
In this work, we studied the basic retinal physiology of C57Bl/6 mice carrying the human L-opsin [henceforth described as Opn1lwLIAIS (Greenwald et al. 2014)]. We asked if the silent substitution method is indeed useful to measure rod- and L-cone-driven responses in these mice and so presented photoreceptor subtype-specific ERGs from mice. The data were compared with those obtained from wild-type and heterozygous mice. For this purpose, we created rod- and L-cone-isolating sinusoidal stimuli and measured ERG responses. We further studied the characteristics (amplitudes and phases) of the responses as a function of temporal frequency, stimulus contrast, and mean luminance. In the wild-type mice, only a very limited data set could be obtained. The phase data in the wild-type and the mutant mice were compared to determine whether the functional integrity of the retina is similar in the two groups. A similar approach of using the silent substitution method in a mouse strain containing the human L-cone pigment was recently employed for studying the influence of melanopsin-containing intrinsically photosensitive retinal ganglion cells (Allen et al. 2014).
MATERIALS AND METHODS
Ethical approval.
All animal experiments were performed in accordance with the principles regarding the care and use of animals adopted by the Association for Research in Vison and Ophthalmology and the Society for Neuroscience. The experiments were approved by the local animal welfare authorities (Regierungspräsidium Mittelfranken, Ansbach, Germany).
Mice.
Embryos of the Opn1lwLIAIS (short: LIAIS) strain sourced from the University of Washington were sent to the Animal Physiology Department of the University of Erlangen-Nürnberg, where they were raised by embryo transfer. A more detailed description of the strain can be found in Greenwald et al. (2014). Briefly, LIAIS mice express a relatively common human L-cone opsin (with the amino acids leucine, isoleucine, alanine, isoleucine, and serine located at positions 153, 171, 174, 178, and 180, respectively, and therefore denoted as LIAIS) instead of the endogenous mouse M-cone opsin. Otherwise, the animals express normal mouse rhodopsin and S-opsin. Greenwald et al. (2014) showed that this opsin substitution does not influence cone structure or function, except for the sensitivity shift of the M-cones into the red, with a maximum sensitivity at about 561 nm instead of 508 nm (Jacobs 1991; Lyubarsky et al. 1999; Nikonov et al. 2006; Sun et al. 1997). We performed ERG measurements in 27 animals: 8 heterozygous (LIAIS+/−; all female, 5.5 and 9 mo of age), 15 LIAIS (hemizygous male, 4–5 and 9 mo), and 4 C57Bl/6 wild-type mice (WT; female, 4–6 mo).
Silent substitution stimuli.
The appropriate double silent substitution stimuli that isolated rod, M-cone, or human L-cone responses (hereafter denoted as L*) were created to be used during in vivo recordings of retinal function with the ERG. These were sinusoidal stimuli using the red, green, and blue light-emitting diode (LED) arrays of a Q450SC Ganzfeld stimulator (Roland Consult, Brandenburg, Germany). The red, green, and blue LEDs had peak wavelengths of 625, 525, and 470 nm, respectively. The spectral outputs of the LEDs were measured using a CAS 140 spectroradiometer (Instrument Systems, Munich, Germany). Overall output of the LEDs was checked using a Minolta LS-110 photometer. To calculate the absorption spectra, we used the nomograms of Lamb (1995). The nomograms need the wavelength at maximum absorbance (λmax) of the photoreceptor types as input. We used the following λmax values: S-cones, 355 nm (Lyubarsky et al. 1999); rods, 498 nm; native M-cones, 506 nm; and L*-cones, 565 nm. We did not apply a correction for lens absorption for the human L-cones because we expected no influence at large wavelengths (Douglas and Jeffery 2014). For the other photoreceptor classes we used the correction for preretinal absorption given by Lyubarsky et al. (1999). The excitation of each photoreceptor type by each of the LEDs was calculated by the integral of the multiplication of the photoreceptor absorption spectra and the emission spectra of the LEDs (Kremers et al. 1999). The correction for preretinal absorption is only valid down to 410 nm. However, none of the LEDs emitted light at wavelengths below 425 nm. Therefore, a correction at wavelengths below 410 nm did not have any influence on the resulting photoreceptor sensitivities. The total photoreceptor excitation equaled the sum of the photoreceptor excitations to each of the LEDs. Rod and cone contrasts (C) were calculated as Michelson contrast using the maximal and minimal excitations (Emax and Emin, respectively) during stimulation (Kremers et al. 1999):
Table 1 summarizes the LED contrasts and the corresponding photoreceptor contrasts of the rod-, M-cone-, and L*-cone-isolating stimuli for WT, LIAIS, and heterozygous (LIAIS+/−) mice. Please observe that the calculations of the silent substitution paradigm are actually on the level of the photopigments. Therefore, cones that double express S-opsin and the M-/L*-opsins are treated as cones that express exclusively M-/L*-opsins. Stimulation of the S-cone pigment was silenced. However, because the emission spectra of the LEDs and the absorption spectra of the S-opsin hardly overlapped, a stimulation of the S-opsin would be very small anyway. Negative LED or photoreceptor contrasts indicate counter phase modulation relative to those having positive contrasts. As a result, rods and cones in the two stimulus conditions were modulated in counter phase (Table 1: rod contrasts were negative, whereas cone contrasts were positive). In the ERG phase plots, a correction was applied by shifting the phases of the rod-driven responses by 180°. The ERG measurements were performed at three different mean luminance levels (13, 39, and 130 cd/m2; see also Table 2) and at different frequencies (3, 4, 6, 8, 12, 14, 18, 22, 26, and 30 Hz). S-cones were not stimulated in any of the stimulus conditions. Hence, data labeled as “cones” for simplicity represent those from either M- or L-types, unless specified.
Table 1.
Stimulus settings for the isolation of rod, M-cone, or L*-cone responses in LIAIS
| LED Contrast, % |
Photoreceptor Contrast, % |
||||||
|---|---|---|---|---|---|---|---|
| LEDs | 1 | 2 | 3 | Photoreceptor Types | 1 | 2 | 3 |
| Rod isolation | |||||||
| LIAIS | |||||||
| Red | 95.03 | 63.36 | 31.68 | S-cones | 0.00 | 0.00 | 0.00 |
| Green | −66.28 | −44.19 | −22.09 | L*-cones | 0.00 | 0.00 | 0.00 |
| Blue | −85.46 | −56.98 | −28.49 | Rods | −75.00 | −50.00 | −25.00 |
| LIAIS+/− | |||||||
| Red | 95.03 | 63.36 | 31.68 | S-cones | 0.00 | 0.00 | 0.00 |
| Green | −66.28 | −44.19 | −22.09 | L*-/M-cones | 0.00/−72.92† | 0.00/−48.62† | 0.00/−24.31† |
| Blue | −85.46 | −56.98 | −28.49 | Rods | −75.00 | −50.00 | −25.00 |
| WT | |||||||
| Red | 91.50 | S-cones | 0.00 | ||||
| Green | 37.02 | M-cones | 0.00 | ||||
| Blue | 42.82 | Rods | −5.00 | ||||
| M-cone (WT and LIAIS+/−) or L*-cone isolation (LIAIS and LIAIS+/−) | |||||||
| LIAIS | |||||||
| Red | 97.06 | 70.59 | 35.30 | S-cones | 0.00 | 0.00 | 0.00 |
| Green | 47.23 | 34.35 | 17.17 | L*-cones | 55.00 | 40.00 | 20.00 |
| Blue | −42.31 | −30.77 | −15.39 | Rods | 0.00 | 0.00 | 0.00 |
| LIAIS+/− | |||||||
| Red | 97.06 | 70.59 | 35.30 | S-cones | 0.00 | 0.00 | 0.00 |
| Green | 47.23 | 34.35 | 17.17 | L*-/M-cones | 55.00/5.54† | 40.00/4.03† | 20.00/2.01† |
| Blue | −42.31 | −30.77 | −15.39 | Rods | 0.00 | 0.00 | 0000 |
| WT | |||||||
| Red | 87.60 | S-cones | 0.00 | ||||
| Green | 42.62 | M-cones | 5.00 | ||||
| Blue | −38.18 | Rods | 0.00 | ||||
Values are light-emitting diode (LED) and photoreceptor contrasts in Opn1lwLIAIS mutant (LIAIS), heterozygous (LIAIS+/−), and wild-type (WT) mice.
Contrasts for the L*- and native M-cones calculated with the LIAIS stimulus settings.
Table 2.
Mean stimulus luminance levels
| LED | Luminance, cd/m2 | ||
|---|---|---|---|
| Red | 6 | 18 | 60 |
| Green | 6 | 18 | 60 |
| Blue | 1 | 3 | 10 |
| Total mean luminance | 13 | 39 | 130 |
In LIAIS and heterozygous mice, the rod measurements were performed using three LED contrasts, resulting in 75, 50, and 25% rod contrast. The contrasts in the two cone types were 0% (double silent substitution). In WT mice, only a rod contrast of 5% could be reached because of the above-mentioned similarity between the native mouse rod and M-cone opsin absorption spectra. In the heterozygous animals, we used stimulus conditions that were identical to those for the LIAIS mice. As a result, any native M-cone pigments would also be stimulated with the rod-isolating stimulus (see values indicated by dagger for M-cones in Table 1), making rod isolation not complete.
Cone-isolating stimuli reached a maximal contrast of 55% for L*-cones in LIAIS and in heterozygous mice. Additionally, L*-cone responses were obtained at 40 and 20% cone contrasts (Table 1). Mouse native M-cones, on the other hand, were stimulated with a contrast of only 5% in WT mice. These conditions were silent substitutions for S-cones and rods for all three mouse strains (i.e., both set at 0; Table 1). As a result, the ratios of LED contrasts were identical in all stimulus conditions (i.e., 97.06:47.23:−42.31 equals 87.60:42.62:−38.18). Observe that the stimuli resulting in 55% cone contrast in the LIAIS mice and those resulting in 5% cone contrast in the WT mice were physically nearly identical despite an 11-fold difference in cone contrast. As in the “rod-isolating” conditions, the LIAIS+/− mice were measured with the same stimulus settings used for LIAIS mice. Therefore, the cone contrast is given for both the L*-cone and endogenous M-cones.
Rod and cone responses were measured at three different total mean luminance levels as shown in Table 2. Each LED set was modulated around a specific mean luminance with a ratio of 6:6:1 for red:green:blue. The luminance values are given in photometric units and are thus weighted by the human luminosity function Vλ. This is not appropriate for mice unless the luminance values are used in relative terms. However, the photopic spectral sensitivities are probably different for WT, LIAIS, and heterozygous mice. Therefore, mean luminance is expressed in units relevant for mouse photopic vision, and thus the state of retinal adaptation is probably different for the three genotypes. Still, the main goal of the measurements was to study the effect of a luminance change and not of luminance per se.
Electroretinography.
Flicker ERG measurements were randomized in each animal type for the 18 stimulus conditions (i.e., 3 mean luminance levels, 3 contrasts at each luminance level, and 2 photoreceptor-isolating settings; see figure legends for respective animal numbers per set). Each animal underwent up to 5 recording sessions, each lasting about 1 h and performed at least 1 wk apart. A detailed description of the procedure for ERG measurements in mice can be found elsewhere (Atorf et al. 2013; Regus-Leidig et al. 2013, 2014). Briefly, the animals were dark-adapted overnight, and all further handling was performed under deep red illumination. Anesthesia was introduced via an intramuscular injection of 50 mg/kg ketamine (Ketavet; Pfizer) and 10 mg/kg xylazine (Rompun, 2%; Bayer). A subsequent subcutaneous injection of saline solution (10 ml/kg, 0.9%) protected the mice from dehydration while anesthetized. Pupils were dilated (ø ≈ 3 mm) with a drop of tropicamide (Mydriaticum Stulln, 5 mg/ml; Pharma Stulln) and phenylephrine hydrochloride (Neosynephrin POS, 5%; Ursapharm). For ERG measurement, the ground needle electrode and reference needle electrodes were inserted subcutaneously at the base of the tail and next to the ears, respectively. Active contact lens electrodes (Mayo, Aichi, Japan), internally covered with Corneregel (Dr. Mann Pharma), were placed on the cornea of each eye. Stimuli were delivered via a Ganzfeld bowl (Q450SC; Roland Consult), with stimulation and data collection controlled using the RetiPort system (Roland Consult).
Mice were adapted to each mean luminance level for 5 min before measurements commenced. At least 40 cycles, each lasting 1 s, were averaged for every stimulus frequency. ERG signals were amplified (100,000×), bandpass filtered (1–300 Hz), and digitized with a sampling frequency of 2,048 Hz. Animals were sedated using isoflurane prior to humane physical euthanasia at the end of the study.
Signal analysis.
ERG signals were Fourier analyzed to extract amplitudes and phases of the first and second harmonic component. Additionally, the noise level was defined as the mean amplitude at frequencies ±1 Hz of the stimulus frequency or double stimulus frequency (for the second harmonic noise). Amplitudes of the first and second harmonic were only accepted if the signal-to-noise ratio was >2. Accepted amplitude values were finally corrected for noise by subtracting the calculated mean noise from the amplitudes. Corresponding timing values of the noise-corrected amplitudes were then also considered.
Statistical analysis.
All experimental data exhibited Gaussian distributions (Komogorov-Smirnov normality test, P > 0.1; Prism v5.00; GraphPad Software, San Diego, CA). As such, parametric methods (t-tests and ANOVA) were employed. An α value of 0.05 was adopted, except when adjusting for multiple comparisons (e.g., between ERG-response functions), when a more conservative level of significance (α ≤ 0.01) was used to protect against false positives associated with repeated-measures design. When a significant interaction arose from comparisons between groups, luminance, or contrast settings, Bonferroni post hoc tests were used. Response phases as a function of temporal frequency were modeled with a linear regression (y = y0 + ax), as previously used by Kremers and Scholl (2001). The parameter a describes the slope (i.e., a measure of phase change with frequency in °/Hz), which was used to compare phase relationships between mouse lines and photoreceptor-isolating conditions. These were compared using one-way ANOVA (Prism v5.00; i.e., setup with mean = a, SE = SD of a, total sample size N = df + 1) and subsequent post hoc tests, which is equivalent to conducting an analysis of covariance (ANCOVA) for comparing linear regressions. The parameter y0 gives the y-intercept of the linear regression, taken as an indicator of whether phases are shifted under the different conditions. If the regression returned a coefficient r2 < 0.70, the slope was disregarded.
RESULTS
Comparison of retinal physiology between transgenic and WT mice.
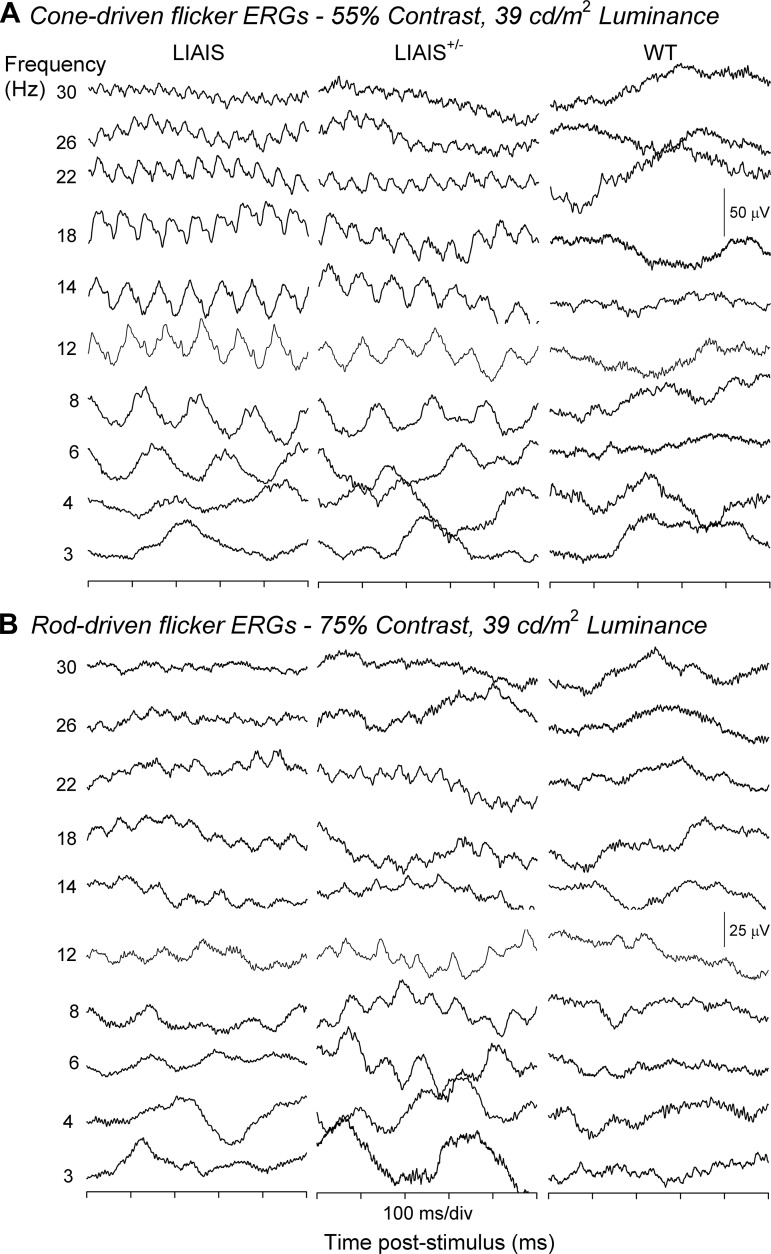
Representative group averaged responses to sinusoidal flickers measured from LIAIS, heterozygous (LIAIS+/−), and WT (B6) mice are displayed in Fig. 1. Episodes of 500 ms are displayed, corresponding to stimulus conditions at which maximal cone (A) and rod contrasts (B) were obtained using double silent substitution (i.e., 55% cone contrast and 75% rod contrast for LIAIS mice, 5% rod and M-cone contrast for WT mice, and mixtures of these in LIAIS+/− mice) at the intermediate mean luminance of 39 cd/m2. Responses to 10 temporal frequencies from 3 to 30 Hz are shown. However, responses to 3-Hz stimuli were often disturbed by low-frequency noise caused by respiration movements and were not further analyzed. Note that the stimuli in the cone-isolating stimuli were physically identical for LIAIS and LIAIS+/− mice and very similar (with identical LED contrast ratios) for WT mice (see Table 1).
Fig. 1.
Flicker electroretinography (ERG) responses recorded from mouse variants. Responses were elicited by M- or L*-cone (A)- and rod-isolating stimuli (B) in mutant (LIAIS, n = 4–11; left), heterozygous (LIAIS+/−, n = 2–5; middle), and wild-type mice (WT, n = 2–4; right). Displayed are identical 500-ms episodes of responses to sinusoidal stimuli of 3–30 Hz (indicated at far left) and at the highest attainable contrast (55% cone contrast and 75% rod contrast for mutants, 5% rod and cone contrasts for WT, and a mixture of contrasts for heterozygous animals; see materials and methods). The mean luminance was 39 cd/m2. L*-cone activity was recordable in both LIAIS and LIAIS+/− mice. M-cone responses elicited from WT mice were substantially smaller and less regular in comparison. Waveforms elicited from rod-isolating conditions are also clearly observable in mutants and heterozygous animals, and less so in WT mice.
In agreement with the expectation that the maximal cone and rod contrasts were larger in the LIAIS mice than in the WT mice, separate cone (Fig. 1A)- and rod-driven responses (Fig. 1B) were indeed recordable in both LIAIS and LIAIS+/− mice (left and middle, respectively). In contrast, these were less obvious in WT mice (right). The cone-driven responses in the LIAIS+/− animals (Fig. 1A, middle) are of intermediate amplitude, whereas rod-driven responses in these animals (Fig. 1B, middle) are sometimes larger than those found in the LIAIS animals, and they display a frequency doubling at certain conditions. This is possibly caused by the fact that not only rods but also native M-cones are stimulated.
In mutant mice (Fig. 1, left and middle), cone-driven responses were overall larger than those driven by the rods (cf. Fig. 1, A and B), despite the lower contrast used in the cone-isolating conditions (75 vs. 55%). In addition, they have similar phases. Taking into account that the stimuli modulated the rods and cones in counter phase (cf. Table 1), this indicates that rod- and cone-driven responses are about 180° apart. Moreover, responses elicited under both rod- and cone-isolating conditions showed an effect of flicker frequency. Specifically, larger responses were measured with low and intermediate temporal frequencies, with their overall amplitude decreasing with increasing temporal frequency above 18 Hz. This change was not as apparent in rod-driven waveforms because they were smaller and less regular.
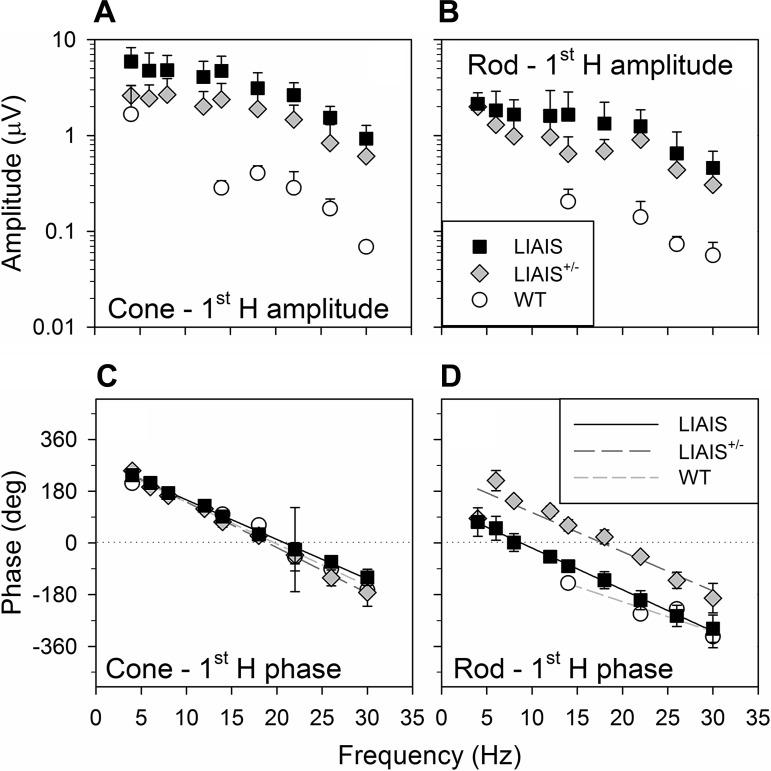
Figure 2 shows noise-corrected average amplitudes (±SD; A and B) and phases (C and D) of the fundamental response components for each photoreceptor-isolating stimulus as a function of temporal frequency. As in Fig. 1, only results from measurements at 39 cd/m2 mean luminance and with the use of maximal rod and cone contrasts are presented.
Fig. 2.
Fundamental ERG parameters (first harmonic, 1st H) of responses from each mouse strain. Data are averages (±SD) plotted as a function of temporal frequency from LIAIS (black squares; L*-cone, n = 11; rod, n = 10), LIAIS+/− (gray diamonds; L*-cone, n = 6; rod, n = 5), and WT mice (open circles; M-cone, n = 2; rod, n = 4) that were obtained with the highest stimulus contrast measured (cone: 55% for mutants and 5% for WT; rod: 75% for mutants and 5% for WT) under a mean luminance condition of 39 cd/m2. Cone (A)- and rod-driven (B) response amplitudes were largest in LIAIS mice. The response amplitudes were relatively constant up to a frequency of about 15 Hz (possibly with a local minimum at about 10 Hz) and decreased with increasing stimulus frequency above 15 Hz. Those obtained from the LIAIS+/− group were approximately half the size, whereas WT mice showed responses that were smaller by about a factor of 10 and just above noise. Phases are shown in C and D, described with linear regressions (LIAIS, black solid lines; LIAIS+/−, dark gray long-dashed lines; WT, light gray short-dashed lines). The phases of the cone-driven responses are shifted by 180° to account for the counter phase modulation of the rods and cones. Similar frequency dependency of the phases is shown in LIAIS and WT mice, whereas those obtained from the LIAIS+/− mice are about 180° shifted.
First harmonic amplitudes (Fig. 2, A and B) elicited by cone- and rod-isolating stimuli exhibited similar trends in both mutant and WT mice as a function of frequency, with relatively constant amplitudes up to about 15 Hz, beyond which the amplitudes became progressively smaller as temporal frequency increased. The frequency dependency differs somewhat from that found by Krishna et al. (2002) for 99% contrast white light stimuli, where a maximal amplitude was found at about 10 Hz, above which the amplitudes decreased. In general, the largest cone- and rod-driven amplitudes could be evoked in LIAIS mice (Fig. 2, A and B, squares), followed by LIAIS+/− mice producing ∼50% smaller responses (Fig. 2, A and B, diamonds), and lastly, WT mice (Fig. 2, A and B, circles), whose responses were smaller by about a factor of 10. Because of the small cone- and rod-driven ERG amplitudes of WT mice, not all values surpassed the noise discrimination criterion, which explains why there are fewer data points for this group. Last, rod-driven responses were smaller than those mediated by cones in all three groups (cf. Fig. 2, B and A), even though the contrast was larger for the mutant animals. Nevertheless, the dependency on temporal frequency is very similar for the different animal groups.
Consistent with the trend in flicker amplitudes observed above, grouped phases show similar frequency dependency for the different photoreceptors and animal groups (Fig. 2, C and D). It should be noted that a 180° phase shift was applied to the cone data set at each temporal frequency to take into account that cones were modulated in counter phase to rods (see materials and methods; Table 1). The data indicate that rod- and cone-driven responses are phase shifted by about 180°. The response phases of the heterozygous animals to “rod-isolating” stimuli are in agreement with this idea. As mentioned in materials and methods, these are not truly rod-driven responses because stimulus conditions for the LIAIS mice were used, which inadvertently stimulated cones with native pigment that are also present in the heterozygotes. In addition to the frequency-doubled responses found in LIAIS+/− mice (Fig. 1), it appears that the cone-driven responses overruled the rod-driven ones, resulting in phases (Fig. 2, C and D, long-dashed lines) that were advanced relative to the rod-driven responses of LIAIS (up to 166° difference) and WT mice (up 201° difference).
Because response phases displayed a linear decrease with increasing temporal frequency, their relationship was modeled with linear regressions (y = y0 + ax). Their slopes were between 11° and 16°/Hz for both rod- and cone-driven responses. Assuming that the phases are exclusively caused by a delay, the slopes indicate delays of between 31 and 44 ms. Comparisons between cone- and rod-mediated signals revealed about 161° (in LIAIS; Fig. 2, C and D, solid lines) to 282° phase differences (in WT; short-dashed lines). Statistical comparisons of regression lines describing phase dependencies on temporal frequency revealed that the phases of both photoreceptor-mediated ERGs were similar between the three groups (1-way ANOVA, P > 0.01). Of these, the rod phase profile of the WT group (Fig. 2D, short-dashed line) showed the least variation with increasing temporal frequency (11°/Hz). However, only two- to four-phase data could be averaged at the four temporal frequencies in WT animals under rod-isolating conditions due to their low signal-to-noise quotient. Given their considerably smaller response, phase data obtained in WT mice were thus less reliable than those from LIAIS and LIAIS+/− mice.
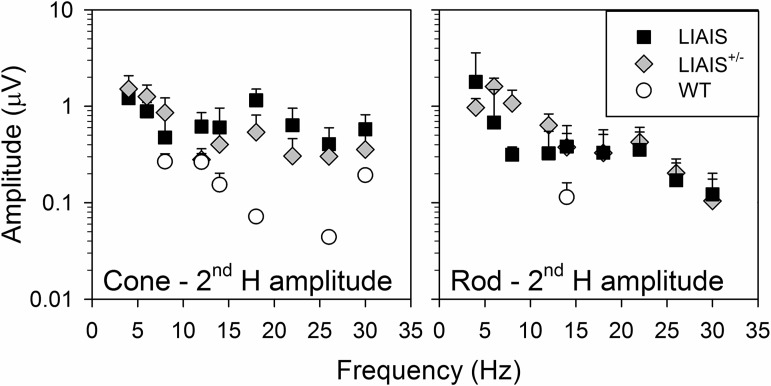
Figure 3 displays the amplitudes of the second harmonic components as a function of temporal frequency. As already noted in the description of Fig. 1, LIAIS+/− animals displayed a strong frequency-doubled response to “rod-isolating” stimuli at temporal frequencies between 5 and 12 Hz, exceeding even those of the LIAIS mice. Again, this indicates that they originated in both rods and the cones with native M-cone pigment. For the LIAIS and WT mice, rod and cone phase profiles were similar between strains.
Fig. 3.
Average (±SD) second harmonic (2nd H) component amplitudes as a function of temporal frequency for LIAIS (black squares; cone, n = 11; rod, n = 10), LIAIS+/− (gray diamonds; cone, n = 6; rod, n = 5), and WT mice (open circles; cone, n = 2; rod, n = 4). Only responses to cone (left)- and rod-isolating stimuli (right) for the highest stimulus contrast measured (cone: 55%, rod for mutants: 75%, rod for WT: 5%) and a mean luminance of 39 cd/m2 are given, as in Fig. 2. Similar to 1st H data, photoreceptor amplitudes generally decreased with increasing stimulus frequency. Note that 2nd H rod amplitudes for LIAIS+/− mice were large at low temporal frequencies, even exceeding those of LIAIS mice.
Influence of contrast and luminance on retinal physiology in LIAIS mice.
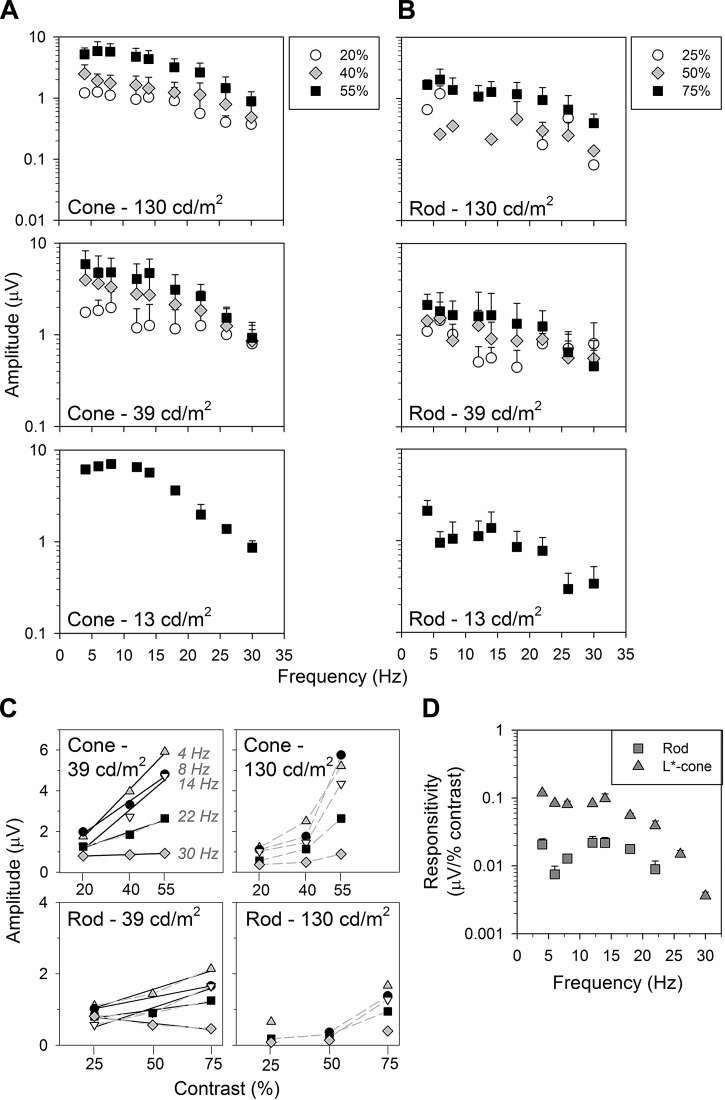
For the few temporal frequencies at which we could record substantial cone- and rod-driven responses in WT mice, they displayed phases like those of the LIAIS mice. This indicates that the replacement of cone pigment in the LIAIS mice did not lead to a physiologically relevant retinal change, in agreement with previous data (Greenwald et al. 2014). We therefore can use the recordings in the LIAIS mice to study how rod- and cone-driven responses depend on contrast and luminance. Thus further frequency-response functions were obtained with lower stimulus contrasts (40 and 20% cone contrasts, 50 and 25% rod contrasts; see Fig. 4, A and B) at 39 cd/m2 and also at 130 and 13 cd/m2.
Fig. 4.
Amplitudes show similar profiles in their dependency on temporal frequency under the same luminance. A and B: data are average (±SD) frequency-response functions of the 1st H components (log) obtained under L*-cone (A; n = 5–11, except at 13 cd/m2, where n = 1–4)- and rod-isolating conditions (B; n = 2–10) in LIAIS mice. Only the highest contrasts (55 and 75%; black squares) were obtained at 13 cd/m2 (bottom), whereas data from all 3 contrasts were collected at 39 (middle) and 130 cd/m2 (top). C: data sampled across temporal frequencies from the top 2 mean luminances are plotted against photoreceptor contrasts, with different symbols denoting various temporal frequencies sampled from A and B. Where applicable (goodness-of-fit; r2 ≥ 0.90), plots were modeled with (solid black) linear regression lines. Amplitude depends approximately linearly on contrast when mean luminance is 39 cd/m2, whereas the relationship is more exponential (gray dashed lines) when mean luminance is 130 cd/m2. This trend is more prominent for cone- than for rod-driven responses. In comparison, the effect of mean luminance on these amplitudes was variable and not as strong. D: L*-cone (triangles; n = 11) vs. rod comparisons (squares; n = 10) as a function of temporal frequency can also be converted to show photoreceptor responsivity.
Cone- (Fig. 4A) and rod-driven responses (Fig. 4B) generally displayed low-pass characteristics, possibly with a dip between 6 and 10 Hz. The signal-to-noise ratio was small at 13 cd/m2. Therefore, only the responses to the highest contrasts are shown (see Fig. 4, A and B, bottom, and Fig. 5, A and B, bottom).
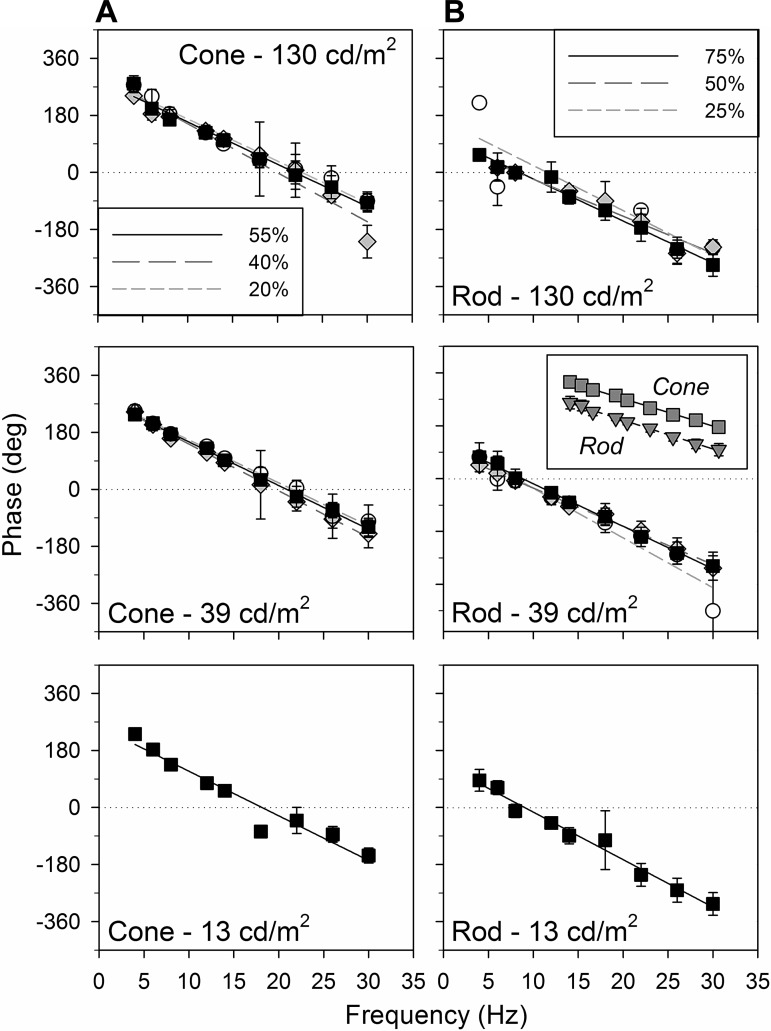
Fig. 5.
Average (±SD) LIAIS mouse frequency-phase profiles from L*-cone (A)- and rod-isolating stimuli (B) are similar under different mean luminance and contrast settings. Plots are modeled with a linear regression. See main text for slope comparisons. Inset: rod vs. cone at top contrast and 39 cd/m2 mean luminance.
The effect of luminance was variable, depending on the isolated photoreceptor response. Cone-driven responses were similar for a mean luminance change by a factor of 3 (top contrast, 39 cd/m2: 4.75 ± 2.52 μV vs. 130 cd/m2: 5.89 ± 2.50 μV, paired t-tests of largest differences between 4 and 18 Hz, P = 0.38, Fig. 4A, top vs. middle; 39 vs. 13 cd/m2, P = 0.02, Fig. 4A, middle vs. bottom). However, when mean luminance was dimmer by a factor of 10 (i.e., 130 vs. 13 cd/m2, Fig. 4A, top vs. bottom), cone amplitudes were surprisingly larger (P < 0.01). Cone amplitudes at 13 cd/m2 also exhibited a faster decrease with increasing temporal frequency. However, the power of this observation is not as high given a smaller N in this group. In contrast, rod-driven response amplitudes were significantly smaller at 13 cd/m2 (P = 0.01), whereas amplitudes elicited with brighter mean luminances were similar (P = 0.06). Unlike cone responses, the frequency dependency of rod responses at 13 cd/m2 was more variable.
Contrast had a strong influence on response amplitudes in the LIAIS mice. This was more prominent in cone- than in rod-driven responses. Mean luminance of the stimuli appears to also affect the effect of contrast (i.e., significant difference between data sets elicited with different photoreceptor contrasts only at 130 cd/m2, under M-cone-isolating conditions; repeated-measures ANOVA: P << 0.01; Bonferroni posttest indicates this difference to be between the 55% and 40% data sets, with no significant difference for 40% vs. 20% data sets).
In Fig. 4C, cone- and rod-driven flicker amplitudes are shown as a function of contrast. The data show that fundamental amplitudes strongly depend on contrast for both conditions. As was previously noted for human observers (Usui et al. 1998a), the relationship between amplitude and contrast was approximately linear when luminance was 39 cd/m2. In contrast, at 130 cd/m2, there seems to be an accelerating, nonlinear relationship between amplitude and contrast. Under both settings, cone-driven responses are increased by a greater proportion with increasing photoreceptor contrast than rod-driven ones (Fig. 4C, top vs. bottom).
Neither mean luminance nor contrast had much influence on phase characteristics (Fig. 5, P > 0.01 for all slope comparisons). The slope of cone-driven phases ranged from 13° to 15°/Hz, suggesting a delay of ∼36 to 42 ms (Fig. 5A), whereas rod-driven responses had phase slopes of 12° to 17°/Hz, corresponding to a 33- to 47-ms delay (Fig. 5B). These differences in phase between the two photoreceptor types were not significant (P > 0.01). What was significant is that their y-intercepts were ∼180° apart (Fig. 5B, inset). This was also the case in WT animals (e.g., at top contrasts for 39 cd/m2 in Fig. 2, C and D, P > 0.05; data at other settings not shown), indicating that the rod-cone similarity seen is innate and not an upshot of human L-cone substitution in LIAIS variant. Besides the poorer fits of the 25% rod contrast and 130 cd/m2 luminance profile (r2 = 0.78), linear regressions of all other frequency-phase behaviors account for at least 94% of the total variance in phase.
Comparison between L*-cones and rod photoreceptor responsivity in LIAIS mice.
Given the approximately linear relationship between ERG amplitudes and their respective photoreceptor contrasts at 39 cd/m2 luminance (Fig. 4C, left), the slope of the linear regressions describing the contrast vs. response amplitude relationship defines the contrast gains or responsivities (Kremers and Pangeni 2012). The nonlinear relationship at 130 cd/m2 prohibits such a simple calculation of contrast gain. In Fig. 4D, we compare averaged cone- and rod-driven responsivities at 39 cd/m2 luminance. The cone-driven responsivities were larger by about a factor of 8 than the rod-driven responsivities.
DISCUSSION
Isolation of medium- to long-wavelength cone function in mice.
In this work, we present mouse rod- and L-cone-driven ERGs obtained using double silent substitution flicker stimuli. Unlike previous rod/cone isolation experiments in mice, mean luminance and stimulus contrast were varied independently in this study, enabling direct comparison of rod- and cone-driven signals to each condition. Below we discuss these signals from the recently engineered (M-cone knockout/L*-cone knockin) LIAIS mouse (Greenwald et al. 2014), making comparisons to similarly evoked responses from humans where possible.
Rod and cone ERG responses were larger in LIAIS mice than in WT and heterozygous LIAIS females, showing that theoretically larger photoreceptor contrasts can be achieved in the LIAIS mice due to a larger separation between the rod and the substituted human L-cone spectra. Not only did these larger photoreceptor contrasts result in larger ERG responses, but also the smaller responses in WT mice to the rod- and cone-isolating stimuli provide evidence that the responses in other genotypes do indeed come from rods and cones, respectively, particularly for the cone-isolating stimuli, which are physically nearly identical for WT and LIAIS mice (Table 1). Despite differences in response magnitude due to differences in their achievable stimulus contrasts (Fig. 4, A, B, and D), response phase profiles measured in LIAIS mice closely matched those measured in WT mice (Fig. 5). In all groups, contrast settings did not influence response phase. These data strongly suggest that the postreceptoral signal processing leading to an ERG response is very similar in LIAIS and WT mice. It therefore can be concluded that the LIAIS mouse is a good model to study the normal physiology of photoreceptor-driven signals in the retina.
It remains to be demonstrated which perceptual consequences arise from this knockout/knockin process in LIAIS mice. It has been shown that they may display an increased sensitivity to long-wavelength light (Jacobs and Deegan Ii 1999; Shaaban et al. 1998; Smallwood et al. 2003), and some color vision may be possible in heterozygous animals (Jacobs et al. 2007).
Retinal physiology of the heterozygotes.
Intermediate ERG response profiles were observed in heterozygous mice (∼65 ± 15% and 54 ± 8% of LIAIS rod and cone ERGs, respectively). Moreover, they displayed frequency-response relationships of rod- and cone-driven amplitudes and phases similar to those of homozygotes and wild types. The only discrepancy was found in their rod-mediated phase profile (Fig. 2D, diamonds), where their phases were shifted and resemble those of the cones (by about 166° vs. LIAIS and 201° vs. WT rod phase). This phenomenon likely originates from the fact that the LIAIS rod-isolating protocol used did not silence the cones containing mouse M-opsin. In fact, the stimuli would modulate them “in phase” with rods with nearly the same contrast as the rods (see values indicated by dagger for LIAIS+/− in Table 1) due to their neighboring λmax values (Lyubarsky et al. 1999; Nikonov et al. 2006). The frequency-doubled ERG response at intermediate temporal frequencies is also a sign of two independent response components occurring simultaneously (Pangeni et al. 2010). In the heterozygous animals, one X chromosome contains the human L-cone pigment gene and the other, the gene for the native M-opsin. It can thus be inferred that a substantial number of cones contain native M-pigment. Given the higher relative strength of cone (vs. rod) contribution to the flicker ERG in mice indicated here [i.e., ∼2.5-fold larger cone vs. rod amplitudes at 20% lower photoreceptor contrast; LIAIS data, despite only ∼3% cone (Carter-Dawson and LaVail 1979; Jeon et al. 1998)] and in humans (Kremers and Pangeni 2012), a substantial response from the cones that overrules physiological behaviors from the rods can be expected in LIAIS+/− mice. This is indeed the case, because an almost equal modulation of a substantially fewer number of M-cones by the rod-isolating stimuli gave rise to cone-like flicker ERGs. This may not be surprising, because cone photoreceptors are more sensitive (than rods) under photopic conditions (e.g., Fig. 2, 39 cd/m2) as well as to rapidly changing light (Fu and Yau 2007).
Luminance and contrast influences on rod and L*-cone processing in the LIAIS mouse.
As mentioned above, this study teased out rod- and medium- to long-wavelength cone subtype-specific contributions to the mouse flicker ERG under identical adaptation conditions. Compared with mice, spectral isolation of individual photoreceptor types in the human retina has received more attention (Bessler et al. 2010; Brainard et al. 2000; Cao et al. 2011; Challa et al. 2010; Kremers et al. 2000, 2009; Kremers and Link 2008; Kremers and Scholl 2001), which has allowed more detailed investigations. Even so, few have examined rod-only contributions to the flicker ERG (Cao et al. 2011; Kremers and Pangeni 2012), because its isolation from S-cones with reasonable strength is difficult [i.e., λmax of S-cone ∼ 435 nm vs. rod ∼ 495 nm (Kraft et al. 1993; Stockman and Sharpe 2000)].
Because LIAIS mice showed robust rod and cone responses to the silent substitution conditions, further characterization of their response properties at more contrasts and luminance levels were performed. We found amplitude and phase behaviors of both photoreceptor types to be quite alike (except for the consistent 180° phase shift between rod- and cone-mediated responses). Generally, similar amplitude and phase profiles were more conserved at the higher mean luminance (130 cd/m2), even with lower photoreceptor contrasts (Figs. 3 and 4, A and B). At the lower luminance (13 cd/m2), rod-driven phases were also similar, but the transition of their fundamental amplitudes with temporal frequency was not as smooth. We found that rod-driven responses could be found at relatively high luminances. In previous studies, it was assumed that rod contribution may not influence ERG data at high luminances (Allen et al. 2014). Our data indicate that this assumption may not always hold. On the other hand, we assumed that melanopsin-driven responses have negligible effects on the ERGs at the measured temporal frequencies. A better separation of rod and melanopsin driven responses will be needed to completely control the output of all photopigments. The overlapping absorption spectra of the rods and the melanopsin-containing retinal ganglion cells make it difficult to achieve such a separation using the silent substitution method.
Photoreceptor contrast and response amplitudes for both rods and cones were positively correlated. A noteworthy feature in this case is that cone-driven response amplitudes changed by a greater extent with incremental contrast than did rod-driven responses (cf. Fig. 4C, top vs. bottom). Furthermore, the relationship between contrast and amplitude at 130 cd/m2 showed an exponential, rather than the expected linear increase as observed at 39 cd/m2 (Fig. 4C, left vs. right) and in human studies [≤78 cd/m2 (Brainard et al. 1999; Cao et al. 2008; Kremers et al. 1999, 2009; Kremers and Link 2008; Usui et al. 1998a)].
Comparison of rod- and L*-cone-driven responses.
As mentioned above, there are large similarities between the rod and cone flicker signals obtained in the current study, especially in LIAIS mice. That their frequency functions are parallel with equal slopes suggests that their processing mechanisms have similar temporal characteristics. Moreover, rod- and cone-driven ERG responses have very similar properties as a function of contrast and mean luminance, as well. However, cones had about a 10-fold higher responsivity than rods (see Fig. 4D). The relative responsivities may change when scotopic conditions are employed. The similarities between rod- and (L*-)cone-driven response characteristics remain intriguing. Rod amplitude profiles in particular, may be more cone-like due to the relatively high luminance used in this study [e.g., vs. putative rod flicker data sans background light from mice devoid of cone function (Tanimoto et al. 2015)]. Possibly, rod-cone similarities found here reflect coupling of rods and cones (Bloomfield and Dacheux 2001; DeVries and Baylor 1995; Tsukamoto et al. 2001). If this proposal is true, then it can be expected that the rod-driven responses have different characteristics when cones (of the medium- to long-wavelength type) are absent.
The fact that rod- and cone-driven responses are counter phase relative to each other (i.e., 180° apart, in LIAIS and WT data,), adds another feature where rod and cone responses can be distinguished postreceptorally. The frequency-doubled response in LIAIS+/− mice also indicates that the two interact in a nonlinear manner, because a simple linear vector addition of the two would lead to a mutual cancelation but not to a frequency doubling. It is difficult to speculate on the retinal mechanisms underlying these results. Possibly, mice rod- and cone-driven signals mainly feed different (on and off) processes, and the signals are rectified. Indeed, results of multielectrode recordings suggest that rod signals in the secondary pathway are mainly linked to a subset of retinal ganglion cells (DeVries and Baylor 1995). Recent data (Szikra et al. 2014) indicated that the rods may relay cone-driven surround inhibition. In the present study, the silent substitution method was used to isolate the responses of rod responses without stimulating the cones. This would mean that also direct rod signals would mediate surround inhibition. This apparent opponency between rod and cone signals could represent the basis of some type of color vision in mice. The behavioral data of Jacobs et al. (2007) indicate that heterozygous females with balanced M/L* ratios were able to discriminate between 500- and 600-nm monochromatic test lights at all intensity levels (as an indication of color vision). Animals with only M-cones or with large M/L* ratios were not able to discriminate between the two colors. These data were interpreted as an indication for opponency between L*- and M-cones. However, a rod-L*-cone opponency cannot be completely excluded on the basis of these data. The critical experiment would be discrimination tasks with homozygous mutants: if color vision were based on L*-M opponency, these animals should not display improved discrimination, whereas the L*-rod opponency would result in a similar or even better discrimination behavior than the heterozygous animals.
The question remains if there is an ecological explanation for similar rod- and cone-driven signals in the mouse, whereas they are different in humans. Perhaps, originally the divergence between rods and cones evolved to transfer on and off signals separately, as is the situation in the mouse. When mammals became diurnal, there was an evolutionary pressure for two photoreceptor systems that were active at different light regimes. The already present divergence between rod and cone pathways might have been a good substrate to accommodate this additional requirement.
Comparison between LIAIS mouse and human ERGs.
Because the correlation between response amplitudes and rod/cone contrast of the stimuli is linear in both LIAIS mice (at 39 cd/m2) and human subjects (Brainard et al. 1999; Kremers et al. 2009; Kremers and Link 2008; Usui et al. 1998a), their responsivities (i.e., the slopes of this contrast-response relationship) can be directly compared (Fig. 6). We assume that resulting retinal illuminances are also similar, because the used intensities are in a similar range (the effects of the smaller mouse eye will be counteracted by the smaller pupil size), and that differences in mean chromaticities in the mouse spectral luminosity function compared with the human Vλ are negligible.
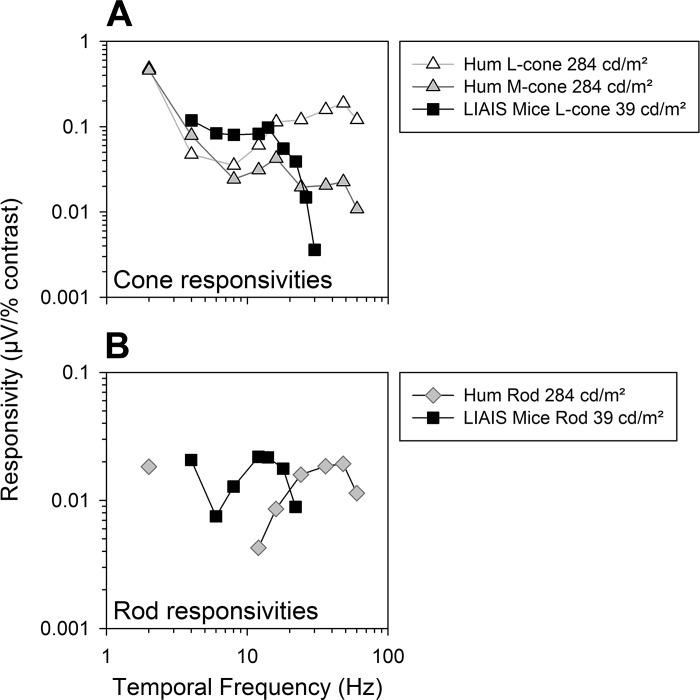
Fig. 6.
Differences are evident between data from LIAIS mice (from Fig. 4D) and human photoreceptor responsivities (n = 5; Kremers and Pangeni 2012). L-cone (A) and rod responsivities (B) for each species are shown as a function of temporal frequency.
There are appreciable differences between photoreceptor-specific human and mouse flicker ERGs, in agreement with previous data obtained with white light (Krishna et al. 2002), despite the mouse expressing human L*-opsin. Species differences in the retinal circuitry may be the cause, which, although not surprising, should nonetheless be explicitly mentioned, since the mouse is still the mainstay model for studying retinal physiology and its disease-related changes. These may need to be considered when extrapolating LIAIS mouse ERG data to the human situation.
The human data indicate that two response mechanisms can be discerned in the ERGs. One is active at frequencies up to about 15 Hz, where L- and M-cone responsivities are similar (i.e., a putatively cone opponent mechanism that may reflect parvocellular activity), whereas the other is active at higher temporal frequencies, measurable up to at least 40 Hz (i.e., reflects magnocellularly based luminance activity). This change in mechanism is also reflected in the human response phases (Kommanapalli et al. 2014). Mouse responsivities, on the other hand, are larger at low temporal frequencies and decrease strongly above about 14 Hz, being barely measurable at 30 Hz. It is possible that the responses in the mouse retina are generally more sluggish or more tonic than in humans. The apparent latencies that explain response phases may also be in agreement with this notion [i.e., estimates in mice: 37–39 ms for cone- and rod-driven signals vs. 15–17 ms in humans for 12–60 Hz (Kommanapalli et al. 2014; Kremers and Pangeni 2012)].
It is difficult to speculate what the mechanisms may be in the mouse. Krishna et al. (2002) proposed a transition from responses dominated by depolarizing bipolar cells at low temporal frequencies to those reflecting activity of hyperpolarizing bipolar cells at high temporal frequencies. An alternative may be a similar transition as in humans: from an opponent to a luminance sensitive mechanism. Of course, the opponent mechanism cannot be parvocellular-like because mice are dichromats with only one cone type in the middle- to long-wavelength range. Possibly, the above-mentioned rod-cone interactions are of importance.
Conclusions.
The mouse M-opsin knockout/human L-pigment knockin LIAIS line created the possibility to effectively use the silent substitution paradigm to study normal physiological activity mediated by any photoreceptor subtype in the mice, the most widely used model organism for studying human disease. Pigment substitution did not alter the physiological properties of the transgenic cones, complementing previous anatomic validations. Similarities not previously seen in humans were revealed between the response properties of mouse rod and medium- to long-wavelength cones, with indications that the two are processed in an opponent manner. Once photoreceptor properties are fully elucidated in the healthy mouse, future research on LIAIS-crossed models of retinal disorders could expand current insights on retinal diseases caused by M-cone or rod malfunction, since an underlying pathophysiological change could be studied for a specific photoreceptor subtype-driven circuitry.
GRANTS
This work was supported by the German Research Foundation (DFG) Grant KR1317/13-1 (to J. Kremers).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.I.T. and J.A. performed experiments; T.I.T. and J.A. analyzed data; T.I.T. and J.K. interpreted results of experiments; T.I.T. prepared figures; T.I.T., J.A., and J.K. drafted manuscript; T.I.T., J.A., and J.K. edited and revised manuscript; T.I.T., J.A., M.N., J.N., and J.K. approved final version of manuscript; J.A., M.N., J.N., and J.K. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. Dieter Engelkamp from the University's Transgenic Mouse Facility, Department of Biology, for transferring mouse embryos to establish a new breeding colony.
REFERENCES
- Allen AE, Storchi R, Martial FP, Petersen RS, Montemurro MA, Brown TM, Lucas RJ. Melanopsin-driven light adaptation in mouse vision. Curr Biol 24: 2481–2490, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atorf J, Scholz M, Garreis F, Lehmann J, Brauer L, Kremers J. Functional protective effects of long-term memantine treatment in the DBA/2J mouse. Doc Ophthalmol 126: 221–232, 2013. [DOI] [PubMed] [Google Scholar]
- Bessler P, Klee S, Kellner U, Haueisen J. Silent substitution stimulation of S-cone pathway and L- and M-cone pathway in glaucoma. Invest Ophthalmol Vis Sci 51: 319–326, 2010. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res 20: 351–384, 2001. [DOI] [PubMed] [Google Scholar]
- Brainard DH, Calderone JB, Nugent AK, Jacobs GH. Flicker ERG responses to stimuli parametrically modulated in color space. Invest Ophthalmol Vis Sci 40: 2840–2847, 1999. [PubMed] [Google Scholar]
- Brainard DH, Roorda A, Yamauchi Y, Calderone JB, Metha AB, Neitz M, Neitz J, Williams DR, Jacobs GH. Functional consequences of the relative numbers of L and M cones. J Opt Soc Am A Opt Image Sci Vis 17: 607–614, 2000. [DOI] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol 8: e1000558, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Tsujimura S, Allen AE, Wynne J, Bedford R, Vickery G, Vugler A, Lucas RJ. Melanopsin-based brightness discrimination in mice and humans. Curr Biol 22: 1134–1141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MA, Lucas RJ. Influence of the rod photoresponse on light adaptation and circadian rhythmicity in the cone ERG. Mol Vis 15: 2209–2216, 2009. [PMC free article] [PubMed] [Google Scholar]
- Cao D, Pokorny J, Grassi MA. Isolated mesopic rod and cone electroretinograms realized with a four-primary method. Doc Ophthalmol 123: 29–41, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Pokorny J, Smith VC, Zele AJ. Rod contributions to color perception: linear with rod contrast. Vision Res 48: 2586–2592, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol 188: 245–262, 1979. [DOI] [PubMed] [Google Scholar]
- Challa NK, McKeefry D, Parry NRA, Kremers J, Murray IJ, Panorgias A. L- and M-cone input to 12Hz and 30Hz flicker ERGs across the human retina. Ophthalmol Physiol Opt 30: 503–510, 2010. [DOI] [PubMed] [Google Scholar]
- Cia D, Bordais A, Varela C, Forster V, Sahel JA, Rendon A, Picaud S. Voltage-gated channels and calcium homeostasis in mammalian rod photoreceptors. J Neurophysiol 93: 1468–1475, 2005. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Baylor DA. An alternative pathway for signal flow from rod photoreceptors to ganglion cells in mammalian retina. Proc Natl Acad Sci USA 92: 10658–10662, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner KO, Rushton WA. Retinal stimulation by light substitution. J Physiol 149: 288–302, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RH, Jeffery G. The spectral transmission of ocular media suggests ultraviolet sensitivity is widespread among mammals. Proc Biol Sci 281: 20132995, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner A, Macleod DI. Flicker photometric study of chromatic adaption: selective suppression of cone inputs by colored backgrounds. J Opt Soc Am 71: 705–717, 1981. [DOI] [PubMed] [Google Scholar]
- Estévez O, Spekreijse H. The “silent substitution” method in visual research. Vision Res 22: 681–691, 1982. [DOI] [PubMed] [Google Scholar]
- Estévez O, Spekreijse H. A spectral compensation method for determining the flicker characteristics of the human colour mechanisms. Vision Res 14: 823–830, 1974. [DOI] [PubMed] [Google Scholar]
- Fu YB, Yau KW. Phototransduction in mouse rods and cones. Pflügers Arch 454: 805–819, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald SH, Kuchenbecker JA, Roberson DK, Neitz M, Neitz J. S-opsin knockout mice with the endogenous M-opsin gene replaced by an L-opsin variant. Vis Neurosci 31: 25–37, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GH. Variations in colour vision in non-human primates. In: Inherited and Acquired Colour Vision Deficiencies: Fundamental Aspects and Clinical Studies, edited by Foster DH. Boca Raton, FL: CRC, 1991, p. 199–214. [Google Scholar]
- Jacobs GH, Deegan JF 2nd. Five distinct M/L photopigments in a New World monkey. Invest Ophthalmol Vis Sci 40: S981, 1999. [Google Scholar]
- Jacobs GH, Williams GA, Cahill H, Nathans J. Emergence of novel color vision in mice engineered to express a human cone photopigment. Science 315: 1723–1725, 2007. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci 18: 8936–8946, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommanapalli D, Murray IJ, Kremers J, Parry NR, McKeefry DJ. Temporal characteristics of L- and M-cone isolating steady-state electroretinograms. J Opt Soc Am A Opt Image Sci Vis 31: A113–A120, 2014. [DOI] [PubMed] [Google Scholar]
- Kraft TW, Schneeweis DM, Schnapf JL. Visual transduction in human rod photoreceptors. J Physiol 464: 747–765, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremers J, Czop D, Link B. Rod and S-cone driven ERG signals at high retinal illuminances. Doc Ophthalmol 118: 205–216, 2009. [DOI] [PubMed] [Google Scholar]
- Kremers J, Link B. Electroretinographic responses that may reflect activity of parvo- and magnocellular post-receptoral visual pathways. J Vis 8: 1–14, 2008. [DOI] [PubMed] [Google Scholar]
- Kremers J, Pangeni G. Electroretinographic responses to photoreceptor specific sine wave modulation. J Opt Soc Am A Opt Image Sci Vis 29: A309–A316, 2012. [DOI] [PubMed] [Google Scholar]
- Kremers J, Rodrigues AR, Silveira LC, da Silva-Filho M. Flicker ERGs representing chromaticity and luminance signals. Invest Ophthalmol Vis Sci 51: 577–587, 2010. [DOI] [PubMed] [Google Scholar]
- Kremers J, Scholl HP. Rod-/L-cone and rod-/M-cone interactions in electroretinograms at different temporal frequencies. Vis Neurosci 18: 339–351, 2001. [PubMed] [Google Scholar]
- Kremers J, Scholl HP, Knau H, Berendschot TT, Usui T, Sharpe LT. L/M cone ratios in human trichromats assessed by psychophysics, electroretinography, and retinal densitometry. J Opt Soc Am A Opt Image Sci Vis 17: 517–526, 2000. [DOI] [PubMed] [Google Scholar]
- Kremers J, Stepien MW, Scholl HP, Saito CA. Cone selective adaptation influences L- and M-cone driven signals in electroretinography and psychophysics. J Vis 3: 146–160, 2003. [DOI] [PubMed] [Google Scholar]
- Kremers J, Usui T, Scholl HP, Sharpe LT. Cone signal contributions to electroretinograms in dichromats and trichromats. Invest Ophthalmol Vis Sci 40: 920–930, 1999. [PubMed] [Google Scholar]
- Krishna VA, Alexander KR, Peachey NS. Temporal properties of the mouse cone electroretinogram. J Neurophysiol 87: 42–48, 2002. [DOI] [PubMed] [Google Scholar]
- Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Guler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron 66: 417–428, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD. Photoreceptor spectral sensitivities: common shape in the long-wavelength region. Vision Res 35: 3083–3091, 1995. [DOI] [PubMed] [Google Scholar]
- Lee BB, Dacey DM, Smith VC, Pokorny J. Horizontal cells reveal cone type-specific adaptation in primate retina. Proc Natl Acad Sci USA 96: 14611–14616, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky AL, Falsini B, Pennesi ME, Valentini P, Pugh EN Jr. UV and midwave-sensitive cone-driven retinal responses of the mouse: a possible phenotype for coexpression of cone photopigments. J Neurosci 19: 442–455, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Kholodenko R, Lem J, Pugh EN Jr. Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol 127: 359–374, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmos P, van Norren D. Cone spectral sensitivity and chromatic adaptation as revealed by human flicker electroretinography. Vision Res 11: 27–42, 1971. [DOI] [PubMed] [Google Scholar]
- Pangeni G, Horn FK, Kremers J. A new interpretation of components in the ERG signals to sine wave luminance stimuli at different temporal frequencies and contrasts. Vis Neurosci 27: 79–90, 2010. [DOI] [PubMed] [Google Scholar]
- Parry NR, Murray IJ, Panorgias A, McKeefry DJ, Lee BB, Kremers J. Simultaneous chromatic and luminance human electroretinogram responses. J Physiol 590: 3141–3154, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regus-Leidig H, Atorf J, Feigenspan A, Kremers J, Maw MA, Brandstatter JH. Photoreceptor degeneration in two mouse models for congenital stationary night blindness type 2. PLoS One 9: e86769, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regus-Leidig H, Ott C, Lohner M, Atorf J, Fuchs M, Sedmak T, Kremers J, Fejtova A, Gundelfinger ED, Brandstatter JH. Identification and immunocytochemical characterization of Piccolino, a novel Piccolo splice variant selectively expressed at sensory ribbon synapses of the eye and ear. PLoS One 8: e70373, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban SA, Crognale MA, Calderone JB, Huang J, Jacobs GH, Deeb SS. Transgenic mice expressing a functional human photopigment. Invest Ophthalmol Vis Sci 39: 1036–1043, 1998. [PubMed] [Google Scholar]
- Shapiro AG, Pokorny J, Smith VC. Cone-rod receptor spaces with illustrations that use the CRT phosphor and light-emitting-diode spectra. J Opt Soc Am A Opt Image Sci Vis 13: 2319–2328, 1996. [DOI] [PubMed] [Google Scholar]
- Smallwood PM, Olveczky BP, Williams GL, Jacobs GH, Reese BE, Meister M, Nathans J. Genetically engineered mice with an additional class of cone photoreceptors: implications for the evolution of color vision. Proc Natl Acad Sci USA 100: 11706–11711, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman A, Sharpe LT. The spectral sensitivities of the middle- and long-wavelength-sensitive cones derived from measurements in observers of known genotype. Vision Res 40: 1711–1737, 2000. [DOI] [PubMed] [Google Scholar]
- Sun H, Macke JP, Nathans J. Mechanisms of spectral tuning in the mouse green cone pigment. Proc Natl Acad Sci USA 94: 8860–8865, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson WH. Chromatic adaptation alters spectral sensitivity at high temporal frequencies. J Opt Soc Am A 10: 1294–1303, 1993. [DOI] [PubMed] [Google Scholar]
- Szikra T, Trenholm S, Drinnenberg A, Juttner J, Raics Z, Farrow K, Biel M, Awatramani G, Clark DA, Sahel JA, da Silveira RA, Roska B. Rods in daylight act as relay cells for cone-driven horizontal cell-mediated surround inhibition. Nat Neurosci 17: 1728–1735, 2014. [DOI] [PubMed] [Google Scholar]
- Szmajda BA, Buzas P, Fitzgibbon T, Martin PR. Geniculocortical relay of blue-off signals in the primate visual system. Proc Natl Acad Sci USA 103: 19512–19517, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto N, Sothilingam V, Kondo M, Biel M, Humphries P, Seeliger MW. Electroretinographic assessment of rod- and cone-mediated bipolar cell pathways using flicker stimuli in mice. Sci Rep 5: 10731, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci 21: 8616–8623, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Kremers J, Sharpe LT, Zrenner E. Flicker cone electroretinogram in dichromats and trichromats. Vision Res 38: 3391–3396, 1998a. [DOI] [PubMed] [Google Scholar]
- Usui T, Kremers J, Sharpe LT, Zrenner E. Response phase of the flicker electroretinogram (ERG) is influenced by cone excitation strength. Vision Res 38: 3247–3251, 1998b. [DOI] [PubMed] [Google Scholar]
- Zele AJ, Smith VC, Pokorny J. Spatial and temporal chromatic contrast: effects on chromatic discrimination for stimuli varying in L- and M-cone excitation. Vis Neurosci 23: 495–501, 2006. [DOI] [PubMed] [Google Scholar]