Abstract
It is not known how changes in skin mechanics affect the responses of cutaneous mechanoreceptors in the finger pads to compression forces. We used venous occlusion to change the stiffness of the fingers and investigated whether this influenced the firing of low-threshold mechanoreceptors to surfaces of differing stiffness. Unitary recordings were made from 10 slowly adapting type I (SAI), 10 fast adapting type I (FAI) and 9 slowly adapting type II (SAII) units via tungsten microelectrodes inserted into the median nerve at the wrist. A servo-controlled stimulator applied ramp-and-hold forces (1, 2, and 4 N) at a constant loading and unloading rate (2 N/s) via a flat 2.5-cm-diameter silicone disk over the center of the finger pad. Nine silicone disks (objects), varying in compliance, were used. Venous occlusion, produced by inflating a sphygmomanometer cuff around the upper arm to 40 ± 5 mmHg, was used to induce swelling of the fingers and increase the compliance of the finger pulp. Venous occlusion had no effect on the firing rates of the SAI afferents, nor on the slopes of the relationship between mean firing rate and object compliance at each amplitude, but did significantly reduce the slopes for the FAI afferents. Although the SAII afferents possess a poor capacity to encode changes in object compliance, mean firing rates were significantly lower during venous occlusion. The finding that venous occlusion had no effect on the firing properties of SAI afferents indicates that these afferents preserve their capacity to encode changes in object compliance, despite changes in skin mechanics.
Keywords: tactile afferents, human, microneurography, sensory coding, skin mechanics
compliance is the ability of an object to deform when subjected to a force; the perceptual attribute of this characteristic is referred to as softness. Humans require knowledge of the softness of objects, which they perceive via the activation of a population of low-threshold mechanoreceptors in the skin of the hands. Sensory input from tactile afferents codes for the physical properties of handled objects (roughness, smoothness, softness, etc.) and controls the motor commands necessary to apply precisely the forces required to manipulate handled objects. The perception of softness is based on interactions between the compliance of the object and tactile afferents in the finger pad and must, therefore, depend on the relative differences in compliance of the object and the skin of the finger pad. We know that skin mechanics of the human finger pad alter as we age; this may be attributed to disorders producing edema of the extremities, or to the dehydration of the skin with age (Lévêque et al. 2000; Verrillo et al. 1999). Interestingly, as an individual ages, there is a decrease in tactile spatial acuity, yet this is improved by application of moisturizer to the skin, indicating that changes in skin mechanics can indeed affect tactile sensibility (Lévêque et al. 2000). Recently, Friedman et al. (2008) performed a psychophysical study that investigated how humans perceive the softness of an object when silicone disks of differing compliance were passively applied to the finger pad, i.e., relying on sensory information from the skin alone: subjects rated the object as “soft” when the compliance of the silicone surface was less than that of the finger pad. Evidently, there is a relationship between perceptual softness, the ability of an object to conform to the finger pad and the encoding of tactile information related to object compliance.
Our laboratory recently explored the capacities of tactile afferents located in the glabrous skin of the hand to encode differences in the compliance of objects brought into contact with the finger pad (Condon et al. 2014). We showed that, of the four classes of tactile afferent in the glabrous skin of the human hand, the slowly adapting (SA) type I (SAI) afferents are the most sensitive class for signaling differences in compliance of an object: they exhibit a linear relationship between compliance and the number of spikes generated during the loading and plateau phases of a ramp-and-hold stimulus. Moreover, they have a high density in the finger pads (Johansson and Vallbo 1979). The fast adapting (FA) type I (FAI) afferents, which also have a high density in the finger pads (Johansson and Vallbo 1979), do contribute, but the slope of the relationship between the number of spikes generated during the loading phase and compliance is significantly lower than that of the SAI afferents. Conversely, the FA type II (FAII) and SA type II (SAII) afferents appear to play negligible roles in signaling changes in object compliance (Condon et al. 2014).
The pulp of the finger pad undergoes considerable compression during contact and the application of force to/by an object, with the pulp being fairly compliant at low forces, but becoming stiffer with higher forces (Pawluk and Howe, 1999; Serina et al. 1997; Westling and Johansson 1987). Because the pulp exhibits viscoelastic properties, including stress relaxation, creep and creep recovery, any change in compliance of the pulp will potentially influence the fingertip deformation that occurs with any stimulus (Jindrich et al. 2003; Pawluk and Howe 1999; Pubols 1982a, 1982b; Serina et al. 1997). Given the interactions between the compliance of an object and the finger pad referred to above, the purpose of the present study is to determine the effects of a change in finger pad compliance on the encoding of object compliance by cutaneous mechanoreceptors. We altered finger pulp compliance experimentally by inflating a sphygmomanometer cuff on the upper arm to a pressure of 40 ± 5 mmHg. This will lower the rate of venous return from the upper limb, consequently altering the mechanical properties of the finger pulp by engorging the highly vascularized finger pads and thereby increasing their compliance. Given that mechanoreceptors in the finger pad eventually respond to deformation of the receptor-bearing tissue, we propose that a change in skin mechanics produced by venous occlusion will affect the sensitivity of tactile afferents to controlled compression stimuli, and thereby affect their capacity to encode changes in object compliance.
METHODS
A total of 23 (18–28 yr) subjects (9 men) participated in the study, which was conducted with the approval of the Human Research Ethics Committee of Western Sydney University and undertaken in accordance with the Declaration of Helsinki. After providing written, informed consent, each subject was seated in a comfortable, semi-reclined position throughout the duration of the experiment. The median nerve at the level of the wrist was located by application of weak cathodal stimuli (1.0–4.5 mA, 0.2 ms, 1 Hz), delivered by a 2-mm-diameter probe via an optically isolated constant-current stimulator (Stimulus Isolator, ADInstruments, Sydney, Australia). A surface Ag/AgCl electrode placed on the dorsum of the wrist served as the anode. An insulated tungsten microelectrode, with a shaft diameter of 200 μm and an exposed tip of ∼5 μm (FHC, Bowdoin, ME) was then inserted percutaneously into the nerve at the level of the wrist; an uninsulated microelectrode inserted subcutaneously ∼1 cm away served as the reference electrode. Intraneural stimulation (0.02–1 mA, 0.2 ms, 1 Hz) via the recording microelectrode was then used to guide the microelectrode into a cutaneous fascicle of the nerve, as judged by the subjects' reports of radiating paraesthesiae. Once the tip of the microelectrode had entered a fascicle, indicated by cutaneous sensations at ≤0.02 mA, neural activity was amplified (×20,000, 0.3–5.0 kHz) using a low-noise headstage (NeuroAmpEX, ADInstruments), and the fascicular innervation zone was explored by stroking and tapping the skin until a single mechanoreceptor was identified, the location of which was identified and marked on the subject's finger pad.
Afferents were classified as FA or SA to a sustained indentation, their receptive field size determined, and mechanical thresholds measured by applying calibrated deformable monofilaments (Semmes-Weinstein Aesthesiometers, Stoelting, Chicago, IL). Afferents were further classified as type I afferents, having small, well-defined receptive fields, or type II, with large, poorly defined receptive fields. FAI afferents were particularly sensitive to stroking across the receptive fields, while FAII afferents responded to blowing across the receptive field and to mechanical percussion remote from the site. SAI afferents possessed a higher dynamic sensitivity than the SAII afferents and often responded with an off-discharge during withdrawal of indentation, while SAII afferents were sensitive to tensile strain in the skin produced by tangential stretch of the skin applied manually remote to the receptive field (Macefield and Birznieks 2009). The present data represent a subset of neural data obtained previously (Condon et al. 2014) in which we have specifically examined the effects of venous occlusion. The receptive field locations of all afferents are shown in Fig. 1.
Fig. 1.
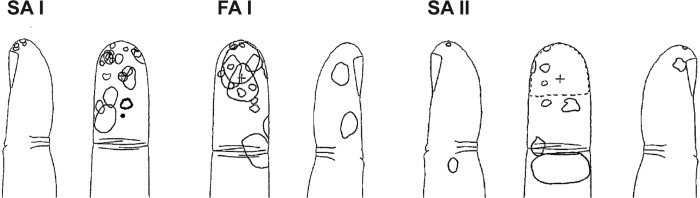
Receptive field locations for the fast adapting FA type I (FAI), slowly adapting (SA) type I (SAI) and SA type II (SAII) afferents.
Stimuli.
Nine silicone surfaces of linearly decreasing compliance (softness) were used; these were the same as those used in the magnitude estimates of softness study undertaken by Friedman and colleagues (2008). The surfaces (obtained from R. H. LaMotte) were made from two-component silicone rubber compounds mixed with varying amounts of diluent to achieve specimens of differing compliance, as described previously (Friedman et al. 2008) and embedded in 36-mm-diameter Petri dishes. The exposed surfaces were then powdered with talc to eliminate stickiness. In turn, each was applied to the surface of the finger pad using a holder attached to the shaft of a linear motor (Baldor), mounted on the vertical (Z) axis of a three-axis linear gantry (Baldor) that was used to locate the position of the stimulating motor in the X- and Y-axes. Displacement in the Z-axis was measured via a linear variable displacement transducer, and force was measured at the base of the silicone rubber holder using a sensitive triaxial transducer (Nano F/T transducer, ATI Industrial Automation). The compliance of the silicone surfaces was determined as the ratio of the amount of surface deformation to an applied force of 1 N (mm/N) and measured in progressive log units of 1.3, ranging from 0.21 to 7.59 mm/N (Friedman et al. 2008).
The surfaces, ranging from the softest (surface 1) to the hardest (surface 9), were presented in the following order: 9, 1, 5, 7, 3, 8, 2, 6 and 4. Each surface was presented to the center of the finger pad of the receptor-bearing digit. The surface was first brought to within 0.5 mm of the finger pad under position control using the linear variable displacement transducer. The control system was then engaged in a force-control mode, in which the Z-force at the probe tip provided the feedback to the control circuitry, and the first stimulus was presented causing the surface to contact the finger pad with a light contact force <0.2 N. Stimuli were then delivered under force-feedback control at a constant rate of 2 N/s and at three amplitudes, 1, 2 and 4 N, returning to the light contact force between the trapezoidal force profiles. The duration of the loading and unloading ramp was 0.5, 1 and 2 s for the 1-, 2- and 4-N loads, with the duration of the plateau phase being 1 s for all loads; the interval between the three loads was 3.5 s. The entire sequence, including the generation of the initial contact force, was repeated for all surfaces, such that the time between the end of one run of three amplitudes and the commencement of the next run required at least 50 s; this time included changing the stimulus surfaces. Each surface was applied once in the control condition and once in the occluded condition. Neural activity was sampled at 10 kHz and stored with force, torque and displacement signals (each at 400 Hz) using a computer-based data acquisition and analysis program (LabChart 7 software and PowerLab16S hardware, ADInstruments).
Venous occlusion was applied after a control sequence had been recorded. A sphygmomanometer cuff, fitted around the arm during the experimental setup, was inflated to a pressure of 40 ± 5 mmHg. This pressure was chosen to prevent ischemia of the hand while maintaining a force sufficient to retard venous return, thereby resulting in engorgement of the fingers and hence increasing the compliance of the fingerpad. A full sequence of stimuli was delivered 5 min following the commencement of venous occlusion, during which the cuff remained inflated. Once the sequence of stimuli was applied, the pressure was released from the sphygmomanometer cuff. Changes in compliance (mm/N) were measured from the recorded force and displacement signals during the plateau phase during the constant-force indentations at each of the three amplitudes (1, 2 and 4 N).
Data analysis.
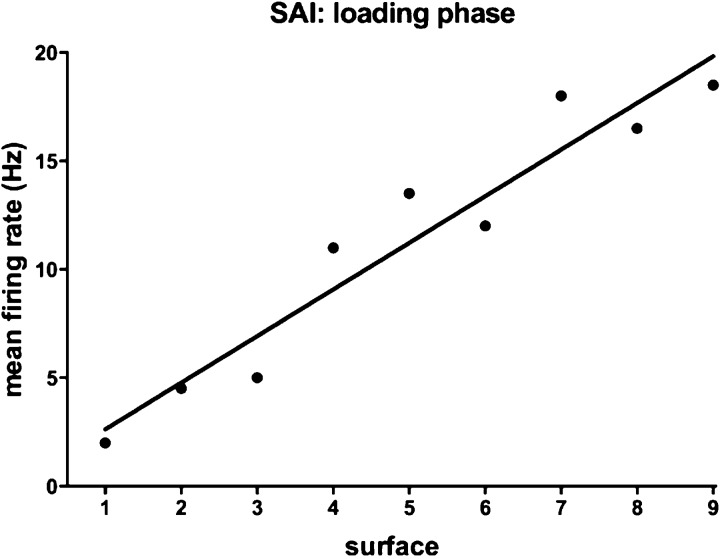
Spike activity was analyzed using IGORPro 6.2 (WaveMetrics). Single units were discriminated, and an instantaneous frequency plot generated. Spontaneous activity was measured as the number of impulses during a 1-s epoch prior to the onset of the stimulus. The number of spikes was counted during the loading, plateau and unloading phases and the peak instantaneous frequency (the inverse of the shortest interspike interval), and the latency at which this occurred, was determined. Finally, the first spike latency was determined as the time difference between the onset of the ramp stimulus and the first detected impulse. For the spontaneously active SAII afferents, this was taken as the latency corresponding to the first short interspike interval following the onset of the ramp. The number of spikes was counted for each phase of the stimulus by collapsing the data into 1) baseline, 2) loading phase, 3) plateau phase and 4) unloading phases. The loading and unloading ramp rates were constant (2 N/s), but because the amplitudes differed (1, 2, 4 N), the duration of the loading ramp increased with increasing amplitude (0.5, 1.0, 2.0 s). Accordingly, mean firing rates during the loading and unloading phases were estimated by dividing the total number of spikes by duration of the stimulation phase (normalized mean firing rate). An example of the relationship between normalized mean firing rate and surface compliance is shown for one SAI afferent in Fig. 2.
Fig. 2.
Relationship between normalized mean firing rate and surface compliance during the loading phase for a single SAI afferent during delivery of the 4-N force ramps to the receptor-bearing finger pad at 2 N/s. The linear regression calculated for the data points is shown.
Statistical analysis.
All analyses were performed using Prism 6 for Mac OS X (GraphPad Software). Two-way ANOVA was performed to establish differences between the control and occlusion conditions at each amplitude and any interaction between the two conditions and the nine silicone surfaces of differing compliance. Linear regression was used to measure the slopes of the relationship between normalized firing rate and object compliance.
RESULTS
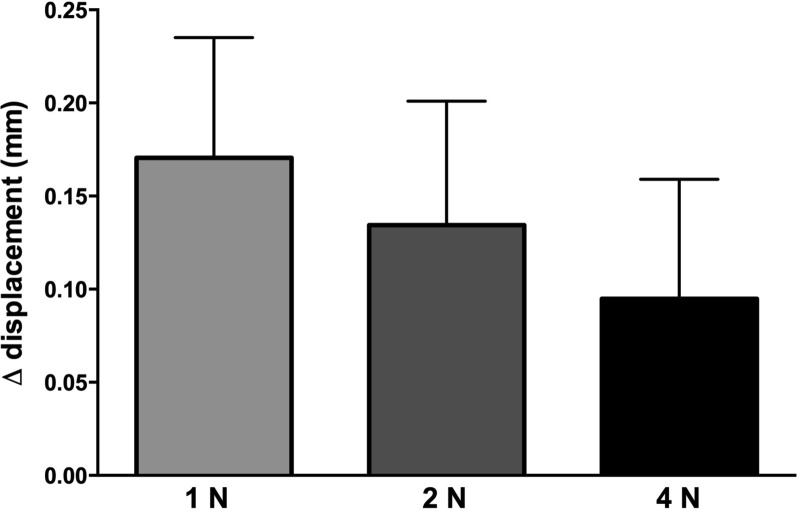
Single-unit recordings were made from 31 tactile afferents in the median nerve at the wrist, a subset of those reported previously (Condon et al. 2014): 10 SAI, 9 FAI, 10 SAII and 2 FAII afferents; the FAII afferents will not be considered further. Recordings were made before and 5 min following the onset of venous occlusion of the arm (40 ± 5 mmHg). Occlusion caused an overt engorgement of the finger pads, but no subject reported discomfort. The displacement of the stimulator probe required to reach the target force (1, 2 or 4 N) across the nine surfaces, ranging from the most compliant silicone disk (surface 1) to the least compliant (surface 9), increased during venous occlusion (paired t-test; P = 0.0012, n = 27), indicating that compliance of the finger pulp had increased. After pooling all surfaces, the increase in displacement following venous occlusion could be seen to be higher for the 1-N than for the 4-N forces (Fig. 3); this can be explained by nonlinear viscoelastic properties of the finger pulp, which is less compliant at higher forces.
Fig. 3.
Increase (Δ) in displacement, measured during the plateau phase of the stimuli, following venous occlusion to 40 mmHg. Data were obtained from 9 subjects.
SAI afferents.
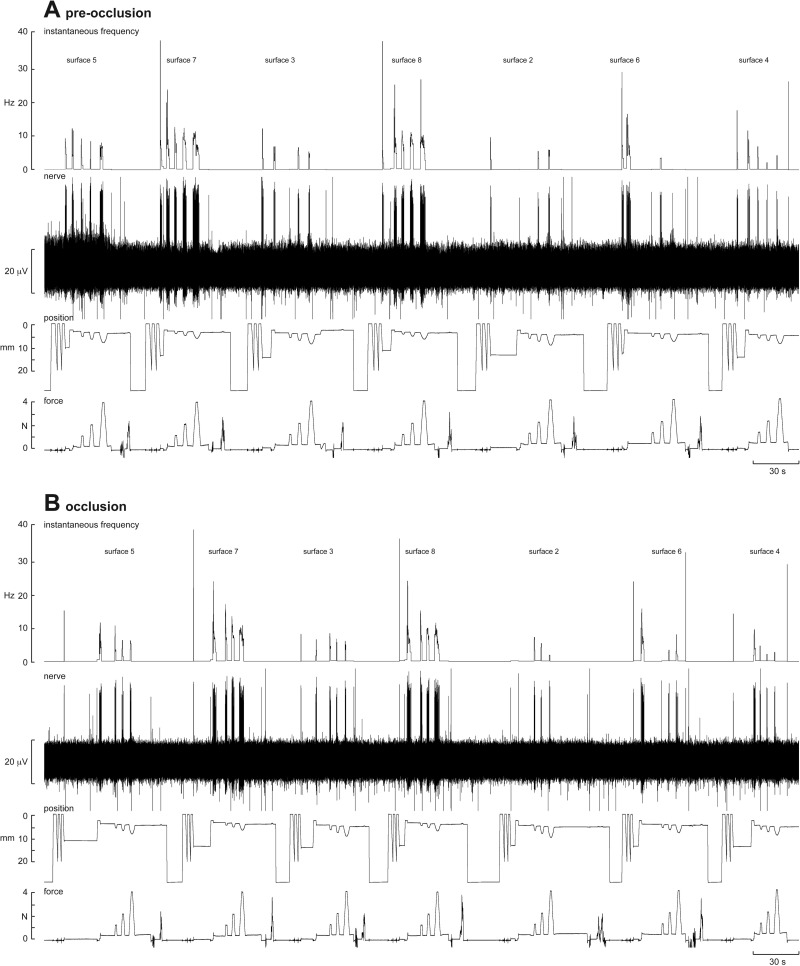
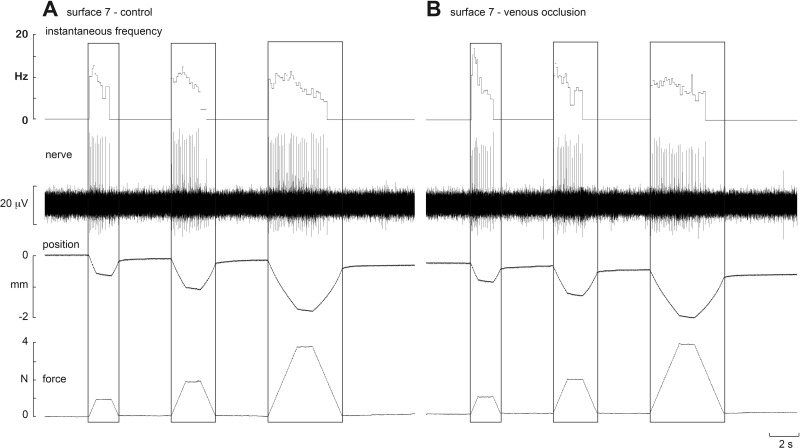
Experimental records from one SAI afferent, located in the finger pad of the index finger, are shown before (A) and during (B) venous occlusion in Fig. 4. Three ramp-and-hold stimuli (1, 2 and 4 N) were applied via seven of the nine silicone disks. Occlusion had little effect on the responses to each of these surfaces. An example from another SAI afferent, also located in the finger pad of the index finger, is shown on an expanded time-base before and during venous occlusion in Fig. 5. Three ramp-and-hold stimuli (1, 2 and 4 N) were applied via one silicone disk (surface 7). Again, it is clear that occlusion had little effect on the responses to this surface; the same was true for the other eight surfaces.
Fig. 4.
Experimental records from an SAI afferent to sets of trapezoidal compression ramps delivered to the pad of the receptor-bearing digit at 2 N/s via flat silicone disks of differing compliance. Responses to 1-, 2- and 4-N forces are shown for seven of the nine compliant surfaces; the softest (surface 1) and hardest (surface 9) are not shown. Data are shown before (A) and after venous occlusion (B). The position trace, obtained from the linear variable displacement transducer signal of the linear motor, indicates part of the initial searching paradigm, by which the control system operated under position control prior to engaging in force feedback on contact with the finger pad. Artifacts in the force trace after the three force ramps indicate manual exchange of one silicone disk to the next one.
Fig. 5.
Experimental records from an SAI afferent to trapezoidal compression ramps delivered to the pad of the receptor-bearing digit at 2 N/s. Responses to 1-, 2- and 4-N forces are shown for a firm surface (surface 7) before (A) and after venous occlusion (B). Venous occlusion had no effect on the sensitivity of the afferent.
As described previously (Condon et al. 2014), as a population the SAI afferents could faithfully encode changes in the compliance of an object brought into contact with the finger pad. Linear relationships between normalized mean firing rate and surface compliance at each amplitude were calculated from individual data, as shown in Fig. 2. Quantitative data from the SAI afferents during the loading phase are shown in Table 1. Mean normalized firing rate increased with increasing stiffness (decreasing compliance) of the surface during the loading phase, but at all amplitudes there were no differences in slope of the relationships before and during venous occlusion. Two-way ANOVA showed that there was no interaction between mean firing rate during the loading phase and the compliance of the surfaces and occlusion; however, venous occlusion did produce significant decreases in first spike onset latency and the latency at which the peak frequency occurred (Table 2). As expected, mean firing rates during the plateau phase were proportional to the amplitude of the force, but at each force venous occlusion had no significant effect on either average firing rate (calculated across all surfaces) or the slope of the linear regression between firing rate and compliance. Slopes during the unloading phase were flat (not significantly different from zero) and were not affected by venous occlusion.
Table 1.
Slopes of the relationship between surface compliance and mean firing rate during the loading phase for SAI, FAI, and SAII afferents, before (control) and during venous occlusion
| Force | Control, 1 N | Occlusion, 1 N | Control, 2 N | Occlusion, 2 N | Control, 4 N | Occlusion, 4 N |
|---|---|---|---|---|---|---|
| SAI | 0.94 ± 0.29 | 0.93 ± 0.25 | 0.84 ± 0.29 | 0.99 ± 0.25 | 0.81 ± 0.31 | 0.92 ± 0.26 |
| P value | 0.0019 | 0.0004 | 0.0048 | 0.0002 | 0.0108 | 0.0006 |
| FAI | 0.39 ± 0.41 | 0.08 ± 0.52 | 0.67 ± 0.29 | 0.23 ± 0.43 | 0.66 ± 0.21 | 0.34 ± 0.29 |
| P value | 0.3361 | 0.8769 | 0.0221 | 0.6031 | 0.0028 | 0.2415 |
| SAII | 0.36 ± 0.49 | 0.20 ± 0.44 | 0.35 ± 0.57 | 0.48 ± 0.41 | 0.50 ± 0.62 | 0.70 ± 0.41 |
| P value | 0.4691 | 0.6508 | 0.5454 | 0.2437 | 0.4247 | 0.0948 |
Values are means ± SE; n = 10 slowly adapting (SA) type I (SAI), 9 fast adapting type I (FAI), and 10 SA type II (SAII) afferents. Significant P values are indicated in bold. Slopes were significant before and after occlusion for the SAI afferents and were not affected by occlusion, while for the FAI afferents slopes were lower following occlusion. Slopes were flat for the SAII afferents and were not affected by occlusion.
Table 2.
Analysis of the influence of the 9 compliant surfaces, and the presence or absence of occlusion, and their interaction, for the firing parameters indicated
| Source of Variation | %Total Variation | P Value |
|---|---|---|
| Mean frequency (loading phase) | ||
| Interaction | 0.91 | >0.99 |
| Surface | 11.56 | <0.0001 |
| Control vs. occlusion | 0.28 | 0.91 |
| Peak frequency | ||
| Interaction | 8.94 | 0.99 |
| Surface | 0.89 | 0.89 |
| Control vs. occlusion | 1.62 | 0.27 |
| First spike latency | ||
| Interaction | 5.96 | 0.9079 |
| Surface | 8.04 | <0.0001 |
| Control vs. occlusion | 6.53 | <0.0001 |
| Peak frequency latency | ||
| Interaction | 3.61 | 0.9862 |
| Surface | 3.16 | 0.0134 |
| Control vs. occlusion | 34.63 | <0.0001 |
Two-way ANOVA for 10 SAI afferents. Significant P values are indicated in bold.
FAI afferents.
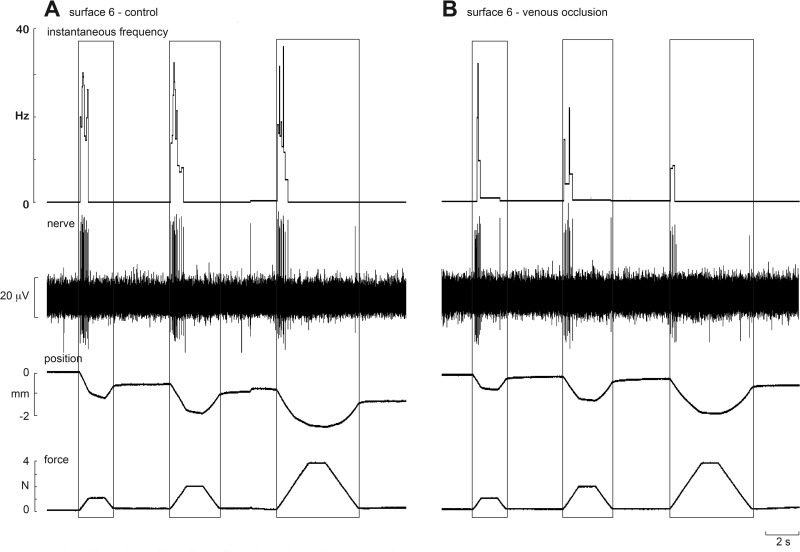
Like the SA afferents, FAI afferents increased their discharge as a function of increasing stiffness during the loading phase; they were silent during the plateau phase. Figure 6 shows experimental records from one FAI afferent, located in the finger pad of the middle finger, during stimulation with one silicone disk (surface 6) before and after venous occlusion. At each amplitude, it can be seen that firing rates were lower following occlusion. While more spikes were generated during the loading phase for the 4-N than the 2- or 1-N ramps, the normalized mean firing rates were actually higher for the 1-N loads. This can be explained by the fact that, for a constant ramp rate (2 N/s), the 4-N loading ramp takes 2 s, and the 1-N ramp 0.5 s, with the normalized mean firing rate being calculated over different times. Given that most of the spikes occurred within the first 0.5 s, normalizing the firing rate will result in artificially different mean values. Nevertheless, it is apparent from Table 1 that venous occlusion significantly reduced the slopes at each amplitude. (Although the slope at the 1-N load was not significantly different from zero before occlusion, it was reduced by the occlusion.) There was a significant increase in first spike latency (P = 0.0005), but two-way ANOVA uncovered no significant interaction between spike latency and surface or occlusion; the same was true for the latency at which the peak frequency occurred during the loading phase (Table 3). As noted above, there was a significant decrease in firing rate following venous occlusion (P = 0.001), but no significant interactions between surfaces and occlusion. Interestingly, peak frequencies during the loading phase were significantly higher during occlusion (P < 0.0001).
Fig. 6.
Experimental records from an FAI afferent to trapezoidal compression ramps delivered to the pad of the receptor-bearing digit at 2 N/s. Responses to 1-, 2- and 4-N forces are shown for a firm surface (surface 6) before (A) and after venous occlusion (B). Venous occlusion reduced the sensitivity of the afferent.
Table 3.
Analysis of the influence of the 9 compliant surfaces, and the presence or absence of occlusion, and their interaction, for the firing parameters indicated
| Source of Variation | %Total Variation | P Value |
|---|---|---|
| Mean frequency (loading phase) | ||
| Interaction | 1.33 | >0.9999 |
| Surface | 2.01 | 0.4241 |
| Control vs. occlusion | 6.42 | 0.0001 |
| Peak frequency | ||
| Interaction | 2.90 | >0.9999 |
| Surface | 1.07 | 0.9275 |
| Control vs. occlusion | 5.91 | 0.0051 |
| First spike latency | ||
| Interaction | 4.88 | 0.9990 |
| Surface | 0.92 | 0.9146 |
| Control vs. occlusion | 6.48 | 0.0005 |
| Peak frequency latency | ||
| Interaction | 6.81 | 0.9672 |
| Surface | 1.92 | 0.5444 |
| Control vs. occlusion | 16.05 | <0.0001 |
Two-way ANOVA for 9 FAI afferents. Significant P values are indicated in bold.
SAII afferents.
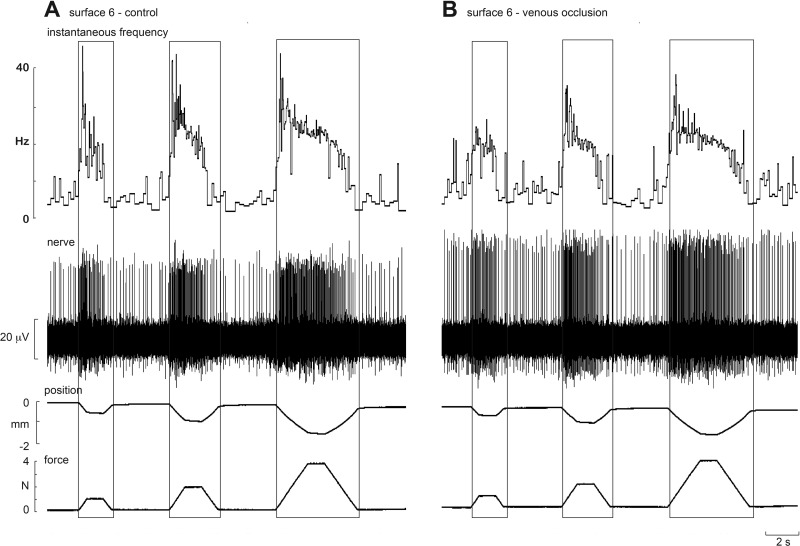
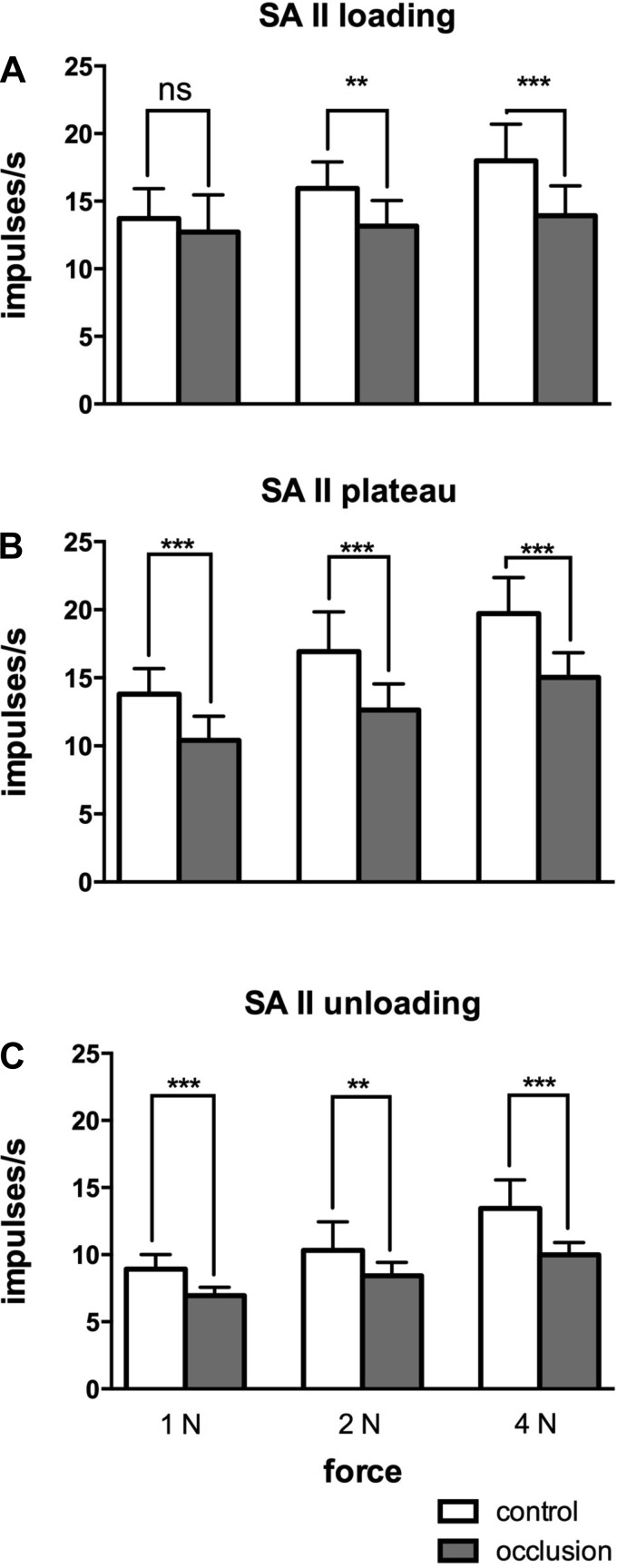
The SAII afferents showed the greatest changes during venous occlusion. Experimental records from one SAII afferent, located adjacent to the nail bed of the middle finger, during stimulation with one silicone disk (surface 6), before and after venous occlusion, are shown in Fig. 7. Like the FAI afferent illustrated in Fig. 6, firing rates of the SAII afferent were lower following occlusion. While the slope of the relationship between normalized mean firing rate and compliance was not significantly affected by occlusion, which was not significantly different from zero at any amplitude (Table 1), at each amplitude the slopes were shifted downwards during the loading, plateau and unloading phases. This was because mean firing rates at each phase of the stimulus were lower during occlusion, as shown in Fig. 8. Two-way ANOVA showed that mean frequencies during the plateau and unloading phases were significantly lower following occlusion. Mean firing rates were also lower during the loading phase, although this failed to reach statistical significance, and peak frequencies were significantly lower. At all phases there were no interactions between surfaces and occlusion (Table 4). Peak frequencies generated during the loading phase were also significantly lower during occlusion. There was no significant change in background discharge rate of the spontaneously active SAII afferents during occlusion.
Fig. 7.
Experimental records from an SAII afferent to trapezoidal compression ramps delivered to the pad of the receptor-bearing digit at 2 N/s. Responses to 1-, 2- and 4-N forces are shown for a firm surface (surface 6) before (A) and after venous occlusion (B). Venous occlusion reduced the sensitivity of the afferent. Note that the spike amplitude was slightly higher following occlusion due to a mechanical shift in the tip of the microelectrode.
Fig. 8.
Mean (±SE) normalized firing rates of the SAII afferents before and during venous occlusion. Significant differences during the loading (A), plateau (B) and unloading (C) phases are indicated. **P < 0.01, ***P < 0.001 (paired t-tests). ns, Not significant.
Table 4.
Analysis of the influence of the 9 compliant surfaces, and the presence or absence of occlusion, and their interaction, for the firing parameters indicated
| Source of Variation | %Total Variation | P Value |
|---|---|---|
| Mean frequency (loading phase) | ||
| Interaction | 1.516 | >0.9999 |
| Surface | 2.086 | 0.3753 |
| Control vs. occlusion | 2.434 | 0.0752 |
| Mean frequency (plateau phase) | ||
| Interaction | 1.007 | >0.9999 |
| Surface | 1.510 | 0.4942 |
| Control vs. occlusion | 17.91 | <0.0001 |
| Mean frequency (unloading phase) | ||
| Interaction | 2.15 | >0.9999 |
| Surface | 2.50 | 0.2058 |
| Control vs. occlusion | 3.55 | 0.0088 |
| Peak frequency | ||
| Interaction | 2.62 | >0.9999 |
| Surface | 0.74 | 0.9346 |
| Control vs. occlusion | 3.43 | 0.0181 |
Two-way ANOVA for 10 SAII afferents. Significant P values are indicated in bold.
DISCUSSION
We have shown that increases in the compliance of the finger pads, produced by vascular engorgement of the finger pulp by application of venous occlusion, affect the firing properties of some, but not all, classes of low-threshold mechanoreceptor in the finger pad. SAI afferents were not affected by this manipulation, whereas at all amplitudes the slopes of the relationship between mean firing rate and surface compliance were significantly reduced for the FAI afferents, and significantly shifted downwards for the SAII afferents. That there were significant changes in the firing properties of the FAI and SAII afferents during venous occlusion indicates that there were indeed physiological changes in skin mechanics. We do not believe a change in blood flow per se will affect cutaneous mechanoreceptors, given that there is no change in blood delivery: no subjects reported parasethesiae either during or following release of the cuff, and pulsatile blood flow was maintained during the occlusion. A similar approach, inflation of a sphygmomanometer cuff proximal to the hand to 40 mmHg, was used by Bowden and McNulty (2013) to increase compliance of the finger pad; these authors also reported no neural signs indicative of ischemic block. Our laboratory has previously shown that many tactile afferents in the finger pad exhibit pulse-related modulation of their discharge, owing to their proximity to blood vessels (Macefield 2003), and demonstrated that any sympathetically-mediated effects on the firing properties of tactile afferents are exerted indirectly through changes in skin blood flow (Macefield and Elam 2004).
We should point out that increasing the hydration of the outer layer of the skin, through application of a cosmetic moisturizer, has been shown to decrease the estimation of roughness (Verrillo et al. 1999), but decrease two-point discrimination thresholds (Bowden and McNulty 2013; Lévêque et al. 2000). Moreover, moisturizing the skin has been shown to reduce the mean firing rates of FAI afferents to brushing stimuli and of SAI afferents to sinusoidal indentation, at least in the hairy skin of the leg (Lévêque et al. 2000); the authors suggested that hydration decreased the mechanical transfer function of the skin by reducing the elasticity of the stratum corneum. This is, of course, a different means of looking at the effects of skin compliance on tactile function than the approach adopted in the present study: we did not directly influence the softness of the outer layers of the skin but rather changed the compliance of the underlying pulp. Nevertheless, both studies emphasize the importance of skin mechanics on the transduction of a mechanical stimulus into a pattern of neural impulses by cutaneous mechanoreceptors.
Effects of changes in finger pad compliance on SAI afferents.
SAI afferents have small receptive fields and are found in high densities in the fingertips (Johansson and Vallbo 1979), thereby enabling their ability to discriminate the shape, friction and texture of an object. Given their small receptive fields, engorgement of the finger pads during venous occlusion may not be expected to have as great an effect as seen in afferents with larger receptive fields (i.e., SAII afferents). The regression lines between mean firing rate and object compliance were not significantly different in control conditions and during venous occlusion conditions. This indicates that the firing rate of SAI afferents may be a very sensitive measure of changes in object compliance (Condon et al. 2014), one that is evidently not affected by changes in compliance of the finger pad itself. It may be that the histological structure and tissue environment of the Merkel disks, which are innervated by the SAI afferents, renders these mechanoreceptors less sensitive to changes in mechanical properties of the skin (Halata et al. 2003).
Effects of changes in finger pad compliance on FAI afferents.
Our laboratory has previously shown that the sensitivity of FAI afferents to changes in object compliance is lower than that for the SAI afferents (Condon et al. 2014). Moreover, unlike the SAI afferents, which can also encode static levels of object compliance (i.e., during the plateau phase), the FAI afferents only respond during the loading phase, and to a lower extent during the unloading phase. The present data show that any contribution provided by the FAI afferents to encoding changes in object compliance is lost when the compliance of the fingers is increased: the slope of the regression line between mean firing rate and compliance became flat during venous occlusion. Additionally, the intersection of the linear regression lines (occurring between surfaces 4 and 5) is very interesting, given the psychophysical investigation conducted by Friedman et al. (2008); these authors showed that when the finger pad of a subject was subjected to the same silicone surfaces used in the present study, the subject frequently identified the compliance of a surface as “soft” when the true compliance of the surface was less than that of the subject's finger pulp. Within the range of stimuli used in the present study, the silicone surfaces that were approximately equivalent to the compliance of the human finger pulp were surfaces 4 and 5, at which point the regression lines intersect each other during the loading phase. Thus it can be assumed that the perception of softness/hardness of an object would not change due to the changes in mechanical properties of the fingertip pulp. This finding supports the results obtained by Bowden and McNulty (2013), who showed that venous occlusion to 40 mmHg had no effect on two-point discrimination.
Effects of changes in finger pad compliance on SAII afferents.
Occluding the venous return increased the compliance of the finger pads through engorgement of the finger pulp, in turn increasing the circumference of the finger pad; this would be expected to have had an effect on the background spontaneous discharge rate of the Ruffini organs (the mechanoreceptor endings of the SAII afferents) due to their sensitivity to tensile strain in the skin (Iggo and Muir 1969). However, although there was a small fall in the background firing rate of these afferents, this was not significant. Nevertheless, venous occlusion did reduce the overall firing rates during the application of the stimulus and shifted the slopes of the linear regressions between mean firing rate and compliance downwards. The net result of venous occlusion therefore was to decrease the responsiveness of the Ruffini organs to the mechanical stimuli. It is clear that venous occlusion caused an alteration in the skin mechanics of the finger pads, which will have caused a change in the internal environment surrounding the Ruffini organs. A detailed histological and mechanical model built to fit with our neurophysiological data would be required to address this. However, given that venous occlusion emulates what occurs in edema, it is likely that the sensitivity of SAII afferents to forces applied to the skin would be reduced in edema. A recent psychophysical study tested the hypothesis that altering the skin mechanics using venous occlusion would affect the ability of a subject to perceive different forces applied by punctate stimuli (von Frey hairs) applied with varying forces: Bowden and McNulty (2013) concluded that venous occlusion had no significant effect on how humans perceive such stimuli. However, the results from the current investigation may be able to explain why Bowden and McNulty (2013) reached this conclusion. The stimuli that these investigators used (von Frey hairs) were more suited to stimulate type I afferents (i.e., SAI and FAI), which have small receptive fields and lower mechanical thresholds. SAII afferents have the highest threshold to mechanical stimulation, have very large receptive areas and respond to lateral skin stretch, and so would have been less likely to be stimulated during this psychophysical study. Accordingly, it is likely that the results obtained by Bowden and McNulty (2013) primarily reflected the effects of venous occlusion on type I afferents, which, as we have seen, are little affected.
Conclusions.
This study has shown that experimentally induced changes in skin mechanics affects the firing of some, but not all, classes of low-threshold cutaneous mechanoreceptor during the application of controlled compression stimuli to the finger pad. Specifically, we have shown that increasing the compliance of the finger pad by venous occlusion had no effect on the capacity of the SAI afferents to encode changes in object compliance, whereas the FAI afferents were no longer able to encode changes in compliance following venous occlusion. The SAII afferents exhibited a decrease in sensitivity to forces applied to the finger pad, but, given that these afferents are insensitive to changes in object compliance, this would have little consequence. Whether these changes in skin mechanics affect the capacity to discriminate differences in softness remains to be determined.
GRANTS
This work was supported in part by an National Health and Medical Research Council of Australia/ARC Thinking Systems grant to V. G. Macefield.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.M.H., M.C., R.A., H.O., V.G.M., and I.B. performed experiments; K.M.H., M.C., V.G.M., and I.B. analyzed data; K.M.H., M.C., F.M., H.O., V.G.M., and I.B. interpreted results of experiments; K.M.H. drafted manuscript; K.M.H., M.C., R.A., F.M., H.O., V.G.M., and I.B. edited and revised manuscript; K.M.H., M.C., R.A., F.M., H.O., V.G.M., and I.B. approved final version of manuscript; V.G.M. and I.B. conception and design of research; V.G.M. prepared figures.
ACKNOWLEDGMENTS
We are grateful to Prof. Robert H. LaMotte for providing the compliant surfaces.
REFERENCES
- Bowden J, McNulty PA. Age-related changes in cutaneous sensation in the healthy human hand. Age (Dordr) 35: 1077–1089, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon M, Birznieks I, Hudson K, Chelvanayagam DK, Mahns D, Olausson H, Macefield VG. Differential sensitivity to surface compliance by tactile afferents in the human finger pad. J Neurophysiol 111: 1308–1317, 2014. [DOI] [PubMed] [Google Scholar]
- Friedman RM, Hester KD, Green BG, LaMotte RH. Magnitude estimation of softness. Exp Brain Res 191: 133–142, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol 271: 225–239, 2003. [DOI] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol 200: 763–796, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindrich DL, Zhou Y, Becker T, Dennerlein JT. Non-linear viscoelastic models predict fingertip pulp force-displacement characteristics during voluntary tapping. J Biomech 36: 497–503, 2003. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo ÅB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol 286: 283–300, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévêque JL, Dresler J, Ribot-Ciscar E, Roll JP, Poelman C. Changes in tactile spatial discrimination and cutaneous coding properties by skin hydration in the elderly. J Invest Dermatol 115: 454–458, 2000. [DOI] [PubMed] [Google Scholar]
- Macefield VG. Cardiovascular and respiratory modulation of tactile afferents in the human finger pad. Exp Physiol 88, 617–625, 2003. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Birznieks I. Cutaneous mechanoreceptors, functional behavior. In: Encyclopedia of Neuroscience, edited by Binder MD, Hirokawa N, and Windhorst U. Berlin: Springer, 2009, p. 914–922. [Google Scholar]
- Macefield VG, Elam M. Does sympathetic nerve discharge affect the firing of myelinated cutaneous afferents in humans? Autonom Neurosci 111: 116–126, 2004. [DOI] [PubMed] [Google Scholar]
- Pawluk DT, Howe RD. Dynamic lumped element response of the human fingerpad. J Biomech Eng 121: 178–183, 1999. [DOI] [PubMed] [Google Scholar]
- Pubols BH., Jr Factors affecting cutaneous mechanoreceptor response. I. Constant force versus constant displacement stimulation. J Neurophysiol 47: 515–529, 1982a. [DOI] [PubMed] [Google Scholar]
- Pubols BH., Jr Factors affecting cutaneous mechanoreceptor response. II. Changes in mechanical properties of skin with repeated stimulation. J Neurophysiol 47: 530–542, 1982b. [DOI] [PubMed] [Google Scholar]
- Serina ER, Mote CD Jr, Rempel D. Force response of the fingertip pulp to repeated compression–effects of loading rate, loading angle and anthropometry. J Biomech 30: 1035–1040, 1997. [DOI] [PubMed] [Google Scholar]
- Verrillo RT, Bolanowski SJ, Checkosky CM, McGlone FP. Effects of hydration on tactile sensation. Somatosens Mot Res 16: 352–360, 1999. [DOI] [PubMed] [Google Scholar]
- Westling G, Johansson RS. Responses in glabrous skin mechanoreceptors during precision grip in humans. Exp Brain Res 66: 128–140, 1987. [DOI] [PubMed] [Google Scholar]