Abstract
Active control of the mediolateral location of the feet is an important component of a stable bipedal walking pattern, although the roles of sensory feedback in this process are unclear. In the present experiments, we tested whether hip abductor proprioception influenced the control of mediolateral gait motion. Participants performed a series of quiet standing and treadmill walking trials. In some trials, 80-Hz vibration was applied intermittently over the right gluteus medius (GM) to evoke artificial proprioceptive feedback. During walking, the GM was vibrated during either right leg stance (to elicit a perception that the pelvis was closer mediolaterally to the stance foot) or swing (to elicit a perception that the swing leg was more adducted). Vibration during quiet standing evoked leftward sway in most participants (13 of 16), as expected from its predicted perceptual effects. Across the 13 participants sensitive to vibration, stance phase vibration caused the contralateral leg to be placed significantly closer to the midline (by ∼2 mm) at the end of the ongoing step. In contrast, swing phase vibration caused the vibrated leg to be placed significantly farther mediolaterally from the midline (by ∼2 mm), whereas the pelvis was held closer to the stance foot (by ∼1 mm). The estimated mediolateral margin of stability was thus decreased by stance phase vibration but increased by swing phase vibration. Although the observed effects of vibration were small, they were consistent with humans monitoring hip proprioceptive feedback while walking to maintain stable mediolateral gait motion.
Keywords: biomechanics, locomotion, sensory feedback, vibration
stable human walking requires appropriate mechanical interactions with the environment influenced in part by the locations of external forces acting on the body. The particular importance of adjustments in the mediolateral location of the feet for maintaining bipedal gait stability has been supported by human walking experiments (MacKinnon and Winter 1993; Bauby and Kuo 2000), model simulations (Townsend 1985; Redfern and Schumann 1994; Kuo 1999), and the development of walking robots (Hobbelen and Wisse 2009). By appropriately adjusting their mediolateral foot placement relative to their center of mass (CoM) (hereby referred to simply as mediolateral foot placement), humans may seek to maintain a “margin of stability” between their base of support and their ongoing CoM motion (Hof et al. 2005; Hof 2008). In fact, trunk or pelvis mechanics (proxies for CoM mechanics) can be used to predict both step width and mediolateral foot placement during an ongoing step (Hurt et al. 2010; Wang and Srinivasan 2014).
We have recently reported direct evidence for a neuromechanical gait stabilization strategy based on the active control of mediolateral foot placement (Rankin et al. 2014). The mechanical state of the stance leg and pelvis predicts hip abductor [gluteus medius (GM)] activity in the contralateral swing leg, which in turn predicts mediolateral foot placement. Specifically, large mediolateral displacements between the sacrum and stance foot at the start of a step are typically accompanied by relatively strong swing leg GM activation and lateral foot placement, whereas small sacrum displacements are followed by weak GM activation and more medial foot placement. The same strategy of adjusting swing leg abductor activity and foot placement in response to changes in pelvis or CoM mechanics is present (albeit scaled up) when external perturbations are applied to either the torso (Hof and Duysens 2013) or the swing foot (Rankin et al. 2014). In addition to the adjustments in foot placement, large mediolateral displacements between the pelvis and stance foot early in a step predict large displacements between these locations at the end of the step (Wang and Srinivasan 2014), although it is not clear whether this relationship is a function of active control or can simply be attributed to passive mechanics of an inverted pendulum. The combination of these results suggests that during walking, humans 1) establish an appropriate foot placement target based on the perception of their pelvis mechanics and 2) adjust their perceived swing leg position toward this target. However, it is presently unclear how humans execute either of these tasks.
Various sources of sensory feedback could potentially contribute to an individual's perception (whether conscious or subconscious) of their body's mechanical state, thereby influencing the control of their mediolateral gait motion. To generate a perception of the mechanical state of the pelvis and trunk relative to the stance foot, humans could use vestibular feedback, visual feedback, cutaneous feedback from the sole of the stance foot, or proprioceptive feedback from the stance leg ankle, knee, or hip. Each of these types of sensory feedback appears to make some level of contribution to the maintenance of standing postural balance (van der Kooij et al. 1999; Maurer et al. 2006; Bingham et al. 2011; Goodworth and Peterka 2012) and may also be important in ensuring stability of the torso over the stance leg while walking. Perceiving the mediolateral position of the swing leg may rely more exclusively on proprioceptive feedback from the swing leg hip, as feedback from other sensory sources would be less likely to provide useful information about this limb position. Therefore, hip proprioception could logically play a role in the perception of both the mechanical state of the pelvis and the position of the swing leg, potentially contributing in two ways to the proposed gait stabilization strategy.
Musculotendon vibration can be used to probe the role of proprioceptive feedback during functional tasks. Vibration of a muscle or its associated tendon elicits small, repetitive changes in muscle length. These length changes are preferentially sensed by muscle spindles and perceived as motion in which the vibrated muscle lengthens (Goodwin et al. 1972; Gilhodes et al. 1986). For example, Achilles tendon vibration during standing posture causes posterior sway, a response to the perception of an anterior lean that would normally accompany plantarflexor stretch (Eklund 1972; Kavounoudias et al. 1999; Ivanenko et al. 2000). Although it has been studied less extensively, unilateral vibration of the GM during standing causes sway away from the vibrated side, a response to the perception of a more adducted hip angle corresponding to GM stretch (Popov et al. 1999).
To better understand how humans successfully maintain mediolateral stability while walking, we must determine whether motor behavior is shaped by specific sources of sensory feedback providing information about the mechanical state of the body. Such an understanding may allow the future development of techniques to improve gait stability in the many clinical populations with altered sensorimotor function. The purpose of the present experiment was to determine whether proprioceptive feedback from the hip abductors influences the control of mediolateral gait motion. Such control may be evident either through changes in the position of the pelvis relative to the stance foot (indicating active control with the stance leg) or changes in mediolateral foot placement location (active control with the swing leg). We applied brief periods of intermittent vibration to the right GM during either the stance or swing phase. Stance phase vibration was intended to generate the perception that the pelvis was closer mediolaterally to the stance foot, as would be the case with a more adducted stance leg. Swing phase vibration was intended to generate the perception of a more adducted swing leg hip angle.
We hypothesized that GM vibration would influence frontal plane movement during gait in two distinct ways. First, we hypothesized that GM vibration during the stance phase would cause participants to further abduct their stance (vibrated) legs to push their CoM away from their stance foot while placing their contralateral (unvibrated) swing leg more medially. These effects would reflect bilateral responses to the elicited false perception of a smaller displacement between the midline and stance foot. Second, we hypothesized that GM vibration during the swing phase would cause participants to place the swing (vibrated) leg more laterally while reducing the abduction of their stance (unvibrated) leg to keep their midline closer to the stance foot. Such effects would reflect bilateral responses to the false perception of an overly adducted swing leg.
MATERIALS AND METHODS
Participants.
Sixteen healthy adults (13 females, 3 males; age = 23 ± 1 yr; height = 166 ± 9 cm; leg length = 85 ± 5 cm; mass = 65.6 ± 11.9 kg; means ± SD) participated in this experiment. All participants self-reported that they were right leg dominant, as identified by asking which leg they would use to kick a ball. None of the participants had any self-reported neurological or orthopedic injuries or conditions. Participants provided informed consent using forms and procedures approved by the Medical University of South Carolina Institutional Review Board and consistent with the Declaration of Helsinki.
Vibration characteristics.
In some trials, vibration was applied over the right GM using an eccentrically weighted motor. The motor, which we have used previously to investigate the effects of vibration at the ankle (Floyd et al. 2014), was positioned midway between the greater trochanter of the femur and the iliac crest and held in place using an elastic strap around the waist (Fig. 1). Vibration was applied at a constant frequency of 80 Hz and amplitude of 0.25 mm, characteristics that tend to preferentially recruit primary muscle spindles from the vibrated muscle (Roll and Vedel 1982; Fallon and Macefield 2007).
Fig. 1.
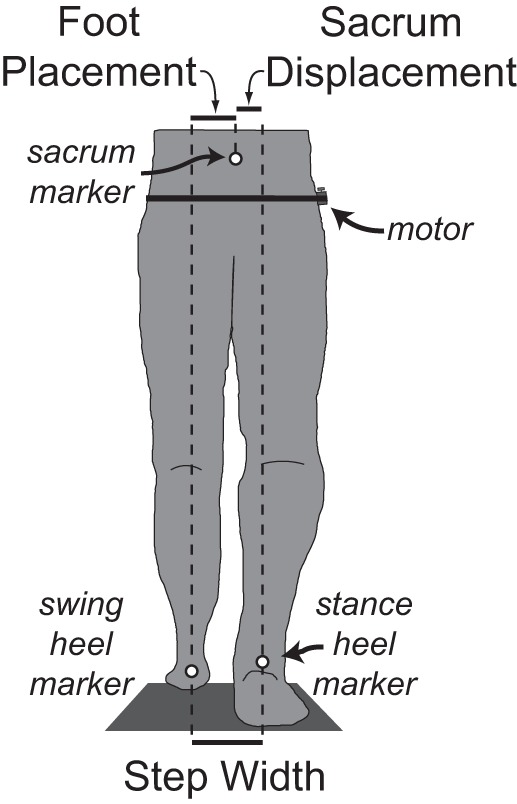
Schematic illustration of the experimental setup and mediolateral gait measures. The body's configuration at the moment of left heel strike is illustrated in the frontal plane from a posterior view. The mediolateral positions of the sacrum, left heel, and right heel markers were used to calculate mediolateral foot placement, sacrum displacement, and step width at this moment in time. A small, eccentrically weighted motor was secured over the right gluteus medius (GM) using elastic straps around the waist and used to deliver periodic vibratory perturbations.
Standing posture trials.
To confirm that our vibratory stimuli were sufficient to elicit a behavioral response, we first quantified the effects of GM vibration on standing posture. Participants completed a series of three 1-min quiet standing trials, standing on a force plate (Bertec, Columbus, OH) with their arms crossed, eyes closed, and feet parallel and as close together as possible without touching. Vibration was applied during the middle 20-s period, preceded and followed by 20-s periods without vibration. Participants were given 1 min of rest between trials, in which they were permitted to move around before returning to the testing position.
We focused on the effects of GM vibration on frontal plane motion, as this muscle appears to be particularly important for lateral postural stability (Gilles et al. 1999). Mediolateral center of pressure (CoP) location was calculated from force plate data collected at 1,000 Hz. Mediolateral CoM location was estimated from the position of a single LED marker (Phase Space, San Leandro, CA) placed over the sacrum and collected at 120 Hz. Both CoP and sacrum position data were low-pass filtered at 10 Hz.
Walking trials.
Participants performed a series of treadmill (Bertec) walking trials at 1.25 m/s, a typical preferred speed during treadmill walking. For all trials, participants wore a harness that did not support body weight but would have prevented a fall in case of a loss of balance. Participants first performed a 10-min walking trial to become accustomed to walking on the treadmill (Zeni and Higginson 2010), followed by four walking trials in which data were collected, each separated by 3 min of rest. The first and final recorded walking trials were 5-min control trials in which vibration was not applied. The purpose of these trials was to determine whether gait behavior changed over the course of the experiment, possibly due to either exposure to vibration or repeated periods of walking. The other two trials included vibration periodically applied to the right GM; in one trial these perturbations occurred when the right leg was in stance (stance phase vibration), and in the other trial these perturbations occurred when the right leg was in swing (swing phase vibration). Both of these trials were 10 min long and performed in randomized order.
During walking trials, the timing of vibration was controlled using pressure-sensitive foot switches (Motion Lab Systems, Baton Rouge, LA) placed under the left and right heels. Stance phase vibration was turned on at right heel strike and off at left heel strike (during the period defined as a left step), so the vibration ended slightly before the right foot left the ground. Swing phase vibration was turned on at left heel strike and off at right heel strike (defined as a right step), so this vibration was also delivered for a short time period while the right leg was in preswing. Although there was a short delay (∼40 ms) between heel strike events and the motors turning on or off, the periods of applied vibration closely corresponded to the intended gait phases. Vibratory perturbations were not delivered in every step but were instead separated by a random period of time (between 6 and 10 s), preventing participants from anticipating their delivery. Approximately 70 perturbations were applied over the course of the 10-min trials. Quantifying the average response to this large number of perturbations would potentially allow us to detect relatively small effects, which may otherwise be masked by the natural variability in gait behavior.
We used a motion capture system (Phase Space) with spatial resolution of 0.1 mm to characterize mediolateral motion during gait. Active LED markers placed on the sacrum, left heel, and right heel were sampled at 120 Hz and low-pass filtered at 10 Hz. Heel strike and toe-off events were identified from anteroposterior velocity of the heel markers (Zeni et al. 2008). The sacrum marker was used to approximate the mediolateral position of the CoM. Although this is a simplification, recent experiments found that the mediolateral position of a single sacrum marker was highly correlated (r > 0.97) with CoM position calculated from full body kinematic data during both normal and mechanically perturbed walking (Yang and Pai 2014). For each step, we calculated the mediolateral foot placement location, sacrum displacement relative to the stance foot, and step width (Fig. 1). The mediolateral foot placement location was calculated as the mediolateral distance between the swing heel and sacrum upon heel strike with the swing foot, serving as a measure of the swing leg position (Balasubramanian et al. 2010; Rankin et al. 2014). Sacrum displacement served as a measure of the relative posture between the stance leg and pelvis. For each step, we also calculated step period and step length.
We also estimated the mediolateral “margin of stability,” a measure of growing popularity that accounts for both the position and velocity of the CoM to predict whether the CoM will move lateral to the base of support and cause a potential loss of balance (Hof et al. 2005; Hof 2008). We calculated an estimate of mediolateral margin of stability for each step upon heel strike, when this metric is typically near-minimal (Hof et al. 2007; Rosenblatt and Grabiner 2010). Our small number of kinematic markers prevented us from using the typical method of calculating CoM position and velocity from a multisegment model and approximations of normal human anthropometry and of quantifying the borders of the base of support from the boundaries of the feet. Instead, we simply used the sacrum marker to approximate CoM motion and the heel markers to approximate the base of support. Our results will thus not be directly comparable with previous reported values (likely underestimating the actual margin of stability). However, our approach will detect changes in margin of stability caused by altering the frontal plane position of the swing leg with respect to the midline, which is the focus of this study. We first approximated the extrapolated center of mass (xCOM) using the sacrum mediolateral position (Xsacrum) and velocity (Vsacrum) (Eq. 1). As is standard with this approach, we accounted for differences in leg length by dividing the sacrum velocity by the natural frequency (ω0) of a pendulum 1.34 times the length of the leg (Hurt and Grabiner 2015):
| (1) |
Our estimated margin of stability (MOS) was then calculated as the difference between the mediolateral position of the heel marker (Xheel) of the leg that just contacted the ground and the extrapolated center of mass (Eq. 2):
| (2) |
Larger MOS values would indicate that the heel marker is farther laterally from the extrapolated center of mass.
Data analysis and statistics.
For standing posture trials, we first identified participants who exhibited the expected qualitative response to vibration, as previous work has demonstrated that some healthy individuals are insensitive to vibratory stimuli (Gurfinkel et al. 1998; Fuentes et al. 2012). To do so, we quantified the average mediolateral CoP and sacrum position during the 20-s periods before, during, and after the vibration across all three trials. An individual was classified as responsive to the vibration if their average CoP and sacrum position during the vibration was farther to the left (more negative in our laboratory reference frame) than the average CoP and sacrum position both before and after the vibration. This pattern of movement would match the expected sway to the left when vibration was turned on and sway to the right when vibration was turned off (Popov et al. 1999). Among participants classified as responsive, the minimum change in average sacrum position was 1.4 mm, a small but clearly detectable shift. Any participants who did not exhibit this pattern of the CoP and sacrum being farthest to the left during the period of vibration were classified as nonresponsive and were not included in subsequent analyses.
Although we measured both CoP and sacrum position, our analyses focused on the sacrum to more closely correspond to the kinematic measures of interest during walking trials. We calculated the average mediolateral sacrum position during the 20-s periods before, during, and after vibration. To account for any differences in initial position on the force plate across participants, we subtracted the average position during the first 20-s period. We used a repeated measures one-way ANOVA to test whether time period (before, during, and after vibration) had a significant effect on average sacrum position. In case of a significant main effect (P < 0.05), we used Tukey-Kramer post hoc tests to identify any significant differences between individual time periods. Fluctuations in the sacrum position over time due to normal sway made identifying the beginning and end of the vibration-evoked sway difficult in individual trials. Therefore, we estimated the time course of the vibration effects using the group average trace of sacrum position over time. We calculated the delay between the onset of vibration and the start of continuous (>0.5 s) leftward sway. We also calculated the duration of this initial sway period before the sacrum stopped moving continuously to the left.
During walking trials, we first investigated whether gait characteristics during unperturbed steps differed across trials and between the right and left legs. For each individual, we quantified average bilateral mediolateral foot placement location, sacrum displacement, step width, estimated margin of stability, step period, and step length for 1) all steps in the first control trial, 2) steps immediately preceding the vibratory stimulation during swing vibration trials (to minimize any potential effects of prior vibration), 3) steps immediately preceding the vibratory stimulation during stance vibration trials, and 4) all steps in the final control trial. We tested whether each of these gait characteristics was influenced by trial number or leg side using a two-way repeated-measures ANOVA with interactions. Where appropriate, we used Tukey-Kramer post hoc tests to identify significant differences between individual trials.
We also investigated the direct effects of the vibratory perturbations. We quantified the average mediolateral foot placement location, sacrum displacement, step width, estimated margin of stability, step period, and step length for the two steps preceding the perturbation (baseline), the perturbed step itself, and the three steps following the perturbation. Because even large mechanical perturbations are corrected within two steps (Rankin et al. 2014), we expected that this would be sufficient to quantify the response to our relatively weak sensory perturbations. A repeated-measures one-way ANOVA was used to test whether step number influenced these metrics for each leg. If a significant main effect (P < 0.05) was present, we used Tukey-Kramer post hoc tests to determine whether individual steps differed significantly from the preceding baseline values.
RESULTS
Standing posture.
Vibration of the right GM typically caused participants to sway away from the applied vibration. Both the sacrum and CoP exhibited similar changes in mediolateral position over time, moving to the left when vibration was turned on and to the right when vibration was turned off (Fig. 2A). Three of the 16 participants did not follow this typical pattern and were excluded from further analysis. These three nonresponsive individuals (1 male and 2 females) had a body mass index similar to that of the responsive participants (24.2 kg/m2 for nonresponders and 23.6 kg/m2 for responders), suggesting that differences between these groups were not simply due to varying amounts of adipose tissue at the hip. For the remaining 13 participants, average sacrum position was significantly (P < 0.0001) influenced by time period (Fig. 2B). Based on the group average sacrum trace, the initial period of sway induced by vibration started 0.27 s after vibration onset and lasted 1.81 s, with the sacrum moving 6.8 mm to the left during this time period. During the remaining period of vibration, there was a trend for participants to sway slightly back toward their initial mediolateral position. After the vibration was turned off, a subsequent period of clear sway lasted 2.05 s, with the sacrum moving 7.5 mm to the right.
Fig. 2.

Effects of vibration on frontal plane postural sway. A: the effects of vibration over the course of the 60-s trial are illustrated for the sacrum (top trace) and CoP (bottom trace). Whereas CoP motion included more high-frequency content, the overall patterns of motion were quite similar. The thick lines and shaded areas illustrate group means ± SE for the participants who exhibited the typical sway pattern (n = 13). The thin lines illustrate the behavior of the other 3 participants. The timing of vibration is indicated by the label under the sacrum and center of pressure (CoP) position traces. B: group average sacrum mediolateral location (n = 13) is illustrated relative to the average sacrum location before vibration (assigning this time period a value of zero). Error bars represent standard deviation. *Significant (P < 0.05) post hoc comparisons between time periods.
Across-trial changes in unperturbed step characteristics.
To determine whether the preferred gait pattern changed across the four walking trials, we compared control trial steps with unperturbed steps during the vibration trials (Table 1). None of the measures of mediolateral gait motion (mediolateral foot placement, sacrum displacement, step width, or estimated margin of stability) varied significantly across walking trials (P ≥ 0.56). In contrast, both step period (P = 0.009) and step length (P = 0.024) were significantly influenced by walking trial, as participants walked with longer, slower steps after the first control trial. Neither the mediolateral gait motion metrics nor step period differed significantly between right and left steps (P ≥ 0.24), although right steps were slightly longer (P = 0.032). Gait symmetry did not change over the course of the experiment, as the interaction between trial number and leg side did not have a significant influence on any of the quantified gait metrics (P ≥ 0.17).
Table 1.
Summary measures of the group average gait characteristics during the 4 walking trials
| Gait Metric | First Control Trial | Stance Vibration Trial | Swing Vibration Trial | Final Control Trial | Trial No. Effect | Step Side Effect | Interaction Effect |
|---|---|---|---|---|---|---|---|
| Mediolateral foot placement, cm | |||||||
| Right steps | 8.0 ± 2.3 | 7.8 ± 2.5 | 8.0 ± 3.0 | 8.0 ± 3.0 | P = 0.87 | P = 0.24 | P = 0.44 |
| Left steps | 7.3 ± 1.8 | 7.0 ± 1.7 | 7.0 ± 2.3 | 7.1 ± 2.4 | |||
| Sacrum displacement, cm | |||||||
| Right steps | 4.6 ± 1.5 | 4.5 ± 1.6 | 4.5 ± 1.9 | 4.5 ± 2.0 | P = 0.92 | P = 0.25 | P = 0.17 |
| Left steps | 5.3 ± 1.8 | 5.2 ± 1.8 | 5.4 ± 2.2 | 5.4 ± 2.3 | |||
| Step width, cm | |||||||
| Right steps | 12.5 ± 2.6 | 12.3 ± 2.7 | 12.5 ± 3.7 | 12.5 ± 3.8 | P = 0.92 | P = 0.54 | P = 0.24 |
| Left steps | 12.5 ± 2.4 | 12.2 ± 2.6 | 12.5 ± 3.7 | 12.5 ± 3.7 | |||
| Estimated margin of stability, cm | |||||||
| Right steps | 1.8 ± 2.0 | 1.7 ± 2.0 | 1.8 ± 2.1 | 1.7 ± 2.2 | P = 0.56 | P = 0.24 | P = 0.52 |
| Left steps | 1.1 ± 1.4 | 0.9 ± 1.3 | 0.9 ± 1.4 | 0.9 ± 1.4 | |||
| Step period, s | |||||||
| Right steps | 0.526 ± 0.025* | 0.528 ± 0.026 | 0.528 ± 0.026 | 0.528 ± 0.027 | P = 0.009 | P = 0.54 | P = 0.17 |
| Left steps | 0.527 ± 0.031* | 0.531 ± 0.031 | 0.531 ± 0.033 | 0.529 ± 0.032 | |||
| Step length, cm | |||||||
| Right steps | 66.4 ± 3.3* | 66.8 ± 3.3 | 66.8 ± 3.5 | 66.6 ± 3.6 | P = 0.024 | P = 0.032 | P = 0.42 |
| Left steps | 65.2 ± 3.8* | 65.4 ± 3.9 | 65.5 ± 4.0 | 65.6 ± 3.8 |
All summary measures are presented as means ± SD. The first and final control trial measures include all steps during these trials. The stance vibration and swing vibration trial measures include only the steps immediately preceding the vibratory perturbations. The results of the 2-way ANOVA are provided, with boldface P values indicating a significant effect (P < 0.05).
Step period and step length during the first control trial were significantly smaller (post hoc test; P < 0.05) than these measures during each of the other 3 trials.
Direct effects of sensory perturbations.
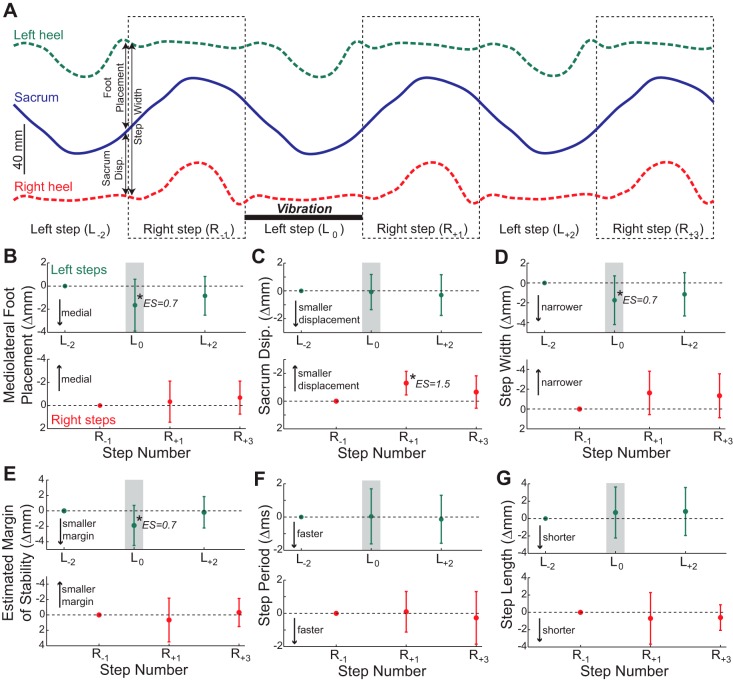
Vibration of the right GM during steps taken with the left leg (stance phase vibration) influenced gait behavior. The results of all statistical tests are presented in the legend to Fig. 3. Here, we describe the notable chronological effects of stance phase vibration through comparisons with the preceding unperturbed steps. During the left step in which vibration was applied, the left foot was placed more medially than normal (Fig. 3B), but the displacement of the sacrum with respect to the stance (right) foot was not altered (Fig. 3C). This caused a narrower than normal step (Fig. 3D) and reduced the estimated margin of stability (Fig. 3E). Due to these changes in the body's frontal plane configuration, the sacrum began the next right step relatively close to the left stance foot. This altered body configuration did not cause significant changes in mediolateral foot placement location during this right step (Fig. 3B) but did cause the sacrum to also end this step relatively close to the left stance foot (Fig. 3C). This combination caused an insignificant trend for this right step to be narrower than normal (Fig. 3D) but had no apparent effect on the estimated margin of stability (Fig. 3E). All of these mediolateral gait motion metrics returned to the baseline level (defined by the steps preceding the perturbation) after a maximum of two steps. Step period and step length were not influenced by the stance phase vibration (Fig. 3, F and G).
Fig. 3.
Effects of stance phase vibration. A: mediolateral locations of the sacrum and both heels are illustrated over 6 consecutive steps, with right steps indicated by dashed outlined boxes. These lines represent the average marker trajectories across participants (n = 13) and across all delivered stance phase vibration perturbations. Vibration was applied to the right GM during a left step (labeled L0). We also illustrate 2 preceding steps (L−2 and R−1) and 3 subsequent steps (R+1, L+2, and R+3) for comparison. Our 3 primary metrics of mediolateral gait motion (foot placement, sacrum displacement, and step width) are indicated for the first left step. Any effects of vibration are not obvious, as the markers appear to follow quite similar trajectories across all 6 steps. B: stance phase vibration had a significant effect on mediolateral foot placement for left steps (P = 0.016) but not for right steps (P = 0.39). C: vibration did not significantly influence sacrum displacement for left steps (P = 0.79) but did have a significant effect for right steps (P = 0.004). D: vibration had a significant effect on step width for left steps (P = 0.028), but this effect did not reach significance for right steps (P = 0.064). E: vibration significantly influenced the estimated margin of stability for left steps (P = 0.010) but not for right steps (P = 0.41). F: vibration did not have a significant effect on step period for either left steps (P = 0.91) or right steps (P = 0.67). G: vibration also had no significant effect on step length for either left steps (P = 0.49) or right steps (P = 0.50). For B–G, all data are plotted as the change from the unperturbed steps prior to the vibration, with error bars representing standard deviation. The shaded boxes indicate the steps during which the perturbation was applied. Top rows in B–G represents left steps, and the bottom rows represent right steps. *Steps that are significantly different from the unperturbed step with this leg (post hoc test; P < 0.05). For all significant post hoc tests, the estimated effect size (ES) magnitude (calculated from means ± SD) is indicated.
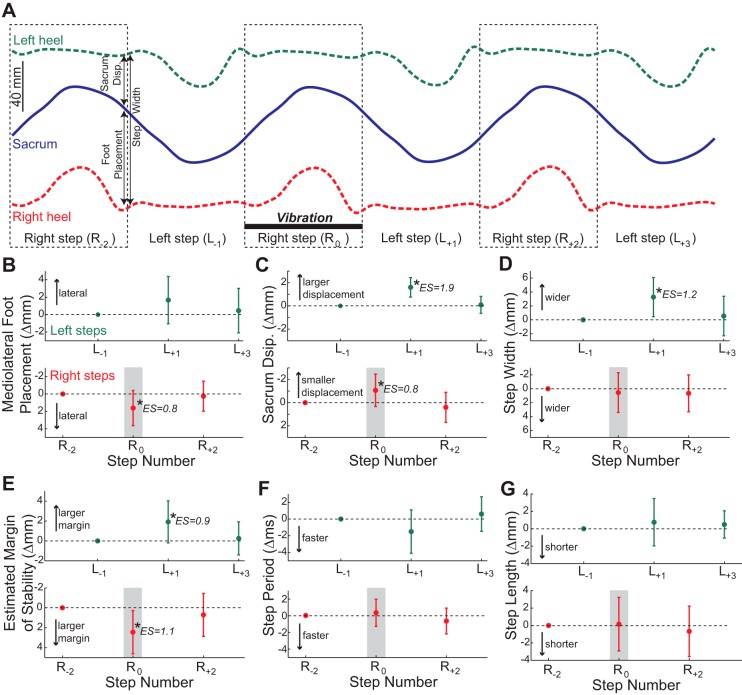
Right GM vibration during steps taken with the right leg (swing phase vibration) also influenced gait behavior, with the results of statistical tests presented in the legend to Fig. 4. During the right step in which vibration was applied, the right foot was placed more laterally than normal (Fig. 4B), whereas the sacrum was closer to the left stance foot at the end of the step (Fig. 4C). This combination had no net effect on step width (Fig. 4D) but significantly increased the estimated margin of stability (Fig. 4E). Due to these adjustments in the body's frontal plane configuration, the next left step began with the sacrum relatively far from the right stance foot. During this next left step, there was an insignificant trend for the left foot to be placed more laterally (Fig. 4B), and the sacrum ended the step relatively far from the right stance foot (Fig. 4C). These changes caused a wider step (Fig. 4D) and a larger estimated margin of stability (Fig. 4E). Additionally, there was a trend that did not reach the level of significance for this left step to have a shorter step period (Fig. 4F). Again, all of the metrics affected by the vibration were not significantly different from their baseline values after a maximum of two steps. Step length was not influenced by the swing phase vibration (Fig. 4G).
Fig. 4.
Effects of swing phase vibration. A: group average mediolateral locations of the sacrum and both heels are illustrated over 6 consecutive steps, as in Fig. 3. Again, any effects of the applied vibration are subtle. B: effects of swing phase vibration did not reach the level of significance for mediolateral foot placement for left steps (P = 0.067) but significantly influenced right steps (P = 0.008). C: vibration significantly influenced sacrum displacement for both left (P < 0.0001) and right (P = 0.004) steps. D: vibration had a significant effect on step width for left steps (P = 0.0008) but not right steps (P = 0.65). E: vibration had a significant effect on estimated margin of stability for both left steps (P = 0.007) and right steps (P = 0.003). F: vibration had a significant main effect (P = 0.023) on left step periods, but no post hoc comparisons reached the level of statistical significance. Vibration did not influence right step period (P = 0.11). G: vibration did not significantly influence left step length (P = 0.43) or right step length (P = 0.55). For B–G, all data are plotted as the change from the unperturbed steps prior to the vibration, and error bars represent standard deviation. The shaded boxes indicate the steps during which the perturbation was applied. The top rows in B–G represent left steps, and the bottom rows represents right steps. *Steps that are significantly different from the unperturbed step with this leg (post hoc test; P < 0.05), with estimated ES indicated.
DISCUSSION
Proprioceptive perturbations, delivered in the form of GM vibration, influenced the control of mediolateral motion during both standing posture and walking. The postural sway caused by vibration indicates that the delivered stimuli were sufficient to elicit an altered perception of pelvis motion. Vibration also influenced mediolateral motion during walking in two distinct ways, partially supporting our hypotheses. Stance phase vibration caused the contralateral leg to be placed more medially, whereas swing phase vibration caused the vibrated leg to be placed more laterally and the pelvis to be held closer to the contralateral stance foot.
The pattern of postural sway induced by vibration is consistent with previous work. The majority of participants swayed away from the vibration when it was turned on, likely as a corrective response to the perception of a more adducted right hip that would normally accompany stretch of the vibrated muscle (Eklund 1972; Kavounoudias et al. 1999; Popov et al. 1999; Ivanenko et al. 2000). Turning the vibration off was followed by sway back toward the vibrated leg beyond the original posture. This overshoot is likely due to relatively long-lasting effects on perception that can follow sustained periods of vibration (Rogers et al. 1985; Wierzbicka et al. 1998). Some participants (3 of 16) did not exhibit this typical sway pattern, similar to previous work which has found that a subset of healthy individuals is insensitive to this type of musculotendon vibration (Gurfinkel et al. 1998; Fuentes et al. 2012).
Our results indicate that proprioceptive feedback from the stance leg influenced the control of mediolateral gait motion, albeit only of the contralateral swing leg. Stance phase GM vibration caused the contralateral (unvibrated) swing leg to be placed more medially, indicating the presence of a neuromechanical link between the stance and swing legs. Specifically, vibration likely caused the perception of a more adducted stance hip with the midline closer mediolaterally to the stance foot, just as during standing posture. The perception of this smaller displacement between the pelvis and the stance foot then caused participants to place their contralateral leg more medially, directly following the previously observed relationship linking the mechanical state of the pelvis to the subsequent foot placement (Rankin et al. 2014; Wang and Srinivasan 2014). In contrast, stance phase vibration did not influence the position of the stance leg during the vibrated step, as the displacement of the sacrum from the stance foot at the end of the step did not change. Therefore, participants apparently did not respond to the perception of a more adducted stance leg by “correcting” this hip position, which would involve abducting the stance hip and pushing the midline away from the stance foot (Pandy et al. 2010). Although it is somewhat surprising that a proprioceptive perturbation did not elicit a response at the joint where it was delivered (the stance hip), this can perhaps be explained by the relative ease of repositioning the swing hip. Adjusting only the swing leg position does not require the inertia of the torso to be accelerated and can occur gradually over the entire step period and is thus predicted by model simulations to require little energy (Kuo 1999). The combination of this more medial foot placement and no adjustment of pelvis motion caused a narrower step and a smaller estimated margin of stability, although these changes were never sufficient to cause an obvious loss of balance. The lack of an apparent response at the stance hip may also be partially due to the altered mechanical state compared with during bipedal standing posture. Specifically, the ongoing frontal plane joint motion and increased abductor contraction strength at the stance hip may have reduced the perceptual effects of the vibration, although the simultaneous swing leg adjustments indicate that this perception was still somewhat perturbed.
Although likely intended as a corrective response to the false perception of pelvis motion, the altered foot placement that accompanied stance phase vibration may itself be considered a mechanical perturbation. The more medial left foot placement during the vibrated step caused participants to begin the next step in a slightly different configuration, with the sacrum closer to the new (left) stance foot. Likely due to this altered initial position, participants also ended the subsequent right step with the sacrum remaining closer to the stance foot. However, this smaller displacement between the pelvis and left stance foot did not cause significantly more medial right foot placement during this right step, as would be expected from the relationship described above (Rankin et al. 2014; Wang and Srinivasan 2014). The simplest explanation for this lack of an effect is that the relatively small change in pelvis location (∼2 mm) was not sufficient to require a contralateral response. Of course, this explanation implies that our stance phase vibration must have evoked the perception of a larger change in pelvis displacement, which we are presently unable to quantify directly.
Swing leg proprioceptive feedback also contributed to the control of mediolateral gait motion, influencing both the swing and stance legs. Swing phase GM vibration caused the vibrated swing leg to be placed more laterally, suggesting that humans monitor GM proprioceptive feedback during the process of taking a step. Vibration likely generated the perception of a more adducted hip angle during the swing phase, which participants then responded to by repositioning the leg to a more abducted angle, resulting in more lateral foot placement. Such a result is similar to the use of proprioceptive feedback during accurate upper extremity reaching tasks (Sarlegna and Sainburg 2009). Simultaneously with this adjustment of swing leg position, swing phase GM vibration also influenced behavior of the unvibrated stance leg, as the sacrum was held in closer proximity mediolaterally to the stance foot. This contralateral response can also be explained by the perception of a more adducted swing leg; participants may have sought to prevent the pelvis from moving too far toward the right leg due to the perceived risk of a more medial foot placement location. The dual responses to swing phase vibration (more lateral foot placement and holding the pelvis closer to the stance leg) combined to have no net effect on step width, although the estimated margin of stability increased significantly.
As with stance phase vibration, the corrective responses to swing phase vibration appear to have perturbed the subsequent step. Due to the lateral foot placement during the vibrated step, the next left step began with the pelvis slightly farther mediolaterally from the right stance foot. This increased displacement persisted through the left step, which ended with the pelvis still slightly farther from the stance foot than during unperturbed steps. Although neither effect reached the level of post hoc significance, there was a trend for this left step to be placed more laterally and to have a shorter stride period than normal. Both of these mechanical changes could contribute to an increase in the margin of stability (Hof 2008; Hak et al. 2013), a possible response to the altered mechanical state of the pelvis.
A comparison of the two types of vibratory perturbations raises the question of why stance phase vibration caused only an adjustment of foot placement, whereas swing phase vibration elicited a bilateral response. This result may be due to the potential negative consequences of not adequately responding to the perceived changes in the body's mechanical state. A decrease in the mediolateral displacement between the pelvis and stance foot (the perception evoked by stance phase vibration) would normally be followed by a more medial foot placement (Rankin et al. 2014; Wang and Srinivasan 2014). Ignoring this perception would simply cause the foot to be placed more laterally than necessary. Although this may somewhat increase the mechanical or metabolic costs (Donelan et al. 2001) and increase the need for stance phase GM activity during the next step (Kubinski et al. 2015), it would not cause an immediate risk to stability. Humans may be content to use only economical adjustments in swing leg position to respond to this perception. In contrast, an overly adducted swing leg (the perception evoked by swing phase vibration) would lead to an overly medial foot placement location if not corrected. If it is of a sufficient magnitude to cause the CoM to pass laterally to this foot, this error could cause an immediate loss of balance. Humans may seek to prevent such an outcome by both placing the swing foot more laterally and contracting the stance leg musculature to slow the mediolateral motion of the pelvis away from the stance foot. This type of conservative behavior when facing errors that could cause a lateral loss of balance has been observed previously with mechanical perturbations, as humans walk with significantly more lateral foot placement when expecting the swing foot to be perturbed (Rankin et al. 2014).
Although our discussion thus far has focused on the potential of applied vibration to elicit false perceptions of the body's mechanical state, an alternative explanation for the observed effects involves a simpler reflexive mechanism. It is possible that the vibration-evoked sensory feedback directly caused a GM contraction through a tonic vibration reflex (Eklund and Hagbarth 1966), thus producing a hip abduction moment. This type of reflexive contraction could logically contribute to the more lateral foot placement of the vibrated leg caused by swing phase vibration, and we are unable to definitively rule out this possibility from the present results. However, stance phase vibration had no measurable effect on the motion of the stance leg, indicating that any direct reflexive effects in this context are relatively minor. Additionally, the effects of both swing and stance phase vibration on the contralateral leg are not easily explained by simple reflexes but would seem to require some level of central processing. Therefore, we believe that our overall results are more likely due to a behavioral response to false perceptions than simple reflexes.
Unlike during standing posture, no after-effects of vibration were clearly evident during walking trials. Within sensory perturbation trials, any significant effects of the perturbations were dissipated within two steps. This rapid return to the unperturbed gait pattern is consistent with the response to larger mechanical perturbations of foot placement (Rankin et al. 2014). The lack of any long-lasting effects may be attributed to the short duration (∼0.5 s) of the applied vibration. Across walking trials, we observed no long-term changes in mediolateral gait motion, which we would expect to be most strongly influenced by repetitive GM vibration. The preference for longer, slower strides after the first control trial may be due to participants simply becoming more comfortable performing the treadmill walking task (Monsch et al. 2012; IJmker et al. 2014).
The effects of GM vibration on mediolateral gait motion, although significant, were quite small. Stance phase vibration altered both foot placement and step width during the vibrated steps by ∼2 mm, whereas swing phase vibration changed foot placement by ∼2 mm and had no significant effect on step width. In comparison, step widths vary considerably more than this during even steady-state walking, as within-subject standard deviations are typically around 2–3 cm (Bauby and Kuo 2000; Helbostad and Moe-Nilssen 2003). Similarly, work based on the extrapolated center of mass predicts that humans would remain stable even with foot placement errors of several centimeters (Krishnan et al. 2013). Therefore, these relatively small effects of the proprioceptive perturbations are clearly insufficient in isolation to explain the natural variability in frontal plane mechanics during gait. The small effects of vibration are likely due to the small magnitude of the perturbations themselves (0.25-mm amplitude), which were intended to influence perception without having a direct mechanical effect. In contrast, studies that seek to investigate the roles of proprioception or somatosensation during human walking using mechanical perturbations (Chien et al. 2014; Yen et al. 2014; Frost et al. 2015) are limited in their ability to distinguish between the effects of altered proprioceptive feedback and the direct effects of the mechanical perturbation during the ongoing task performance. The observed sizes of the effects during walking seem reasonable compared with the standing posture results, as the sacrum underwent a displacement of ∼7 mm after a longer period of ∼2 s of vibration. It is quite possible that larger sensory perturbations would have larger effects on foot placement, just as the response to small, naturally occurring foot placement perturbations “scaled up” in the presence of larger, externally applied perturbations (Rankin et al. 2014).
The relatively small effects of the applied proprioceptive perturbations may also be partially due to multisensory integration. Although proprioceptive feedback from the stance hip could logically contribute to the perception of pelvis mechanics, other feedback sources could also influence this perception. For example, vestibular feedback (Bent et al. 2005), visual feedback (O'Connor and Kuo 2009), cutaneous feedback from the sole of the foot (Kavounoudias et al. 2001), and proprioceptive feedback from the ankle (Kavounoudias et al. 1999) or knee (Cammarata and Dhaher 2011) could all contribute to the perception of pelvis mechanics relative to the stance foot. The human nervous system is well equipped to combine sensory information from multiple sources, often with the apparent goal of improving perception accuracy (Green and Angelaki 2010). Because our perturbations would be expected only to alter sensory feedback from one of these sources, the conflict between available sensory information may have prevented the perturbations from having a larger effect. It is less clear whether other sources of sensory information could also contribute to the perception of swing leg position. Future work should investigate whether other sources of sensory feedback influence the observed two-part gait stabilization strategy, possibly using perturbation methods similar to those described here.
In the present study, we quantified several metrics of mediolateral gait motion, which can provide varying types of insight into how humans maintain mediolateral gait stability. Step width (the mediolateral distance between the feet) is commonly quantified in gait studies, as it can be measured solely by identifying the points of contact with the ground (e.g., using commercially available pressure-sensitive mats). However, the present results demonstrate that changes in the body's mediolateral configuration are not always apparent from step width. Although it has been suggested that walking with wider steps will increase the mediolateral margin of stability (Hof et al. 2007), this has not consistently been the case in experimental studies (Rosenblatt and Grabiner 2010; Rosenblatt et al. 2012). Based on visual inspection of Figs. 3 and 4, it appears that changes in the estimated margin of stability are more closely related to mediolateral foot placement than step width. Indeed, correlating the group average measures reveals that changes in the estimated margin of stability are well predicted by changes in mediolateral foot placement (r2 = 0.82) but not as well predicted by changes in step width (r2 = 0.38). This result suggests that wider steps will only increase the margin of stability if they are the result of more lateral foot placement relative to the CoM, as would be expected from the equation used to calculate margin of stability. Although measuring motion of the sacrum during walking provided us with more information than step width alone, the present work is somewhat limited by its lack of detailed kinematic measurements. First, we were unable to calculate the actual CoM location or velocity, which would require many more kinematic markers on the limbs and torso. We were also unable to calculate the actual lateral boundary of the base of support, including possible adjustments to foot heading (through medial or lateral rotation) that may contribute to gait stabilization (Rebula 2014). Despite these limitations, previous work has indicated that control over the body's next effective point of contact with the environment (i.e., CoP) is dominated by a stepping foot placement strategy (Hof et al. 2010) that we were able to quantify.
In conclusion, proprioceptive feedback from the hip appears to contribute to the control of mediolateral gait motion during human walking. These results are consistent with humans using proprioception to execute the neuromechanical gait stabilization strategy ensuring mediolateral stability while walking, although this suggestion requires further investigation. Hip proprioception appears to contribute to the process of establishing an appropriate foot placement target based on the mechanical state of the pelvis as well as accurately controlling the swing leg and stance leg configuration. Future work should investigate whether other sensory sources also contribute to execution of this strategy in conjunction with hip proprioception. A clearer understanding of how uninjured humans meet the mechanical demands of stable bipedal gait could contribute to the development of interventions for clinical populations with reduced gait stability.
GRANTS
This study was partially supported by the Department of Veterans Affairs, Office of Research and Development, Rehabilitation Research and Development Service, through Grant No. 1IK2RX000750-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.C.R.-R., M.H.W., C.R.W., and J.C.D. performed experiments; D.C.R.-R., M.H.W., C.R.W., and J.C.D. interpreted results of experiments; D.C.R.-R., M.H.W., C.R.W., and J.C.D. drafted manuscript; D.C.R.-R., M.H.W., C.R.W., and J.C.D. edited and revised manuscript; D.C.R.-R., M.H.W., C.R.W., and J.C.D. approved final version of manuscript; J.C.D. conception and design of research; J.C.D. analyzed data; J.C.D. prepared figures.
REFERENCES
- Balasubramanian CK, Neptune RR, Kautz SA. Foot placement in a body reference frame during walking and its relationship to hemiparetic walking performance. Clin Biomech 25: 483–490, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech 33: 1433–1440, 2000. [DOI] [PubMed] [Google Scholar]
- Bent LR, McFadyen BJ, Inglis JT. Vestibular contributions during human locomotor tasks. Exerc Sport Sci Rev 33: 107–113, 2005. [DOI] [PubMed] [Google Scholar]
- Bingham JT, Choi JT, Ting LH. Stability in a frontal plane model of balance requires coupled changes to postural configuration and neural feedback control. J Neurophysiol 106: 437–448, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarata ML, Dhaher YY. Proprioceptive acuity in the frontal and sagittal planes of the knee: a preliminary study. Eur J Appl Physiol 111: 1313–1320, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien JH, Anthony Eikema DJ, Mukherjee M, Stergiou N. Locomotor sensory organization test: a novel paradigm for the assessment of sensory contributions in gait. Ann Biomed Eng 42: 2512–2523, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD. Mechanical and metabolic determinants of the preferred step width in human walking. Proc R Soc Lond B 268: 1985–1992, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund G. General features of vibration-induced effects on balance. Ups J Med Sci 77: 111–124, 1972. [DOI] [PubMed] [Google Scholar]
- Eklund G, Hagbarth KE. Normal variability of tonic vibration reflexes in man. Exp Neurol 16: 80–92, 1966. [DOI] [PubMed] [Google Scholar]
- Fallon JB, Macefield VG. Vibration sensitivity of human muscle spindles and Golgi tendon organs. Muscle Nerve 36: 21–29, 2007. [DOI] [PubMed] [Google Scholar]
- Floyd LM, Holmes TC, Dean JC. Reduced effects of tendon vibration with increased task demand during active, cyclical ankle movement. Exp Brain Res 232: 283–292, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost R, Skidmore J, Santello M, Artemiadis P. Sensorimotor control of gait: a novel approach for the study of the interplay of visual and proprioceptive feedback. Front Hum Neurosci 9: 14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes CT, Gomi H, Haggard P. Temporal features of human tendon vibration illusions. Eur J Neurosci 36: 3709–3717, 2012. [DOI] [PubMed] [Google Scholar]
- Gilhodes JC, Gurfinkel VS, Roll JP. Role of Ia muscle spindle afferents in post-contraction and post-vibration motor effect genesis. Exp Brain Res 61: 395–402, 1986. [DOI] [PubMed] [Google Scholar]
- Gilles M, Wing AM, Kirker SGB. Lateral balance organization in human stance in response to a random or predictable perturbation. Exp Brain Res 124: 137–144, 1999. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralyzing joint afferents. Brain 95: 705–748, 1972. [DOI] [PubMed] [Google Scholar]
- Goodworth AD, Peterka RJ. Sensorimotor integration for multisegmental frontal plane balance control in humans. J Neurophysiol 107: 12–28, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AM, Angelaki DE. Multisensory integration: resolving sensory ambiguities to build novel representations. Curr Opin Neurobiol 20: 353–360, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurfinkel VS, Levik YS, Kazennikov OV, Selionov VA. Locomotor-like movement evoked by leg muscle vibration in humans. Eur J Neurosci 10: 1608–1612, 1998. [DOI] [PubMed] [Google Scholar]
- Hak L, Houdijk H, Beek PJ, van Dieen JH. Steps to take to enhance gait stability: the effect of stride frequency, stride length, and walking speed on local dynamic stability and margins of stability. PLoS One 8: e82842, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbostad JL, Moe-Nilssen R. The effect of gait speed on lateral balance control during walking in healthy elderly. Gait Posture 18: 27–36, 2003. [DOI] [PubMed] [Google Scholar]
- Hobbelen DG, Wisse M. Active lateral foot placement for 3D stabilization of a limit cycle walker prototype. Int J Hum Rob 6: 93–116, 2009. [Google Scholar]
- Hof AL. The ‘extrapolated center of mass’ concept suggests a simple control of balance in walking. Hum Mov Sci 27: 112–125, 2008. [DOI] [PubMed] [Google Scholar]
- Hof AL, Duysens J. Responses of human hip abductor muscles to lateral balance perturbations during walking. Exp Brain Res 230: 301–310, 2013. [DOI] [PubMed] [Google Scholar]
- Hof AL, Gazendam MG, Sinke WE. The condition for dynamic stability. J Biomech 38: 1–8, 2005. [DOI] [PubMed] [Google Scholar]
- Hof AL, van Bockel RM, Schoppen T, Postema K. Control of lateral balance in walking. Experimental findings in normal subjects and above-knee amputees. Gait Posture 25: 250–258, 2007. [DOI] [PubMed] [Google Scholar]
- Hof AL, Vermerris SM, Gjaltema WA. Balance responses to lateral perturbations in human treadmill walking. J Exp Biol 213: 2655–2664, 2010. [DOI] [PubMed] [Google Scholar]
- Hurt CP, Grabiner MD. Age-related differences in the maintenance of frontal plane dynamic stability while stepping to targets. J Biomech 48: 592–597, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt CP, Rosenblatt N, Crenshaw JR, Grabiner MD. Variation in trunk kinematics influences variation in step width during treadmill walking by older and younger adults. Gait Posture 31: 461–464, 2010. [DOI] [PubMed] [Google Scholar]
- IJmker T, Lamoth CJ, Houdijk H, van der Woude LH, Beek PJ. Postural threat during walking: effects on energy cost and accompanying gait changes. J Neuroeng Rehab 11: 71, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R, Lacquaniti F. Influence of leg muscle vibration on human walking. J Neurophysiol 84: 1737–1747, 2000. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Gilhodes JC, Roll R, Roll JP. From balance regulation to body orientation: two goals for muscle proprioceptive information processing? Exp Brain Res 124: 80–88, 1999. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol 532: 869–878, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Rosenblatt NJ, Latash ML, Grabiner MD. The effects of age on stabilization of the mediolateral trajectory of the swing foot. Gait Posture 38: 923–928, 2013. [DOI] [PubMed] [Google Scholar]
- Kubinski SN, McQueen CA, Sittloh KA, Dean JC. Walking with wider steps increases stance phase gluteus medius activity. Gait Posture 41: 130–135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AD. Stabilization of lateral motion in passive dynamic walking. Int J Robot Res 18: 917–930, 1999. [Google Scholar]
- MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech 26: 633–644, 1993. [DOI] [PubMed] [Google Scholar]
- Mauer C, Mergner T, Peterka RJ. Multisensory control of human upright stance. Exp Brain Res 171: 231–250, 2006. [DOI] [PubMed] [Google Scholar]
- Monsch ED, Franz CO, Dean JC. The effects of gait strategy on metabolic rate and indicators of stability during downhill walking. J Biomech 45: 1928–1933, 2012. [DOI] [PubMed] [Google Scholar]
- O'Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. J Neurophysiol 102: 1411–1419, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandy MG, Lin YC, Kim HJ. Muscle coordination of mediolateral balance in normal walking. J Biomech 43: 2055–2064, 2010. [DOI] [PubMed] [Google Scholar]
- Popov KE, Kozhina GV, Smetanin BN, Shlikov VY. Postural responses to combined vestibular and hip proprioceptive stimulation in man. Eur J Neurosci 11: 3307–3311, 1999. [DOI] [PubMed] [Google Scholar]
- Rankin BL, Buffo SK, Dean JC. A neuromechanical strategy for mediolateral foot placement in walking humans. J Neurophysiol 112: 374–383, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebula JR. Mechanisms of Stability and Energy Expenditure in Human Locomotion (doctoral dissertation). Ann Arbor, MI: Department of Mechanical Engineering, Univ. of Michigan, 2014. [Google Scholar]
- Redfern MS, Schumann T. A model of foot placement during gait. J Biomech 27: 1339–1346, 1994. [DOI] [PubMed] [Google Scholar]
- Rogers DK, Bendrups AP, Lewis MM. Disturbed proprioception following a period of muscle vibration in humans. Neurosci Lett 57: 147–152, 1985. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47: 177–190, 1982. [DOI] [PubMed] [Google Scholar]
- Rosenblatt NJ, Grabiner MD. Measures of frontal plane stability during treadmill and overground walking. Gait Posture 31: 380–384, 2010. [DOI] [PubMed] [Google Scholar]
- Rosenblatt NJ, Hurt CP, Grabiner MD. Sensitivity of dynamic stability to changes in step width during treadmill walking in adults. J Appl Biomech 28: 616–621, 2012. [DOI] [PubMed] [Google Scholar]
- Sarlegna FR, Sainburg RL. The roles of vision and proprioception in the planning of reaching movement. In: Progress in Motor Control: A Multidisciplinary Perspective, edited by Sternad D. New York: Springer, 2009, p. 317–339. [Google Scholar]
- Townsend MA. Biped gait stabilization via foot placement. J Biomech 18: 21–38, 1985. [DOI] [PubMed] [Google Scholar]
- van der Kooij H, Jacobs R, Koopman B, Grootenboer H. A multisensory integration model of human stance control. Biol Cybern 80: 299–308, 1999. [DOI] [PubMed] [Google Scholar]
- Wang Y, Srinivasan M. Stepping in the direction of the fall: the next foot placement can be predicted from current upper body state in steady-state walking. Biol Lett 10: 20140405, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka MM, Gilhodes JC, Roll JP. Vibration-induced postural post effects. J Neurophysiol 79: 143–150, 1998. [DOI] [PubMed] [Google Scholar]
- Yang F, Pai YC. Can sacral marker approximate center of mass during gait and slip-fall recovery among community-dwelling older adults? J Biomech 47: 3807–3812, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen SC, Landry JM, Wu M. Augmented multisensory feedback enhances locomotor adaptation in humans with incomplete spinal cord injury. Hum Mov Sci 35: 80–93, 2014. [DOI] [PubMed] [Google Scholar]
- Zeni JA, Higginson JS. Gait parameters and stride-to-stride variability during familiarization to walking on a split-belt treadmill. Clin Biomech 25: 383–386, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeni JA, Richards JG, Higginson JS. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 27: 710–714, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]