Synopsis
The spectrum of primary immunodeficiency disorders (PID) is expanding. It includes typical disorders that primarily present with defective immunity as well as disorders that predominantly involve other systems and exhibit few features of impaired immunity. The rapidly growing list of new immunodeficiency disorders and treatment modalities makes it imperative for the provider to stay abreast of the latest and best management strategies. We present a brief overview of recent clinical advances in the field of PIDs.
Keywords: immunodeficiency, antibody deficiency, autoimmunity, immune defect, innate immune defect, lymphoproliferation, immune dysregulation
Introduction
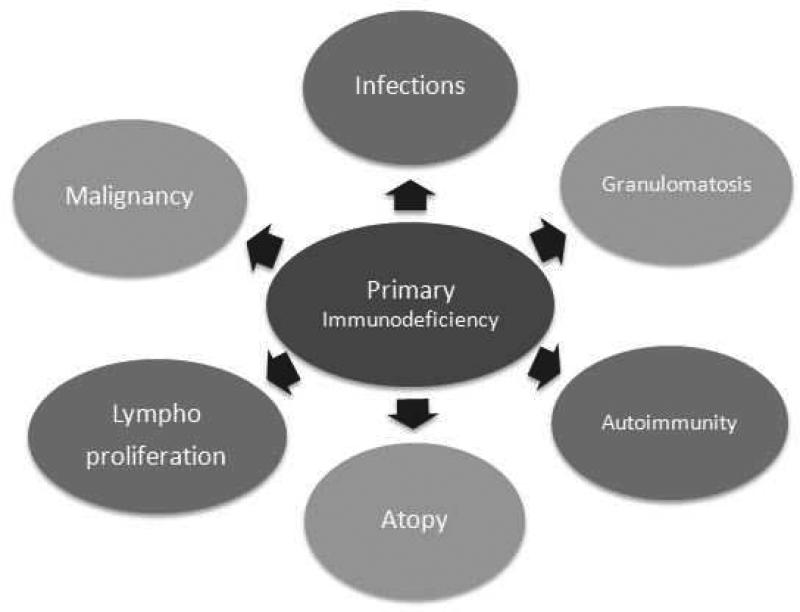
Traditionally, primary immunodeficiency disorders (PIDs) have been described as diseases caused by one or more defects of the immune system, leading to increased susceptibility to infections. It is now known that PIDs are a group of heterogeneous disorders with immune system abnormalities characterized by various combinations of recurrent infections, autoimmunity, lymphoproliferation, granulomatous process, atopy, and malignancy (See Figure 1). The overall clinical picture is dictated by the specific type of underlying immune defect. Based on the type of PID, the types of infections can vary. While bacterial infections may be a key feature of B cell defects, infections with diverse pathogens (e.g., viruses, fungi, and bacteria) are a feature of combined T and B cell immunodeficiencies. Similarly, autoimmune manifestations can range from autoimmune cytopenias secondary to B cell defects to systemic lupus erythematosus in complement disorders. Some PIDs (e.g., X-Linked lymphoproliferative disease) are characterized by lymphoproliferation while others (such as those associated with chronic granulomatous disease) manifest with cutaneous, respiratory, or gastrointestinal tract granulomas due to immune dysregulation. While lymphomas and leukemias are the most common malignancies noted, other types of malignancies may also be seen. Atopic features such as asthma, atopic dermatitis, and food allergies can be observed in some patients with T cell defects. Hence, the types of manifestations and involvement of other systems can provide a clue to the type of PID.
Figure 1.
Features of Primary Immunodeficiencies
History
A few immune disorders like ataxia teleangiectasia (1926) and Wiskott Aldrich syndrome (1937) were discovered in early half of 20th century. But the landmark in the history of PIDs was marked by the discovery of agammaglobulinemia by Colonel Ogden Bruton in 1952. In 1950, Eduard Glanzmann and Paul Riniker found that Candida albicans infections are associated with an absence of lymphocytes. 1 Two Swiss groups from Bern and Zurich (Hassig Cottier, R.Tobler and Walter Hitzig) discovered similar patients in 1958 and recognized it to be an immunodeficiency. This condition that was initially coined as Swiss type agammaglobulinemia was renamed as severe combined immunodeficiency (SCID) by world health organization (WHO) in 1970. 1 In 1954, Robert Good discovered a fatal granulomatous disease that is now known as chronic granulomatous disease (CGD). 1 Over the last 65 years, the field of PIDs has advanced exceedingly. With the advent of cutting-edge genetic technology, more than 240 PIDs have been discovered and the number continues to increase.2
Epidemiology
The prevalence of PID varies depending on the type of immunodeficiency. While Selective IgA deficiency is common (1 in 223 to 1 in 1000),3 other immunodeficiencies such as severe combined immunodeficiency are fortunately rare (1 in 58,000).4 As many immunodeficiencies are continually being discovered, the exact prevalence is unknown, though it is estimated to be low.
Classification
Immune deficiencies can be described as primary or secondary. While primary immune deficiencies are due to inherent dysfunction of the immune system and are chiefly genetic in etiology, secondary immune deficiencies are consequent to other underlying causes (See Box 1).
Box 1: Causes of Secondary Immune deficiencies.
- Age:
- Prematurity
- Infancy
- Old age
- Medications
- Immunosuppressants
- Corticosteroids
- Procedures
- Splenectomy
- Anesthesia use
- Post-stem cell transplant
- Infections
- HIV infection, AIDS
- Cytomegalovirus
- Epstein Barr virus
- Temporary during other infections
- Metabolic
- Diabetes Mellitus
- Uremia
- Nutrition related
- Malnutrition
- Zinc deficiency
- Vitamin/ other mineral deficiencies
- Protein losing conditions
- Nephrotic syndrome
- Protein losing enteropathy
- Alcoholic cirrhosis
- Hereditary Conditions
- Chromosomal abnormalities
- Sickle cell disease
- Miscellaneous
- Systemic lupus erythematosus
- Burns
- Malignancies
- Radiation therapy
From Stiehm RE, Ochs, H. D, Winkelstein, J. A. Immunodeficiency
Disorders: General Considerations. 5 ed: Saunders; 2004; with permission.
Various classifications for PIDs have been suggested. The 2014 International Union of Immunodeficiency Society (IUIS) stratifies PIDs into nine categories based on the type of immune defect 2 (See Box 2). Notably, some of the PIDs fit the criteria for more than one category in this schema. International Consensus (ICON) classification of PIDs recognizes the disorders of immune dysregulation and auto inflammation to be separate entities since their immune manifestations may be secondary to the dysregulation or autoimmunity.5
Box 2: Categories of PIDs as per IUIS 2014 classification.
Predominantly Antibody deficiencies
T cell immunodeficiencies or combined immunodeficiencies
Syndromic immunodeficiencies
Complement defects
Phagocytic defects
Defects of Innate Immunity
Diseases of immune dysregulation
Autoinflammatory disorders
Phenocopies of PIDs
Data from Al-Herz W, Bousfiha A, Casanova JL, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Frontiers in immunology. 2014;5:162.
Predominantly antibody deficiencies
Antibody deficiencies are typically characterized by a predisposition to infectious diseases. Infections are predominantly bacterial in origin but can be viral or protozoal as well. These deficiencies range from complete absence of antibody to varying degrees of functional antibody abnormalities (Table 1).
Table 1.
| Type of PID | Clinical features | Infections | Genetic Mutations (Other names) (OMIM numbers) |
|---|---|---|---|
| Antibody Deficiencies | |||
| Severely reduced serum immunoglobulins (Igs) with profoundly decreased or absent B cells (agammaglobulinemia)2,27,28 | • Defect in early development of B cells in the bone marrow • Complete or near-total absence of B cells in peripheral blood. • Lack of peripheral lymphoid tissue like lymph nodes and tonsils. • Almost all of them (90%) present by preschool age, with over half presenting in infancy following the depletion of maternal immunoglobulins, and a few in adulthood |
• Recurrent otitis media, pneumonia, and sinusitis • Chronic enteroviral meningoencepha litis and neutropenia have been reported in XLA |
• BTK −85% (Bruton's or XLA) (300300) • Autosomal recessive Agam: deficiency of μ heavy chain (147020), λ5 (146770), CD79α(Igα) (112205) and CD79β (Igβ (147245), BLNK (604615), PIK3R1 (171833), and TCF3 (147141) • Other causes: myelodysplasia with hypogammaglobuline mia (monosomy 7, trisomy 8, or dyskeratosis congenita), and thymoma with immunodeficiency with unknown genetic defect |
| Severely reduced serum Ig isotypes (at least two) with normal or low numbers of B cells: Commonly classified as common variable immunodeficiency (CVID)2,29 |
• Present after two years of age with IgG 2SD below normal for age, low IgA and/ or IgM AND defective specific or functional antibodies • Other causes of hypogammaglobuline mia should be ruled out • Heterogeneous manifestations like autoimmunity, granulomatous disease, gastrointestinal disease, lymphoid hyperplasia, lymphoma, and other malignancies |
• Recurrent infections: commonly sinopulmonary • Protozoal infections like Giardia |
• ICOS (604558) , • CD19 (107265) , • CD81 (186845), • CD20 (112210) , • CD21 (614699), • TACI (604907) , • LRBA (606453) , BAFF-R (606269) , TWEAK (602695), NFKB2 (615577), and CXCR4 (WHIM syndrome) (193670) • Most patients do not have a causative gene identified • Once the causative gene is identified, it is appropriate to use the genetic name rather than CVID |
| Severe reduction in IgG and IgA, with normal or elevated IgM, and normal number of B cells (commonly termed as Hyper IgM Syndrome) | • AID/ UNG: 30 Patients present with lymphoid hyperplasia, and increased risk for autoimmunity | Bacterial infections | AID (605257) and UNG (191525) defects mainly affect class switch recombination and somatic hypermutation |
| • CD40/CD40L30,31 lack secondary germinal centers in lymph nodes. They also have increased susceptibility to sclerosing cholangitis and hepatocellular carcinomas | Opportunistic infections (Cryptosporidium parvum, Histoplasma, Bartonella, Candida, and Cryptococcus sp) | CD40 (109535), CD40L (300386) affect T and B lymphocyte interaction and resulting signaling | |
| Other causes of high IgM: NEMO defect (300248) PI3K p110delta deficiency, Other diseases: PMS2, ataxia teleangiectasia (AT), and Nijmegen Breakage Syndrome (NBS). | |||
| Isotype or light chain deficiencies with generally normal number of B cells | • Selective IgA deficiency (SIgAD) can occur in isolation, with IgG subclass, or specific antibody, deficiency • SIgAD can be associated with autoimmunity, atopic diseases, and rarely lymphoid and gastrointestinal malignancies 3 • May evolve into CVID later in life |
Most patients are asymptomatic and diagnosed incidentally, such as while testing for celiac disease but SIgAD can present with recurrent sinopulmonary infections, | The cause is not known but associated TACI mutations have been found |
| • Isolated IgG subclass deficiency is mostly asymptomatic unless associated with other immune defect like specific antibody deficiency | |||
| • PI3K p110delta deficiency 32 can be associated with lymphomas | CMV, EBV | • PIK3CD, PI3K-δ Other PIDs in this category include Ig heavy chain, kappa constant deficiency, PIK3CD, PRKCD mutations | |
| Specific antibody deficiencies (SAD) with normal number of B cells | • Inability to produce sufficient antibodies to specific microbes, commonly those with protective polysaccharide capsules such as pneumococcus and hemophilus influenzae | Recurrent sinopulmonary infections or as per the specific antibody that is defective | |
| Transient hypogammaglobulinemia of infancy (THI) with normal number of B cells | • Decreased IgG (2SD lower than normal level for age), low IgA and/ or IgM once the transplacentally transferred maternal IgG wanes • Typically resolves during toddlerhood but can persist till 5 years of age 27, and rarely, evolve into lasting PID. |
While THI can be asymptomatic, the toddler can have recurrent sinopulmonary infections, or less commonly, severe invasive infections, triggering evaluation. | |
| Combined Immunodeficiencies | |||
| Low CD8 subset: | • Severe ZAP70 33 deficiency can present with features of SCID • Hypomorphic mutations cause low CD8 counts and elevated IgE |
Recurrent bacterial (sinopulmonary), fungal, and viral infections | ZAP70 (269840) |
| • CD8 deficiency 34 and those with MHC Class I deficiency can present during childhood or adulthood • Vasculitis and pyoderma gangrenosum noted in MHC Class I deficiency • Bronchiectasis, and skin granulomas are noted 35 |
Chronic sinusitis and respiratory infections | CD8 (186910), TAP1, TAP2, TAPBP (604571) | |
| Low CD4 T cell subset: | MHC Class II deficiency, 36 also known as bare lymphocyte syndrome, presents with protracted diarrhea, failure to thrive, and autoimmunity | Recurrent bacterial, fungal, viral, protozoal infections | CIITA, RFX5, RFXAP, RFXANK (209920) |
| • MAGT1 deficiency 37 can present with chronic EBV infection and related malignancies. • Also called X-linked EBV associated neoplasia (XMEN) |
Chronic EBV infection | MAGT1 (300715) | |
| LCK 38 and LRBA 39 deficiency can present with autoimmunity and inflammatory manifestations | Recurrent infections | LCK (153390) and LRBA (606453) | |
| UNC119 deficiency 40 can present with bronchiectasis | Recurrent infections (viral, fungal, and bacterial) | UNC119 (604011) | |
| Low CD27+ B cells | CD27 deficiency 41 is associated with hypogammaglobulinemia. | Persistent EBV viremia | CD27 (615122) |
| Progressive decrease in T cell numbers, with normal B cell numbers, and normal or decreased serum Igs | • ITK (IL2 inducible T cell kinase) deficiency42 have lymphadenopathy, hepatosplenomegaly, cytopenias, EBV associated malignancies, and other viral and fungal infections • Neurological impairment and autoimmune hemolytic anemia are noted in purine nucleoside phosphorylase (PNP) deficiency 2 |
ITK (613011), PNP (164050) | |
| Combined Immunodeficiencies with associated Syndromic Features | |||
| Congenital thrombocytopenia and eczema | • Seen in Wiskott Aldrich Syndrome/WAS 43 or WASP interacting protein (WIP) deficiency 44 • Can present with autoimmunity and malignancy |
||
| DNA repair defects 45 | • AT presents with progressive neurological impairment, later onset of teleangiectasia • AT patients can develop malignancies like leukemia and lymphoma • Immune defects noted are low IgG and IgA with high IgM • Alpha feto protein level is elevated |
Ataxia teleangiectasia (AT) (604391), Nijmegen breakage syndrome (NBS) (251260), and Bloom syndrome (210900) | |
| • NBS patients present with growth retardation, microcephaly, and have increased risk of malignancy like lymphoma • Alpha feto protein level is normal in NBS |
|||
| Thymic defects | • Chromosome 22qll.2 deletion syndrome25,26 (congenital heart defect, hypocalcemia, cleft lip/ palate, behavioral problems) • Immune system can range from completely normal, or partial combined deficiency causing recurrent sinopulmonary infections to profound T cell deficiency leading to SCID- like features |
Chromosome 22qll.2 deletion (188400) | |
| • CHARGE syndrome 25 presents with coloboma, heart defect, choanal atresia, growth and developmental retardation, genital anomalies, and ear anomalies • Varying degrees of immune defects ranging from normal immune system to complete absence of T cells |
CHD7(608892), SEMA3E (2l4800) | ||
| Osseous dysplasias 46 | • Cartilage hair hypoplasia: short-limbed short stature, joint laxity, metaphyseal chondrodysplasia, hair hypoplasia, neuronal dysplasia of intestine, and increased risk of malignancy • Both cellular and humoral immune defects are noted |
RMRP (250250) | |
| • Schimke syndrome presents with dysmorphic facies, thin hair, spondyloepiphyseal dysplasia, short spine, renal failure and hyper pigmented macules along with cellular immune defects | Opportunistic infections | SMARCAL1 (242900) | |
| Hyper IgE syndromes 47 | • STAT3 deficiency eczema, pneumtoceles, coarse facies, skeletal anomalies like scoliosis | Recurrent skin abscesses | STAT3(147060), Tyk2 (611521), DOCK8 (243700), PGM3 (172100) |
| • DOCK8 lacks the skeletal features but is more often associated with allergies • Impaired T cell (CD8) and NK cell function, low NK cell count and low CD27+ memory B cells |
Viral infections like human papilloma virus (HPV), Varicella zoster, molluscum contagiosum, herpes simplex virus (HSV) | Other disorders like WAS, Omenn's syndrome, and can have high IgE levels | |
| • Tyk2 deficiency can present with severe eczema | Staphylococcus infections, intracellular infections like Mycobacteria, Salmonella, fungi, and viruses | ||
| • PGM3 deficiency can present with impaired neurocognition, autoimmunity, and severe eczema | |||
| Phagocytic defects | |||
| Motility defects | Leukocyte adhesion deficiency present with poor wound healing, delayed separation of umbilical cord, and periodontitis, neutrophilia, and leukocytosis. • LAD1 presents with delayed separation of umbilical cord, poor wound healing with paucity of pus, in early infancy and is marked by lack of CD18 on flow cytometry 48 • LAD2 is characterized by psychomotor and growth retardation but decreasing infections with age. It is marked by the presence of Bombay blood group and lack of fucosylated glycoprotein moieties like Sialyl Lewis X 48. • LAD3 features bleeding tendency and sometimes osteopetrosis in early infancy 5,48 |
ITGB2 (116920), FUCT1 (266265), KINDLIN3 (612840) Other motility defect: RAC2 (602049) | |
| Respiratory burst Defects: Chronic granulomatous disease(CGD) 5 | • Defective NADPH oxidase system. • Associated with granulomatous disease of gastrointestinal, respiratory, or urinary tracts. |
Catalase positive organisms like Staphylococcus aureus, Nocardia, Pseudomonas, Serratia species, Burkholderia cepacia Fungi like Candida, and Aspergillus | CYBB (306400), CYBA (233690), NCF1 (233700), NCF2 (233710), and NCF4 (601488) |
| Mendelian susceptibility to mycobacterial disease or MSMD 5 | Defects in IL12/ IFN gamma pathway lead to susceptibility to specific organisms | Infection by Salmonella spp, Mycobacteria and Cryptococcus neoformans | IL12RB1 (209950), IL12B (161561), IFNGR1 (209950), IFNGR2 (147569), STAT1 (600555), CYBB (306400), IRF8 (601565), ISG15 (147571) |
| GATA2 deficiency 49 | • Low B cell, NK cell, and monocyte counts. • CD4 and neutrophil counts can be low but are less remarkable. • Lymph edema, pulmonary alveolar proteinosis, warts, skin malignancies, sensorineural hearing loss, miscarriage, hypothyroidism myelodysplasia, and leukemias are other features |
Severe viral infections, mycobacterial infections, fungal infections | GATA2 (137295) |
| Complement Defects 2,5,6 | |||
| Early complement defects | • C1q ,C1r , C1s, C4, C2 deficiency present with SLE • C3, Factor I, H deficiency, CD46 deficiency can present with infections, glomerulonephritis, and atypical hemolytic-uremic syndrome (HUS) |
Infections with encapsulated organisms | 120550, 601269, 120575, 216950, 120580, 120810, 120820, 217000, 120700, 610984, 609814, 120920 |
| Late complement and alternative pathway deficiency like | C5, C6, C7, C8 (C8A, C8G, C8B), C9 (mild), Factor D, Properdin deficiency | Present with infections caused by Neisserial species. | 120900, 217050, 217070, 120950, 120960, 613825, 134350, 312060 |
| Other complement defects | Inflammatory lung disease, and autoimmunity Ficolin 3 can present with necrotizing enterocolitis | • MASP2 deficiency: respiratory and pyogenic infections • Ficolin 3 deficiency (FCN3) can present with respiratory infections and abscesses. |
605102, 604973 |
Combined Immunodeficiencies
SCID is associated with markedly reduced T cells and variable amounts of B cells. It is uniformly fatal if untreated. Typically, patients present early in life with failure to thrive, recurrent diarrhea, rashes, and serious bacterial, fungal, and viral infections. An assay developed to detect T cell receptor excision circles (TRECs) has been used to screen newborns for SCID since 2009.4 Currently, 26 states in the United States employ this technique resulting into prompt recognition and successful treatment.
Other combined immunodeficiencies are associated with less severely affected T and B cell subsets. Some of them present with distinct associated features giving rise to a syndrome (Table 1).
Phagocytic Defects
This category of PIDs includes disorders with either insufficient numbers of phagocytic cells or defective function. Patients with these conditions often have delayed wound healing and infectious granulomas with paucity of pus formation (Table 1).
Complement deficiencies
Complement defects include defects in classical pathway, alternative pathway, or lectin pathway. 6 Patients with classical pathway defects often have some concomitant features of autoimmunity in addition to immunodeficiency, while patients with the alternate and lectin pathway abnormalities commonly present with severe pyogenic infections (Table 1).
Disorders of innate immunity
Innate immunodeficiencies include Toll like receptor (TLR) defects and natural killer (NK) cell defects along with some other disorders with predisposition to viral / fungal infections included in Table 1, 2, and 3.
Table 2.
Typical Infections in PIDs
| Infection/ Infective agent | Associated primary immunodeficiency |
|---|---|
| Recurrent Epstein barr virus (EBV) | XLP1, XIAP, CD27, ITK, MAGT1, Coronin1-A def, PI3K-delta, PRKCD |
| Herpes simplex virus (HSV) encephalitis | TLR3 signaling pathway defects |
| Neisseria Meningitides | Terminal complement defects |
| Serratia marascens, Burkholderia cepacia, Staphylococcus aureus, Listeria monocytogenes, Granulibacter bethesdensis, Chromobacterium violaceum, Francisella philomiragia, Mycobacteria, Nocardia, Aspergillus, Candida albicans, Paecilomyces species | Chronic granulomatous disease |
| Enteroviral encephalitis | X-linked agammaglobulinemia |
| Mycobacteria | CGD, NEMO/ NFKB1 pathway defects, IL12 pathway defects, Tyk2, GATA2, IRF8, Macrophage gp91 phox, ISG15 deficiencies |
| HSV, EBV, cytomegalovirus, Varicella | CNKD, FNKD, DOCK8 deficiency |
| Cryptococcus neoformans | Anti GMCSF Abs, IFN gamma pathway defects |
| Cryptosporidium parvum | HyperIgM syndrome, IL21-R defect |
| Staphylococcus aureus | Anti IL6 Abs, DOCK8 deficiency |
| Chronic mucocutaneous candidiasis | IL17 signaling pathway defects, STAT1, VODI, IRF8, APECED, Act1 deficiency |
| Invasive candidiasis or other fungal ds | CARD9 |
| Salmonella sp | IL12 pathway defects, IFN gamma pathway defects, STAT1, Tyk2, IRF8, ISG15 deficiencies |
| Human papilloma virus | DOCK8, EVER1, EVER2, GATA2, WHIM, RHOH, STK4 deficiencies |
| Trypanosomiasis | APOL1 |
| HSV | STAT1, CNKD, FNKD |
| Pneumocystis jiroveci | SCID, HyperIgM, VODI, CARD11, IL21-R |
| Histoplasma | GATA2 deficiency |
| Molluscum contagiosum | RHOH deficiency |
XLP1, X-linked lymphoproliferative disease 1; XIAP, X-linked inhibitor-of-apoptosis; ITK, Interleukin-2- inducible T cell kinase; MAGT1, magnesium transporter 1; PI3K-delta, phosphoinositide 3-kinase; PRKCD, protein kinase C delta; TLR3, toll like receptor 3; CGD, chronic granulomatous disease; NEMO, NF-kappa B essential modulator; NFKB1, NF-kappa B1; IL, interleukin; Tyk2, tyrosine kinase 2; GATA2, GATA binding protein-2; IRF8, interferon regulatory factor 8; gp91 phox, glycoprotein 91 phagocytic oxidase; ISG15, interferon stimulated gene 15; CNKD, classical NK cell deficiency; FNKD, functional NK cell deficiency; DOCK8, dedicator of cytokinesis; anti GM-CSF Abs, anti granulocyte macrophage colony stimulating factor antibodies; IFN, interferon; IL21-R, interleukin 21-receptor; STAT1, signal transducer and activator of transcription 1; VODI, venoocclusive disease with immunodeficiency; APECED, autoimmune polyendocrinopathy candidiasis ectodermal dystrophy; CARD, caspase recruitment domain-containing protein; SCID, severe combined immunodeficiency; EVER, epidermodysplasia verruciformis gene; WHIM, warts hypogammaglobulinemia, infections, myelokathexis; RHOH, ras homolog gene family member H; STK4, serine/ threonine protein kinase 4.
Table 3.
Typical features of some primary Immunodeficiencies:
| Clinical Feature | Associated Primary immunodeficiency |
|---|---|
| Ectodermal dysplasia | NEMO/ NFKB1 defect, IKBA, ORAI-1, STIM-1 deficiencies, Comel-Netherton Syndrome |
| Alopecia | RAG1/ 2 (Omenn's), FOXN1 defects |
| Myopathy | ORAI-1, STIM-1 deficiencies, Barth Syndrome |
| Hypocalcemia | Chromosome 22q 11.2 deletion |
| Neurological impairment | PNP deficiency, Chediak Higashi, Kostmann Syndrome |
| Thrombocytopenia | CD40LG, CD40, IKAROS defects |
| Neutropenia | Hermansky Pudlak, TWEAK, CD40, CD40L defects, WAS, XLA |
| Dwarfism | STAT5b, MCM4 deficiencies, FILS Syndrome, Cartilage- Hair hypoplasia, Shwachmann-Diamond Syndrome, Bloom Syndrome, Schimke Syndrome |
| Multiple intestinal atresia | TTC7A defect |
| Deafness/ Hearing impairment | Reticular dysgenesis, ADA, GATA2 deficiencies |
| Conjunctivitis/ uveitis | Periodic fevers |
| Lymphoid hypertrophy | UNG, AID deficiencies |
| Eczema | WAS, DOCK8, STAT3, ITCH, Tyk2, STAT5b deficiencies |
| Food allergies | DOCK8 deficiency |
| Vasculitis | C7, C2 deficiency, MHC1 deficiency |
| Cold Urticaria | NLRP3 defects, PLCG2 deficiency |
| PAP | GATA2, CSF2RA deficiencies, Auto abs to GM-CSF |
| IBD/ Enteropathy | IL10, CGD, IPEX, CVID, STXBP2/MUNC 18-2 (FHL), XLP2, NOD2, LRBA deficiencies |
| Endocrinopathy | APECED, IPEX, Chromosome 22q11.2 deletion |
| Lung Disease | LRBA, ITCH deficiencies |
| Pneumtoceles | STAT3 deficiencies |
| Periodontitis | LAD, localized juvenile periodontitis, HyperIgM syndrome |
| Lymph edema | GATA2 deficiency |
| Adrenal insufficiency | MCM4 deficiency |
| Cleft lip/ palate | Chromosome 22q11.2 deletion, MASP1, 3MC Syndrome |
| Kaposi sarcoma | OX40 deficiency |
| Osteomyelitis | IL-1RN (Deficiency of Interleukin 1 receptor antagonist DIRA), LPIN2 (Chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anemia or Majeed Syndrome) |
| Psoriasis | DITRA (IL36RN), CAMPS (CARD14) |
| Panniculitis | CANDLE (PSMB8) |
NEMO, NF-kappa essential modulator; NFKB1-NF-kappa B1; STIM1, stromal interaction molecule 1; RAG1, recombination activating gene; FOXN1, forkhead box protein N1; PNP, purine nucleotide phosphorylase; CD40LG, CD40 ligand; TWEAK, TNF-like weak inducer of apoptosis; WAS, Wiskott Aldrich syndrome; XLA, X-linked agammaglobulinemia; STAT, signal transducer and activator of transcription; FILS syndrome, facial dysmorphism, immunodeficiency, livedo, and short stature syndrome; TTC7A, tetratricopeptide repeat domain-7A; ADA, adenosine deaminase; UNG, uracil DNA glycosylase; AID, activation-induced cytidine deaminase; DOCK8, dedicator of cytokinesis; MHC, major histocompatibility complex; NLRP3, Nod-like receptor PYD family; PLCG, phospholipase gamma; autoab to GM-CSF, autoantibodies to granulocyte macrophage colony stimulating factor; IL, interleukin; CGD, chronic granulomatous disease; IPEX, immunodeficiency polyendocrinopathy enteropathy X-linked; CVID, common variable immunodeficiency; FHL, familial hemophagocytic lymphohistiocytosis; XLP, X-linked lymphoproliferative syndrome; NOD2, nucleotide-binding oligomerization domain receptor; LRBA, lipopolysaccharide-responsive and beige-like anchor protein; APECED, autoimmune polyendocrinopathy candidiasis ectodermal dystrophy; LAD, leukocyte adhesion defect; CNKD, classical NK cell deficiency; MASP1, mannose associated serine protease-1; DITRA, deficiency of IL36 receptor antagonist; CARD, caspase recruitment domain; CAMPS, CARD14 mediated psoriasis; CANDLE, chronic atypical neutrophilic dermatitis with lipodystrophy.
NF-κB pathway defect/Anhidrotic ectodermal dysplasia (EDA) – X-linked NEMO (Online Mendelian inheritance in man or OMIM# 300248), autosomal dominant IKBA (OMIM# 612132) gain of function (GOF) mutation, and autosomal recessive IKBKB mutations can all cause EDA characterized by hypohidrosis, sparse hair, conical teeth and early loss of teeth 5. Hypogammaglobulinemia with impaired specific antibody production is noted in these defects. NEMO defect presents with Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, Haemophilus influenza, mycobacterial, and less frequently, viral and fungal infections. NEMO defect may be marked by high IgM level in about 15% of the patients. Patients with IKBA defects are prone to Pneumocystis jirovecii and mucocutaneous candidiasis. 5,7 IKBKB defect presents as SCID with the most common infections being Listeria monocytogenes, Serratia marascens, Escherichia coli, mucocutaneous candidiasis, and parainfluenza 5,8.
- TLR signaling pathway deficiency
- ○ Pyogenic infections: are noted in IRAK-4, MYD88, HOIL1 deficiencies. 7 IRAK4 (OMIM# 607676) and MyD88 (OMIM# 612260) present with infections due to Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa but exhibit normal resistance to most other bacteria, viruses, fungi, and parasites. The infections are marked by the absence of fever and normal C-reactive protein levels though pus formation has been noted. 7
NK Cell deficiency 10: can be classified as classical NK cell deficiency (CNKD) with absence of NK cells or functional NK cell deficiency (FNKD) with normal number of NK cells but impaired function; secondary NK cell deficiency must be ruled out. CKND (GATA2 (OMIM# 137295) and MCM4 (OMIM# 609981) patients can present with HSV, Epstein barr virus (EBV), cytomegalovirus (CMV), Varicella zoster virus (VZV), human papilloma virus (HPV), and less commonly fungal infections. They have increased risk of malignancies. FNKD (FCGR3A, (OMIM# 146740) has been associated most commonly with HSV infections and rarely with VZV, EBV, and recurrent respiratory viral infections.
Disorders of immune dysregulation
These disorders are associated with autoimmune manifestations and lymphoproliferation. Cytokine/interleukin (IL) pathway defects involve mutations that can result in gain or loss of function.
Familial hemophagocytic lymphohistiocytosis (FHL): 11 is a disease of defective cytotoxic cells and lysosomes leading to excessive cytokine release, T cell and macrophage related inflammation. It typically presents with fever, cytopenias, and hepatosplenomegaly. It is distinguished by the presence of hemophagocytosis, hypofibrinogenemia, hypertriglyceridemia, elevated ferritin level and soluble IL2 (CD25), abnormal liver functions, and decreased NK cell activity. FHL without hypo pigmentation is caused by defects in cytotoxic granule priming and fusion. The genetic mutations identified to date include PRF1 (OMIM# 603553), UNC13D (OMIM# 608898), STX11 (OMIM# 603552), and STXBP2 (OMIM# 613101). FHL with hypo pigmentation results in partial oculocutaneous albinism and immune defects; examples of such conditions are Chediak Higashi (LYST (OMIM# 214500)), Griscelli type 2 (RAB27A (OMIM# 607624)), and Hermansky Pudlak type 2 (AP3B1 (OMIM# 608233)) syndromes. Defective secretory lysosomes in various cells cause features such as bleeding, neutropenia, and immunodeficiency.
Immunodeficiency, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX like disorders: most commonly present with autoimmunity/ cytopenias, diabetes mellitus, enteropathy, eczema, and bacterial infections. While IPEX is caused by defective regulatory T cells due to mutations in FOXP3 (OMIM# 304790), mutations in CD25 deficiency (IL2RA, (OMIM# 606367)), STAT5B (OMIM# 245590), STAT1 (MIM# 614162), and LRBA (OMIM# 606453) 12,13 cause IPEX like syndromes.
Lymphoproliferative disorders associated with EBV: X-linked lymphoproliferative syndrome 1 (XLP1) (SH2D1A (OMIM# 308240)), XLP2 (BIRC4 or XIAP, (OMIM# 300635)), ITK (OMIM# 613011), and CD27 (OMIM# 615122) deficiency can present with EBV induced lymphoproliferation. These conditions predispose to the development of lymphomas, except in XLP2 patients who are more likely to develop colitis. Patients with XLP1, XLP2, or CD27 defects can develop hemophagocytic lymphohistiocytosis (HLH).14
Autoimmunity without lymphoproliferation is noted in APECED (OMIM# 240300) and ITCH (OMIM# 613385) syndromes. APECED is autoimmune polyendocrinopathy, candidiasis, ectodermal dystrophy caused by mutation in AIRE.2 ITCH defect can cause hepatosplenomegaly, dysmorphism, failure to thrive, developmental delay. Lungs, liver, and gastrointestinal tract can be infiltrated by inflammatory cells.15
Autoimmune lymphoproliferative syndrome (ALPS): ALPS is a disorder with lymphadenopathy and splenomegaly of at least 6 months duration, cytopenias, and predisposition to lymphomas. It is marked by the presence of double negative T lymphocytes (TCRαβ CD4-CD8- B220+ cells)16. Defects in FAS (OMIM# 601859), FASLG (OMIM# 134638), and Caspase 10 (OMIM# 603909) can cause ALPS. Caspase 8 (OMIM# 607271), FADD deficiency (OMIM# 613759), CARD 11 GOF (OMIM# 606445), PRKCD (OMIM# 615559) defects display overlapping features. Recurrent infections are seen in caspase 8 deficiency.16
Autoinflammatory disorders
Periodic fever Syndrome: These disorders are characterized by periodic fevers 17. Depending on the causative genes, other manifestations include arthritis, abdominal pain, and urticarial or other rash. Cryopyrin associated periodic fevers are caused by mutations in NALP3 and are associated with conjunctivitis and amyloidosis and various syndromes (e.g., Muckle-Wells Syndrome (OMIM# 191900), Familial cold autoinflammatory syndrome (OMIM# 120100), Neonatal onset multisystem inflammatory disease ((OMIM# 607115), NOMID)). Monogenic causes of periodic fevers caused by mutations in MEFV ((OMIM# 249100), Familial Mediterranean fever), MVK ((OMIM# 260920), Hyper IgD), and TRAPS(TNFRSF1, (OMIM# 142680)) occur in specific ethnicities. Pyogenic infections, fever, apthous ulcers, pharyngitis, and adenopathy (PFAPA) is a periodic fever syndrome with unknown gene defect.
Inflammatory bowel disease, chronic osteomyelitis, psoriasis, panniculitis, and pyoderma gangrenosum can be manifestations of various auto inflammatory disorders.2
Phenocopies of PIDs:2
These disorders behave and present like primary PIDs, but they are acquired secondary to occurrence of autoantibodies or somatic mutations.
Somatic mutations: ALPS-sFAS, RAS associated autoimmune leukoproliferative disease (RALD) (KRAS or NRAS).
Autoantibodies: chronic mucocutaneous candidiasis (IL17, IL22), adult onset immunodeficiency with mycobacterial and salmonella infections (interferon gamma), recurrent skin infections (IL6), pulmonary alveolar proteinosis (granulocyte macrophage-colony stimulating factor (GM-CSF)) and acquired angioedema (C1 inhibitor)
Diagnosis
The diagnosis of PIDs is challenging for multiple reasons.
Since PIDs are rare, a high index of suspicion is needed
Individuals with the same PID can have different manifestations and most PIDs lack pathognomonic characteristics
Individuals with a defect in the same gene can have different presentations
Screening tests are non specific
Normal results of the screening test do not rule out PID
Some of the diagnostic laboratory tests use specialized techniques that are not available in commercial laboratories
History and Physical Examination
A meticulous history and comprehensive physical examination is critical to diagnose PIDs. Clues to the correct diagnosis may include history of recurrent infections, presence of affected family members, physical findings related to growth and development, and presence or absence of peripheral lymphatic tissue. The 10 warning signs published by the Jeffery Modell Foundation help to alert providers ensuring screening is initiated in situations where these criteria are met.
Causes of secondary PIDs such as medications, HIV, malnutrition, and protein losing conditions should be ruled out. Other conditions predisposing to recurrent infections should be considered in the differential diagnosis. For instance, recurrent sinopulmonary infections can be a feature of cystic fibrosis, primary ciliary dyskinesia, bronchiectasis, or environmental allergies. Similarly recurrent skin infections can be secondary to atopic dermatitis.
Table 2 provides a list of infectious agents and associated PIDs. Table 3 provides a list of clinical features and associated PIDs. These lists may serve as a guide but are not exhaustive.
Laboratory Investigations 18
The laboratory evaluation of patients with suspected PIDs is typically two or three tiered, starting with screening labs and followed by definitive diagnostic and molecular tests. Some of the useful but often forgotten tests include peripheral blood smear, ESR, and CRP. Over the last five years, newborn screening for TRECs has changed the paradigm of screening evaluation.
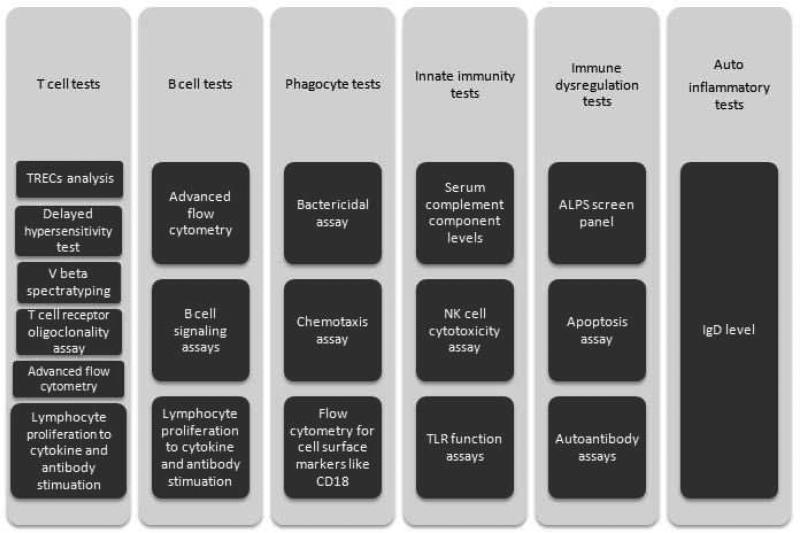
Screening tests: These tests are listed in Figure 2A. Test results can be affected by various external factors like age or specimen handling. For example, isohemagglutinins testing cannot be reliably performed till 6 months age; lymphocyte proliferation assays to antigen stimulation may be impaired before the age of 1 year; and response to polysaccharide antigen is suboptimal till after 24 months of age. Hence, test results should always be compared to age appropriate reference values. Specimen handling can affect some of the laboratory tests. For instance, a low serum complement level can be a result of specimen handling issues and delay in running the assay.
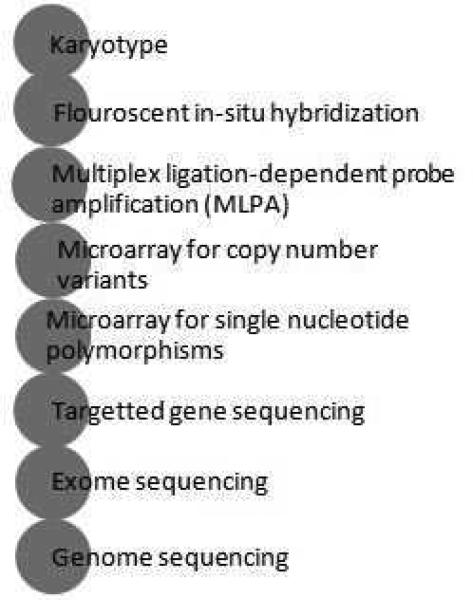
Figure 2.
Diagnostic tests in evaluation of Primary Immunodeficiencies
Data from Locke BA, Dasu T, Verbsky JW. Laboratory diagnosis of primary immunodeficiencies. Clinical reviews in allergy & immunology. Apr 2014;46(2):154-168.
A. Basic Immunology work up
B. Advanced immunology work up based on clinical and laboratory clues
TRECs, T cell receptor excision circles; NK, natural killer; TLR, toll-like receptor; ALPS, autoimmune lymphoproliferative syndrome, IgD, immunoglobulin D.
C. Molecular testing
Second tier tests (Figure 2B): These tests are performed based on clinical features and results of the screening tests. Particular attention should be paid to the laboratory techniques used as the results and normal ranges can vary depending on the technique. Some of these tests are performed in research laboratories and have limited availability in clinical laboratories. For example, though the basic T, B, and NK cell markers by flow cytometry are available in most laboratories, the tests to evaluate lymphocyte signaling have limited availability. This limited availability also limits the experience and expertise in the interpretation of these tests to limited academic centers.
Definitive molecular diagnosis is pursued more often now that next-generation sequencing (NGS) has made it possible to pinpoint the molecular etiology. The various methods of genetic testing are shown in Figure 2C.
Treatment
Definitive treatment
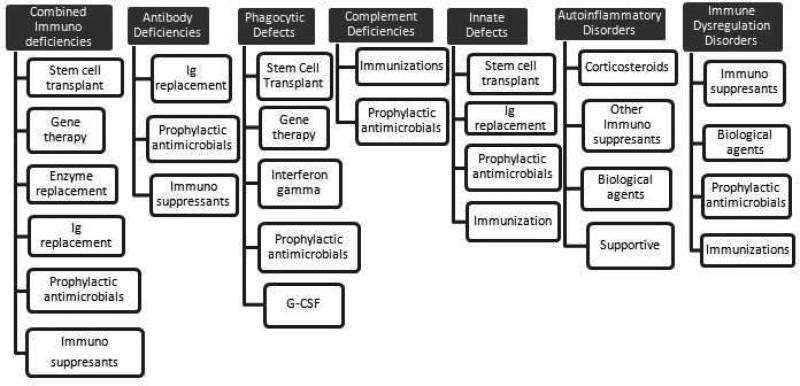
The type of treatment offered depends on the type of immunodeficiency (See Figure 3).
Figure 3.
Therapies for various types of Primary Immunodeficiencies
Ig, immunoglobulin; G-CSF, granulocyte-colony-stimulating factor
For antibody deficiencies, the options range from a) monitoring (asymptomatic SIgAD) b) prophylactic antibiotics (symptomatic SIgAD) to c) immunoglobulin replacement therapy (Agammaglobulinemia, CVID, and SAD). The dose of immunoglobulin replacement should be individualized based on clinical response.19
Immunosuppressants (for example: hydroxychloroquine, infliximab, and a combination of azathioprine and Rituximab) are used for treatment of granulomatous lymphocytic interstitial lung disease (GLILD) associated with CVID.20 High mortality was observed in the clinical trials of CVID patients who were treated with hemopoetic stem cell transplantation (HSCT); though benefit was noted in the surviving patients suggesting a need for further studies.21
For severe combined immunodeficiency, the definitive treatment options include enzyme replacement (e.g. PEG-ADA), gene therapy, and HSCT. Gene therapy with retroviral vectors was previously attempted but was reported to increase the risk for development of leukemia and myelodysplasia.22 Recent studies have shown some promise for gene therapy using lentivirus vectors 22. HSCT outcomes depend on several factors like the age at the time of HSCT, active infections, type of donor (matched/ mismatched, related/ unrelated, bone marrow/ cord blood), and conditioning regimen.23
Phagocytic defects are managed with prophylactic antimicrobials and interferon gamma infusions. Some disorders with neutropenia can be treated with granulocyte- colony stimulating factor (G-CSF).
Some well-defined syndromes such as Wiskott Aldrich Syndrome (WAS), hyper IgM syndrome caused by CD40 and CD40L defects, DOCK8 defect, IPEX, HLH, XLP, and, CGD can also be treated with HSCT.24 Gene therapy has been attempted for X-linked SCID, ADA deficiency, WAS, and CGD.22
Chromosome 22q11.2 deletion syndrome (OMIM# 188400) and CHARGE Syndrome (CHD7 (OMIM# 608892), SEMA3E (OMIM# 214800)) with profound T cell immunodeficiency have shown to be treated with thymic transplantation in a clinical trial.25,26
Auto inflammatory disorders can be treated depending on the etiopathogenesis. Corticosteroids can be used for some periodic fevers syndromes. IL1 receptor antagonists such as anakinra can be used in inflammasome associated periodic fevers. HSCT has been used for IL-10 defects causing early onset inflammatory bowel disease.
Symptomatic treatment
Prompt recognition and treatment of infections and other complications is the key to keeping PID patients healthy. Every effort should be made to obtain the microbiological diagnosis; however, institution of empirical therapy should not be delayed.
Prevention of manifestations/ complications
Lifestyle changes to prevent infections and other complications can significantly improve morbidity of PID patients. The importance of hand washing and overall hygiene cannot be overstated. Patients should be educated about avoiding sick contacts and unimmunized contacts. A discussion with the health care provider regarding immunization is important. Depending on the type of PID, patients can receive some or all of the routine immunizations; in particular, ensuring annual protection against influenza vaccine needs to be emphasized. Compliance with medications and office visits can play an important role in the care of these patients. A healthy lifestyle with nutritious diet and aerobic exercise is important to ensuring optimal quality of life. Along with the guidance on physical health, resources should be provided for maintaining mental and emotional well-being.
Follow up/ monitoring
Regular follow up with the primary care provider as well as the immunologist should be encouraged. Depending on the type of PID, interval laboratory evaluation and imaging should be used to monitor for the complications of the PID or its treatment.
Prognosis
Discussions with the patient/ family should include elaboration on the possible outcomes of the condition including rare complications and complications of the treatment modalities. While prognosis varies depending on the type of PID, some of the common factors affecting the overall prognosis include:
Age at diagnosis
Presence of infections
Presence of non-infectious complications such as lung disease, lymphoproliferation, granulomatous disease or autoimmune complications
Age at the time of definitive treatment (e.g. HSCT)
Other co morbidities
With the advances in discovery of genetic causes of PIDs, it is important to counsel families about the implications for future family planning and the patient's probability of passing on the defect to future generations.
Future of the PID field
With rapidly improving genetic technology, the field of PIDs is expanding exponentially. As next-generation sequencing continues to discover new defects, there is a growing need to improve the functional immunological assays that uncover the underlying pathophysiology and molecular mechanisms.
Gene therapy, newer biological agents, and possibly RNA interference (iRNA) therapies are on the horizon and promise to dramatically improve outcomes for individuals with PIDs and their families.
Key points.
Primary Immunodeficiencies lead to various combinations of recurrent infections, autoimmunity, lymphoproliferation, granulomatous disease, atopy, and malignancy.
Immunodeficiency caused by defects in more than one gene can lead to similar manifestations and defect in the same gene can cause varied manifestations.
High index of suspicion along with meticulous history and physical examination are a key to early diagnosis of primary immunodeficiency.
Early PID diagnosis can be achieved with improved access to validated immunologic laboratory tests.
Acknowledgments
Disclosures:
“This work was supported by a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # UL1TR000001 (formerly #UL1RR033179). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ochs HD, Hitzig WH. History of primary immunodeficiency diseases. Curr Opin Allergy Clin Immunol. 2012 Dec;12(6):577–587. doi: 10.1097/ACI.0b013e32835923a6. [DOI] [PubMed] [Google Scholar]
- 2.Al-Herz W, Bousfiha A, Casanova JL, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Frontiers in immunology. 2014;5:162. doi: 10.3389/fimmu.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yel L. Selective IgA deficiency. J Clin Immunol. 2010 Jan;30(1):10–16. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwan A, Abraham RS, Currier R, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014 Aug 20;312(7):729–738. doi: 10.1001/jama.2014.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Routes J, Abinun M, Al-Herz W, et al. ICON: the early diagnosis of congenital immunodeficiencies. J Clin Immunol. 2014 May;34(4):398–424. doi: 10.1007/s10875-014-0003-x. [DOI] [PubMed] [Google Scholar]
- 6.Grumach AS, Kirschfink M. Are complement deficiencies really rare? Overview on prevalence, clinical importance and modern diagnostic approach. Mol Immunol. 2014 Oct;61(2):110–117. doi: 10.1016/j.molimm.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clinical microbiology reviews. 2011 Jul;24(3):490–497. doi: 10.1128/CMR.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pannicke U, Baumann B, Fuchs S, et al. Deficiency of innate and acquired immunity caused by an IKBKB mutation. N Engl J Med. 2013 Dec 26;369(26):2504–2514. doi: 10.1056/NEJMoa1309199. [DOI] [PubMed] [Google Scholar]
- 9.Frazao JB, Errante PR, Condino-Neto A. Toll-like receptors’ pathway disturbances are associated with increased susceptibility to infections in humans. Archivum immunologiae et therapiae experimentalis. 2013 Dec;61(6):427–443. doi: 10.1007/s00005-013-0243-0. [DOI] [PubMed] [Google Scholar]
- 10.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013 Sep;132(3):515–525. doi: 10.1016/j.jaci.2013.07.020. quiz 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faitelson Y, Grunebaum E. Hemophagocytic lymphohistiocytosis and primary immune deficiency disorders. Clin Immunol. 2014 Nov;155(1):118–125. doi: 10.1016/j.clim.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Charbonnier LM, Janssen E, Chou J, et al. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immunol. 2015 Jan;135(1):217–227. e219. doi: 10.1016/j.jaci.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Current opinion in pediatrics. 2013 Dec;25(6):708–714. doi: 10.1097/MOP.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veillette A, Perez-Quintero LA, Latour S. X-linked lymphoproliferative syndromes and related autosomal recessive disorders. Curr Opin Allergy Clin Immunol. 2013 Dec;13(6):614–622. doi: 10.1097/ACI.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 15.Lohr NJ, Molleston JP, Strauss KA, et al. Human ITCH E3 ubiquitin ligase deficiency causes syndromic multisystem autoimmune disease. American journal of human genetics. 2010 Mar 12;86(3):447–453. doi: 10.1016/j.ajhg.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira JB, Bleesing JJ, Dianzani U, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010 Oct 7;116(14):e35–40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rigante D, Vitale A, Lucherini OM, et al. The hereditary autoinflammatory disorders uncovered. Autoimmunity reviews. 2014 Sep;13(9):892–900. doi: 10.1016/j.autrev.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Locke BA, Dasu T, Verbsky JW. Laboratory diagnosis of primary immunodeficiencies. Clinical reviews in allergy & immunology. 2014 Apr;46(2):154–168. doi: 10.1007/s12016-014-8412-4. [DOI] [PubMed] [Google Scholar]
- 19.Berger M. Choices in IgG replacement therapy for primary immune deficiency diseases: subcutaneous IgG vs. intravenous IgG and selecting an optimal dose. Curr Opin Allergy Clin Immunol. 2011 Dec;11(6):532–538. doi: 10.1097/ACI.0b013e32834c22da. [DOI] [PubMed] [Google Scholar]
- 20.Verbsky JW, Routes JM. Sarcoidosis and common variable immunodeficiency: similarities and differences. Seminars in respiratory and critical care medicine. 2014 Jun;35(3):330–335. doi: 10.1055/s-0034-1376862. [DOI] [PubMed] [Google Scholar]
- 21.Wehr C, Gennery AR, Lindemans C, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015 Jan 14; doi: 10.1016/j.jaci.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Farinelli G, Capo V, Scaramuzza S, et al. Lentiviral vectors for the treatment of primary immunodeficiencies. Journal of inherited metabolic disease. 2014 Jul;37(4):525–533. doi: 10.1007/s10545-014-9690-y. [DOI] [PubMed] [Google Scholar]
- 23.Pai SY, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N Engl J Med. 2014 Jul 31;371(5):434–446. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de la Morena MT, Nelson RP., Jr. Recent advances in transplantation for primary immune deficiency diseases: a comprehensive review. Clinical reviews in allergy & immunology. 2014 Apr;46(2):131–144. doi: 10.1007/s12016-013-8379-6. [DOI] [PubMed] [Google Scholar]
- 25.Jyonouchi S, McDonald-McGinn DM, Bale S, et al. CHARGE (coloboma, heart defect, atresia choanae, retarded growth and development, genital hypoplasia, ear anomalies/deafness) syndrome and chromosome 22q11.2 deletion syndrome: a comparison of immunologic and nonimmunologic phenotypic features. Pediatrics. 2009 May;123(5):e871–877. doi: 10.1542/peds.2008-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gennery AR. Immunological aspects of 22q11.2 deletion syndrome. Cellular and molecular life sciences : CMLS. 2012 Jan;69(1):17–27. doi: 10.1007/s00018-011-0842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stiehm RE, Ochs HD, Winkelstein JA. Immunodeficiency Disorders: General Considerations. 5 ed Saunders; 2004. [Google Scholar]
- 28.Driessen G, van der Burg M. Educational paper: primary antibody deficiencies. Eur J Pediatr. 2011 Jun;170(6):693–702. doi: 10.1007/s00431-011-1474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkelstein JA, Marino MC, Lederman HM, et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 2006 Jul;85(4):193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham-Rundles C. The many faces of common variable immunodeficiency. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2012;2012:301–305. doi: 10.1182/asheducation-2012.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qamar N, Fuleihan RL. The hyper IgM syndromes. Clinical reviews in allergy & immunology. 2014 Apr;46(2):120–130. doi: 10.1007/s12016-013-8378-7. [DOI] [PubMed] [Google Scholar]
- 32.Winkelstein JA, Marino MC, Ochs H, et al. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine (Baltimore) 2003 Nov;82(6):373–384. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]
- 33.Crank MC, Grossman JK, Moir S, et al. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. J Clin Immunol. 2014 Apr;34(3):272–276. doi: 10.1007/s10875-014-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Picard C, Dogniaux S, Chemin K, et al. Hypomorphic mutation of ZAP70 in human results in a late onset immunodeficiency and no autoimmunity. Eur J Immunol. 2009 Jul;39(7):1966–1976. doi: 10.1002/eji.200939385. [DOI] [PubMed] [Google Scholar]
- 35.de la Calle-Martin O, Hernandez M, Ordi J, et al. Familial CD8 deficiency due to a mutation in the CD8 alpha gene. J Clin Invest. 2001 Jul;108(1):117–123. doi: 10.1172/JCI10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmer J, Andres E, Donato L, et al. Clinical and immunological aspects of HLA class I deficiency. QJM : monthly journal of the Association of Physicians. 2005 Oct;98(10):719–727. doi: 10.1093/qjmed/hci112. [DOI] [PubMed] [Google Scholar]
- 37.Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annual review of immunology. 2001;19:331–373. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- 38.Li FY, Lenardo MJ, Chaigne-Delalande B. Loss of MAGT1 abrogates the Mg2+ flux required for T cell signaling and leads to a novel human primary immunodeficiency. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2011 Sep;24(3):S109–114. doi: 10.1684/mrh.2011.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hauck F, Randriamampita C, Martin E, et al. Primary T-cell immunodeficiency with immunodysregulation caused by autosomal recessive LCK deficiency. J Allergy Clin Immunol. 2012 Nov;130(5):1144–1152. e1111. doi: 10.1016/j.jaci.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Herrera G, Tampella G, Pan-Hammarstrom Q, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. American journal of human genetics. 2012 Jun 8;90(6):986–1001. doi: 10.1016/j.ajhg.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorska MM, Alam R. A mutation in the human Uncoordinated 119 gene impairs TCR signaling and is associated with CD4 lymphopenia. Blood. 2012 Feb 9;119(6):1399–1406. doi: 10.1182/blood-2011-04-350686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Montfrans JM, Hoepelman AI, Otto S, et al. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J Allergy Clin Immunol. 2012 Mar;129(3):787–793. e786. doi: 10.1016/j.jaci.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh S, Bienemann K, Boztug K, et al. Interleukin-2-inducible T-cell kinase (ITK) deficiency - clinical and molecular aspects. J Clin Immunol. 2014 Nov;34(8):892–899. doi: 10.1007/s10875-014-0110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchbinder D, Nugent DJ, Fillipovich AH. Wiskott-Aldrich syndrome: diagnosis, current management, and emerging treatments. The application of clinical genetics. 2014;7:55–66. doi: 10.2147/TACG.S58444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanzi G, Moratto D, Vairo D, et al. A novel primary human immunodeficiency due to deficiency in the WASP-interacting protein WIP. J Exp Med. 2012 Jan 16;209(1):29–34. doi: 10.1084/jem.20110896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Driscoll M. Diseases associated with defective responses to DNA damage. Cold Spring Harbor perspectives in biology. 2012 Dec;4(12) doi: 10.1101/cshperspect.a012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baradaran-Heravi A, Thiel C, Rauch A, et al. Clinical and genetic distinction of Schimke immuno-osseous dysplasia and cartilage-hair hypoplasia. Am J Med Genet A. 2008 Aug 1;146A(15):2013–2017. doi: 10.1002/ajmg.a.32406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farmand S, Sundin M. Hyper-IgE syndromes: recent advances in pathogenesis, diagnostics and clinical care. Current opinion in hematology. 2015 Jan;22(1):12–22. doi: 10.1097/MOH.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 49.Etzioni A. Genetic etiologies of leukocyte adhesion defects. Curr Opin Immunol. 2009 Oct;21(5):481–486. doi: 10.1016/j.coi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Hsu AP, McReynolds LJ, Holland SM. GATA2 deficiency. Curr Opin Allergy Clin Immunol. 2015 Feb;15(1):104–109. doi: 10.1097/ACI.0000000000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]