Abstract
Cdc42 is a member of the Rho GTPase protein family that plays key roles in local F-actin organization through a number of kinase and non-kinase effector proteins. The myotonic dystrophy kinase-related Cdc42-binding kinases (MRCKs), and the RhoA binding coiled-coil containing kinases (ROCKs) are widely expressed members of the Dystrophia myotonica protein kinase (DMPK) family. The MRCK proteins are ∼190 kDa multi-domain proteins expressed in all cells and coordinate certain acto-myosin networks. Notably MRCK is a key regulator of myosin18A and myosin IIA/B, and through phosphorylation of their common regulatory light chains (MYL9 or MLC2) to promote actin stress fiber contractility. The MRCK kinases are regulated by Cdc42, which is required for cell polarity and directional migration; MRCK links to the acto-myosin complex through interaction with a coiled-coil containing adaptor proteins LRAP35a/b. The biological activities of MRCK in model organisms such as worms and flies confirm it as a myosin II activator. In mammalian cell culture MRCK can be critical for cancer cell migration and neurite outgrowth. We review the current literatures regarding MRCK and highlight the similarities and differences between MRCK and ROCK kinases.
Keywords: Rho GTPase, Cdc42, cell adhesion, acto-myosin
Background
The Rho family in mammals represents a group of 23 gene products 1 that in general are activated by guanine nucleotide exchange factors and inactivated by GTPase-activating proteins.2 Target (effector) proteins for these Rho proteins often contain recognizable sequence motifs involved with GTPase interaction that allow identification via database searches.3 The Rho proteins are hubs for cytoskeletal regulation: as such they are known to regulate F-actin assembly through formins,4 F-actin branching through WAVE and WASP proteins,5 septin assembly through Borg proteins,6 and intermediate filament reorganization through kinases such as Pak1.7
One of the key contractile systems in non-muscle cells is the acto-myosin network comprising myosin II heavy chains (different genes encode A, B orand C isoforms also termed MYH9, 10 and 14) which are regulated by a common regulatory light chain (MYL9 /, or MLC2).8 The myosin II system comprises alternating units of bipolar myosin filaments and actin filaments bundled by α-actinin. Recent evidence suggests that the different myosin isoforms can co-assemble into mixed bipolar filaments.9 MLC2 phosphorylation at Ser19 sometimes in combination with Thr18, controls the ATPase activity of the myosin II heavy chain, to generate contractile forces. Besides the classical myosin light chain kinases (MLCK) which is regulated by calcium/calmodulin,10 multiple Rho GTPase effector kinases are now implicated in this process, including Rho kinase (ROK/ROCK/Rho-kinase),11,12 citron kinase (CRIK),13 myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK),14 and p21-activated kinase 1 (PAK1).15
The founding member of these MLC2 kinases is Dystrophia myotonica protein kinase (DMPK) which belongs to the AGC super-family and is most closely related by sequence to MRCKα, sharing ∼60% sequence identity in the kinase domain (Fig. 1). There are 6 different DMPK isoforms 16 of which 4 are expressed independently of tissue type, while 2 are predominantly present in smooth muscle. All have molecular weights of 68–74 kDa which are much smaller than other family members. Changes within in the DMPK gene locus give rise to myotonic dystrophy type 1 (DM1), the most prevalent muscular dystrophy in adults. Genetic defects in DM1 cause amplification of a trinucleotide repeat in the 3' untranslated region of DMPK. It is thought that the disease results from both gain-of-function of the DMPK RNA and from decreased DMPK expression in the cytoplasm, as previously reviewed.17
Figure 1.
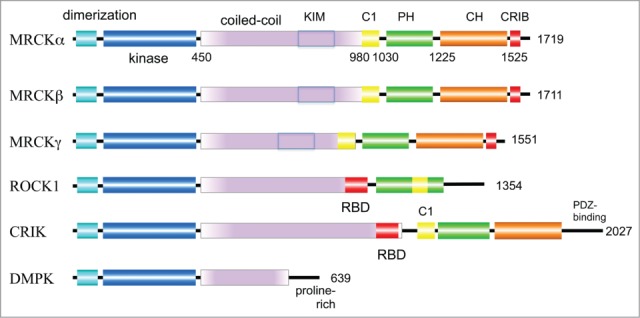
Domain organization of MRCK and related kinases.The size of the human proteins (number of residues) is indicated on the right of the schematic. All DMPK-like kinases contain an N-terminal region that forms a tight dimer and packs against the hydrophobic motif found at the C-terminal of the kinase domain (see Fig. 2). The length of the coiled-coil region that lies C-terminal to the kinase domain is variable between the various kinases; in the case of MRCK this region contain the kinase-inhibitory motif (KIM) that serves to autoinhibit the kinase. The three human MRCK isoforms have very similar domain structures, with MRCKγ being the smallest and most divergent. ROCK and CRIK contain a RhoA binding domain (RBD) whose coiled-coil structure serves to interact with the GTP-bound form of RhoA. The Cdc42 and Rac1 binding region (CRIB) by contrast is located at the C-terminal region of the polypeptide. The C1 domain of MRCK (yellow) interacts with diacylglycerol; the related C1 domain of ROCK, which however does not bind diacylglycerol, splits the PH domain (green). The citron homology domain (CH, brown) is found in MRCK and CRIK.
RhoA-ROCK activity is well established for maintaining actin stress fibers and stabilizing associated focal adhesion formation in cultured cell.18 ROCK also plays a key role in allowing tail retraction of migrating cells and prevention of cell rear protrusion.19 The roles of ROCK in this context has been recently reviewed in some detail.20 In some cell types MLCK has been shown to regulate focal complex formation during lamellipodial extension,21,22 but subsequently MRCK was identified as a key regulator of lamellar acto-myosin dynamics independent of MLCK and ROCK.23 The ability of these different MLC kinases to promote protrusion, traction and retraction must relate to both their unique localization in the cell and different target selectivity.24 The precise coordination of these kinases downstream of their respective Rho GTPase activators allows efficient cell movement.
Signaling pathways that arise from ‘classical’ phospholipase-C initiated signaling pathway allows Ca2+mobilization to activate MLCK,25 and diacylglycerol to activate MRCK.26 In non-muscle cells there is a great deal known about the RhoA-ROCK pathway that drives MLC phosphorylation because of the existence of the potent and selective small molecule inhibitors of ROCKs such as Y27632.27 The activity of this compound on ROCK was described in classic paper in 1997.28 In addition to ROCK and CRIK, the Myotonic dystrophy-related Cdc42-binding kinases MRCKα and MRCKβ14 and less widely expressed MRCKγ 29 contribute to MLC phosphorylation downstream of Cdc42.
The structural and primary sequence features of MRCK
Full-length rat MRCKα and MRCKβ are ∼180 kDa proteins that were identified through expression screening 14 using a strategy that previously uncovered the Cdc42 effector ACK1.30 Human MRCKα and MRCKβ kinases are closely related,31,32 and share the same functional domains with each other and MRCKγ.9,29 The MRCK proteins contain a C-terminally located Cdc42/Rac interaction binding (CRIB) domain (Fig. 1); MRCKα can also bind to Rac1.33 Interestingly the CRIB region of MRCKγ, which is expressed predominantly in muscle cells binds preferentially to TC10 versus Cdc42.29 This is consistent with TC10 being a muscle enriched Cdc42-like GTPase.34 Although Cdc42 is clearly upstream of MRCK, the potential for other Cdc42-like Rho GTPases (TCL, Chp, and Wrch) to recruit MRCK has not been studied in detail.
The organization of the kinase domain and flanking N-terminal and C-terminal sequences are similar to the myotonic dystrophy kinase (DMPK) family of kinases.35 The region N-terminal to the kinase domain is conserved across the family; MRCKα contains a functional binding site for phorbol 12-myristate 13-acetate (PMA; an analog of diacylglycerol, DAG) that is similar to the zinc finger C1 domains of PKC and chimaerin.14 The human gene for MRCKα is located at chromosome band 1q42.1 and extends over approximately 250–300 kb 31; no genetic lesions have been identified in this gene to date. The (C1) domains (Fig. 1) of MRCKα and MRCKβ are similar to conventional PKC isoforms and bind phorbol esters with nanomolar affinities.36,37 The binding of DAG to the C1 domains can drive kinase activation through a mechanism that appears to involve the flanking kinase-inhibitory motif (KIM).37 However the lower affinities of MRCK C1 domain for phorbol esters compared to homologous PKC C1 domains mean that this interaction alone not sufficient to translocate the MRCK to the plasma membrane.36 The Pleckstrin Homology (PH) domains (Fig. 1) are generally used in targeting proteins various lipid compartments, but the nature and function of the MRCK PH domain is not understood. All three MRCK proteins have a Citron Homology domain, which derives its name from the citron kinase CRIK, where the domain is similarly located next to the PH domain.38
Biochemical regulation of MRCK activity
The regulation of related ROCK kinases has been more extensively studied; auto-inhibition of the ROCK kinase domain (Fig. 1) via intramolecular interactions between the catalytic and the C-terminus of the kinase can be relieved via proteolytic cleavage, binding of lipids to a PH domain, or indeed binding of RhoA.GTP to the central coiled-coil region of ROCK.39 Although auto-phosphorylation of MRCKα was suggested for activation loop on sites including Ser234 based on substitution,37 activation-loop phosphorylation is not annotated in Phosphosite. The lack of documented auto-phosphorylation events (upon kinase activation) has prevented the development of phospho-specific antibodies capable of assessing MRCK activity in vivo. This has hampered proper analysis of the subcellular localization of active MRCK (the same situation prevails for ROCK and Citron kinases).
The crystal structure of the MRCKβ kinase domain has revealed a dimeric kinase structures highly related to ROCK1 and DMPK.40 The kinase domains of MRCKβ40 DMPK 41 and ROCK1 42 indicates its can adopt a compact ‘active’ conformation in an unphosphorylated state. The activation (A)-loop occupies a well-ordered conformation that does not impede access to the nucleotide or substrate binding sites (see red loop in Fig. 2). The MRCKβ A-loop lies below the αC helix (yellow), which holds ATP in a conformation competent for catalysis.40 As for the crystal structure of ROCK kinases,42 a C-terminal extension of the kinase domain termed the hydrophobic motif (dark blue in Fig. 2) serves to correctly position the αC helix (yellow). This motif contains 3 phenylalanine residues which contact the αC helix and also makes multiple contacts with the N-terminal extension of the kinase (MRCK residues 5–65) through both intramolecular (gray helices) and inter-molecular interactions (green helices) of the other subunit (b) of the dimer. Thus the N-terminal region likely plays a direct role in positioning the αC helix. Many AGC kinases (including DMPKs, the RhoA regulated PKNs and atypical PKCs contain the regulatory site termed the hydrophobic motif or “PIF-pocket” which in some kinases serves as a docking site for substrates. This site is also involved in the allosteric regulation of N-terminal domains of several AGC kinases. Recently it has been suggested that the ‘master regulator’ of AGC kinases PDK1 positively modulates MRCKα activity,43 although this does not involve the phosphorylation activity of PDK1.
Figure 2.
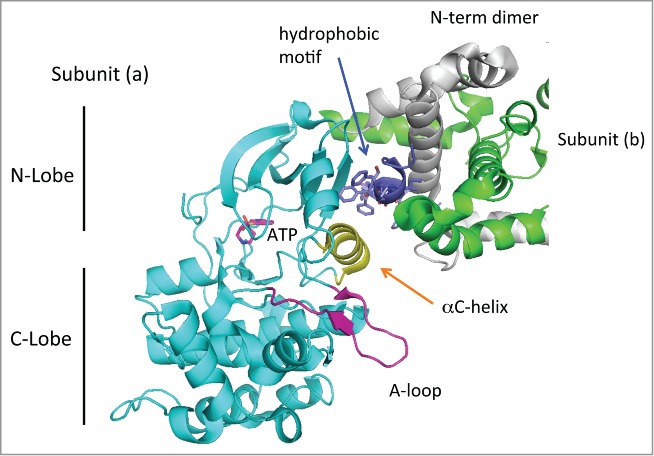
Structure of the dimeric MRCKβ catalytic domain. The dimeric MRCKβ is based on the coordinates in 3TKU. The catalytic domain reveals a typical structure, with the disposition of C- and N-lobe as indicated. The activation loop (purple) is fully ordered, and the aC-helix (yellow) lies in a position that would be compatible with catalysis. The position of ATP as indicated is based on binding of Fasudil in this crystal structure.40 The hydrophobic motif (dark blue) binds to the N-lobe as is typical for other kinases, but this region makes substantial contacts also with the N-terminal helices that are involved in dimerization. Thus the dimerization domain may be required for holding the hydrophobic motif.
MRCKα forms oligomers which by gel filtration chromatography correspond primarily to inactive tetramers of ∼900 kDa.37 The coiled-coil domain arrangement of the region downstream of the kinase domain indicates parallel intermolecular interactions as for ROCK.44 It is unclear whether Cdc42.GTP binding to MRCK plays any direct role in kinase activation, whereas RhoA.GTP binding to ROCK directly regulates kinase activity.45 The C-terminal domains of MRCK (including the CRIB) do interact with the kinase-containing N-terminal region.37 An auto-inhibitory region positioned within the coiled-coil region can bind and inhibit the MRCK catalytic domain29,37; this has been termed the kinase inhibitory motif (KIM, see Fig. 1). Removal of this sequence leads to elevated kinase activity.
Based on careful in vitro and in vivo analysis of MRCKα, kinase activation appears in part to require diacylglycerol (DAG) binding to the zinc finger region, leading to ∼3-fold MRCK activation.37 In co-transfection experiments no enhancement of MRCK activity is seen when Cdc42V12 is expressed with full-length kinase.37 Nonetheless siRNA mediated knockdown of Cdc42 in cells causes a loss of MRCK association with the acto-myosin network.23 This is associated with loss of circumferential myosin II filaments at the lamella region that are regulated by Cdc42, while the ventral stress fibers are unaffected. In summary, the mechanism by which the (active) kinase domains of MRCK, ROCK and Citron are regulated by their cognate Rho proteins remains largely unresolved.
The intracellular localization of MRCK
The tight interaction of Cdc42.GTP with the CRIB domain(s) has been shown as promoting the proper localization of MRCK. Depletion of Cdc42 by siRNA treatment leads to a profound loss of MRCK clusters associated with acto-myosin filaments in lamella of Hela cells.23 The association of MRCKα with myosin at this site is dependent on a small adaptor protein LRAP35a which allows it to form a direct tripartite complex with the heavy chain of myosin 18a designated MYO18A.23 The LRAP35a C-terminus is bound to the MYO18A PDZ domain, while its central coiled-coil region binds the KIM motif of MRCK. It seems likely therefore that oligomeric MRCK is localized through MYO18A to acto-myosin clusters, which in turn regulates the more abundant myosin IIA isoform (although MRCK may not directly bind to these 2 proteins) which is found in the lamella.23 Interestingly a smaller protein adaptor LRAP25, which shares sequence homology in the coiled-coil region with LRAP35a, has been reported to target MRCK to the cell edge or lamellipodium and allows it to interact with LIM kinase.46 It seems likely that MRCK is therefore targeted primarily by LRAP-like adaptors, with additional contributions Cdc42 or Rac1 binding and diacylglycerol interaction with the C1 domain. The ability of PDK1 and MRCKα to associate with each other in lamellipodia43 may require LRAP25.
The identification of Shroom3 as an adaptor for ROCK has been a key finding.47 It seems likely that the various Shroom proteins are essential for cellular targeting of ROCK1/2. The Shroom binding site on ROCK lies within the coiled-coil domain (in a similar position to that for LRAP35a in MRCK), and N-terminal to the RhoA binding site.48 This Shroom binding region is essential for ROCK-dependent apical constriction of epithelial cells. Interestingly the Drosophila CRIK ortholog (Sticky) localizes to the cleavage furrow of dividing cells independent of RhoA,49 while the coiled-coil region is necessary and sufficient for its localization. In post-mitotic neurons the mammalian Citron kinase similarly becomes concentrated at postsynaptic sites through interactions of the coiled-coil region.50 Thus several strands of evidence point to the coiled-coil domain as critical for the localization and function of DMPK family kinases, mediated by selective adaptor proteins.
MRCK kinase inhibitors
The ROCK and MRCK kinase domains being highly related are able to phosphorylate common substrates when tested in vitro. The widely used ROCK inhibitor Y-27632 and related compounds have been crucial to the identification of ROCK targets in vivo.27 Although Y-27632 is considered to be 10-fold less effective against MRCK isoforms than ROCK,40 there are no robust bio-markers for these kinases (for example phospho-speciific antibodies directed to the kinases themselves or specific substrates). Thus it is problematic to accurately assess MRCK in vivo selectivity. Even though Y-27632 is regarded as ROCK-selective, it inhibits the RhoA target PRK2 with similar affinity to ROCK.51,52 The ROCK inhibitor fasudil binds to MRCK, and crystals of the MRCKβ kinase domain in complex with fasudil and TCPA-1 40 revealed how these act as ATP-mimetics. Recently a potent MRCK selective inhibitor has been reported (abbreviated as BDP5290) that is more than 50-fold selective over ROCK.53 The X-ray structure of the MRCKß kinase domain in complex with BDP5290 indicates the basis of its selectivity.
The benzophen-anthridine alkaloid chelerythrine has been found to be selective pharmacological inhibitor for MRCK.24 No significant activity was found for PKCα nor other MLC kinases such as ROCK and MLCK,24 Chelerythrine does show some activity in vitro toward CRIK at 10 μM. With an IC50 for MRCK of 1.8 μM in vitro, and an effective concentration of 5.0 μM in cells, chelerythrine can pass through biological membranes and elicits cellular phenotypes that are consistent with MRCK inhibition.24 Chelerythrine does not target the ATP binding pocket and is potentially more specific than ATP-competitive inhibitors. Remarkably >1000 publications have used chelerythrine as a PKCα inhibitor since it was first described.54 However chelerythrine (at 10 μM) does not inhibit PKCα family kinases in vitro.51 It should be noted that chelerythrine has a number of non-kinase targets can complicate interpretation of phenotypes (Brunhofer et al., 2012; Basu et al., 2013). The KIM domain of MRCK can inhibit MRCK kinases in trans; over-expressing the MRCKγ KIM domain suppresses MRCKα in vivo.37 This genetically encoded pan-MRCK inhibitor has the potential to validate less selective chemical inhibitors, much like the PAK1 auto-inhibitory domain.55
MRCK substrates
There has been no systematic search for MRCK substrates, but based on structural and primary similarities kinase between ROCK and MRCK one might assume they can share targets. It was established early on that ROCK directly regulates myosin II through phosphorylation of its regulatory light chain (MLC2).56 Among the various DMPK family kinases, it is notable that ROCK and CRIK are capable of di-phosphorylation of MLC2 Thr18/Ser19, whereas MRCK (and MLCK) can only mono-phosphorylate Ser1924; thus one can partly differentiate the various MLC2 kinase activities in vivo.24 The double phosphorylation of MLC2 leads to changes in the contractility of the myosin II heavy chain.57 Surprisingly DMPK itself has no activity toward MLC2, although it can modify MYPT1 at Thr654, which is a key site to inhibit its phosphatase activity.58 Conversely in smooth muscle MLC2 is convincingly shown to be a direct target of the muscle enriched zipper-interacting protein kinase (ZIPK), but this kinase cannot phosphorylate MYPT1.59
Changes in MLC2 phosphorylation status induced by MRCKα 60 result from its direct phosphorylation, as well as inhibition of MYPT1 61,62 which is part of the myosin light chain phosphatase (MLCP) complex (Fig. 3). MLCP is composed of 3 subunits: a 38-kDa catalytic subunit (PP1δ), the 130-kDa myosin targeting subunit (MYPT1), and an additional 20-kDa subunit (M20) that has homology to the C-terminal portion of MYPT1. Both MYPT1 and the smaller p85 MYPT3 are substrates of MRCK.63 Phosphorylation of a centrally located conserved motif termed the phosphatase inhibitory motif (PIM) allows it to bind and inhibit (the associated) PP1δ.64 Therefore inhibition of MLCP activity can result from MYPT phosphorylation by multiple DMPK family kinases potentially over 3 sites that are conserved across the various isoforms and equivalent to MYPT1 Ser472, Thr697 and Thr855.65-67 The phosphorylation of Ser472 allows binding of 14-3-3 68 which blocks MYPT1 interaction with myosin heavy chain (Fig. 3). Thr697 phosphorylation inhibits MLCP activity by inactivating the catalytic PP1δ.69 The role of Thr850 phosphorylation is less clear but this modification also down-regulates MLCP activity.67,70
Figure 3.
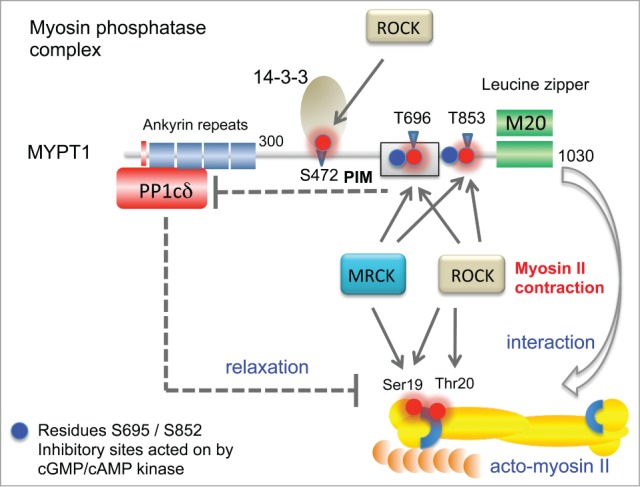
Features of the MYPT1 (PPP1R12A) phosphatase complex and its activity toward myosin regulatory light chain (MLC2). The catalytic PP1δ subunit binds MYPT1 at an RVxF motif found at amino acids 35–38 (red bar). Ankyrin repeats (x8) are further involved to bind the protein phosphatase catalytic subunit PP1c. When the complex is in the vicinity of the myosin complex, it can act on MLC2. MYPT1 can be phosphorylated at Thr696 (PIM, phosphatase inhibitory motif) by both ROCK and MRCK, which allows this region to inhibit the PP1c (dotted line). Although MYPT1 Thr853 phosphorylation is also inhibitory, the mechanism is not so well understood. Other important regulatory sites marked as blue spheres are regulated by cGMP- or cAMP-dependent kinases 71; these help maintain MYPT1 activity by preventing ROCK phosphorylation of neighboring residues. Phosphorylation of Ser472 generates a site that allows for recruitment of 14–3–3, which prevents myosin interaction. The third subunit of the MYPT1 holoenzyme is termed M20 which binds to and indeed resembles MYPT1 residues 934–1006. This region of the complex is thought to allow targeting the bipolar myosin heavy chain complexes as indicated (arrow).
The situation is further complicated by the observation that the Thr697 and Thr855 sites containing flanking serine residues which can be co-phosphorylated.71 Dual phosphorylation of MYPT1 occurs in vitro and in situ at both Ser696–Thr697 and Ser854–Thr855 (Fig. 3). Pre-phosphorylation of Ser696 and Ser854 inhibits the ability of ROCK to phosphorylate the neighboring Thr697 and Thr855. The role of Ser696 phosphorylation has been studied for some time.72,73 The mono-phosphorylation of Thr697 and Thr855 (by ROCK and presumably MRCK and CRIK) precludes the ability of PKAc to phosphorylate the neighboring Ser residues. Thus the interplay of inhibitory (Thr) and dis-inhibitory (Ser) neighboring modifications of MYPT1 regulate its activity.
Genetic experiments in Caenorhabditis elegans revealed that MRCK and ROCK orthologues contributed to phosphorylation of MLC and MYPT1 proteins, though a constitutively-active form of MLC could complement only loss of MRCK, but not ROCK.74 Thus the primary function of MRCK in this C. elegans developmental context is MLC regulation, while ROCK must have additional targets. In other situations MRCKα can act on LIM kinases 1 and 2, by phosphorylating their activation loop.75,76 This serves to stabilize F-actin by inactivating cofilin.77: cofilin serves to fragment and destabilize F-actin. Mammalian LIMK1 is required for synaptic architecture,78 while knocking out LIMK2 results in an eyelid defect in mice.79 Recently an adaptor LRAP25 has been described which links MRCK to LIMK1 46 in a similar way to LRAP35 coupling MRCK with MYO18a. The lamellipodium-localized LRAP25-MRCK complex allows activation of LIMK1, which promotes F-actin stability and allows membrane protrusion. In B16 cells inhibition of either MRCK or LRAP25 resulted in suppression of LIMK1 activity.46
Biological activities of MRCK
Since MRCK is a direct target of Cdc42, there has been strong interest uncovering those aspects of Cdc42 function that result from MRCK coupling. MRCK has been found in a number of contexts to promote cancer cell motility and invasiveness as recently reviewed.80 Cdc42 plays an important role in establishing cell polarity in a number of contexts, and its disregulation can be a factor in cancer progression.81 The actin-myosin contraction regulated by MRCK aids re-orientation of cell nuclei relative to microtubule-organizing center (MTOC), and this help establish the forward polarity of the Golgi apparatus in migrating cells.82 Cdc42 has other direct targets that are needed in this process including Par6 that recruits the atypical-PKC complex.83 MRCK forms clusters at the leading edge of cells and undergoes retrograde flow as is typical for acto-myosin associated proteins.23 This activity promotes re-orientation of cell nuclei relative to microtubule-organizing centers (MTOC) to establish polarity in migrating cells in a monolayer.82 In epithelial adherens junctions(AJs), circumferential actin bundles that align along the cell–cell junctions; non-muscle myosin IIA(NM-IIA) generates tension required for proper formation of AJs alignment.84,85 In contrast to epithelial cells, the junctional fibers of endothelial cells rely on NM-IIB recruited in a Rap1-dependent manner.86 This pathway requires MRCKβ to form circumferential actin bundles proximal to the plasma membrane that are important for its barrier function.87 By contrast Rho-ROCK promotes radial stress fibers and adherens junction clustering which reduces endothelial barrier function.87
To date no knockout mice targeting any of the 3 MRCK genes have been reported. In Drosophila, mutations of gek (a Drosophila homolog of MRCK) cause abnormal F-actin accumulation and, as expected, gek interacts genetically with Drosophila Cdc42.88 MRCKα activity can enhance the ability of Cdc42 to induced filopodia 14 which could relate to the ability of the kinase to activate ERM proteins61 Interestingly MRCK (like Cdc42) is permissive for neurite outgrowth in PC12 cells,89 an activity opposite to that of RhoA-ROCK, which is commonly down-regulated during neuritogenesis in the central nervous system.90
Among the multiple kinases that contribute to MLC phosphorylation, MRCK and ROCK are likely expressed in all non-muscle cells. Several studies have found somewhat overlapping functions for MRCK and ROCK kinases; for example the ability of ephrinB to induce endothelial cell retraction required the combined blockade of ROCK and MRCK.91 Similarly reducing the level of both ROCK and MRCK can block MDA MB 231 breast cancer cell invasion into 3-dimensional protein matrices.62 This study showed that single cells move through a 3-dimensional matrix with either a rounded morphology, characterized by Rho-ROCK dependence, or with an elongated morphology characterized by Cdc42-MRCK. The kinase is also implicated in in collective cancer cell invasion.92 Thus Cdc42-MRCK and Rho-ROCK signals can drive in vivo cancer cell motility albeit via quite different modes of migration.80
Conclusions
MRCK kinases belong to a relatively restricted group of kinases that are key regulators acto-myosin contractility; this activity in turn play critical roles in cell adhesion, motility and division. It is notable that ROCK and MRCK are probably targeted by interaction with selective adaptor proteins such as Shroom and LRAP35, which serve to independently target these kinases in the cell. The localization of such kinases also involves membrane-binding domains, notably the zinc finger C1-like and PH domains. The future development of tools to identify active forms of MRCK (and ROCK) will be essential to better understand these abundant and important kinases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Wherlock M, Mellor H. The Rho GTPase family: a Racs to Wrchs story. J Cell Sci 2002; 115:239-40; PMID:11839775 [DOI] [PubMed] [Google Scholar]
- 2.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629-35; PMID:12478284; http://dx.doi.org/ 10.1038/nature01148 [DOI] [PubMed] [Google Scholar]
- 3.Pirone DM, Carter DE, Burbelo PD. Evolutionary expansion of CRIB-containing Cdc42 effector proteins. Trends Genet 2001; 17:370-3; PMID:11418196; http://dx.doi.org/ 10.1016/S0168-9525(01)02311-3 [DOI] [PubMed] [Google Scholar]
- 4.Daou P, Hasan S, Breitsprecher D, Baudelet E, Camoin L, Audebert S, Goode BL, Badache A. Essential and nonredundant roles for Diaphanous formins in cortical microtubule capture and directed cell migration. Mol Biol Cell 2014; 25:658-68; PMID:24403606; http://dx.doi.org/ 10.1091/mbc.E13-08-0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blundell MP, Worth A, Bouma G, Thrasher AJ. The Wiskott-Aldrich syndrome: the actin cytoskeleton and immune cell function. Dis Markers 2010; 29:157-75; PMID:21178275; http://dx.doi.org/ 10.1155/2010/781523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinoshita M. Assembly of mammalian septins. J Biochem (Tokyo) 2003; 134:491-6; http://dx.doi.org/ 10.1093/jb/mvg182 [DOI] [PubMed] [Google Scholar]
- 7.Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res 2007; 313:2098-109; PMID:17498690; http://dx.doi.org/ 10.1016/j.yexcr.2007.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 2008; 9:690-701; PMID:18719708; http://dx.doi.org/ 10.1038/nrm2476 [DOI] [PubMed] [Google Scholar]
- 9.Beach JR, Shao L, Remmert K, Li D, Betzig E, Hammer JA, 3rd. Nonmuscle myosin II isoforms coassemble in living cells. Curr Biol: CB 2014; 24:1160-6; PMID:24814144; http://dx.doi.org/ 10.1016/j.cub.2014.03.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 2001; 276:4527-30; PMID:11096123; http://dx.doi.org/ 10.1074/jbc.R000028200 [DOI] [PubMed] [Google Scholar]
- 11.Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem 1997; 272:12257-60; PMID:9139666; http://dx.doi.org/ 10.1074/jbc.272.19.12257 [DOI] [PubMed] [Google Scholar]
- 12.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 1996; 16:5313-27; PMID:8816443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madaule P, Furuyashiki T, Eda M, Bito H, Ishizaki T, Narumiya S. Citron, a Rho target that affects contractility during cytokinesis. Microsc Res Tech 2000; 49:123-6; PMID:10816250; http://dx.doi.org/ 10.1002/(SICI)1097-0029(20000415)49:2%3c123::AID-JEMT3%3e3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- 14.Leung T, Chen XQ, Tan I, Manser E, Lim L. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol Cell Biol 1998; 18:130-40; PMID:9418861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol 1999; 145:837-49; PMID:10330410; http://dx.doi.org/ 10.1083/jcb.145.4.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groenen PJ, Wansink DG, Coerwinkel M, van den Broek W, Jansen G, Wieringa B. Constitutive and regulated modes of splicing produce six major myotonic dystrophy protein kinase (DMPK) isoforms with distinct properties. Hum Mol Genet 2000; 9:605-16; PMID:10699184; http://dx.doi.org/ 10.1093/hmg/9.4.605 [DOI] [PubMed] [Google Scholar]
- 17.Wansink DG, van Herpen RE, Coerwinkel-Driessen MM, Groenen PJ, Hemmings BA, Wieringa B. Alternative splicing controls myotonic dystrophy protein kinase structure, enzymatic activity, and subcellular localization. Mol Cell Biol 2003; 23:5489-501; PMID:12897125; http://dx.doi.org/ 10.1128/MCB.23.16.5489-5501.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett 1997; 404:118-24; PMID:9119047; http://dx.doi.org/ 10.1016/S0014-5793(97)00107-5 [DOI] [PubMed] [Google Scholar]
- 19.Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol 2001; 154:147-60; PMID:11448997; http://dx.doi.org/ 10.1083/jcb.200103048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases 2014; 5:e29846; PMID:25010901; http://dx.doi.org/ 10.4161/sgtp.29846; 279: 34156-64; PMID:1519468415016377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannone G, Dubin-Thaler BJ, Dobereiner HG, Kieffer N, Bresnick AR, Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell 2004; 116:431-43; PMID:15016377; http://dx.doi.org/ 10.1016/S0092-8674(04)00058-3 [DOI] [PubMed] [Google Scholar]
- 22.Katoh K, Kano Y, Amano M, Kaibuchi K, Fujiwara K. Stress fiber organization regulated by MLCK and Rho-kinase in cultured human fibroblasts. Am J Physiol Cell Physiol 2001; 280:C1669-79; PMID:11350763 [DOI] [PubMed] [Google Scholar]
- 23.Tan I, Yong J, Dong JM, Lim L, Leung T. A tripartite complex containing MRCK modulates lamellar actomyosin retrograde flow. Cell 2008; 135:123-36; PMID:18854160; http://dx.doi.org/ 10.1016/j.cell.2008.09.018 [DOI] [PubMed] [Google Scholar]
- 24.Tan I, Lai J, Yong J, Li SF, Leung T. Chelerythrine perturbs lamellar actomyosin filaments by selective inhibition of myotonic dystrophy kinase-related Cdc42-binding kinase. FEBS Lett 2011; 585:1260-8; PMID:21457715; http://dx.doi.org/ 10.1016/j.febslet.2011.03.054 [DOI] [PubMed] [Google Scholar]
- 25.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 2003; 83:1325-58; PMID:14506307 [DOI] [PubMed] [Google Scholar]
- 26.Hall C, Lim L, Leung T. C1, see them all. Trends Biochem Sci 2005; 30:169-71; PMID:15817391; http://dx.doi.org/ 10.1016/j.tibs.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 27.Rath N, Olson MF. Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep 2012; 13:900-8; PMID:22964758; http://dx.doi.org/ 10.1038/embor.2012.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997; 389:990-4; PMID:9353125; http://dx.doi.org/ 10.1038/40187 [DOI] [PubMed] [Google Scholar]
- 29.Ng Y, Tan I, Lim L, Leung T. Expression of the human Myotonic Dystrophy kinase-related Cdc42-binding kinase gamma is regulated by promoter DNA methylation and Sp1 binding. J Biol Chem 2004; 279:34156-64; PMID:151946848497321 [DOI] [PubMed] [Google Scholar]
- 30.Manser E, Leung T, Salihuddin H, Tan L, Lim L. A non-receptor tyrosine kinase that inhibits the GTPase activity of p21cdc42. Nature 1993; 363:364-7; PMID:8497321; http://dx.doi.org/ 10.1038/363364a0 [DOI] [PubMed] [Google Scholar]
- 31.Moncrieff CL, Bailey ME, Morrison N, Johnson KJ. Cloning and chromosomal localization of human Cdc42-binding protein kinase beta. Genomics 1999; 57:297-300; PMID:10198171; http://dx.doi.org/ 10.1006/geno.1999.5769 [DOI] [PubMed] [Google Scholar]
- 32.Tan I, Cheong A, Lim L, Leung T. Genomic organization of human myotonic dystrophy kinase-related Cdc42-binding kinase alpha reveals multiple alternative splicing and functional diversity. Gene 2003; 304:107-15; PMID:12568720; http://dx.doi.org/ 10.1016/S0378-1119(02)01185-X [DOI] [PubMed] [Google Scholar]
- 33.Schwarz J, Proff J, Havemeier A, Ladwein M, Rottner K, Barlag B, Pich A, Tatge H, Just I, Gerhard R. Serine-71 phosphorylation of Rac1 modulates downstream signaling. PLoS One 2012; 7:e44358; PMID:22970203; http://dx.doi.org/ 10.1371/journal.pone.0044358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neudauer CL, Joberty G, Tatsis N, Macara IG. Distinct cellular effects and interactions of the Rho-family GTPase TC10. Curr Biol: CB 1998; 8:1151-60; PMID:9799731 [http://dx.doi.org/ 10.1016/S0960-9822(07)00486-1 [DOI] [PubMed] [Google Scholar]
- 35.Garcia P, Ucurum Z, Bucher R, Svergun DI, Huber T, Lustig A, Konarev PV, Marino M, Mayans O. Molecular insights into the self-assembly mechanism of dystrophia myotonica kinase. Faseb J 2006; 20:1142-51; PMID:16770013; http://dx.doi.org/ 10.1096/fj.05-5262com [DOI] [PubMed] [Google Scholar]
- 36.Choi SH, Czifra G, Kedei N, Lewin NE, Lazar J, Pu Y, Marquez VE, Blumberg PM. Characterization of the interaction of phorbol esters with the C1 domain of MRCK (myotonic dystrophy kinase-related Cdc42 binding kinase) alpha/beta. J Biol Chem 2008; 283:10543-9; PMID:18263588; http://dx.doi.org/ 10.1074/jbc.M707463200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan I, Seow KT, Lim L, Leung T. Intermolecular and intramolecular interactions regulate catalytic activity of myotonic dystrophy kinase-related Cdc42-binding kinase alpha. Mol Cell Biol 2001b; 21:2767-78; PMID:11283256; http://dx.doi.org/ 10.1128/MCB.21.8.2767-2778.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madaule P, Eda M, Watanabe N, Fujisawa K, Matsuoka T, Bito H, Ishizaki T, Narumiya S. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature 1998; 394:491-4; PMID:9697773; http://dx.doi.org/ 10.1038/28873 [DOI] [PubMed] [Google Scholar]
- 39.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 2003; 4:446-56; PMID:12778124; http://dx.doi.org/ 10.1038/nrm1128 [DOI] [PubMed] [Google Scholar]
- 40.Heikkila T, Wheatley E, Crighton D, Schroder E, Boakes A, Kaye SJ, Mezna M, Pang L, Rushbrooke M, Turnbull A, et al. Co-crystal structures of inhibitors with MRCKbeta, a key regulator of tumor cell invasion. PLoS One 2011; 6:e24825; PMID:21949762; http://dx.doi.org/ 10.1371/journal.pone.0024825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elkins JM, Amos A, Niesen FH, Pike AC, Fedorov O, Knapp S. Structure of dystrophia myotonica protein kinase. Protein Sci: Pub Protein Soc 2009; 18:782-91; PMID:19309729; http://dx.doi.org/ 10.1002/pro.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobs M, Hayakawa K, Swenson L, Bellon S, Fleming M, Taslimi P, Doran J. The structure of dimeric ROCK I reveals the mechanism for ligand selectivity. J Biol Chem 2006; 281:260-8; PMID:16249185; http://dx.doi.org/ 10.1074/jbc.M508847200 [DOI] [PubMed] [Google Scholar]
- 43.Gagliardi PA, di Blasio L, Puliafito A, Seano G, Sessa R, Chianale F, Leung T, Bussolino F, Primo L. PDK1-mediated activation of MRCKalpha regulates directional cell migration and lamellipodia retraction. J Cell Biol 2014; 206:415-34; PMID:25092657; http://dx.doi.org/ 10.1083/jcb.201312090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dvorsky R, Blumenstein L, Vetter IR, Ahmadian MR. Structural insights into the interaction of ROCKI with the switch regions of RhoA. J Biol Chem 2004; 279:7098-104; PMID:14660612; http://dx.doi.org/ 10.1074/jbc.M311911200 [DOI] [PubMed] [Google Scholar]
- 45.Chen XQ, Tan I, Ng CH, Hall C, Lim L, Leung T. Characterization of RhoA-binding kinase ROKalpha implication of the pleckstrin homology domain in ROKalpha function using region-specific antibodies. J Biol Chem 2002; 277:12680-8; PMID:11815607; http://dx.doi.org/ 10.1074/jbc.M109839200 [DOI] [PubMed] [Google Scholar]
- 46.Lee IC, Leung T, Tan I. Adaptor protein LRAP25 mediates MRCK regulation of LIMK1 in lamellipodial F-actin dynamics. J Biol Chem 2014; 289:26989-7003; PMID:25107909; http://dx.doi.org/ 10.1074/jbc.M114.588079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimura T, Takeichi M. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development 2008; 135:1493-502; PMID:18339671; http://dx.doi.org/ 10.1242/dev.019646 [DOI] [PubMed] [Google Scholar]
- 48.Mohan S, Das D, Bauer RJ, Heroux A, Zalewski JK, Heber S, Dosunmu-Ogunbi AM, Trakselis MA, Hildebrand JD, Vandemark AP. Structure of a highly conserved domain of Rock1 required for Shroom-mediated regulation of cell morphology. PLoS One 2013; 8:e81075; PMID:24349032; http://dx.doi.org/ 10.1371/annotation/f9aef9a9-5488-4492-8b8c-1cdded2e3d79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bassi ZI, Verbrugghe KJ, Capalbo L, Gregory S, Montembault E, Glover DM, D'Avino PP. Sticky/Citron kinase maintains proper RhoA localization at the cleavage site during cytokinesis. J Cell Biol 2011; 195:595-603; PMID:22084308; http://dx.doi.org/ 10.1083/jcb.201105136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W, Benson DL. Targeting and clustering citron to synapses. Mol Cell Neurosci 2006; 31:26-36; PMID:16202622; http://dx.doi.org/ 10.1016/j.mcn.2005.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000; 351:95-105; PMID:10998351; http://dx.doi.org/ 10.1042/0264-6021:3510095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SJ, Kim JH, Sun JM, Kim MG, Oh JW. Suppression of hepatitis C virus replication by protein kinase C-related kinase 2 inhibitors that block phosphorylation of viral RNA polymerase. J Viral Hepat 2009; 16:697-704; PMID:19243496; http://dx.doi.org/ 10.1111/j.1365-2893.2009.01108.x [DOI] [PubMed] [Google Scholar]
- 53.Unbekandt M, Croft DR, Crighton D, Mezna M, McArthur D, McConnell P, Schuttelkopf AW, Belshaw S, Pannifer A, Sime M, et al. A novel small-molecule MRCK inhibitor blocks cancer cell invasion. Cell Commun Signal 2014; 12:54; PMID:25288205; http://dx.doi.org/ 10.1186/s12964-014-0054-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 1990; 172:993-9; PMID:2244923; http://dx.doi.org/ 10.1016/0006-291X(90)91544-3 [DOI] [PubMed] [Google Scholar]
- 55.Zhao ZS, Manser E, Chen XQ, Chong C, Leung T, Lim L. A conserved negative regulatory region in alphaPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol 1998; 18:2153-63; PMID:9528787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Begum N, Duddy N, Sandu O, Reinzie J, Ragolia L. Regulation of myosin-bound protein phosphatase by insulin in vascular smooth muscle cells: evaluation of the role of Rho kinase and phosphatidylinositol-3-kinase-dependent signaling pathways. Mol Endocrinol 2000; 14:1365-76; PMID:10976915; http://dx.doi.org/ 10.1210/mend.14.9.0522 [DOI] [PubMed] [Google Scholar]
- 57.Mizutani T, Haga H, Koyama Y, Takahashi M, Kawabata K. Diphosphorylation of the myosin regulatory light chain enhances the tension acting on stress fibers in fibroblasts. J Cell Physiol 2006; 209:726-31; PMID:16924661; http://dx.doi.org/ 10.1002/jcp.20773 [DOI] [PubMed] [Google Scholar]
- 58.Muranyi A, MacDonald JA, Deng JT, Wilson DP, Haystead TA, Walsh MP, Erdodi F, Kiss E, Wu Y, Hartshorne DJ. Phosphorylation of the myosin phosphatase target subunit by integrin-linked kinase. Biochem J 2002; 366:211-6; PMID:12030846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moffat LD, Brown SB, Grassie ME, Ulke-Lemee A, Williamson LM, Walsh MP, MacDonald JA. Chemical genetics of zipper-interacting protein kinase reveal myosin light chain as a bona fide substrate in permeabilized arterial smooth muscle. J Biol Chem 2011; 286:36978-91; PMID:21880706; http://dx.doi.org/ 10.1074/jbc.M111.257949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan I, Ng CH, Lim L, Leung T. Phosphorylation of a novel myosin binding subunit of protein phosphatase 1 reveals a conserved mechanism in the regulation of actin cytoskeleton. J Biol Chem 2001a; 276:21209-16; PMID:11399775; http://dx.doi.org/ 10.1074/jbc.M102615200 [DOI] [PubMed] [Google Scholar]
- 61.Nakamura N, Oshiro N, Fukata Y, Amano M, Fukata M, Kuroda S, Matsuura Y, Leung T, Lim L, Kaibuchi K. Phosphorylation of ERM proteins at filopodia induced by Cdc42. Genes Cells: Dev Mol Cell Mech 2000; 5:571-81; PMID:10947843; http://dx.doi.org/ 10.1046/j.1365-2443.2000.00348.x [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson S, Paterson HF, Marshall CJ. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol 2005; 7:255-61; PMID:15723050; http://dx.doi.org/ 10.1038/ncb1230 [DOI] [PubMed] [Google Scholar]
- 63.Yong J, Tan I, Lim L, Leung T. Phosphorylation of myosin phosphatase targeting subunit 3 (MYPT3) and regulation of protein phosphatase 1 by protein kinase A. J Biol Chem 2006; 281:31202-11; PMID:16920702; http://dx.doi.org/ 10.1074/jbc.M607287200 [DOI] [PubMed] [Google Scholar]
- 64.Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem 2004; 259:197-209; PMID:15124925; http://dx.doi.org/ 10.1023/B:MCBI.0000021373.14288.00 [DOI] [PubMed] [Google Scholar]
- 65.Ichikawa K, Ito M, Hartshorne DJ. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. J Biol Chem 1996; 271:4733-40; PMID:8617739; http://dx.doi.org/ 10.1074/jbc.271.9.4733 [DOI] [PubMed] [Google Scholar]
- 66.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996; 273:245-8; PMID:8662509; http://dx.doi.org/ 10.1126/science.273.5272.245 [DOI] [PubMed] [Google Scholar]
- 67.Muranyi A, Derkach D, Erdodi F, Kiss A, Ito M, Hartshorne DJ. Phosphorylation of Thr695 and Thr850 on the myosin phosphatase target subunit: inhibitory effects and occurrence in A7r5 cells. FEBS Lett 2005; 579:6611-5; PMID:16297917; http://dx.doi.org/ 10.1016/j.febslet.2005.10.055 [DOI] [PubMed] [Google Scholar]
- 68.Koga Y, Ikebe M. A novel regulatory mechanism of myosin light chain phosphorylation via binding of 14-3-3 to myosin phosphatase. Mol Biol Cell 2008; 19:1062-71; PMID:18094049; http://dx.doi.org/ 10.1091/mbc.E07-07-0668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terrak M, Kerff F, Langsetmo K, Tao T, Dominguez R. Structural basis of protein phosphatase 1 regulation. Nature 2004; 429:780-4; PMID:15164081; http://dx.doi.org/ 10.1038/nature02582 [DOI] [PubMed] [Google Scholar]
- 70.Velasco G, Armstrong C, Morrice N, Frame S, Cohen P. Phosphorylation of the regulatory subunit of smooth muscle protein phosphatase 1M at Thr850 induces its dissociation from myosin. FEBS Lett 2002; 527:101-4; PMID:12220642; http://dx.doi.org/ 10.1016/S0014-5793(02)03175-7 [DOI] [PubMed] [Google Scholar]
- 71.Grassie ME, Sutherland C, Ulke-Lemee A, Chappellaz M, Kiss E, Walsh MP, MacDonald JA. Cross-talk between Rho-associated kinase and cyclic nucleotide-dependent kinase signaling pathways in the regulation of smooth muscle myosin light chain phosphatase. J Biol Chem 2012; 287:36356-69; PMID:22948155; http://dx.doi.org/ 10.1074/jbc.M112.398479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sriwai W, Zhou H, Murthy KS. G(q)-dependent signalling by the lysophosphatidic acid receptor LPA(3) in gastric smooth muscle: reciprocal regulation of MYPT1 phosphorylation by Rho kinase and cAMP-independent PKA. Biochem J 2008; 411:543-51; PMID:18237278; http://dx.doi.org/ 10.1042/BJ20071299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem 2004; 279:34496-504; PMID:15194681; http://dx.doi.org/ 10.1074/jbc.M405957200 [DOI] [PubMed] [Google Scholar]
- 74.Gally C, Wissler F, Zahreddine H, Quintin S, Landmann F, Labouesse M. Myosin II regulation during C. elegans embryonic elongation: LET-502/ROCK, MRCK-1 and PAK-1, three kinases with different roles. Development 2009; 136:3109-19; PMID:19675126; http://dx.doi.org/ 10.1242/dev.039412 [DOI] [PubMed] [Google Scholar]
- 75.Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem 2001a; 276:670-6; PMID:11018042; http://dx.doi.org/ 10.1074/jbc.M007074200 [DOI] [PubMed] [Google Scholar]
- 76.Sumi T, Matsumoto K, Shibuya A, Nakamura T. Activation of LIM kinases by myotonic dystrophy kinase-related Cdc42-binding kinase alpha. J Biol Chem 2001b; 276:23092-6; PMID:11340065; http://dx.doi.org/ 10.1074/jbc.C100196200 [DOI] [PubMed] [Google Scholar]
- 77.Mizuno K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signalling 2013; 25:457-69; PMID:23153585; http://dx.doi.org/ 10.1016/j.cellsig.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 78.Meng Y, Zhang Y, Tregoubov V, Falls DL, Jia Z. Regulation of spine morphology and synaptic function by LIMK and the actin cytoskeleton. Rev Neurosci 2003; 14:233-40; PMID:14513866; http://dx.doi.org/ 10.1515/REVNEURO.2003.14.3.233 [DOI] [PubMed] [Google Scholar]
- 79.Rice DS, Hansen GM, Liu F, Crist MJ, Newhouse MM, Potter D, Xu N, Abuin A, Vogel PJ, Zambrowicz BP. Keratinocyte migration in the developing eyelid requires LIMK2. PLoS One 2012; 7:e47168; PMID:23071748; http://dx.doi.org/ 10.1371/journal.pone.0047168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Unbekandt M, Olson MF. The actin-myosin regulatory MRCK kinases: regulation, biological functions and associations with human cancer. J Mol Med 2014; 92:217-25; PMID:24553779; http://dx.doi.org/ 10.1007/s00109-014-1133-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stengel K, Zheng Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signalling 2011; 23:1415-23; PMID:21515363; http://dx.doi.org/ 10.1016/j.cellsig.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 2005; 121:451-63; PMID:15882626; http://dx.doi.org/ 10.1016/j.cell.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 83.McCormack J, Welsh NJ, Braga VM. Cycling around cell-cell adhesion with Rho GTPase regulators. J Cell Sci 2013; 126:379-91; PMID:23547086; http://dx.doi.org/ 10.1242/jcs.097923 [DOI] [PubMed] [Google Scholar]
- 84.Gomez GA, McLachlan RW, Yap AS. Productive tension: force-sensing and homeostasis of cell-cell junctions. Trends Cell Biol 2011; 21:499-505; PMID:21763139; http://dx.doi.org/ 10.1016/j.tcb.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 85.Ratheesh A, Priya R, Yap AS. Coordinating Rho and Rac: the regulation of Rho GTPase signaling and cadherin junctions. Prog Mol Biol Transl Sci 2013; 116:49-68; PMID:23481190; http://dx.doi.org/ 10.1016/B978-0-12-394311-8.00003-0 [DOI] [PubMed] [Google Scholar]
- 86.Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol 2010; 12:696-702; PMID:20543839; http://dx.doi.org/ 10.1038/ncb2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ando K, Fukuhara S, Moriya T, Obara Y, Nakahata N, Mochizuki N. Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J Cell Biol 2013; 202:901-16; PMID:24019534; http://dx.doi.org/ 10.1083/jcb.201301115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luo L, Lee T, Tsai L, Tang G, Jan LY, Jan YN. Genghis Khan (Gek) as a putative effector for Drosophila Cdc42 and regulator of actin polymerization. Proc Natl Acad Sci U S A 1997; 94:12963-8; PMID:9371783; http://dx.doi.org/ 10.1073/pnas.94.24.12963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen XQ, Tan I, Leung T, Lim L. The myotonic dystrophy kinase-related Cdc42-binding kinase is involved in the regulation of neurite outgrowth in PC12 cells. J Biol Chem 1999; 274:19901-5; PMID:10391936; http://dx.doi.org/ 10.1074/jbc.274.28.19901 [DOI] [PubMed] [Google Scholar]
- 90.Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron 2000; 26:431-41; PMID:10839361; http://dx.doi.org/ 10.1016/S0896-6273(00)81175-7 [DOI] [PubMed] [Google Scholar]
- 91.Groeger G, Nobes CD. Co-operative Cdc42 and Rho signalling mediates ephrinB-triggered endothelial cell retraction. Biochem J 2007; 404:23-9; PMID:17300218; http://dx.doi.org/ 10.1042/BJ20070146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 2007; 9:1392-400; PMID:18037882; http://dx.doi.org/ 10.1038/ncb1658 [DOI] [PubMed] [Google Scholar]


