Abstract
Lessons from surface-initiated polymerization are applied to grow cell-penetrating poly(disulfide)s directly on substrates of free choice. Reductive depolymerization after cellular uptake should then release the native substrates and minimize toxicity. In the presence of thiolated substrates, propagators containing a strained disulfide from asparagusic or, preferably, lipoic acid and a guanidinium cation polymerize into poly(disulfide)s in less than 5 min at room temperature at pH 7. Substrate-initiated polymerization of cationic poly(disulfide)s and their depolymerization with dithiothreitol causes the appearance and disappearance of transport activity in fluorogenic vesicles. The same process is further characterized by gel-permeation chromatography and fluorescence resonance energy transfer.
Cell-penetrating peptides (CPPs) are short, polycationic peptides or protein domains that are used by viruses to enter cells.1,2 Their unique ability to transport linked substrates across lipid bilayer membranes has attracted great interest in biomedical applications. Substrates of varying sizes and properties, e.g., small fluorophores to proteins and quantum dots, have been successfully transported into cells using CPPs. The mechanism of cellular uptake is under debate, currently favored are endocytosxis (i.e., macropinocytosis) or passive diffusion across the membrane, depending on conditions. Multiple, moderately hydrophobic cations seem to be all that is needed. Guanidinium cations, as in arginine, are most common, alternatives include ammonium or phosphonium cations.1 The originally peptidic oligomer backbone has been extensively varied, covering oligocarbamates, β-peptides and several variations of synthetic polymers.1 Currently, cell-penetrating poly(disulfide)s are emerging as the cell-penetrating molecules of the future because their cytosolic degradation liberates the substrate and eliminates toxicity, one of the key disadvantages associated with CPPs.3-5 However, cell-penetrating poly-(disulfide)s have so far been used mainly in noncovalent polyplexes for gene transfection, and covalent attachment of substrates would be difficult with their preparation methods. We have found recently that poly(disulfide)s can be grown directly on solid substrates by surface-initiated ring-opening disulfide-exchange polymerization.6 Therefore, we wondered whether the same methodology could be used to prepare cell-penetrating poly(disulfide)s with covalently attached substrates in solution (Figure 1). Probes or drugs that contain thiol group but cannot penetrate cells without assistance are the ideal substrates, which could serve as an initiator to be appended with a membrane-active poly(disulfide). Thiolated siRNA, for instance, is commercially available. The generality of this approach promises a conceptually innovative solution for a central current challenge, i.e., the noninvasive, nontoxic delivery of unmodified substrates in well-defined, covalent systems rather than complex, noncovalent formulations. In this initial report on the topic, we describe the design, synthesis and evaluation of propagators for the substrate-initiated synthesis of cell-penetrating poly(disulfide)s. Their formation in less than 5 min at pH 7 and their depolymerization with 10 mM dithiothreitol (DTT) can be followed directly as appearance and disappearance of transport activity in fluorogenic vesicles.
Figure 1.
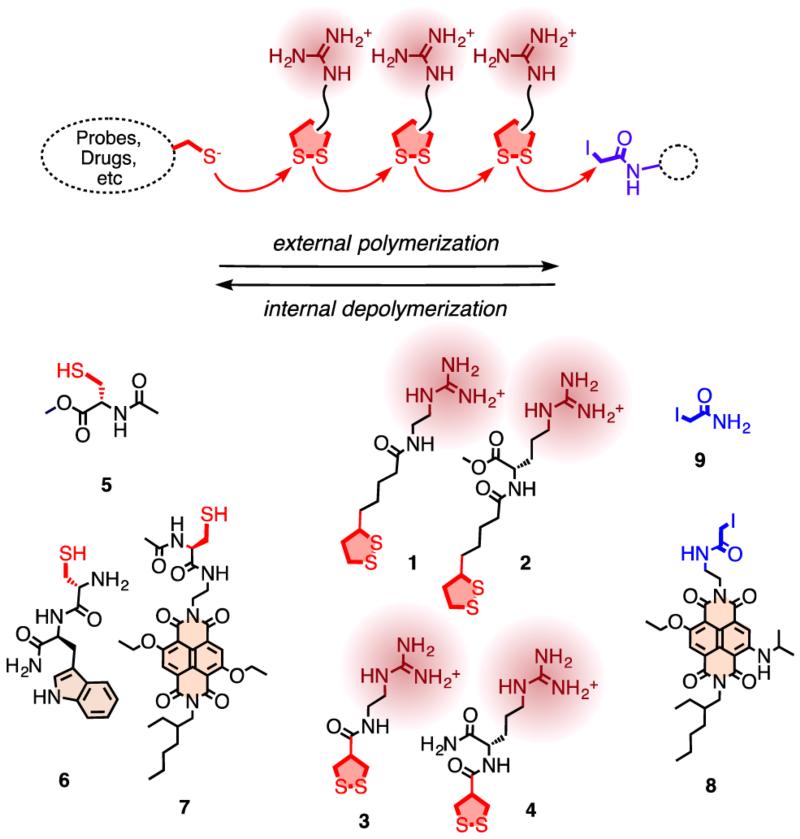
For the covalent delivery of unmodified probes, guanidinium-containing propagators (e.g., 1–4) are polymerized on thiolated substrates (e.g., 5–7) and terminated with iodoacetamides (e.g., 8, 9). After uptake, cell-penetrating poly(disulfide)s are degraded by reductive depolymerization to eliminate toxicity and release the unmodified substrate.
To ultimately combine surface-initiated polymerization6 with cellular uptake,1-5 we prepared the strained disulfides 1–4 as possible propagators, thiols 5–7 as initiators, and the iodoacetamides 8 and 9 as terminators (Figure 1). The synthesis of all new compounds was straightforward and is described in detail in the Supporting Information (SI Schemes S1–S7, Figures S10–S23).7 Only freshly prepared material was used.
Fluorogenic vesicles are convenient analytical tools to follow reactions with minimal effort and maximal speed.8 Here, EYPC-LUVs⊃CF, i.e., large unilamellar vesicles (LUVs) composed of egg yolk phosphatidylcholine (EYPC) and loaded with 5(6)-carboxyfluorescein (CF), were used. EYPC-LUVs⊃CF report CF release as fluorescence recovery because local dilution reduces self-quenching.
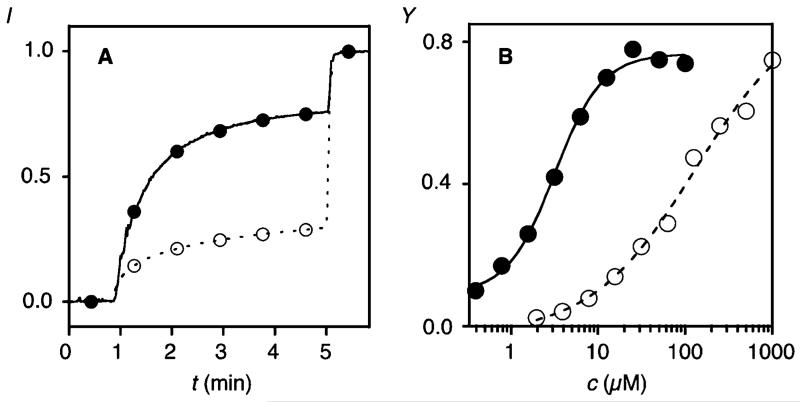
The addition of propagator 1 to EYPC-LUVs⊃CF caused CF release only above a relatively high EC50 = 129.9 ± 0.7 μM (Figures 2A, B, ○, S1–S3, S6). Ring-opening disulfide exchange polymerization3 of 1 (100 mM, pH 7, 1 M triethanolamine (TEOA) buffer), initiated with thiol 5 (5 mM), was followed by adding aliquots of the reaction mixture to EYPC-LUVs⊃CF after termination with iodoacetamide 9. Rapid fluorescence recovery was observed with increasing reaction time (Figure 3A, ●). At saturation, dose response curves were recorded for the obtained polymers (Figures 2A, B, ●, S1–S4). An EC50 = 3.2 ± 1.6 μM calculated to a 40-fold increase in activity upon substrate-initiated polymerization of propagator 1 with initiator 5 and terminator 9 (Figures 2B, ●, S2–S4).
Figure 2.
Activity of monomers (○) and polymers (●). (A) Change in CF emission intensity I during the addition of reaction mixtures with and without initiator 5 (50 s, 75 μM final guanidinium (monomer 1) concentration) and excess Triton X100 (300 s) to EYPC-LUVs⊃CF. Reaction mixtures: 100 mM 1, 0 (○) or 5 mM 5 (●), 1 M TEOA, pH 7, 10 min; termination: 5 mM 9. (B) Transport activity Y of 1 before (○) and after polymerization (●, 5 mM 5) with increasing concentration of guanidinium cations (Y = I before lysis in A (~5 min)).
Figure 3.
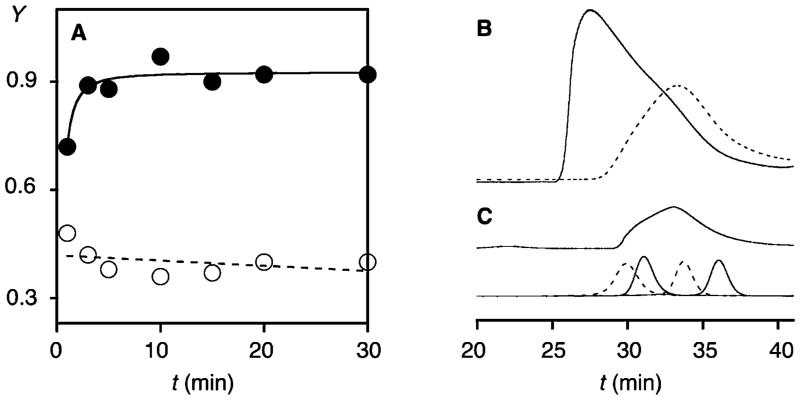
Polymers of 1 made with (●, solid) and without (○, dashed) initiator 5. (A) Y during polymerization of 1 (100 mM) with (●) and without 5 (○, 5 mM, pH 7). (B) GPC of 1 (100 mM) polymerized with (solid) and without 5 (dashed, 5 mM, pH 7), compared to (C) polyarginine (top) and standards (bottom, MW 43, 25, 13.7, and 6.5 kDa); Superdex 75, 30% acetonitrile in acetate buffer, pH 6.5.
According to activity in EYPC-LUVs⊃CF, substrate-initiated polymerization of propagator 1 was accomplished in less than 5 min (Figure 3A, ●). Polymerization was better in the presence than in the absence of initiator 5 (Figures 3A, S2–S4). This conclusion was supported by gel-permeation chromatography (GPC). Polymers obtained from propagator 1 in the presence of initiator 5 were of high molecular weight (Mw = 62.7 kDa) and dispersity (PDI = 1.83, Figure 3B, solid). Considering increasing transport activity with polymer length but less predictable length-dependence of cellular uptake of CPPs, high molecular weight and dispersity compared to commercially available polyarginine (Mw = 16.7 kDa, PDI = 1.7, Figure 3C, solid) were both very desirable characteristics. The same was true for the disappearance of all activity in EYPC-LUVs⊃CF within minutes of incubation with 10 mM DTT (Figure S7).7 This is in the range of cytosolic glutathione (~5 mM) and thus confirms the previously reported biodegradability of cell-penetrating poly(disulfide)s.3,4 Without initiator, weaker absorbance, i.e., lower yield of polymer, at lower Mw ~ 16.2 kDa was observed (PDI = 2.1, Figure 3B, dashed).
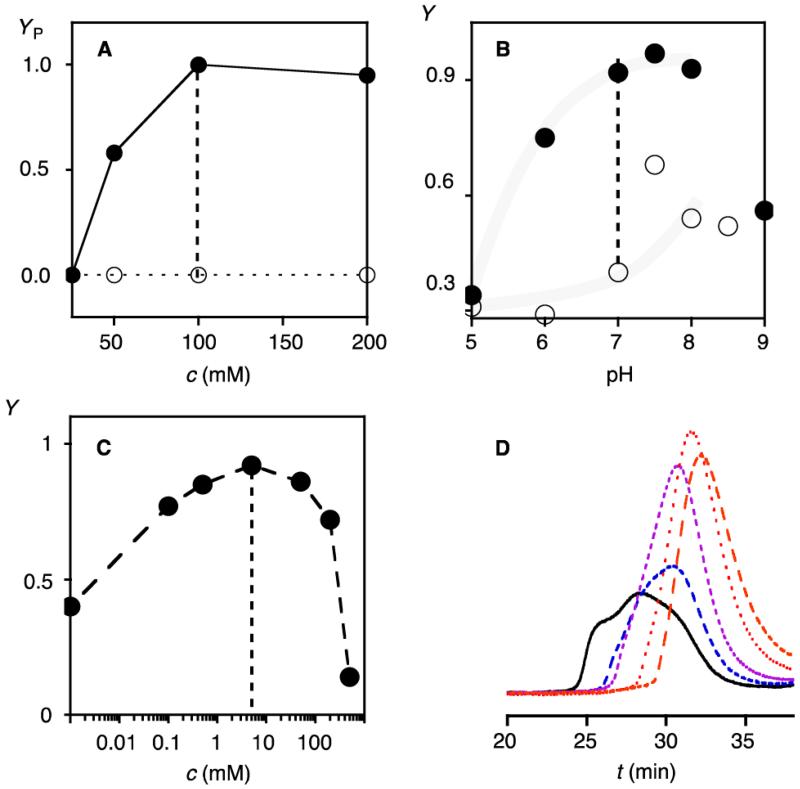
The polymerization of propagator 1 was analyzed systematically from pH 5 to pH 9 and concentrations from 25 to 200 mM in the presence and the absence of 5 mM initiator 5 (pKa ~ 9.5, Figure 4A, B). Best results were obtained with 100 mM 1 in 1 M TEOA, pH 7 (Figure 4A, B, dotted lines). At lower pH and concentrations, the substrate-initiate polymerization was incomplete, at higher pH, random polymerization without initiator as well as precipitation started to interfere.
Figure 4.
Dependence on propagator concentration (A), pH (B) and initiator concentration (C, D). (A) YP after polymerization of increasing concentration c of 1 with (●) and without 5 (○, 5 mM, 1 M TEOA, pH 7, 10 min; assay: 75 μM guanidinium each, YP = Y normalized to Y = 0 before polymerization and Y = 1 for maximal activity). (B) Y after polymerization with increasing pH (5 mM 5 (●), 0 mM 5 (○), 100 mM 1, 1 M buffer).7 (C) Y after polymerization with increasing concentration of 5 (100 mM 1, pH 7). (D) GPC after polymerization with 5 (with increasing tR: 0.5, 1, 2, 5, 10 mM; 200 mM 2, pH 7.5).
The bell-shaped dependence on initiator concentration was in agreement with the formation of fewer polymers at low and more but shorter and thus less active ones at high initiator concentrations (Figure 4C). Corroborative evidence for the incorporation of the initiators into the polymers was obtained by GPC. Polymers obtained from propagator 2 and increasing concentrations of initiator 5 gave polymers with decreasing molecular weight and dispersity (Figure 4D). Moreover, polymers obtained with Cys-Trp initiator 6 showed the tryptophan emission in the GPC peak. The relative Trp emission increased with decreasing molecular weight, that is decreasing polymer/initiator ratio (Figure S8).7
The substrate-initiated polymerization of propagators 1 and 2 with the strained disulfides from lipoic acid was straightforward to control and optimize. The disulfides from asparagusic acid are ideal for surface-initiated polymerization6 but turned out to be too reactive9 for substrate-initiated polymerization in solution. Independent of their backbone, propagators 3 and 4 more easily polymerized with less difference between substrate-initiated and random polymerization without initiator (Figure S6). Moreover, cell-penetrating poly(disulfide)s obtained from lipoyl propagators 1 and 2 were active in EYPC LUVs, whereas the less lipophilic polymers from asparagusyl propagators 3 and 4 were poorly active. However, like arginine-rich CPPs,2 all polymers could be activated in EYPC LUVs by counterions such as pyrenebutyrate (Figures S5, S6).7
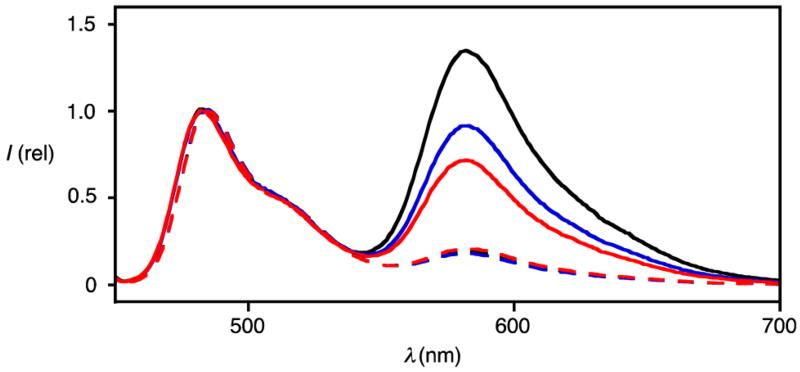
To probe for substrate-initiated polymerization also with the less perfect asparagusyl propagators, fluorescence resonance energy transfer (FRET) was considered as a method complementary to the functional studies with fluorogenic LUVs and GPC described above for the preferable lipoyl propagators. Polymerization of propagator 4 in CHCl3/DMF 3:1 was initiated with the yellow, green-fluorescent naphthalenediimide (NDI)6 fluorophore 7 (λex = 469 nm, λem = 484 nm) and 0.25% Hünig base (DIEA) as base, and terminated with the red NDI 8 (λex = 552 nm, λem = 582 nm, Figure 5). With increasing polymerization time, the FRET emission at λem = 582 nm in CHCl3 decreased (Figure 5). This decrease was consistent with increasing distance between initiator and terminator with increasing reaction time. Depolymerization with DTT caused nearly complete disappearance of FRET (Figure 5, dotted). These results further demonstrated the incorporation of the terminator in the polymer.
Figure 5.
FRET from initiator 7 to terminator 8. Emission spectra (λex = 445 nm, CHCl3) after polymerization of 4 (25 mM) with 7 (1 mM) for 5 s (black), 30 s (blue) and 60 s (red) in CHCl3/DMF 3:1 (0.25% DIEA), terminated with 8 (2 mM, solid) and depolymerized with DTT (10 mM, dashed).
In summary, substrate-initiated polymerization of cell-penetrating poly(disulfide)s is introduced as a conceptually new approach to cellular uptake. Two types of propagators and four unrelated methods to prove direct growth of polymers on substrates are described. Namely, (a) polymers obtained with and without initiators are different, (b) the dependence on initiator concentration is bell-shaped, (c) labeled initiators are eluted with polymers in GPC, and (d) FRET between donating initiators and accepting terminators decreases with polymerization time. Ring-opening disulfide exchange polymerization with propagators derived from lipoic acid is facile to control (pH, concentration of initiators, propagators, etc.) and gives polymers with high, stimuli-responsive transport activity in neutral lipid bilayers, whereas propagators derived from asparagusic acid are too reactive and give polymers that require counterion activation for function. With these complete, clear and consistent results, the newly introduced system is ready for cellular uptake experiments10 and copolymerization studies1a,6b,f to modulate the properties of the cell-penetrating poly(disulfide)s.
Supplementary Material
ACKNOWLEDGMENTS
We thank D. Jeannerat, A. Pinto and S. Grass for NMR measurements, the Sciences Mass Spectrometry (SMS) platform for mass spectrometry services, and the University of Geneva, the European Research Council (ERC Advanced Investigator), the National Centre of Competence in Research (NCCR) in Chemical Biology and the Swiss NSF for financial support.
Footnotes
ASSOCIATED CONTENT
Details on experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Tezgel AO, Gonzalez-Perez G, Telfer JC, Osborne BA, Minter LM, Tew GN. Mol. Ther. 2013;21:201–209. doi: 10.1038/mt.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Som A, Reuter A, Tew GN. Angew. Chem., Int. Ed. 2012;51:980–983. doi: 10.1002/anie.201104624. [DOI] [PubMed] [Google Scholar]; (c) Appelbaum JS, LaRochelle JR, Smith BA, Balkin DM, Holub JM, Schepartz A. Chem. Biol. 2012;19:819–830. doi: 10.1016/j.chembiol.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ornelas-Megiatto C, Wich PR, Fréchet JM. J. Am. Chem. Soc. 2012;134:1902–1905. doi: 10.1021/ja207366k. [DOI] [PubMed] [Google Scholar]; (e) Treat NJ, Smith D, Teng C, Flores JD, Abel BA, York AW, Huang F, McCormick CL. ACS Macro Lett. 2012;1:100–104. doi: 10.1021/mz200012p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Budhathoki-Uprety J, Peng L, Melander C, Novak BM. ACS Macro Lett. 2012;1:370–374. doi: 10.1021/mz200116k. [DOI] [PubMed] [Google Scholar]; (g) Song A, Walker SG, Parker KA, Sampson NS. ACS Chem. Biol. 2011;6:590–599. doi: 10.1021/cb100413w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Tezgel AO, Telfer JC, Tew GN. Biomacromolecules. 2011;12:3078–3083. doi: 10.1021/bm200694u. [DOI] [PubMed] [Google Scholar]; (i) Gorska K, Manicardi A, Barluenga S, Winssinger N. Chem. Commun. 2011;47:4364–4366. doi: 10.1039/c1cc10222b. [DOI] [PubMed] [Google Scholar]; (k) Dix AV, Fischer L, Sarrazin S, Redgate CPH, Esko JD, Tor Y. ChemBioChem. 2010;11:2302–2310. doi: 10.1002/cbic.201000399. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Inomata K, Ohno A, Tochio H, Isogai S, Tenno T, Nakase I, Takeuchi T, Futaki S, Ito Y, Hiroaki H, Shirakawa M. Nature. 2009;458:106–109. doi: 10.1038/nature07839. [DOI] [PubMed] [Google Scholar]; (m) McNaughton BR, Cronican JJ, Thompson DB, Liu DR. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6111–6116. doi: 10.1073/pnas.0807883106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Trantow BM, Cooley CB, Nederberg F, Kiesewetter MK, Hedrick JL, Waymouth RM, Wender PA. J. Am. Chem. Soc. 2009;131:16401–16403. doi: 10.1021/ja907363k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Kolonko EM, Pontrello JK, Mangold SL, Kiessling LL. J. Am. Chem. Soc. 2009;131:7327–7333. doi: 10.1021/ja809284s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Wender PA, Galliher WC, Goun EA, Jones LR, Pillow TH. Adv. Drug Delivery Rev. 2008;60:452–472. doi: 10.1016/j.addr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Esbjorner EK, Lincoln P, Norden B. Biochem. Biophys. Acta. 2007;1768:1550–1558. doi: 10.1016/j.bbamem.2007.03.004. [DOI] [PubMed] [Google Scholar]; (r) Shimanouchi T, Walde P, Gardiner J, Mahajan YR, Seebach D, Thomae A, Kraemer SD, Voser M;R, Kuboi R. Biochem. Biophys. Acta. 2007;1768:2726–2736. doi: 10.1016/j.bbamem.2007.07.011. [DOI] [PubMed] [Google Scholar]; (s) Kale AA, Torchilin VP. Bioconjugate Chem. 2007;18:363–370. doi: 10.1021/bc060228x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Martinell M, Salvatella X, Fernández-Carneado J, Gordo S, Feliz M, Menéndez M, Giralt E. ChemBioChem. 2006;7:1105–1113. doi: 10.1002/cbic.200500555. [DOI] [PubMed] [Google Scholar]; (t) Schmidt D, Jiang QX, MacKinnon R. Nature. 2006;444:775–779. doi: 10.1038/nature05416. [DOI] [PubMed] [Google Scholar]; (u) Fischer R, Fotin-Mleczek M, Hufnagel H, Brock R. ChemBioChem. 2005;6:2126–2142. doi: 10.1002/cbic.200500044. [DOI] [PubMed] [Google Scholar]; (v) Pantarotto D, Briand J-P, Prato M, Bianco A. Chem. Commun. 2004:16–17. doi: 10.1039/b311254c. [DOI] [PubMed] [Google Scholar]; (w) Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (x) Umezawa N, Gelman MA, Haigis MC, Raines RT, Gellman SH. J. Am. Chem. Soc. 2002;124:368–369. doi: 10.1021/ja017283v. [DOI] [PubMed] [Google Scholar]
- (2).(a) Sakai N, Matile S. J. Am. Chem. Soc. 2003;125:14348–14356. doi: 10.1021/ja037601l. [DOI] [PubMed] [Google Scholar]; (b) Takeuchi T, Kosuge M, Tadokoro A, Sugiura Y, Nishi M, Kawata M, Sakai N, Matile S, Futaki S. ACS Chem. Biol. 2006;1:299–303. doi: 10.1021/cb600127m. [DOI] [PubMed] [Google Scholar]
- (3).Bang E-K, Lista M, Sforazzini G, Sakai N, Matile S. Chem. Sci. 2012;3:1752–1763. [Google Scholar]
- (4).(a) Kim TI, Rothmund T, Kissel T, Kim SW. J. Controlled Release. 2011;152:110–119. doi: 10.1016/j.jconrel.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kim T, Kim SW. React. Funct. Polym. 2011;71:344–349. doi: 10.1016/j.reactfunctpolym.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lin C, Engbersen JF. J. Expert Opin. Drug Delivery. 2009;6:421–439. doi: 10.1517/17425240902878010. [DOI] [PubMed] [Google Scholar]; (d) Son S, Namgung R, Kim J, Singha K, Kim W. J. Acc. Chem. Res. 2011;152:110–119. doi: 10.1021/ar200248u. [DOI] [PubMed] [Google Scholar]; (e) Meng F, Hennink WE, Zhong Z. Biomaterials. 2009;30:2180–2198. doi: 10.1016/j.biomaterials.2009.01.026. [DOI] [PubMed] [Google Scholar]
- (5).Thiols at the cell surface have been proposed to further contribute to the uptake of disulfide-containing systems.; Torres AG, Gait MJ. Trends Biotechnol. 2012;30:185–190. doi: 10.1016/j.tibtech.2011.12.002. [DOI] [PubMed] [Google Scholar]
- (6).(a) Sakai N, Lista M, Kel O, Sakurai S, Emery D, Mareda J, Vauthey E, Matile S. J. Am. Chem. Soc. 2011;133:15224–15227. doi: 10.1021/ja203792n. [DOI] [PubMed] [Google Scholar]; (b) Lista M, Areephong J, Sakai N, Matile S. J. Am. Chem. Soc. 2011;133:15228–15230. doi: 10.1021/ja204020p. [DOI] [PubMed] [Google Scholar]; (c) Sakai N, Matile S. J. Am. Chem. Soc. 2011;133:18542–18545. doi: 10.1021/ja207587x. [DOI] [PubMed] [Google Scholar]; (d) Charbonnaz P, Sakai N, Matile S. Chem. Sci. 2012;3:1492–1496. [Google Scholar]; (e) Areephong J, Orentas E, Sakai N, Matile S. Chem. Commun. 2012;48:10618–10620. doi: 10.1039/c2cc35773a. [DOI] [PubMed] [Google Scholar]; (f) Orentas E, Lista M, Lin N-T, Sakai N, Matile S. Nat. Chem. 2012;4:746–750. doi: 10.1038/nchem.1429. [DOI] [PubMed] [Google Scholar]
- (7).See SI.
- (8).(a) Takeuchi T, Matile S. Chem. Commun. 2013;49:19–29. doi: 10.1039/c2cc36729g. [DOI] [PubMed] [Google Scholar]; (b) Litvinchuk S, Tanaka H, Miyatake T, Pasini D, Tanaka T, Bollot G, Mareda J, Matile S. Nat. Mater. 2007;6:576–580. doi: 10.1038/nmat1933. [DOI] [PubMed] [Google Scholar]; (c) Sakai N, Sordé N, Matile S. J. Am. Chem. Soc. 2003;125:7776–7777. doi: 10.1021/ja029845w. [DOI] [PubMed] [Google Scholar]; (d) Sordé N, Das G, Matile S. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11964–11969. doi: 10.1073/pnas.2132894100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Das G, Talukdar P, Matile S. Science. 2002;298:1600–1602. doi: 10.1126/science.1077353. [DOI] [PubMed] [Google Scholar]
- (9).Burns JA, Whitesides GM. J. Am. Chem. Soc. 1990;112:6296–6303. [Google Scholar]
- (10).Preliminary results with cell-penetrating poly(disulfide)s grown with 1 and 2 on fluorescent probes as initiators revealed efficient uptake into HeLa cells. These ongoing studies will be reported in due course.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.