Abstract
The dorsolateral prefrontal and posterior parietal cortex are two brain areas involved in cognitive functions such as spatial attention and working memory. When tested with identical tasks, only subtle differences in firing rate are present between neurons recorded in the two areas. In this article we report that major differences in neuronal variability characterize the two areas during working memory. The Fano factors of spike counts in dorsolateral prefrontal neurons were consistently lower than those of the posterior parietal cortex across a range of tasks, epochs, and conditions in the same monkeys. Variability differences were observed despite minor differences in firing rates between the two areas in the tasks tested and higher overall firing rate in the prefrontal than in the posterior parietal sample. Other measures of neuronal discharge variability, such as the coefficient of variation of the interspike interval, displayed the same pattern of lower prefrontal variability. Fano factor values were negatively correlated with performance in the working memory task, suggesting that higher neuronal variability was associated with diminished task performance. The results indicate that information involving remembered stimuli is more reliably represented in the prefrontal than the posterior parietal cortex based on the variability of neuronal responses, and suggest functional differentiation between the two areas beyond differences in firing rate.
Keywords: Fano factor, intraparietal sulcus, monkey, principal sulcus, working memory
the prefrontal cortex has long been viewed as the seat of higher cognitive processing, controlling executive functions such as working memory and task switching (Goldman-Rakic 1987; Miller and Cohen 2001). However, neurophysiological responses of prefrontal neurons are often strikingly similar to those of neurons in cortical areas connected with the prefrontal cortex (Katsuki and Constantinidis 2012). For example, neurons in the posterior parietal cortex (PPC) exhibit neural correlates of a host of cognitive functions (Bisley and Goldberg 2010; Fitzgerald et al. 2012; Rawley and Constantinidis 2009), which resemble greatly those of the dorsolateral prefrontal cortex (dlPFC), the part of the frontal lobe connected with it (Cavada and Goldman-Rakic 1989).
Early neurophysiological experiments in the two areas pointed to a specialization of function in terms of working memory, suggesting that dorsolateral prefrontal neurons are better able to represent stimuli required to be held in memory and to resist the effect of behaviorally irrelevant distractors, whereas posterior parietal neurons appear to represent the most recent stimulus whether it is a target or distractor (Constantinidis and Steinmetz 1996; di Pellegrino and Wise 1993; Qi et al. 2010; Suzuki and Gottlieb 2013). Recent reports paint a more complex picture. Although differential responses between PPC and dlPFC are evident in various tasks, representation of distractor stimuli appears to be task dependent, as well, and prefrontal neurons may readily respond to distractor stimuli in the context of some tasks (Jacob and Nieder 2014; Qi et al. 2015). Prior studies also demonstrate that prefrontal (Hoshi et al. 1998; Wallis et al. 2001; Wallis and Miller 2003; White and Wise 1999) as well as posterior parietal neurons (Rawley and Constantinidis 2010; Stoet and Snyder 2004) are modulated by task rules. When compared directly in the same animals executing the same tasks, differences in mean firing rate between dlPFC and PPC are subtle, as in a recent study from our laboratory (Qi et al. 2015). This finding cannot account for the profound deficits in the ability to maintain task information in memory and switch between tasks specifically after prefrontal lesions (Buckley et al. 2009; Rossi et al. 2007).
This lack of consistent functional specialization in the neurophysiological literature motivated us to examine differences in discharge patterns between areas, beyond the mean firing rate. We specifically focused on the variability of spike counts during the task. Discharge rates of cortical neurons are highly variable from trial to trial during the presentation of identical sensory stimuli or execution of motor movements to the same targets, which has important implications about information encoding in neuronal populations (Averbeck et al. 2006; Cohen and Kohn 2011; Faisal et al. 2008; Shadlen and Newsome 1998). Furthermore, it has been recognized that variability can be an important indicator of neuronal computations and cognitive states (Churchland et al. 2011; Cohen and Maunsell 2009; Mitchell et al. 2007; Scholvinck et al. 2015). Previous studies have reported systematic differences in variability and regularity of firing rate between cortical areas (Maimon and Assad 2009; Shinomoto et al. 2009). We therefore compared measures of neuronal variability in the dlPFC and PPC.
METHODS
Two male, rhesus monkeys (Macaca mulatta) weighing 7–9 kg were used in this study. All surgical and animal use procedures in this study followed guidelines by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals and the National Research Council's Guide for the Care and Use of Laboratory Animals, and were reviewed and approved by the Wake Forest University Institutional Animal Care and Use Committee.
Surgery and neurophysiology.
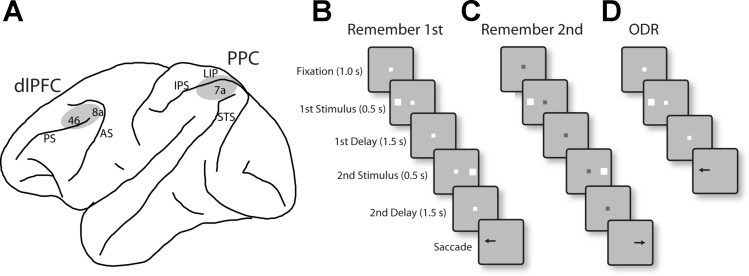
Details of surgical and recording procedures were reported previously (Qi et al. 2015). Briefly, two 20-mm-diameter recording cylinders were implanted over the dlPFC and the PPC of the same hemisphere in each monkey (Fig. 1A). Extracellular activity of single units was recorded from areas 8a and 46 of dlPFC and areas 7a and the lateral intraparietal area (LIP) of the PPC using arrays of two to four microelectrodes in each cylinder. The majority of recordings in the PPC analyzed in the present study were obtained in area 7a; only six neurons with delay period activity in the diametric distractor task and two in the randomized distractor and oculomotor delayed response tasks were recorded from area LIP. The electrical signal from each electrode was amplified, bandpass filtered between 500 Hz and 8 kHz, and recorded with a modular data acquisition system at 25-μs resolution (APM system; FHC, Bowdoin, ME). The anatomic location of electrode penetrations was determined on the basis of MR imaging.
Fig. 1.
A: schematic diagram of the monkey brain, outlining areas where recordings were performed. Recordings in dorsolateral prefrontal cortex (dlPFC) sampled areas 8a and 46. Recordings in posterior parietal cortex (PPC) sampled areas 7a and the lateral intraparietal area (LIP). AS, arcuate sulcus; PS, principal sulcus; IPS, intraparietal sulcus; STS, superior temporal sulcus. B: remember-first task. The white fixation point instructed the monkeys to remember the first stimulus (which could appear at 1 of 8 locations arranged on a ring of 12° eccentricity). The monkeys were required to ignore the second stimulus, which appeared after an intervening delay period. After a second delay period, the fixation point turned off cuing the monkey to saccade to location of the first stimulus. C: remember-second task. A blue fixation point instructed the monkey to remember and saccade to the location of the second stimulus. In an initial set of experiments, the second stimulus appeared always diametric to the first (diametric distractor set), for both the remember-first and remember-second tasks. In a second series of recordings, the second stimulus appeared at a randomized location relative to the first stimulus (randomized distractor set). D: oculomotor delayed response (ODR) task. In this task, only one stimulus was presented, and after a delay period the monkey was required to saccade to its remembered location.
Behavioral tasks.
The tasks used in the present study were variations of the oculomotor delayed response (ODR) task (Funahashi et al. 1989), which involves presentation of a visual stimulus followed by a delay period, at the end of which the fixation point turns off and the monkey is required to make an eye movement to the location of the remembered stimulus (Fig. 1D). We used a variant of this task involving sequential presentation of two stimuli. The task required the monkey to remember and make an eye movement to either the first stimulus, if the fixation point was white (Fig. 1B), or the second stimulus, if the fixation point was blue (Fig. 1C). The first stimulus (S1) was displayed pseudorandomly at one of eight locations arranged along a circular ring of 12° eccentricity, with a 45° angular separation between neighboring stimuli, followed by a delay period (D1). The second stimulus then appeared (S2), and after a second delay period of 1.5 s (D2), the monkeys were required to saccade to the location of the first or second stimulus. To minimize the uncertainty about the stimulus to be remembered, the remember-first and remember-second conditions were presented in blocks of trials. The monkeys were rewarded with fruit juice after making a correct saccade. Breaking fixation exceeding a 2° window led to the immediate termination of the trial without reward.
In an initial set of experiments, the second stimulus always appeared at the diametric location relative to the first stimulus. We refer to this as the diametric distractor set of experiments. In a second series of recordings, the location of the second stimulus varied with respect to the first stimulus and was either the same as S1 or offset (either clockwise or counterclockwise, in different sets) by 45°, 90°, or 180°. We refer to this as the randomized distractor set of experiments.
Neural data analysis.
Recorded spike waveforms were sorted into separate units using an automated cluster analysis method referred to as the KlustaKwik algorithm (Harris et al. 2000), which applied principal component analysis of the waveforms. Mean firing rate was then determined in each task epoch. Neurons with significant elevation of firing rate during the presentation of delay periods were identified by comparing the firing rate in the 1.5-s interval of the delay period with the 1-s interval of fixation (paired t-test; P < 0.05). Most analyses rely on data from correct trials. Most analyses were also performed on neurons with significantly elevated responses during the first delay period. The stimulus location that elicited the best response in this period was identified, and this location was used for comparison between tasks.
Fano factor.
The Fano factor of a neuron's spike count (defined as the variance divided by the mean) was estimated in different task periods. We relied on the method of Churchland et al. (2010), because we have applied it previously to data recorded from the prefrontal cortex (Qi and Constantinidis 2012). Data for each neuron were treated separately. Spike counts were computed in a 100-ms sliding window moving in 20-ms steps, separately for each stimulus location. This analysis was performed in a series of bins across the length of an epoch. We computed the variance and mean of the spike count of each neuron across trials and performed a regression of the variance to the mean (Churchland et al. 2010). The slope of this regression represents the reported Fano factor. Fano factors were determined separately for each neuron, and then averaged across neurons, for all analyses.
To discount the effect of firing rate on Fano factor, data from the dlPFC and PPC were rate-matched with the following procedure: Firing rates from all stimulus conditions were first averaged together for each neuron. Neurons were then grouped in 1 spike/s bins based on firing rate. In each bin, equal numbers of neurons recorded from the dlPFC and PPC were randomly selected for analysis. The procedure produced two samples with equal numbers of neurons, matched for firing rate during the stimulus period.
We considered whether the Fano factor accurately captures neuronal variability across a range of firing rates by comparing it with a quadratic model of variance of the form:
where var represents the variance of spike counts, m is the speak count mean, and β is a fitting parameter for the variance of the gain signal (Goris et al. 2014). We used an F-test to compare the two models, on a neuron-by-neuron basis, in each time bin.
Coefficient of variation.
We computed the coefficient of variation (CV) of interspike intervals (ISI) from the series formed by the intervals between successive spikes in a spike train, as we have done previously for parietal data (Joelving et al. 2007). CV is obtained directly from the ISI histogram by dividing its SD by its mean. The CV provides a measure of how close the spike train is to an ideal Poisson spike train (for which CV = 1). Highly regular neuronal spiking patterns will show lower CV values, whereas irregular (e.g., bursty) spiking patterns will show greater CV values.
RESULTS
Neurophysiological data were collected from areas 8a and 46 of the dlPFC and areas 7a and LIP of the PPC of two monkeys as they performed working memory tasks (Fig. 1A). The monkeys were trained to remember one of two stimuli presented in sequence depending on the color of the fixation point and to make a saccade toward it (Fig. 1, B and C). A white fixation point instructed them to remember the location of the first stimulus; we refer to this as the “remember-first” condition. A blue fixation point indicated that the monkeys needed to saccade toward the second stimulus (“remember-second” condition). We used two stimulus sets, one always involving a second stimulus that appeared at a location diametric to the first; we refer to this as the diametric distractor set. In a subsequent set of recordings, the second stimulus could appear at a location that was randomized relative to the first; we refer to this as the randomized distractor set. We additionally recorded from neurons in the single-stimulus version of the ODR task, which does not include a distractor (Fig. 1D). We have previously reported a comparison of firing rates between areas and task conditions (Qi et al. 2015). We focus here on the variability of neuronal responses.
Database.
A total of 552 neurons were recorded from the PPC (300 and 252 from monkeys GR and HE, respectively) and 426 neurons from the dlPFC (158 and 268 from GR and HE, respectively) across all experiments and task conditions. For each of the three tasks used, we focused mostly on a sample of neurons that displayed significantly elevated discharges in the first delay period after the offset of the cue stimulus (Fig. 1B) compared with the baseline period (paired t-test, P < 0.05), which provides a neural correlate of spatial working memory (Funahashi et al. 1989). This sample consisted of 107 neurons in PFC and 95 neurons in PPC in the diametric distractor set. Additionally, 85 neurons in PFC and 75 neurons in PPC exhibited significantly elevated delay period activity in the randomized distractor task. In the ODR task, 44 neurons in PFC and 66 neurons in PPC exhibited delay period activity.
Overview of firing rates.
Measures of neural variability such as the Fano factor and CV are dependent on firing rate (Goris et al. 2014). It was essential therefore to determine the firing rate of the sample of neurons we analyzed. Mean discharge rate of the samples of neurons from dlPFC and PPC we analyzed are shown in Fig. 2 (only responses for the best location in the receptive field are shown for each neuron, in the randomized distractor set, for simplicity). We performed a two-way ANOVA to compare responses, using as factors brain area (dlPFC or PPC) and task epoch, for the task epochs that were common across all tasks (fixation, stimulus presentation, and delay period). As expected, the firing rate differed significantly between task epochs, being maximal during the stimulus presentation (2-way ANOVA, P < 0.05 for each of the ODR, diametric distractor, and randomized distractor tasks). We therefore analyze variability separately for each task epoch.
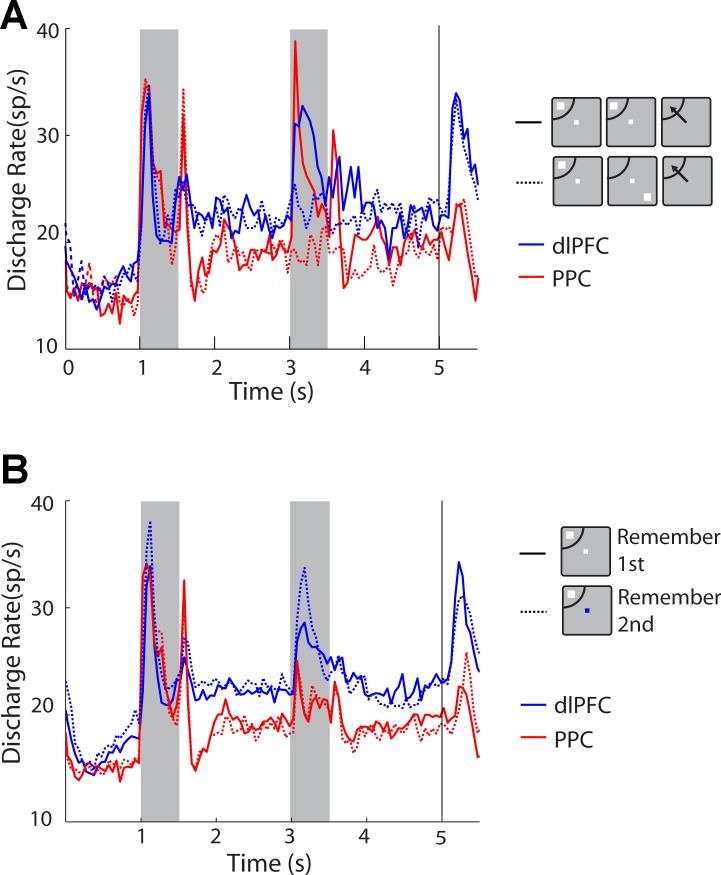
Fig. 2.
Population peristimulus time histograms (PSTH) averaging activity from dlPFC and PPC neurons with significant delay period activity in the randomized distractor task. A: discharge rate data are plotted for the remember-first task with the first stimulus appearing in the receptive field and the distractor appearing either in the receptive field (solid lines) or out of the receptive field (dotted lines). Blue and red lines represent dlPFC and PPC, respectively (dlPFC, n = 75; PPC, n = 85). B: population PSTH for the same set of neurons as in A comparing activity in the remember-first (solid lines) and remember-second (dotted lines) tasks. sp/s, Spikes per second.
Comparison of firing rates between areas revealed higher overall rates in our sample of dlPFC neurons compared with the sample of PPC neurons, although the difference did not reach significance across all conditions. In the randomized distractor set, no significant difference was present between firing rates (2-way ANOVA, P > 0.2). In the diametric distractor set, no significant differences were present during the remember-first task (2-way ANOVA, P > 0.7), but dlPFC neurons had a slightly higher firing rate in the remember-second task (2-way ANOVA, P = 0.04). A post hoc test revealed that firing rate during the cue presentation differed significantly between PPC and dlPFC (post hoc Tukey's test, P < 0.05). Finally, in the ODR task, dlPFC neurons exhibited a significantly higher firing rate from PPC (2-way ANOVA, P < 0.05).
Comparison of firing rates revealed only subtle differences between areas (Fig. 2A), as we have previously reported (Qi et al. 2015). Additionally, more dlPFC activity was elicited by the remember-second task than by the remember-first task (blue dotted vs. solid line in the first delay period of Fig. 2B), whereas the opposite was true for PPC (red dotted vs. solid line), although this difference too was minor.
Neuronal variability.
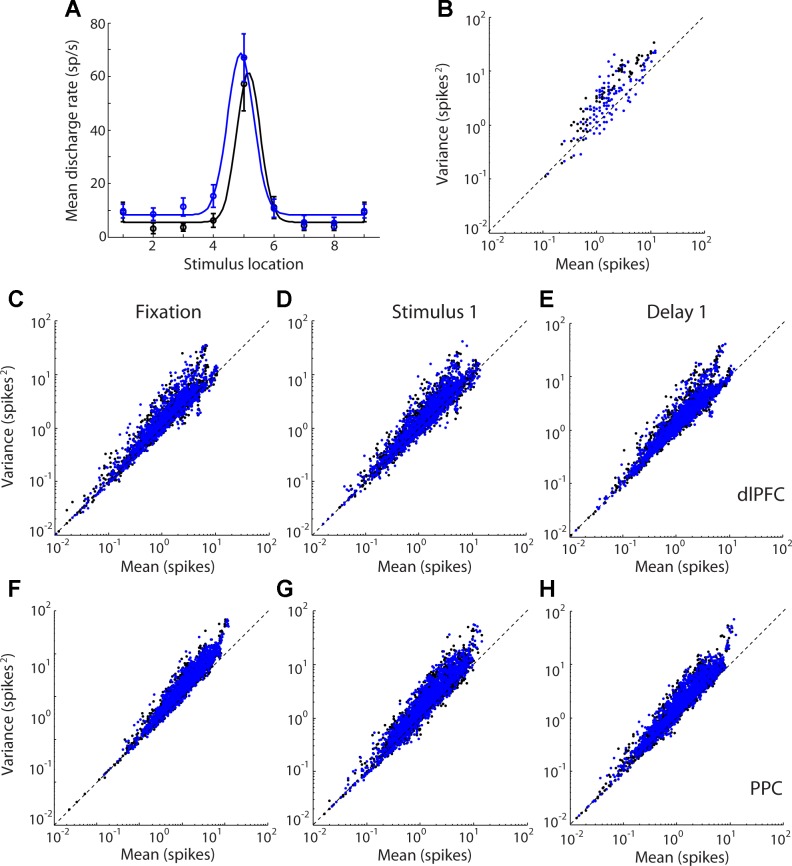
We next sought to characterize the relationship between firing rate and variability in our sample. A spiking process obeying a Poisson distribution would be expected to display a variance of spike counts that is equal to its mean; however, experimental results across multiple cortical areas indicate that neuronal spike counts deviate from this Poisson model, with variances that are almost always greater than the mean (Constantinidis and Goldman-Rakic 2002; Dean 1981; McAdams and Maunsell 1999; Softky and Koch 1993; Tolhurst et al. 1983; Vogels et al. 1989). This has led to proposals that variance of spike counts can be best decomposed into two components, one equal to the mean and one proportional to the square of the mean (Goris et al. 2014). Examining the relationship between the variance and mean in our sample confirmed the deviation from linearity (Fig. 3). The benefits of using a metric of variance that includes a quadratic form were modest, however, particularly for the dlPFC, because our sample involved conditions with low overall spike counts. Among neurons with significant responses in any task epoch, only 1.5% of dlPFC neurons favored significantly the quadratic model (F-test, P < 0.05), whereas 2% favored the linear model. In PPC, 2% of neurons favored the quadratic model and 1% favored the linear model. We rely primarily on the Fano factor (variance divided by the mean) for comparisons of variability between the two areas, rather than a quadratic model of variance, because the former also allows direct comparison with previous reports in the literature.
Fig. 3.
A: tuning curve of an example neuron. Firing rate is plotted for each stimulus location of the remember-first and remember-second tasks in the diametric stimulus set. B: spike counts in 100-ms bins are plotted against variance in the same interval for the neuron in A. C–H: scatter plots of variance vs. mean spike count for neurons with significant response in any task epoch (dlPFC, n = 180; PPC, n = 209). Each data point corresponds to a 100-ms time window for 1 neuron for 1 stimulus location. Data points from the prefer-first task (black dots) and prefer-second task (blue dots) of the diametric distractor set are plotted together. Data are shown separately for each task epoch for dlPFC (C–E) and PPC neurons (F–H).
Fano factor.
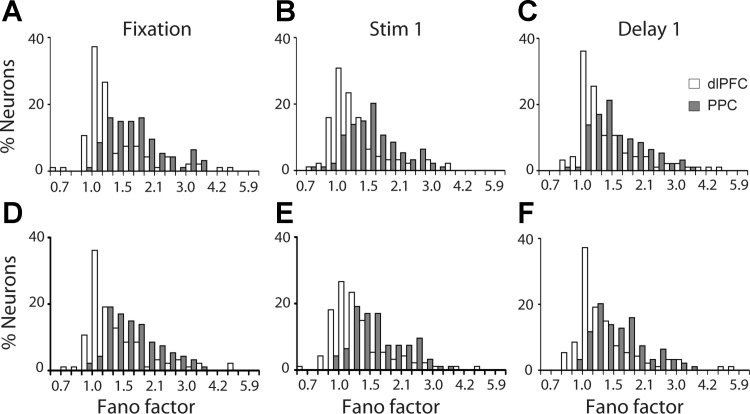
We first analyzed the Fano factor of spike counts in each epoch of the diametric distractor set, separately for each neuron. The Fano factor was determined separately for each epoch (1, 0.5, and 1.5 s for the fixation, stimulus, and delay periods, respectively). We obtained a wide range of Fano factor values across prefrontal neurons, though ∼70% of the neurons fell in the range of 1.0–1.5. For PPC, the same approximate percentage was represented in the range of 1.5–2.1 (Fig. 4).
Fig. 4.
A: distribution of Fano factor values during the fixation period for neurons with significant delay period activity in the remember-first task of the diametric distractor set (dlPFC, n = 107; PPC, n = 95). B and C: distribution of Fano factor values during the stimulus (B) and delay periods (C) for the same group of neurons. D and E: distribution of Fano factors of the same group of neurons during the fixation, stimulus, and delay periods in the remember-second task.
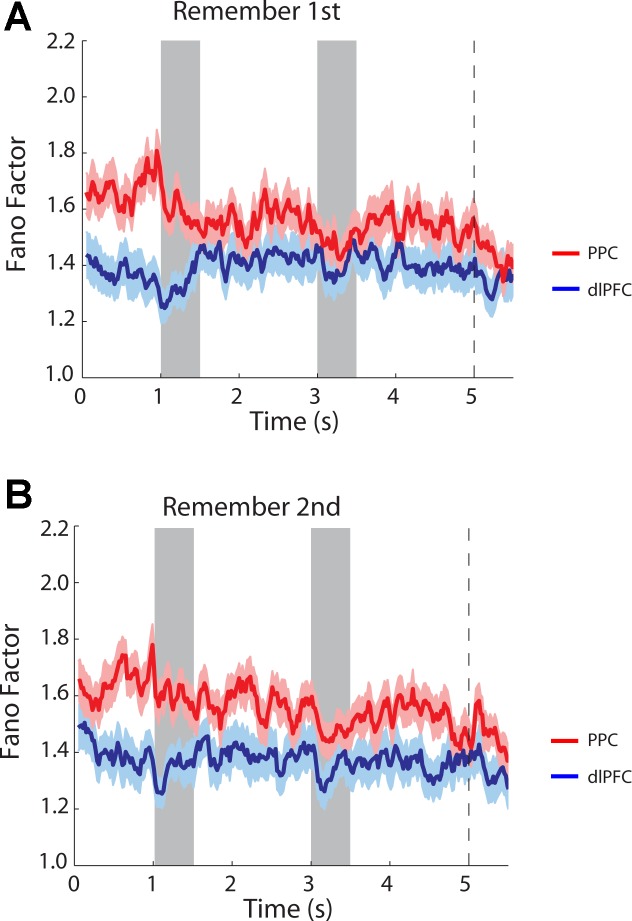
A two-way ANOVA comparing Fano factors across task epochs and areas revealed that mean values of Fano factor were significantly lower in dlPFC than in PPC (2-way ANOVA, P < 0.0001 for main effect of area in both remember-first and remember-second tasks). In contrast, we observed small differences across epochs (2-way ANOVA, P > 0.5 for main effect of epoch in both remember-first and remember-second tasks). A time-resolved calculation of the Fano factor further illustrated that higher values of Fano factor in the PPC were already present in the fixation period, before the appearance of the stimulus. The variability gap narrowed during the course of the trial, particularly at the onset of each stimulus appearance, which is known to quench variability across a variety of brain areas and tasks (Churchland et al. 2010), but persisted throughout the trial, for both the remember-first (Fig. 5A) and remember-second tasks (Fig. 5B). No significant interaction was present in the two-way ANOVA of task and area (P > 0.3 for remember-first task; P > 0.7 for remember-second task).
Fig. 5.
Time course of Fano factor in successive 100-ms bins for dlPFC and PPC neurons tested with the diametric-distractor set (dlPFC, n = 107; PPC, n = 95). A: remember-first task. B: remember-second task. Shaded area around each curve represents the SE. Shaded bars in the plot represent the time of stimulus presentation; vertical dotted line indicates the time of fixation point turning off, which represents the cue for the eye movement.
This analysis included only correct trials. We did not have sufficient trials to analyze variability in error trials alone, and the incidence of errors was not uniform across task conditions and stimulus location. However, the difference between parietal and prefrontal variability was unaffected when we repeated the analysis by including error trials (2-way ANOVA, P < 0.00001 for main effect of area in both remember-first and remember-second tasks).
To confirm that the difference in Fano factor was consistent across tasks, we repeated the same analysis in the randomized distractor set and the ODR task. We should note that neurons analyzed in these tasks constitute an entirely separate sample of neurons, recorded several months apart from the neurons obtained in the diametric distractor set. In this case too, Fano factors were again significantly lower in dlPFC than in PPC (2-way ANOVA, P < 0.001 for both remember-first and remember-second tasks). As was the case with the diametric distractor data set, there was no significant difference between different task epochs (2-way ANOVA, P > 0.5 for both remember-first and remember-second tasks). The result confirms that across two independent samples of neurons, neuronal variability was lower in the dlPFC than in the PPC.
The results were also consistent when we expanded our sample to include all neurons recorded in the task or limited our sample to only spatially selective neurons, separately for each monkey. When we repeated our analysis to include not only the neurons with significant increase of firing rate in the delay period, as we have done so far, but all neurons with any type of response in the task (n = 180 for dlPFC, n = 209 for PPC), we again saw higher levels of Fano factors in PPC. The difference was also significant (2-way ANOVA, main effect of area, P < 0.001). Additionally, when we examined separately Fano factors in dlPFC and PPC of each monkey, nearly identical differences between areas were present in the two subjects (P < 0.001 for the difference between areas in both monkeys). Finally, we limited our analysis to neurons with spatially selective responses during the delay period (n = 87 for dlPFC, n = 55 for PPC). In this case too, a significant difference was present (2-way ANOVA, main effect of area, P < 0.0001).
As discussed earlier, Fano factor values are generally dependent on firing rate, and it was important to ascertain that the differences between areas that we reported were not an artifact of systematic differences in firing rate between areas. However, higher Fano factors are expected for higher firing rates (Goris et al. 2014), and dlPFC neurons consistently exhibited higher firing rates than PPC neurons (Fig. 2). This suggested that the lower values of dlPFC variability we found may have been somewhat inflated by the higher firing rate of this sample, and therefore the difference between areas we report may be underestimated. When we computed Fano factors for samples of neurons from the two brain areas matched for firing rate, the results confirmed a significantly higher Fano factor in PPC than in dlPFC; two-way ANOVA indicated a significantly higher Fano factor in PPC than in dlPFC (P < 0.001) and no significant difference between task epochs (P > 0.4). We additionally compared the values of a quadratic measure of variance (Goris et al. 2014) between the two areas. This too revealed a significantly higher value for the PPC than for the dlPFC (2-way ANOVA, P < 10−10 for both remember-first and remember-second tasks of the diametric stimulus set).
Coefficient of variation of interspike interval.
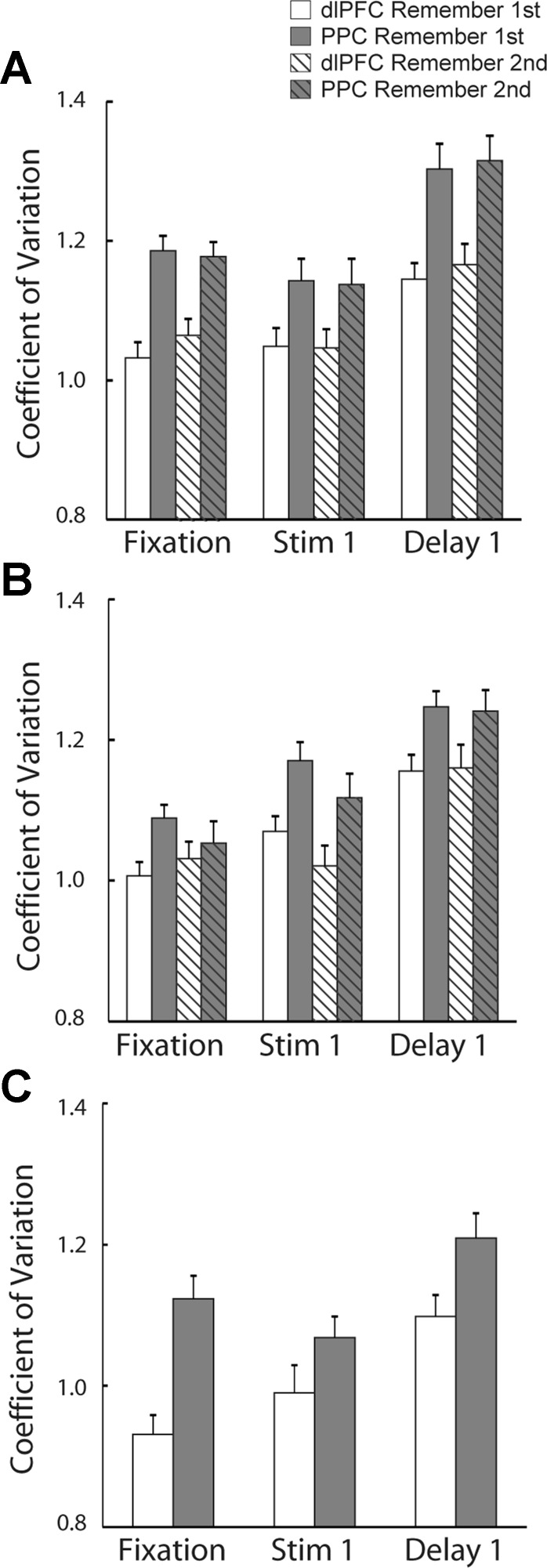
We proceeded to test if the systematic difference between areas was robust across other measures of neuronal discharge variability, beyond the Fano factor of spike counts. We therefore calculated another measure of variability, the CV of the ISI (standard deviation divided by the mean). It has previously been shown that CV too is modulated at different task epochs of cognitive tasks, and higher values of CV have been reported in the delay period relative to the fixation period, independent of changes in firing rate, and following the presentation of stimuli both in the receptive field and out of the receptive field (Compte et al. 2003; Joelving et al. 2007). Modulation of the CV can be indicative of recurrent dynamics of working memory (Compte et al. 2003).
We again focused on neurons that exhibited significant delay period activity (n = 107 neurons in PFC and n = 95 neurons in PPC, in the diametric-distractor set). We calculated CV values for each neuron, separately for each task epoch, and compared mean values (Fig. 6). The analysis replicated the findings of previous studies in the dlPFC: a significant increase in the CV was evident in the delay period compared with the fixation period (t-test, P < 10−5). Furthermore, this increase was present in the delay period following both the preferred and nonpreferred locations. We obtained nearly identical results when we repeated this analysis in the PPC. Significant elevation of the CV was observed for both the preferred and nonpreferred locations in the delay period compared with the fixation period (t-test, P < 10−5).
Fig. 6.
A: average coefficient of variation (CV) of the interspike interval (ISI) during the fixation, stimulus presentation, and delay periods for neurons with significant delay period activity in the diametric distractor task (dlPFC, n = 107; PPC, n = 95). B: average CV in the random distractor task (dlPFC, n = 75; PPC, n = 85). C: average CV in the ODR task (dlPFC, n = 44; PPC, n = 66). Error bars represent SE.
We then performed CV comparisons between areas in the same fashion as for the Fano factor (Fig. 6A). We used two-way ANOVA to compare CV in different task epochs between brain areas and found that mean values of CV in dlPFC were lower than in PPC for both the remember-first and remember-second tasks (2-way ANOVA, P < 0.0001). Unlike the Fano factor, CV values across different task epochs varied significantly (2-way ANOVA, P < 0.0001 for both remember-first and remember-second tasks).
Similar results were obtained when we repeated the analysis in the sample of neurons recorded while the monkeys were tested with the random distractor task and ODR task (Fig. 6, B and C). Mean values of CV during fixation, cue presentation, and delay period were significantly lower in dlPFC than in PPC (2-way ANOVA, P < 0.001 for both remember-first and remember-second tasks). A significant difference was also present between different task epochs (2-way ANOVA, P < 0.0001).
Relationship between variability and task performance.
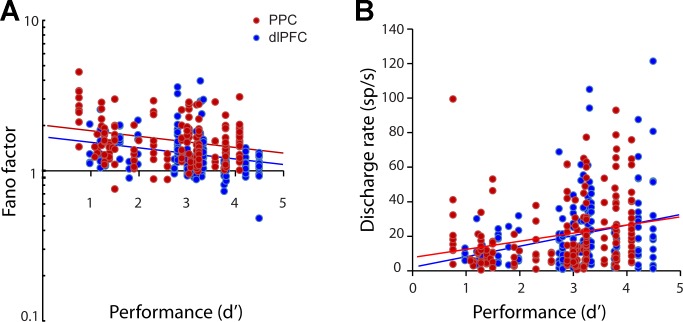
Lower levels of variability in sensory cortex have been associated with higher levels of performance (Cohen and Maunsell 2009). To unveil the relationship between variability and performance in the context of our working memory tasks, we performed an analysis on a session-by-session basis, determining the (log transformed) Fano factor of neurons recorded at each session as a function of the session's level of behavioral performance (expressed as d′). A linear regression revealed a significant relationship between the two variables for both the dlPFC and PPC in the remember-first (Fig. 7A) and remember-second tasks (P < 0.05 for dlPFC in remember-first; P < 0.0005 for PPC in remember-first; P < 0.05 for dlPFC in remember-second task; P < 0.05 for PPC in remember-second task). In other words, sessions in which lower variability of neuronal responses were present resulted in higher levels of performance. No difference in the slope of regression line was evident between dlPFC and PPC (F-test, P > 0.3), whereas dlPFC levels of variability were consistently lower than PPC levels. The effect was not the result of familiarity with the task, either. The monkeys were well familiar with the task before recordings commenced, and performance was stable across sessions; the difference in performance of the second half of sessions relative to the first was −3% for monkey GR and +1% for monkey HE. A regression analysis revealed no significant effect of sequential session number on performance (P > 0.1 for both monkeys). We did not have sufficient data to perform all possible comparisons separately for each monkey; however, a significant relationship between performance and Fano factor was present for monkey GR in the PPC (regression analysis, P < 0.05 for remember-first task and P < 0.05 for remember-second task) and for monkey HE in the dlPFC (P < 0.05 for remember-first task and P < 0.05 for remember-second task). When we included sequential session number and individual monkey as independent variables (in addition to performance) in the linear regression model (Fig. 7A), the performance coefficient remained significantly higher than zero for the remember-first task in the PPC (P < 0.005), the remember-second task in the PPC (P < 0.005), and the remember-second task in the dlPFC (P < 0.05); it approached the margin of statistical significance for the remember-first task in the dlPFC (P = 0.07).
Fig. 7.
A: Fano factor in the stimulus presentation period is plotted as a function of behavioral performance (expressed as d′) in dlPFC (blue dots) and PPC (red dots). Each point represents the Fano factor of a single neuron, plotted against the performance during the session in which the neuron was recorded. Regression lines are shown (dlPFC, blue lines; PPC, red lines). B: discharge rate as a function of behavioral performance, plotted in the same fashion as in A.
This relationship between spike count variability and behavioral performance could be accounted for, in principle, by a negative correlation between firing rate and performance. To test for this possibility, we also analyzed performance as a function of mean firing rate during the delay period for the same neurons (Fig. 7B). A linear regression revealed a significant relationship between the two variables, which, however, moved in the opposite direction: lower levels of performance were observed for sessions with lower levels of activity, in agreement with previous studies (Qi et al. 2011). The results indicated that higher variability is associated with lower performance, independent of firing rate.
DISCUSSION
Our study compared the variability of neuronal discharges in the PPC and dlPFC, two cortical areas implicated in the maintenance of working memory and planning of future actions based on task rule (Constantinidis and Procyk 2004). Analysis of firing rates in our working memory tasks revealed only subtle differences between areas (Fig. 2). Higher incidence of neurons with significant modulation for either the remember-first or remember-second task in the dlPFC compared with the PPC was also previously reported (Qi et al. 2015), a result that is obscured in the grand average of Fig. 2. Nonetheless, differences in neuronal variability that we report presently were much more pronounced between areas and could not have been predicted on the basis of firing rate modulation by tasks or by the absolute level of firing rate of our dlPFC and PPC samples. We found differences in variability to be consistent across the remember-first and remember-second tasks. We also replicated the main finding in two independent samples of neurons, tested with the diametric and randomized distractor sets.
Our findings are in agreement with preliminary reports suggesting high variability in area LIP during a memory-guided saccade task (Johnston et al. 2009). A previous comparison of Fano factors in LIP and dlPFC, tested in a different task in our laboratory, failed to reveal a difference between areas; however, a smaller neuron sample was available for that analysis, and a trend toward higher Fano factors in area LIP was present in that data set, too (Katsuki et al. 2014). The variability of area LIP, in turn, appears to be greater than that of upstream visual cortical areas, such as MT and MST (Maimon and Assad 2009). Previous studies also suggest that prefrontal cortex neurons display low spiking regularity compared with neurons in various other areas (Shinomoto et al. 2009), roughly equivalent to the lower values of CV we report presently for the prefrontal cortex. Our current results identify variability as a factor that differentiates functionally prefrontal and posterior parietal areas.
Influence of cognitive functions on neuronal variability.
Firing rate of cortical neurons is highly variable even when identical stimuli are presented under well-controlled experimental conditions, which has traditionally been thought of as the result of noisy computations by neural circuits (Dean 1981; McAdams and Maunsell 1999; Softky and Koch 1993; Tolhurst et al. 1983; Vogels et al. 1989). Some inherent variability in sensory systems can be beneficial, for example, in enhancing the sensitivity to near-threshold signals, or can be used in a probabilistic fashion combined with prior knowledge to optimize decisions (Knill and Pouget 2004; Loeb and Fishel 2014). Recently, it was recognized that variability of neuronal responses is modulated systematically by a number of factors, including the presentation of sensory stimuli (Churchland et al. 2010) and preparation of motor movements (Churchland et al. 2006), both of which decrease variability, across multiple brain areas. Furthermore, variability is modulated by attention shifted toward or away from a stimulus in the receptive field (Cohen and Maunsell 2009; Mitchell et al. 2007) and by decision making, requiring a judgment between alternative options (Churchland et al. 2011; Hussar and Pasternak 2010; Qi and Constantinidis 2012). Neuronal variability, therefore, can be both an indicator of neuronal coding and a determinant of behavioral output.
Discharge variability in cortical areas may be viewed as simply reflecting internal noise. An alternative idea views neuronal responses resulting from doubly stochastic processes such that measured variance includes the variability of sensory inputs as well as the variability of modulatory inputs that affect the gain of their propagation along the cortical hierarchy (Goris et al. 2014). Reduced variability could be a signature of attention, which modulates the gain of responses, consistent with the quenching of variability produced by the onset of sensory stimuli (Churchland et al. 2010). Higher variability of neuronal responses has also been generally observed in conditions with more difficult judgments (Churchland et al. 2011; Cohen and Maunsell 2009) and has been shown to decrease after training in a cognitive task (Qi and Constantinidis 2012). Lower levels of variability, therefore, could be viewed as a signature of more stereotypical state of attention. Our present results also show that lower variability was associated with higher behavioral performance in the working memory task.
Few comparisons of neuronal variability are available between brain areas (Maimon and Assad 2009; Shinomoto et al. 2009), and the interpretation of differences between areas outside the sensory cortex is not straightforward. Our results could be interpreted, in principle, as suggestive of an additional source of firing rate modulation in the PPC that is not present in the dlPFC. Factors such as attention or reward expectation may vary from trial to trial, and the consequent modulation of firing rate they cause may translate as the variability we measure here. However, firing rate changes across experimental conditions were remarkably similar in the two areas in this data set (Qi et al. 2015), and factors such as attention, working memory, and reward expectation have been shown to modulate firing rate in both areas, with no weaker effects on prefrontal than parietal cortex (Katsuki and Constantinidis 2012). Theoretical models generally predict higher levels of variability tied to cortical state at subsequent stages of cortical processing (Goris et al. 2014; Scholvinck et al. 2015; Shadlen et al. 1996). Our results may be best interpreted along these lines. Internal state variables, such as attention, certainty, and motivation, which are more stereotypical at sessions of higher performance, appear to influence prefrontal firing to a greater extent.
Prefrontal and parietal specialization.
In principle, functional differences between the dlPFC and PPC areas may be distinguished into three nonmutually exclusive categories (Katsuki and Constantinidis 2012). First, dlPFC may be viewed as an output area that translates the outcome of cognitive operations in the parietal lobe into action plans. Second, the two brain areas may be uniquely specialized so that there is a division of labor in terms of cognitive operations between them. Third, the distinguishing feature of dlPFC may be the capacity for plasticity, which is essential for flexible behavior depending on context. Comparison of dlPFC and PPC discharges in tasks that require remembering the location of the first of two stimuli presented in sequence has consistently revealed that prefrontal activity can better represent the initial remembered stimulus and resist the effect of distractors, which argues in favor of at least some specialization (Qi et al. 2010, 2015; Suzuki and Gottlieb 2013). However, responses in the remember-second task, where the most recent stimulus is the one to be remembered, do not differentiate well between prefrontal and parietal activity (Qi et al. 2015). It should also be noted that a considerable population of prefrontal neurons has been shown to respond to distractors preferentially in the context of various tasks (Jacob and Nieder 2014; Qi et al. 2015). Therefore, although specialization exists, it is difficult to generalize regarding the role of the two areas with respect to working memory.
Anatomic and physiological specializations between these two areas have been identified in terms of not only the preferential dopaminergic innervation of the frontal lobe but also intrinsic properties of the prefrontal and parietal circuits (Katsuki and Constantinidis 2012). Experimental evidence suggests that dopamine serves to enhance excitatory persistent activity after the appearance of the preferred stimulus of a neuron and to suppress background excitation of nonpreferred stimuli (Gao et al. 2001; Paspalas and Goldman-Rakic 2005; Seamans et al. 2001; Vijayraghavan et al. 2007; Williams and Goldman-Rakic 1995). Computational models therefore suggest an improved signal-to-noise ratio and enhanced stability in networks that incorporate dopamine inputs (Durstewitz et al. 2000; Lew and Tseng 2014). Specialized interneuron types that are unique or more abundant in the prefrontal cortex could serve a similar role in stabilizing prefrontal activity compared with other areas (Wang et al. 2004; Zhou et al. 2012). The lower levels of variability we observed in the prefrontal cortex may be directly related to these properties of the prefrontal circuit. Our results offer direct experimental evidence for more robust neuronal activity in the prefrontal cortex and offer an explanation of how prefrontal activity could both be modulated to a greater extent by cognitive factors and exert greater influence on behavior by virtue of its reliability.
GRANTS
This research was supported by National Eye Institute Grant R01 EY-16773 and by the Tab Williams Family Endowment and Harry O'Parker Neurosciences Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.-L.Q. and C.C. conception and design of research; X.-L.Q. performed experiments; X.-L.Q. and C.C. analyzed data; X.-L.Q. and C.C. interpreted results of experiments; X.-L.Q. and C.C. prepared figures; C.C. drafted manuscript; X.-L.Q. edited and revised manuscript; X.-L.Q. and C.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the contributions of Bryce Lambert and Anthony Elworthy in the collection of the data set analyzed in this work, Kathini Palaninathan for technical help, and Samson King for helpful comments on the manuscript.
REFERENCES
- Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci 7: 358–366, 2006. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33: 1–21, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, Mansouri FA, Hoda H, Mahboubi M, Browning PG, Kwok SC, Phillips A, Tanaka K. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science 325: 52–58, 2009. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol 287: 422–445, 1989. [DOI] [PubMed] [Google Scholar]
- Churchland AK, Kiani R, Chaudhuri R, Wang XJ, Pouget A, Shadlen MN. Variance as a signature of neural computations during decision making. Neuron 69: 818–831, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Afshar A, Shenoy KV. A central source of movement variability. Neuron 52: 1085–1096, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Cunningham JP, Sugrue LP, Cohen MR, Corrado GS, Newsome WT, Clark AM, Hosseini P, Scott BB, Bradley DC, Smith MA, Kohn A, Movshon JA, Armstrong KM, Moore T, Chang SW, Snyder LH, Lisberger SG, Priebe NJ, Finn IM, Ferster D, Ryu SI, Santhanam G, Sahani M, Shenoy KV. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat Neurosci 13: 369–378, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Kohn A. Measuring and interpreting neuronal correlations. Nat Neurosci 14: 811–819, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 12: 1594–1600, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Constantinidis C, Tegner J, Raghavachari S, Chafee MV, Goldman-Rakic PS, Wang XJ. Temporally irregular mnemonic persistent activity in prefrontal neurons of monkeys during a delayed response task. J Neurophysiol 28: 3441–3454, 2003. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J Neurophysiol 88: 3487–3497, 2002. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Procyk E. The primate working memory networks. Cogn Affect Behav Neurosci 4: 444–465, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Neuronal activity in posterior parietal area 7a during the delay periods of a spatial memory task. J Neurophysiol 76: 1352–1355, 1996. [DOI] [PubMed] [Google Scholar]
- Dean AF. The variability of discharge of simple cells in the cat striate cortex. Exp Brain Res 44: 437–440, 1981. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Wise SP. Effects of attention on visuomotor activity in the premotor and prefrontal cortex of a primate. Somatosens Mot Res 10: 245–262, 1993. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol 83: 1733–1750, 2000. [DOI] [PubMed] [Google Scholar]
- Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci 9: 292–303, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JK, Swaminathan SK, Freedman DJ. Visual categorization and the parietal cortex. Front Integr Neurosci 6: 18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349, 1989. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA 98: 295–300, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. In: Handbook of Physiology, edited by Plum F, Mountcastle VB. Bethesda, MD: American Physiological Society, 1987, p. 373–417. [Google Scholar]
- Goris RL, Movshon JA, Simoncelli EP. Partitioning neuronal variability. Nat Neurosci 17: 858–865, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84: 401–414, 2000. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Shima K, Tanji J. Task-dependent selectivity of movement-related neuronal activity in the primate prefrontal cortex. J Neurophysiol 80: 3392–3397, 1998. [DOI] [PubMed] [Google Scholar]
- Hussar C, Pasternak T. Trial-to-trial variability of the prefrontal neurons reveals the nature of their engagement in a motion discrimination task. Proc Natl Acad Sci USA 107: 21842–21847, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob SN, Nieder A. Complementary roles for primate frontal and parietal cortex in guarding working memory from distractor stimuli. Neuron 83: 226–237, 2014. [DOI] [PubMed] [Google Scholar]
- Joelving FC, Compte A, Constantinidis C. Temporal properties of posterior parietal neuron discharges during working memory and passive viewing. J Neurophysiol 97: 2254–2266, 2007. [DOI] [PubMed] [Google Scholar]
- Johnston KD, Brunamonti E, Thomas NW, Pare M. Posterior parietal cortex persistent activity and visuospatial working memory. Program No. 356.16 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2009. Online. [Google Scholar]
- Katsuki F, Constantinidis C. Unique and shared roles of the posterior parietal and dorsolateral prefrontal cortex in cognitive functions. Front Integtr Neurosci 6: 17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki F, Saito M, Constantinidis C. Influence of monkey dorsolateral prefrontal and posterior parietal activity on behavioral choice during attention tasks. Eur J Neurosci 40: 2910–2921, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knill DC, Pouget A. The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci 27: 712–719, 2004. [DOI] [PubMed] [Google Scholar]
- Lew SE, Tseng KY. Dopamine modulation of GABAergic function enables network stability and input selectivity for sustaining working memory in a computational model of the prefrontal cortex. Neuropsychopharmacology 39: 3067–3076, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb GE, Fishel JA. Bayesian action&perception: representing the world in the brain. Front Neurosci 8: 341, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimon G, Assad JA. Beyond Poisson: increased spike-time regularity across primate parietal cortex. Neuron 62: 426–440, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on the reliability of individual neurons in monkey visual cortex. Neuron 23: 765–773, 1999. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202, 2001. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55: 131–141, 2007. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Presynaptic D1 dopamine receptors in primate prefrontal cortex: target-specific expression in the glutamatergic synapse. J Neurosci 25: 1260–1267, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Constantinidis C. Variability of prefrontal neuronal discharges before and after training in a working memory task. PLoS One 7: e41053, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Elworthy AC, Lambert BC, Constantinidis C. Representation of remembered stimuli and task information in the monkey dorsolateral prefrontal and posterior parietal cortex. J Neurophysiol 113: 44–57, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Katsuki F, Meyer T, Rawley JB, Zhou X, Douglas KL, Constantinidis C. Comparison of neural activity related to working memory in primate dorsolateral prefrontal and posterior parietal cortex. Front Syst Neurosci 4: 12, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Meyer T, Stanford TR, Constantinidis C. Changes in prefrontal neuronal activity after learning to perform a spatial working memory task. Cereb Cortex 21: 2722–2732, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawley JB, Constantinidis C. Effects of task and coordinate frame of attention in area 7a of the primate posterior parietal cortex. J Vis 10: 1–16, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawley JB, Constantinidis C. Neural correlates of learning and working memory in the primate posterior parietal cortex. Neurobiol Learn Mem 91: 129–138, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AF, Bichot NP, Desimone R, Ungerleider LG. Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. J Neurosci 27: 11306–11314, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Saleem AB, Benucci A, Harris KD, Carandini M. Cortical state determines global variability and correlations in visual cortex. J Neurosci 35: 170–178, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci USA 98: 301–306, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Britten KH, Newsome WT, Movshon JA. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J Neurosci 16: 1486–1510, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci 18: 3870–3896, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomoto S, Kim H, Shimokawa T, Matsuno N, Funahashi S, Shima K, Fujita I, Tamura H, Doi T, Kawano K, Inaba N, Fukushima K, Kurkin S, Kurata K, Taira M, Tsutsui K, Komatsu H, Ogawa T, Koida K, Tanji J, Toyama K. Relating neuronal firing patterns to functional differentiation of cerebral cortex. PLoS Comput Biol 5: e1000433, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Softky WR, Koch C. The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J Neurosci 13: 334–350, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoet G, Snyder LH. Single neurons in posterior parietal cortex of monkeys encode cognitive set. Neuron 42: 1003–1012, 2004. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Gottlieb J. Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat Neurosci 16: 98–104, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst DJ, Movshon JA, Dean AF. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res 23: 775–785, 1983. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 10: 376–384, 2007. [DOI] [PubMed] [Google Scholar]
- Vogels R, Spileers W, Orban GA. The response variability of striate cortical neurons in the behaving monkey. Exp Brain Res 77: 432–436, 1989. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature 411: 953–956, 2001. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol 90: 1790–1806, 2003. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci USA 101: 1368–1373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IM, Wise SP. Rule-dependent neuronal activity in the prefrontal cortex. Exp Brain Res 126: 315–335, 1999. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376: 572–575, 1995. [DOI] [PubMed] [Google Scholar]
- Zhou X, Katsuki F, Qi XL, Constantinidis C. Neurons with inverted tuning during the delay periods of working memory tasks in the dorsal prefrontal and posterior parietal cortex. J Neurophysiol 108: 31–38, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]