Abstract
Novel polar functionalities containing 6-nitro-2,3-dihydroimidazooxazole (NHIO) analogues were synthesized to produce a compound with enhanced solubility. Polar functionalities including sulfonyl, uridyl, and thiouridyl-bearing NHIO analogues were synthesized and evaluated against Mycobacterium tuberculosis (MTB) H37Rv. The aqueous solubility of compounds with MIC values ≤0.5 μg/mL were tested, and six compounds showed enhanced aqueous solubility. The best six compounds were further tested against resistant (RifR and MDR) and dormant strains of MTB and tested for cytotoxicity in HepG2 cell line. Based on its overall in vitro characteristics and solubility profile, compound 6d was further shown to possess high microsomal stability, solubility under all tested biological conditions (PBS, SGF and SIF), and favorable oral in vivo pharmacokinetics and in vivo efficacy.
Keywords: Mycobacterium tuberculosis; MTB H37Rv; multidrug resistant-TB; 6-nitro-2,3-dihydroimidazooxazole; structure−activity relationship
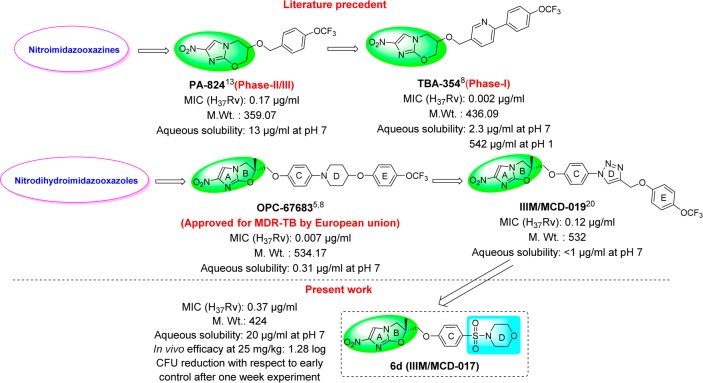
Tuberculosis is a deadly infectious disease that has infected approximately one-third of the world’s population. The current lengthy treatment for TB and the development of resistance toward existing drugs have further complicated the treatment of this disease.1 New TB drugs that can shorten and simplify current treatment regimes and demonstrate potency against both drug-sensitive and drug-resistant TB are urgently needed.2 In the past decade, the nitroimidazole skeleton has been of great interest to TB researchers,3 which led to the identification of one drug, delamanid or OPC-676834−6 (a nitrodihydroimidazooxazole analogue approved by the European union for MDR-TB) and two clinical candidates, pretomanid or PA-8247 and TBA-3548,9 (nitroimidazooxazine analogues in phase-II/III and phase-I clinical trials, respectively) (Figure 1). Both of the advanced candidates, delamanid and pretomanid, are lipophilic, which may aid their entry through the highly lipophilic cell wall of MTB and lead to their high potency.10−12 The highly lipophilic nature of these compounds has presented absorption issues during clinical trials, which have been managed by altering the formulation of the compound. To address these absorption issues, several groups have studied the nitroimidazooxazine scaffold (PA-824)13−19 and synthesized a newer generation analogue, TBA-354, with improved physicochemical properties and in vivo efficacy.8 Despite the comparatively better anti-TB profile of OPC-67683, no efforts were made to improve the physicochemical properties of OPC-67683, and delamanid was approved for MDR-TB with a recommended dose of 100 mg twice daily.6 The recommendation of a twice-daily dose might limit its introduction in a first-line drug regimen and efforts to improve its physicochemical properties are needed.
Figure 1.
Nitroimidazole-containing anti-TB agents.
We therefore generated novel triazolyl- and isoxazolyl-containing nitrodihydroimidazooxazole (NHIO) analogues, of which the molecule IIIM/MCD-019 demonstrated good in vitro and in vivo profiles but high lipophilicity and poor aqueous solubility.20 In this study, we synthesized polar functionalities containing nitrodihydroimidazooxazole (NHIO) analogues to improve the aqueous solubility. The present study also provides further insight into existing structure–activity relationships (SARs) of this class, as well as a new compound with a good activity profile, improved aqueous solubility, and favorable oral PK and in vivo efficacy.
Three series of analogues were synthesized with different polar functional moieties (sulfonyl, uridyl, and thiouridyl) (Figure 2). In Series 1, a sulfonyl moiety was placed between ring C and ring D. In Series 2, a sulfonyl moiety was introduced between ring D and ring E. In Series 3, uridyl/thiouridyl moieties were introduced between rings C and D.
Figure 2.
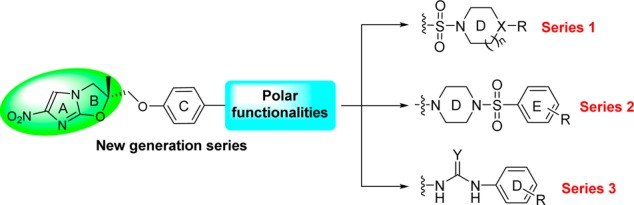
Medicinal chemistry approach.
Results and Discussion
Chemistry
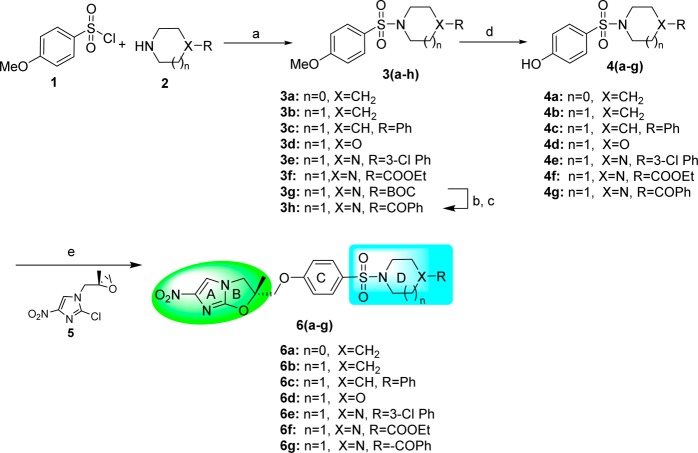
First, the key phenolic intermediate 4 was synthesized from 4-methoxybenzenesulfonyl chloride 1 in two steps by coupling with secondary amines 2 in the presence of triethylamine and DMAP followed by demethylation using boron tribromide.21 Then, a key epoxy intermediate 5 was synthesized from 4-nitroimidazole in seven steps, as previously reported.20 Finally, phenolic intermediate 4, when treated with epoxide 5 in the presence of sodium hydride, afforded NHIO analogues 6a–g of Series 1 (Scheme 1).
Scheme 1. Synthesis of Sulfonyl-Containing NHIO Analogues of Series 1.
Reagents and conditions: (a) Et3N, DMAP, DCM, overnight, rt, 85–95%; (b) TFA, DCM, rt, 1 h; (c) benzoyl chloride, TEA, DMAP, DCM, rt, 3 h, 90%; (d) BBr3 DCM, 12h, rt, 60–80%; (e) NaH, DMF, 0 to 50 °C, 6 h, 25–35%.
Next, NHIO analogues 13a–e of Series 2 were synthesized as illustrated in Scheme 2. The key phenolic intermediate 10 for this series was synthesized in two steps wherein (i) N-Boc piperazine 8 coupled with THP protected 4-bromophenol 7 under Buchwald conditions22 gave product 9 and; (ii) in the second step, THP-deprotection in the presence of pyridinium p-toluenesulfonate furnished key phenolic intermediate 10. Intermediate 10 was coupled with intermediate 5 in the presence of sodium hydride to give compound 11, followed by Boc deprotection and subsequent coupling with substituted benzenesulfonyl chlorides 12 in the presence of triethyl amine and DMAP to produce NHIO analogues 13a–e.
Scheme 2. Synthesis of Sulfonyl-Containing NHIO Analogues of Series 2.
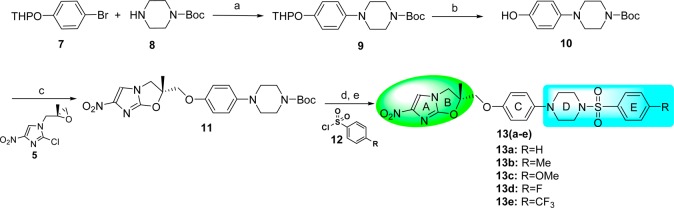
Reagents and conditions: (a) Pd(OAc)2, rac-BINAP, Cs2CO3, toluene, reflux, 1 h, 65%; (b) pyridinium p-toluenesulfonate, EtOH, 70 °C, 24 h, 85%; (c) NaH, DMF, 50 °C, 2 h, 32%; (d) TFA, DCM, 1 h; (e) TEA, DMAP, DCM, rt, 12 h, 80–90%.
The synthesis of uridyl- and thiouridyl-containing NHIO analogues 18a–n of Series 3 was performed as illustrated in Scheme 3. The key phenolic intermediate 15 was synthesized from 4-aminophenol 14. Treatment of intermediate 15 with intermediate 5 in the presence of sodium hydride gave the coupled product 16. Boc-deprotection of compound 16 followed by coupling with substituted phenyl isocyanates or isothiocyanates 17 produced the NHIO analogues 18a–n of Series 3.
Scheme 3. Synthesis of Uridyl/Thiouridyl-Containing NHIO Analogues of Series 3.
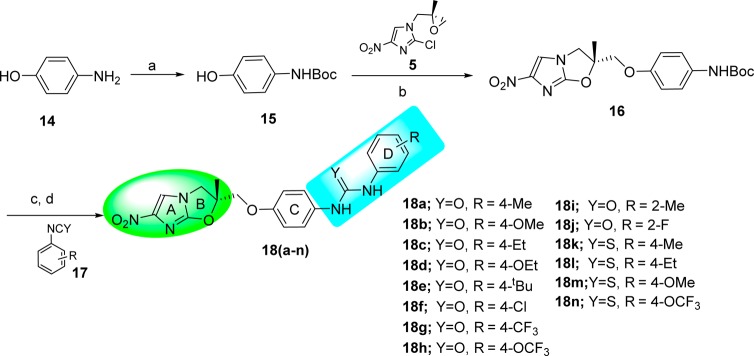
Reagents and conditions: (a) Boc2O, NaOH, H2O, rt, 4 h, 95%; (b) NaH, DMF, 0 °C to 50 °C, 12 h, 35%; (c) TFA, DCM, rt, 2 h; (d) TEA, DCM, rt, 5 h, 60–80%.
Biological Evaluation
A total of 28 new NHIO analogues, 6a–g, 11, 13a–e, 16, and 18a–n, were screened for in vitro activity against MTB H37Rv (ATCC27294 strain) using the agar dilution method. The MIC was determined as the minimum concentration of the compound required to inhibit 90% of bacterial growth. The aqueous solubility was determined at pH 7. ClogP values were calculated by Schrodinger software. The MIC, aqueous solubility, and ClogP values of all of the synthesized compounds are summarized in Table 1.
Table 1. In Vitro Activity and Aqueous Solubility of 6a–g, 11, 13a–e, 16, and 18a–na.
| compd | MIC (H37RV) (μg/mL) | solubilityb (μg/mL) | ClogPc | compd | MIC (H37RV) (μg/mL) | solubilityb (μg/mL) | ClogPc |
|---|---|---|---|---|---|---|---|
| 6a | 1 | 1.43 | 18c | 0.5 | <1 | 3.47 | |
| 6b | 0.5 | 13.33 | 1.8 | 18d | 0.5 | 6.66 | 3.49 |
| 6c | 0.67 | 3.52 | 18e | 0.09 | 1.0 | 4.20 | |
| 6d | 0.37 | 20.0 | 0.92 | 18f | 0.25 | 6.66 | 3.44 |
| 6e | 0.83 | 3.48 | 18g | 0.5 | <1 | 3.92 | |
| 6f | 4 | 1.62 | 18h | 0.67 | 2.90 | ||
| 6g | 4 | 2.02 | 18i | 1 | 3.28 | ||
| 11 | 6.67 | 3.93 | 18j | 1.67 | 3.23 | ||
| 13a | 1 | 2.66 | 18k | 0.83 | 4.10 | ||
| 13b | 0.5 | <1 | 3.13 | 18l | 1 | 4.61 | |
| 13c | 0.5 | <1 | 2.72 | 18m | 0.5 | 8.66 | 3.89 |
| 13d | 0.06 | 8.33 | 3.01 | 18n | 0.25 | <1 | 5.06 |
| 13e | 0.25 | <1 | 3.66 | IIIM/MCD-019 | 0.12 | <1 | 5.25 |
| 16 | 1.34 | 2.88 | Delamanid | 0.007 | 0.31 | 6.19 | |
| 18a | 0.21 | <1 | 3.26 | Rifampicin | 0.06 | ||
| 18b | 2 | 4.08 |
Values reported are the average of three individual measurements.
Solubility was determined at pH 7.
ClogP values were calculated by Schrodinger software.
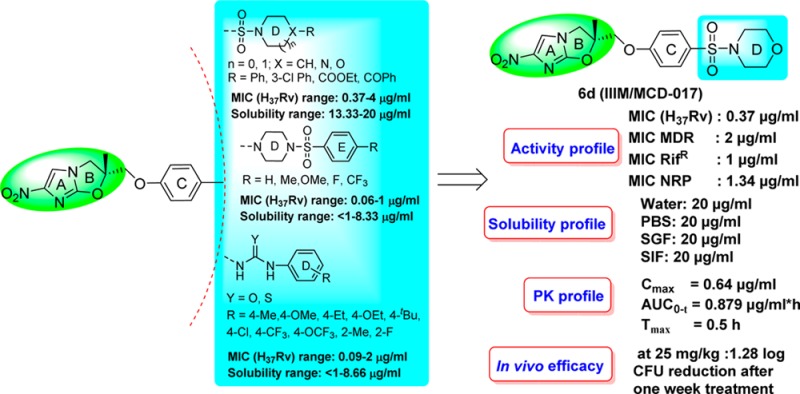
In Series 1, all seven synthesized NHIO analogues with varying cyclic secondary amine rings D attached to the sulfonyl group (6a–g) were screened against MTB H37Rv. The MIC values of these compounds were between 0.37 and 4 μg/mL. Two NHIO analogues, with piperidyl 6b and morpholinyl 6d rings attached to the sulfonyl group, possessed MIC values of 0.5 and 0.37 μg/mL, respectively against MTB H37Rv. The presence of a piperazine ring (6e–g) led to a decrease in activity.
In Series 2, all five synthesized NHIO analogues with varying substitutions on ring E (13a–e) were screened against MTB H37Rv. The MIC values were between 0.06 and 1 μg/mL. All the 4-substituted ring E analogues had high MIC values and two compounds 4-F 13d and 4-CF313e showed the best activity, with MIC values of 0.06 and 0.25 μg/mL, respectively.
In Series 3, all 14 synthesized NHIO analogues with varied substitutions on ring D and a uridyl/thiouridyl linker between the C and D rings (18a–n) were screened against MTB H37Rv and found to have MIC values between 0.09 and 2 μg/mL. Among all of the tested analogues, uridyl-containing NHIO analogues demonstrated comparatively better activity than thiouridyl-containing NHIO analogues. In uridyl-based NHIO analogues, 4-Me 18a, 4-tert-butyl 18e, and 4-Cl 18f substitutions on ring D are favored and demonstrated comparatively better activity. Of the thiouridyl-based NHIO analogues, 4-OCF3 substituted compound 18n demonstrated the best activity with a MIC value of 0.25 μg/mL.
Based on the screening results of sulfonyl- and uridyl/thiouridyl-containing NHIO analogues, key structural features essential for anti-TB activity have been identified as follows: (i) the introduction of polar functionalities is acceptable, (ii) the presence of hydrophobic groups/atoms at the terminal rings are essential for activity, even in truncated analogues (compounds 6b and 6d), (iii) the presence of a sulfonyl group either between rings C and D or between rings D and E is also acceptable, and (iv) among the uridyl/thiouridyl analogues, uridyl-containing NHIO analogues are more favorable (Figure 3).
Figure 3.
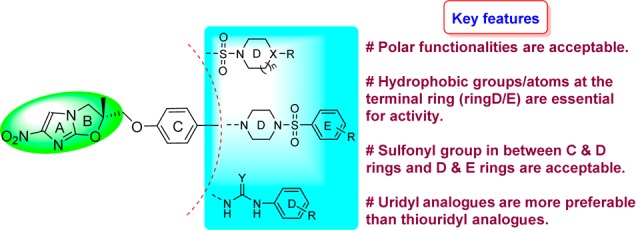
Structural–activity relationship of NHIO analogues and MTB H37Rv.
Based on their preliminary in vitro profiles against MTB H37Rv, NHIO analogues with MIC values ≤0.5 μg/mL were selected for an aqueous solubility study. The NHIO analogues 6b, 6d, 13d, 18d, 18f, and 18m demonstrated comparatively better aqueous solubility between 6.66 and 20 μg/mL. These analogues were further assessed for activity against resistant strains (i.e., RifR and MDR) and nonreplicating strains of MTB, and one analogue, 6d, was found to have single digit MIC values in all of the tested panels. Cytotoxicity was also evaluated in the HepG2 cell line. None of the tested compounds were toxic in HepG2 cell line, and all of the compounds had acceptable safety indices (Table 2). Among the six selected NHIO analogues, 6d had a comparatively better profile across the panel of assays performed.
Table 2. In Vitro Activity against Nonreplicating and Resistant Strains of MTBa and Cytotoxicity.
| compd | MIC (NRP)b (μg/mL) | MIC (RifR) (μg/mL) | MIC (MDR) (μg/mL) | CC50c (μg/mL) | SId |
|---|---|---|---|---|---|
| 6b | 10.67 | 3.34 | >8 | >20 | >40 |
| 6d | 1.34 | 1 | 2 | >20 | >40 |
| 13d | >8 | 1 | >8 | >20 | >333 |
| 18d | 1 | 1 | >8 | >20 | >80 |
| 18f | 4 | 0.67 | >8 | >20 | >80 |
| 18m | >8 | 2 | >8 | >20 | >80 |
| IIIM/MCD-019 | 4 | 0.06 | 0.12 | >20 | >166 |
| Delamanide | 0.37 | 0.005 | 0.02 | 107.5 | 10710 |
| Rifampicin | 2 | 256 | 128 | ||
| GATI | 1 | 0.5 |
Values reported are the average of three individual measurements.
Nonreplicating phase of M.tb.
Cytotoxicity (concentration causing death of 50% of cells; CC50) to HepG2 cells.
Selectivity index: CC50/MIC.
Data for the delamanid was reported in ref (8).
The best NHIO analogue, 6d, was further assessed for solubility, microsomal stability, and in vivo oral pharmacokinetics (data shown in Table 3). Compound 6d demonstrated a promising solubility of 20 μg/mL under all of the tested biological conditions (PBS, SGF, and SIF). In microsomal stability studies, compound 6d was found to have good stability, and 99.32% of the compound remained after 30 min in rat liver microsomes. Compound 6d was evaluated by a snapshot in vivo oral PK study and was found to have a Cmax value of 0.64 μg/mL and absorbed quickly into systemic circulation (Tmax = 0.5 h). This compound also possesses a higher volume of distribution (Vd = 12,970 mL/kg) than both an earlier identified lead (IIIM/MCD-019) and delamanid, moderate exposure with an AUC0-t of 0.879 μg/mL·h, a 2 h half-life (t1/2 = 1.99 h), and moderate clearance (Cl = 75.2 mL/min/kg).
Table 3. Solubility, Microsomal Stability, and in Vivo Pharmacokinetic Studies in Mice.
| Solubility
(μg/mL)a |
Microsomal stabilitya | PK
parameterse |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| compd | PBSb | SGFc | SIFd | % remaining after 30 min in RLM | Cmax(μg/mL) | AUC0-t (μg/mL·h) | Tmax (h) | t1/2 (h) | Vd (mL/kg) | CI (mL/min/kg) |
| 6d | 20 | 20 | 20 | 99.32 | 0.64 | 0.879 | 0.5 | 1.99 | 12,970 | 75.2 |
| IIIM/MCD-019 | <1 | <1 | <1 | 90.84 | 0.54 | 7.428 | 2.00 | 4.01 | 377 | 0.38 |
Data were the average of three individual measurements.
PBS: phosphate buffer solution (pH 7.4).
SGF: simulated gastric fluid (pH 1.2).
SIF: simulated intestinal fluid (pH 6.8).
P.O. at 5 mg/kg and each value represents average of five determinations. Pharmacokinetic parameters were calculated by WINNONLIN software.
The in vivo efficacy of compound 6d was further evaluated in an intranasal model of acute infection in Balb/C mice (Figure 4). One week post-MTB infection, compound 6d was orally administered at 25 mg/kg once daily for 5 days. Rifampicin (20 mg/kg) and IIIM/MCD-019 (100 mg/kg) were used as controls. Compound 6d significantly reduced the bacterial load, demonstrating a 1.28 log CFU reduction compared to the early control (group at the start of treatment) and a 1.61 log CFU reduction compared to the untreated control (late control group run in parallel without drug treatment). Compound 6d demonstrated better in vivo efficacy than our previously identified lead compound, IIIM/MCD-019. Moreover, all of the mice remained healthy during the course of treatment.
Figure 4.
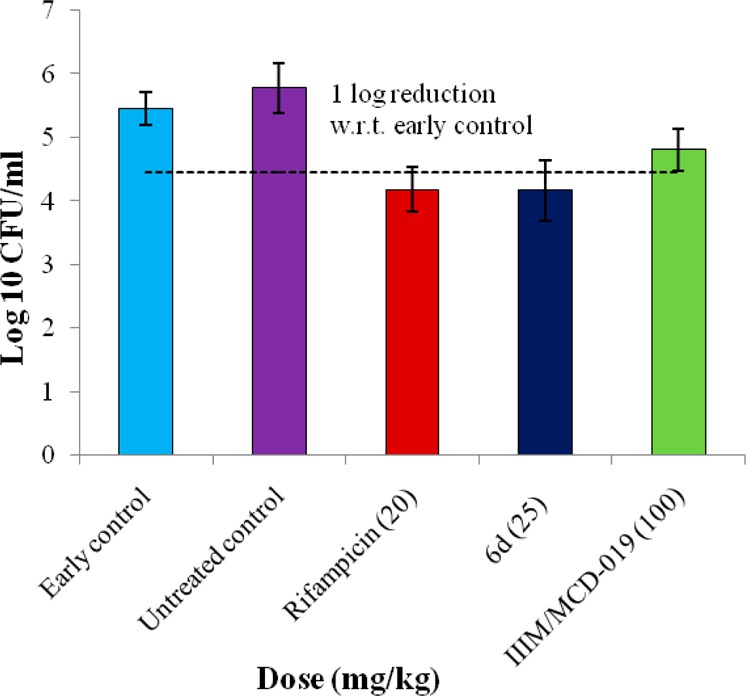
In vivo activity of 6d in intranasal model of acute infection in Balb/c mice. The mice were orally dosed once daily for 5 days (n = 6) starting on the day after infection with 105 CFU of MTB.
In conclusion, the present study suggests that the introduction of polar functionalities between rings D and C/E is acceptable. Overall, 28 new NHIO-based analogues were synthesized and evaluated against Mycobacterium tuberculosis (MTB) H37Rv. Potent compounds with MIC values ≤0.5 μg/mL were further selected for the determination of aqueous solubility. Based on the MIC values (H37Rv) and the aqueous solubility data, six compounds were tested against both resistant (RifR and MDR) and dormant strains of MTB, and cytotoxicity was evaluated in the HepG2 cell line. Compound 6d was found to have a good in vitro profile and to exhibit good aqueous solubility. In in vivo studies, compound 6d exhibited a promising PK profile and showed significant log CFU reductions in an intranasal mouse model of acute infection. The present study presents new opportunities for modifications to compounds with poor physicochemical characteristics and provides a novel compound with a simple structure, low molecular weight, and improved aqueous solubility.
Acknowledgments
K.R.Y., G.M., S.Si,, S,Sh., and S.K. thank CSIR and UGC for their fellowship. The authors thank Prof. Serge Mignani for providing valuable suggestions for the selection of the best compounds to investigate in detail.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00202.
Full experimental details for the synthesized compounds, NMR and MS spectra, and descriptions of biological assays (PDF)
Author Contributions
# K.R.Y., G.M., and S.Si. contributed equally to this work. K.R.Y. and G.M. performed the chemical synthesis. S.Si. performed in vitro and in vivo anti-TB experiments. S.Sh. helped with chemical synthesis. S.K. performed cytotoxic experiments. G.K., R.C., P.W., and G.D.S. performed microsomal stability and in vivo PK studies. S.S.B. performed the solubility study. P.P.S. and K.R.Y. drafted the manuscript. P.P.S., I.A.K., and R.A.V. participated in the design and execution of this study.
This work was supported by the Council of Scientific and Industrial Research (CSIR) and CSIR-OSDD, New Delhi with research grants #BSC0205 and #HCP 0001, respectively. This work is assigned the IIIM Publication No: IIIM/1803/2015.
The authors declare no competing financial interest.
Supplementary Material
References
- Ditiu L. A new era for global tuberculosis control. Lancet 2011, 378, 1293. 10.1016/S0140-6736(11)61567-5. [DOI] [PubMed] [Google Scholar]
- Zumla A.; Nahid P.; Cole S. T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discovery 2013, 12, 388–404. 10.1038/nrd4001. [DOI] [PubMed] [Google Scholar]
- Barry C. E. 3rd; Boshoff H. I.; Dowd C. S. Prospects for clinical introduction of nitroimidazole antibiotics for the treatment of tuberculosis. Curr. Pharm. Des. 2004, 10, 3239–3262. 10.2174/1381612043383214. [DOI] [PubMed] [Google Scholar]
- Matsumoto M.; Hashizume H.; Tomishige T.; Kawasaki M.; Tsubouchi H.; Sasaki H.; Shimokawa Y.; Komatsu M. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006, 3, 2131–2144. 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H.; Haraguchi Y.; Itotani M.; Kuroda H.; Hashizume H.; Tomishige T.; Kawasaki M.; Matsumoto M.; Komatsu M.; Tsubouchi H. Synthesis and antituberculosis activity of a novel series of optically active 6-nitro-2,3-dihydroimidazo[2,1-b]oxazoles. J. Med. Chem. 2006, 49, 7854–7860. 10.1021/jm060957y. [DOI] [PubMed] [Google Scholar]
- Ryan N. J.; Lo J. H. Delamanid: first global approval. Drugs 2014, 74, 1041–1045. 10.1007/s40265-014-0241-5. [DOI] [PubMed] [Google Scholar]
- Stover C. K.; Warrener P.; VanDevanter D. R.; Sherman D. R.; Arain T. M.; Langhorne M. H.; Anderson S. W.; Towell J. A.; Yuan Y.; McMurray D. N.; Kreiswirth B. N.; Barry C. E.; Baker W. R. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 2000, 405, 962–966. 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- Upton A. M.; Cho S.; Yang T. J.; Kim Y.; Wang Y.; Lu Y.; Wang B.; Xu J.; Mdluli K.; Ma Z.; Franzblau S. G. In vitro and in vivo activities of the nitroimidazole TBA-354 against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 136–144. 10.1128/AAC.03823-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TB alliance drug, TBA-354, enters phase-I clinical trials, 2015. http://www.newtbdrugs.org/blog/tb-alliance-drugtba-354-enters-phase-i-clinical-trials/.
- Barry C. E.; Crick D. C.; McNeil M. R. Targeting the formation of the cell wall core of M. tuberculosis. Infect. Disord.: Drug Targets 2007, 7, 182–202. 10.2174/187152607781001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers E. C.; Mancera R. L. New anti-tuberculosis drugs with novel mechanisms of action. Curr. Med. Chem. 2008, 15, 1956–1967. 10.2174/092986708785132906. [DOI] [PubMed] [Google Scholar]
- Kmentova I.; Sutherland H. S.; Palmer B. D.; Blaser A.; Franzblau S. G.; Wan B.; Wang Y.; Ma Z.; Denny W. A.; Thompson A. M. Synthesis and structure-activity relationships of aza- and diazabiphenyl analogues of the antitubercular drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1, 3]oxazine (PA-824). J. Med. Chem. 2010, 53, 8421–8439. 10.1021/jm101288t. [DOI] [PubMed] [Google Scholar]
- Lakshminarayana S. B.; Boshoff H. I.; Cherian J.; Ravindran S.; Goh A.; Jiricek J.; Nanjundappa M.; Nayyar A.; Gurumurthy M.; Singh R.; Dick T.; Blasco F.; Barry C. E. 3rd; Ho P. C.; Manjunatha U. H. Pharmacokinetics-pharmacodynamics analysis of bicyclic 4-nitroimidazole analogs in a murine model of tuberculosis. PLoS One 2014, 9, e105222. 10.1371/journal.pone.0105222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmentova I.; Sutherland H. S.; Palmer B. D.; Blaser A.; Franzblau S. G.; Wan B.; Wang Y.; Ma Z.; Denny W. A.; Thompson A. M. Synthesis and Structure–Activity Relationships of Aza- and Diazabiphenyl Analogues of the Antitubercular Drug (6S)-2-Nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J. Med. Chem. 2010, 53, 8421–8439. 10.1021/jm101288t. [DOI] [PubMed] [Google Scholar]
- Palmer B. D.; Thompson A. M.; Sutherland H. S.; Blaser A.; Kmentova I.; Franzblau S. G.; Wan B.; Wang Y.; Ma Z.; Denny W. A. Synthesis and structure-activity studies of biphenyl analogues of the tuberculosis drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1, 3]oxazine (PA-824). J. Med. Chem. 2010, 53, 282–294. 10.1021/jm901207n. [DOI] [PubMed] [Google Scholar]
- Sutherland H. S.; Blaser A.; Kmentova I.; Franzblau S. G.; Wan B.; Wang Y.; Ma Z.; Palmer B. D.; Denny W. A.; Thompson A. M. Synthesis and structure-activity relationships of antitubercular 2-nitroimidazooxazines bearing heterocyclic side chains. J. Med. Chem. 2010, 53, 855–866. 10.1021/jm901378u. [DOI] [PubMed] [Google Scholar]
- Thompson A. M.; Sutherland H. S.; Palmer B. D.; Kmentova I.; Blaser A.; Franzblau S. G.; Wan B.; Wang Y.; Ma Z.; Denny W. A. Synthesis and structure-activity relationships of varied ether linker analogues of the antitubercular drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1, 3]oxazine (PA-824). J. Med. Chem. 2011, 54, 6563–6585. 10.1021/jm200377r. [DOI] [PubMed] [Google Scholar]
- Kim P.; Kang S.; Boshoff H. I.; Jiricek J.; Collins M.; Singh R.; Manjunatha U. H.; Niyomrattanakit P.; Zhang L.; Goodwin M.; Dick T.; Keller T. H.; Dowd C. S.; Barry C. E. 3rd Structure-activity relationships of antitubercular nitroimidazoles. 2. Determinants of aerobic activity and quantitative structure-activity relationships. J. Med. Chem. 2009, 52, 1329–1344. 10.1021/jm801374t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P.; Zhang L.; Manjunatha U. H.; Singh R.; Patel S.; Jiricek J.; Keller T. H.; Boshoff H. I.; Barry C. E. 3rd; Dowd C. S. Structure-activity relationships of antitubercular nitroimidazoles. 1. Structural features associated with aerobic and anaerobic activities of 4- and 5-nitroimidazoles. J. Med. Chem. 2009, 52, 1317–1328. 10.1021/jm801246z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munagala G.; Yempalla K. R.; Singh S.; Sharma S.; Kalia N. P.; Rajput V. S.; Kumar S.; Sawant S. D.; Khan I. A.; Vishwakarma R. A.; Singh P. P. Synthesis of new generation triazolyl- and isoxazolyl-containing 6-nitro-2,3-dihydroimidazooxazoles as anti-TB agents: in vitro, structure-activity relationship, pharmacokinetics and in vivo evaluation. Org. Biomol. Chem. 2015, 13, 3610–3624. 10.1039/C5OB00054H. [DOI] [PubMed] [Google Scholar]
- McOmie J. F. W.; Watts M. L.; West D. E. Demethylation of aryl methyl ethers by boron tribromide. Tetrahedron 1968, 24, 2289–2292. 10.1016/0040-4020(68)88130-X. [DOI] [Google Scholar]
- Wolfe J. P.; Tomori H.; Sadighi J. P.; Yin J.; Buchwald S. L. Simple, efficient catalyst system for the palladium-catalyzed amination of aryl chlorides, bromides, and triflates. J. Org. Chem. 2000, 65, 1158–1174. 10.1021/jo991699y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.