Abstract
Human implantation is a complex process requiring synchrony between a healthy embryo and a functionally competent or receptive endometrium. Diagnosis of endometrial receptivity (ER) has posed a challenge and so far most available tests have been subjective and lack accuracy and a predictive value. Microarray technology has allowed identification of the transcriptomic signature of the window of receptivity window of implantation (WOI). This technology has led to the development of a molecular diagnostic tool, the ER array (ERA) for diagnosis of ER. Use of this test in patients with recurrent implantation failure (RIF) has shown that the WOI is displaced in a quarter of these patients and use of a personalized embryo transfer (pET) on the day designated by ERA improves reproductive performance. Our results in the Indian population revealed an endometrial factor in 27.5% RIF patients, which was significantly greater than the non-RIF group 15% (P = 0.04). After pET, the overall ongoing pregnancy rate was 42.4% and implantation rate was 33%, which was at par with our in-vitro fertilization results over 1-year. We also performed ERA in patients with persistently thin endometrium, and it was reassuring to find that the endometrium in 75% of these patients was receptive despite being 6 mm or less. A pregnancy rate of 66.7% was achieved in this group. Though larger studies are required to validate these results ERA has become a useful tool in our diagnostic armamentarium for ER.
KEY WORDS: Endometrial receptivity, ERA, in-vitro fertilization, recurrent implantation failure, thin endometrium
INTRODUCTION
Human implantation is a highly complex and multifactorial process. Successful implantation at the very least, requires the presence of a healthy embryo, a receptive endometrium, a synchronized and successful molecular dialogue between the two and immune protection from the host. Due to lack of objective and accurate methods of assessment, endometrial receptivity (ER) is rarely investigated in an infertile patient or even prior to in-vitro fertilization (IVF). Advent of “Omics” that is, the analysis of biological samples using molecular profiling has revived interest in the study of ER particularly in the context of implantation failure (IF) in IVF.
The human endometrium is a dynamic tissue; it undergoes changes at multiple levels during the menstrual cycle in response to ovarian hormones and paracrine secretions. The endocrine and paracrine secretions control gene expression of the different endometrial cell types.[1] The proliferative phase, controlled by estrogen allows for the proliferation of stromal cells and glands and elongation of the spiral arteries. The postovulatory progesterone (P) rise brings about secretory changes and the endometrium acquires a receptive phenotype permitting implantation of the blastocyst. This period of receptivity is known as the “window of implantation” (WOI). The WOI opens on day 19 or 20 of the cycle and remains open for just 4–5 days at the time whenP reaches peak serum concentrations.[2] During the phase of receptivity, the endometrium undergoes morphological, cytoskeletal, biochemical, and genetic changes to become functionally competent. The ability to identify the endometrial WOI in the clinical setting would enhance the outcome of fertility treatments such as IVF.
MARKERS OF ENDOMETRIAL RECEPTIVITY
Diagnosis of ER has posed a challenge because of the lack of an accurate, noninvasive, and clinically applicable test. Histological, biochemical, and ultrasound markers of ER have been proposed for use to improve implantation rates (IRs) in IVF. Unfortunately, most of these methods are invasive and none have any predictive value. Though limited in value, ultrasound markers of ER are used in the clinical setting. ER is assessed on the basis of endometrial thickness, character, volume, and blood flow patterns.[3,4]
HISTOLOGICAL MARKERS
Noyes et al.[5] established morphological criteria to evaluate endometrial development and receptivity. For many decades, these criteria remained the mainstay for defining ER despite the huge inter and intra-cycle variability. The use of Noyes criteria to predict the WOI accurately has been questioned in recent years and randomized studies[6,7,8] have invalidated their use. Endometrial pinopods identified on electron microscopy also generated interest as a marker of ER.[9] Pinopods are cytoplasmic projections of the luminal epithelial cells, abundant during the WOI, thought to promote blastocyst adhesion. The presence of pinopods was demonstrated in post receptive endometrium, and this precluded their use as a useful marker of ER.[10]
BIOCHEMICAL MARKERS
A number of molecules present during the mid-secretory phase have been studied as markers of ER. The ones which have shown significant association with the WOI are the integrins, leukemia inhibitory factor, homeobox A10, mucin 1, calcitonin, and cyclo-oxygenase 2.[11] Many more are being investigated; however, none have found their place in the clinical setting.
MOLECULAR MARKERS
The various molecular approaches for the study of biological samples are collectively called the “Omics” and include - genomics (study of genes), epigenomics (study of epigenetic DNA modifications), transcriptomics (study of gene expression), proteomics (quantification of proteins), metabolomics and lipidomics (composition and quantification of metabolites and lipids). Currently, transcriptomics is considered the most established technology available for evaluation of the endometrial factor.
TRANSCRIPTOMICS OF THE HUMAN ENDOMETRIUM
DNA microarray technology, allows measurement of thousands of genes simultaneously, and its use to study the level of gene transcription in tissues has revolutionized medicine. The transcriptome reflects genes that are actively expressed at any given time within a specific cell population or tissue. Transcriptomics allows characterization of gene expression at the mRNA level, giving rise to a “sample-specific” molecular profile or its “Transcriptomic Signature.” This permits characterization of tissue function or disease phenotype.[12]
Analysis of transcriptomic profiles or signatures is done using exploratory methods and statistical tests. The exploratory methods used are trees, clustering, or principal component analysis (PCA). PCA is a statistical method that allows projecting higher-dimensional data onto a lower-dimensional space. When PCA is applied to data, samples with similar trends in their gene expression profiles tend to cluster close together in the plot[13] [Figure 1]. Hierarchical clustering with visual heat map representation and PCA's are the most widely used methods. Specialized software is used to analyze the microarray data. Visual heat map representations show the differential expression patterns between different samples and genes.[14] For endometrial dating, the phase assignment is made based on previous histological dating by at least two independent pathologists.[15]
Figure 1.
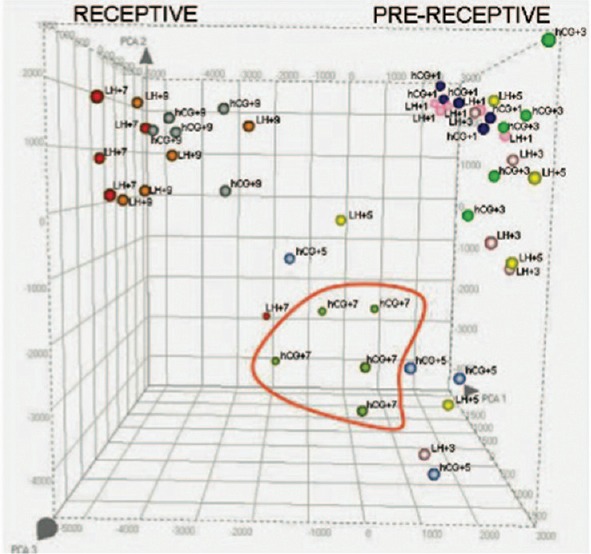
Principal component analysis of human endometrium throughout the development of the luteal phase in natural (LH + n) and controlled ovarian stimulation cycles (human chorionic gonadotropin + n). Reprinted with permission Horjcadas et al. J Clin Endocrinol Metab, November 2008, 93(11):4500-10
The transcriptome of the endometrium has been defined in all phases of the menstrual cycle;[16,17] clustering of genes into four groups was identified and these were consistent with histological phenotypes of proliferative (PE), early secretory (ESE), mid-secretory (MSE), and late-secretory (LSE) phases. The human endometrial transcriptome has been studied in women with a gynecological disease such as endometriosis and cancer.[18,19] Studies on the transcriptomics of human ER also gained impetus and ER transcriptome was studied in the natural cycle[20] and recurrent implantation failure (RIF).[21,22] Gene expression patterns have also been defined during controlled ovarian stimulation (COS) and hormonal replacement therapy (HRT) cycles.[23,24] Haouzi et al. 2010[25] compared the gene expression profile of ER between natural and stimulated cycles for the same patients. They observed that the gene transcription profile of the endometrial WOI under COS was defective for biological functions such as transforming growth factor beta signaling, leukocyte transendothelial migration, and the cell cycle. Extensive research on endometrial transcriptomics has allowed a provisional definition of the genomic signature of human ER and an understanding of alterations in the endometrium and ER that are associated with gynecological pathology and infertility.
At a molecular level, the prereceptive/early secretory phase is characterized by increased metabolic activity in preparation for implantation. This leads to a predominance of products related to cell metabolism (fatty acids, lipids, eicosanoids, and amino alcohols), transport, and germ cell migration.[14] There is an inhibition of mitosis during this phase as suggested by the downregulation of several growth factors.[17]
The receptive phase witnesses a “transcriptional awakening” or upregulation of most gene expression. Apart from a high level of metabolic and secretory activity there is also an upregulation of genes involved in the activation of the immune response.[17] During the late-secretory phase, the WOI closes and in this phase genes related to immune response-both cellular and humoral, blood coagulation, steroid bio-synthesis, and prostaglandin metabolism are regulated[26] [Figure 2].
Figure 2.
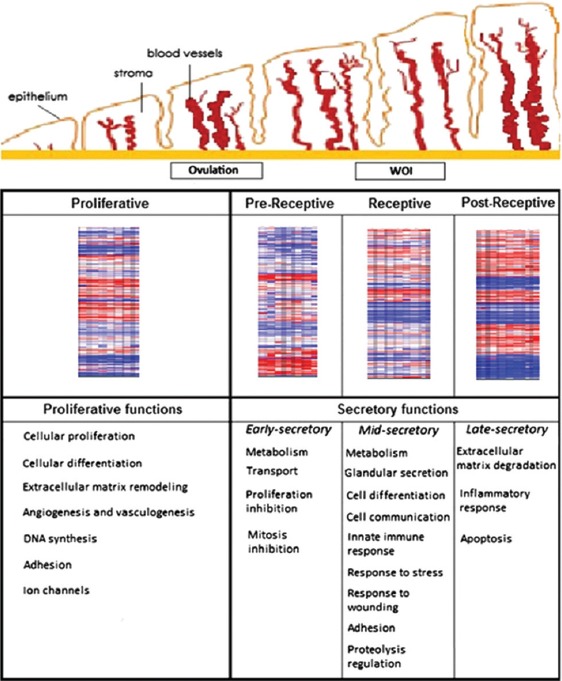
Evolution of endometrial tissue over time and the gene expression profile at each given stage. Heat map showing ERA gene expression profiles in each endometrial cycle stage (PE, prereceptive, receptive, and postreceptive) and the major biological functions regulated in each of these phases. Reprinted with permission from Garrido-Goˇmez. Genomics of endometrial receptivity. Fertil Steril 2013
ENDOMETRIAL RECEPTIVITY ARRAY
Search for an adequate marker of ER, led to the development of a molecular diagnostic test – the endometrial receptivity array (ERA). ERA consists of a customized microarray based on the transcriptomic signature of human ER, specifically when the human endometrium is receptive to blastocyst adhesion.[27]
It has been designed to identify ER by comparing the genetic profile of a test sample with those of luteinizing hormone (LH) + 7 controls in a natural cycle, or on day 5 of P administration (P + 5) after E2 priming in a HRT cycle. The test contains 238 genes that are differentially expressed between these profiles. This array is coupled to a computational predictor that can diagnose the personalized endometrial WOI of a given patient regardless of their endometrial histology.[28]
The gene signature used by the predictor was obtained by selecting those genes whose expression was consistent among three different models of ER: The natural cycle as the optimal model, the COH cycle as suboptimal, and the refractory endometrium as a negative control.[27,29] The bioinformatic predictor classifies an endometrial sample as “receptive” or “nonreceptive.” The “nonreceptive” ERA is further classified as prereceptivity or postreceptive giving an exact status of the endometrium at the time of biopsy[28] [Figures 3 and 4].
Figure 3.
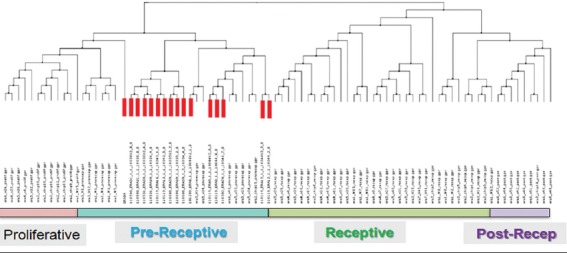
The predictor and clustering analysis indicating “nonreceptive” samples. Courtesy IVIOMICS
Figure 4.
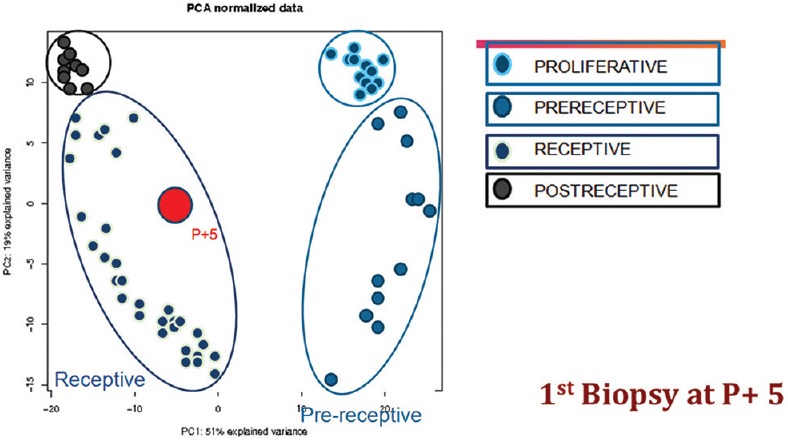
Principal component analysis for personalized Window of implantation. ERA R P + 5. Courtesy IVIOMICS
Accuracy and consistency are the hallmarks of a good diagnostic test. ERA has a sensitivity and specificity of 0.99758 and 0.8857, respectively.[27] It has also been documented to have a high reproducibility. Garrido-Gómez et al. 2013,[28] in their study demonstrated that the transcriptomic profile of the mid-secretory phase endometrium did not change significantly between cycles or over relatively long periods (3 years). They also established that the concordance for ERA endometrial receptivity (ER) dating against the LH peak was 0.922 (0.815–1.000), and the reproducibility was 100%. Comparison between the histologic dating performed by 2 pathologists against a LH peak reference yielded the kappa index values of 0.618 (0.446–0.791) and 0.685 (0.545–0.824), respectively. Interobserver variability was 0.622 (0.435–0.839). These observations confirm the superiority of ERA over histological dating.
The study of the transcriptome of ER has brought to light the fact that the WOI is not fixed, as was believed earlier. Embryo-endometrial synchrony is fundamental to successful implantation, and this has been known since the 1960s. It has also been established that many failures in IVF can be attributed to embryo-endometrial asynchrony. Ovarian stimulation given for follicular recruitment advances endometrial development; by the time embryos are transferred the WOI has closed. Shapiro et al. 2011 compared IRs in fresh and frozen ART cycles and submitted that the attributable risk percentage of IF due to reduced ER in the fresh group was 64.7%.[30] The transcriptomic signature of the WOI can be used to define an individual's personalized receptive window for use in IVF. It could also help in understanding the effect of different infertility treatments on the endometrial WOI and possibly identify the cause of treatment failure. Identifying ER changes in unexplained infertility, endometriosis, and other causes of infertility would help in providing more efficient treatment.
Ruiz-Alonso et al., 2013[31] in a prospective multicentric trial, first proposed the clinical application of this test. She coined the phrase “personalized embryo transfer” (pET) or transferring an embryo based on the woman's personalized WOI. She investigated patients with RIF who had more than three IVF or IVF-OD failures. Controls were patients with no previous IVF failure. RIF and control patients underwent ERA-either on day LH + 7 in a natural cycle or on day P + 5 in an HRT cycle. The results showed that a changed WOI existed in 25.9% of RIF patients. In controls, ERA was nonreceptive in only 12% patients. This suggests that an endometrial factor exists in a quarter of patients with RIF and could contribute to their IF. Correcting the WOI by doing a pET improved the reproductive outcome. A pregnancy rate (PR) of 50.0% and an IR of 38.5% were achieved which was similar to the group in which ERA was found to be receptive on first biopsy (51.7% and 33.9%, respectively). The authors also eliminated the effect of endometrial injury leading to increased IRs.[32] The implantation and PR even after 6 months of the biopsy remained consistent. Since the number of patients in the study was small, the authors have suggested caution in interpreting these clinical results. Ruiz-Alonso et al. 2014[33] reported on a case with the failure of 4 self IVF cycles and 3 IVF-OD cycles. ERA revealed a displaced WOI to P + 7. A pET done subsequently resulted in a successful twin pregnancy and birth. A large multicentric trial is in process from the same group to confirm their initial findings.
ENDOMETRIAL STUDY IN THE INDIAN SETTING
The endometrial factor was studied in patients undergoing IVF treatment at our center once the test became available in India. We performed ERA in patients with RIF, patients with 1 previous IVF failure and patients with persistent thin endometrium <6 mm.
RIF was defined as failure of implantation after two consecutive cycles of IVF, ICSI, frozen embryo transfer (FET), IVF-OD, where the cumulative number of transferred embryos was number less than 4 for cleavage stage embryos and number less than 2 for blastocyst. With all embryos being of good quality and of appropriate developmental stage.[34]
AIM OF THE STUDY
To identify the contribution of the endometrial factor in RIF
To see if PR improved after using the personalized WOI
To find out if patients with persistently thin endometrium have a defective WOI.
TYPE OF STUDY
A retrospective analysis of data.
Primary end point of the study for group I and II were ongoing PR (OPR). Ongoing pregnancy was defined as pregnancy over 12 weeks.
Primary end point for group III was percentage of nonreceptive to receptive endometrium.
MATERIALS AND METHODS
This retrospective study examined 186 infertile women who underwent ER array (ERA) from August 2013 to November 2014.
Patient population was divided into three groups.
Group I - 80 patients with RIF,
Group II - 93 patients with one IVF failed, and
Group III - 13 patients with thin endometrium (≤6 mm).
Patients were in the age group of 25–46 years (34.8 ± 4.8) in group I, 24–46 years (33.3 ± 4) in group II, and 26–40 years (33.4 ± 4.5) in group III. The average body mass index in the three groups was 26.6 ± 4.3 kg/m2, 25.2 ± 3.7 kg/m2, and 27.4 ± 4 kg/m2, respectively. Number of previous IVF failures ranged from 2 to 6.
Inclusion criteria
Patients having a normal ovarian reserve (follicle stimulating hormone <8, antral follicle count >10, and anti-mullerian hormone >2 ng/ml) were included. Only those previous cycles where embryo grading was available for scrutiny were included. These failed cycles had been done in good ART centers with an in-house embryologist and good quality control in the laboratory.
Exclusion criteria
Patients with uncorrected uterine and adnexal pathology, e.g., hydrosalpinx, submucous polyps, or myomas previous difficult ET’s, and thin endometrium in group I and II were excluded from the study.
Apart from the routine infertility work-up a hysteroscopy (when not done earlier), oral glucose tolerance test, and thyroid profile were done for all patients. For RIF patients karyotypes of both partners, thrombophilia profile, lupus anticoagulant, and anticardiolipin antibodies IgG and IgM, were carried out and were normal. An endometrial biopsy (EB) for Koch's culture and DNA polymerase chain reaction was also performed routinely in all patients before IVF. Patients in this retrospective analysis were negative for endometrial Koch’s.
All patients underwent an EB for the ERA test in a hormone replacement cycle (HRT). Transvaginal ultrasound was done on the 2nd day of the period and after ensuring that the endometrium had shed completely HRT was started. Estradiol valerate was started in a dose of 2 mg, this was increased to 6 mg or more till an appropriate endometrial thickness (≥7 mm) was achieved. Vaginal progesterone suppository 400 mg twice a day was then started for a period of 5 days (P + 5). In patients with thin endometrium, we tried to achieve the maximum thickness that had been observed on earlier scans, before starting progesterone. Serum progesterone level was checked when the endometrial thickness was adequate and a cut-off value ≤0.9 ng/ml was taken to start progesterone (P) administration. If serum progesterone level was ≥1 ng/ml, ERA testing was canceled because high endogenous progesterone levels compromise result of the test.
Procedure
Endometrial biopsies were collected from the uterine cavity with the use of Pipelle catheters from Gynetics on day P + 5 in an HRT cycle. The day of the EB in HRT cycle is after five full days of P impregnation that is on 6th day morning. After the biopsy, the endometrial tissue was transferred to a cryotube containing 1.5 mL RNA stabilizing agent (Qiagen), vigorously shaken for a few seconds, and kept at 4°C in refrigerator for 4 h. Care was taken that the tissue was adequate and well-immersed in the fluid provided. If the tissue is too much, there is RNA degradation, and if too little, sufficient RNA is not available for extraction. The samples were then transported at room temperature to IVIOMICS, India and sent for analysis to IVIOMICS Valencia Spain.
The test results were available in 2 weeks. ERA test diagnosed the endometrium to be receptive (R) or nonreceptive (NR). Nonreceptive endometrium was further classified as pre or post receptive. A second ERA sample was taken for these patients on the suggested day (P + 6, P + 7, or P + 4). This result was either receptive or gave a short WOI of 12 h that is, embryo transfer to be done at P + 4.5, 5.5 or 6.5.
Patients with a receptive endometrium (P + 5) underwent FET in a subsequent HRT cycle simulating the ERA cycle. In patients with a changed implantation window FET was done based on the personalized WOI identified by ERA (pET). Two good quality blastocysts were transferred.
Written informed consent was obtained from the patients for performing ERA.
RESULTS GROUP I AND II
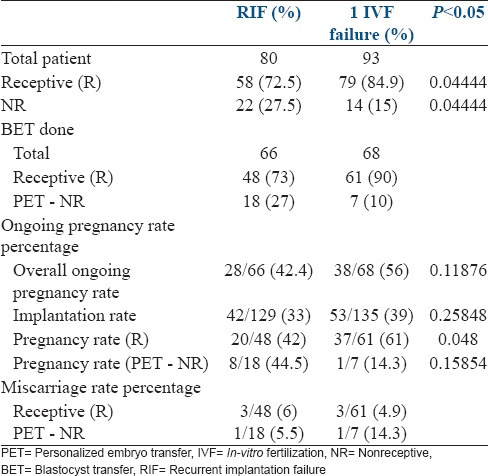
ERA was receptive in 72.5% (58/80) patients in group I and 84.9% (79/93) in group II and nonreceptive (NR) in 27.5% (22/80) patients in group I and 15% (14/93) in group II. In group II, the percentage of patients showing receptive ERA was higher and nonreceptive ERA was lower and this was statistically significant (P = 0.04 R, P = 0.04 NR). This would indicate that a displaced WOI (an endometrial factor) was found more frequent in patients with RIF and could be responsible for their repeated IF.
Sixty-six patients (82.5%) in group I and 68 patients (73%) in group II went for frozen blastocyst transfer (BET). The overall OPR was 42.4% (28/66) in group I and 56% (38/68) in group II. IR was 33% (42/129) in group I and 39% (53/135) in group II. The average PR in our fresh IVF cycles for the year 2014 was 40% and for OD cycles was 61.2%. The IR was 21.1%in fresh cycles and 42.7% in OD cycles for the same period. Thus, the IR and PR in RIF patients after pET improved and was at par with the non-RIF patients.
In patients where 1st ERA (initial ERA) was receptive, the OPR was 42% (20/48) in group I and 61% (37/61) in group II. Group II showed a significantly higher OPR (P = 0.048). In patients with a nonreceptive ERA, the OPR after pET was 44.5% (8/18) in group I and 14.3% (1/7) in group II. This was similar in the two group (P = 0.15).
The miscarriage rate was similar between groups in patients with 1st ERA receptive it was 6% (3/48) in group I and 4.9% (3/61) in group II. MR was 5.5% (1/18) in group I and 14.3% (1/7) in group II after pET in NR group [Table 1].
Table 1.
Comparison group I and II
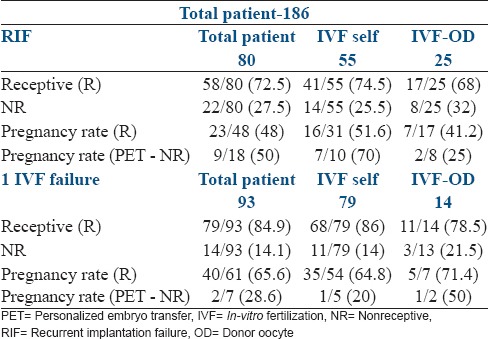
These groups were further divided on the basis of self-IVF cycles and IVF-OD cycles [Table 2]. Of the 80 RIF patients, 55 were self-cycles and 25 were OD cycles. In group 2, distribution was similar with 79 patients in the self IVF group and 14 in the OD group. There was no difference in the proportion of receptive to nonreceptive ERA between self and OD cycles in both groups. In group I, 1st ERA receptive was found in 74.5% (41/55) patients in self-cycles and 68% (17/25) in OD cycles. In group II, this was 86% (68/79) in self-cycles and 78.5% (11/14) in OD cycles. NR ERA occurred in 25.5% (14/55) self and 32% (8/25) OD cycles in group I and 14% (11/79) self and 21.5% (3/14) OD cycles group II. In group I, the PR after pET (NR) was 70% (7/10) in self and 25% (2/8) in OD cycles. In group II, PR was 20% in self-cycles and 50% in OD cycles. In the receptive ERA group, PR was 51.6% (16/31) in self and 41.2% (7/17) in OD cycles in group I and 64.8% (35/54) self and 71.4% (5/7) in OD cycles in group II.
Table 2.
IVF and IVF-OD cycles in group I and II
We found a short ‘WOI’ in 22 patients of these 9 were in group I, 12 in group II, and 1 in group III. Embryo transfer in these patients was done 12 h earlier than the time of EB. The PR in group I was 62.5% (5/8), 1 patient aborted giving an OPR of 50%. In group II, 9/12 patients had a BET the PR was 75% (9/12) and an OPR of 58.3% (7/12). In group III there was only 1 patient she conceived after pET.
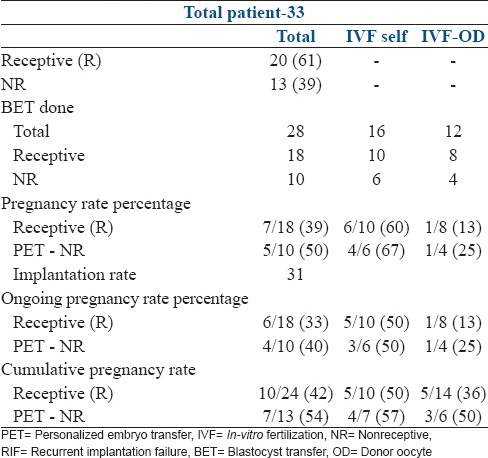
We also analyzed a sub-group of 33 RIF patients who had failed one frozen BET at our center with the transfer of 2 good quality blastocysts, to eliminate operator, and embryo grading bias. These transfers had all been done in an HRT cycle after 5 days of progesterone supplementation (P + 5). Incidence of nonreceptive ERA was 39% (13/33) which was significantly higher than group II (P = 0.003). Twenty-eight patients went for transfer. The PR was 50% (5/10) in the pET NR group and 39% (7/18) in the receptive group. The overall IR was 31%. There was 1 early abortion in both groups giving an OPR of 33% in patients with the initial ERA-receptive and 40% after pET NR. In this group where we had controlled for variables like operator, clinic, embryo grading, type of cycle we found a changed WOI in one-third of the patients. The PR improved in these patients to 50%. In those patients where the initial ERA was receptive at P + 5 in this sub-group, the RIF would have to be attributed to the embryo or immune causes [Table 3].
Table 3.
Subgroup analysis of RIF patients
RESULTS GROUP III PATIENTS WITH THIN ENDOMETRIUM
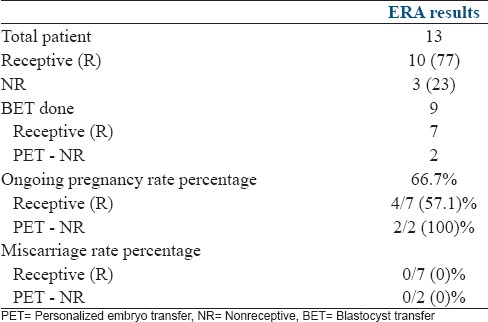
ERA was non-receptive in 23% (3/13) and receptive in 77% (10/13). The proportion of receptive to NR endometrium was similar to group II (P = 0.4). Nine patients (69%) went for BET. The overall OPR was 66.7% (6/9). In patients where 1st ERA was receptive, the OPR was 57.1% (4/7) and in NR group after pET PR was 100% (2/2). There were no miscarriages in this group. Thin endometrium has long been associated with IF. It was reassuring to know that despite the reduced endometrial thickness the changed WOI occurred in the same proportion of patients as the non-RIF group. The knowledge that an embryo is being transferred into a receptive environment in such patients lends comfort to the patient and the physician [Table 4].
Table 4.
Thin endometrium group III
DISCUSSION
The introduction of microarray technology has enabled rapid progress in the understanding of many biological functions and disease processes computing Omics with bioinformatic predictors has improved the diagnosis and subsequent treatment in diseases such as cancer.[35] Success in this area coupled with identification of the transcriptomics of the receptive endometrium during natural and stimulated cycles,[36] led to the development of a molecular diagnostic test to identify the WOI – ERA.[27]
In the era of personalized medicine, a “one size fits all” policy is no longer acceptable. In IVF individualized ovarian stimulation, protocols are being promoted to optimize treatment. So far, for lack of an objective and accurate test ER remained a gray area. ERA is a step forward in improving IVF results through identification of the WOI and personalizing embryo transfer. The test has been shown to be accurate and reproducible and does not have the limitation of inter cycle variability. The clinical application of the test has been applied only in some clinics, and hence larger studies are required to validate it.
In our study, we found that 27.5% women with RIF showed a displaced WOI. The paper by Ruiz-Alonso et al. 2013[31] suggested an increased percentage of NR endometrium in the RIF group, but this did not reach statistical significance. Our results, however, showed that this difference was statistically significant. A larger number of women in the RIF group had a NR endometrium. The PR, OPR, and IR in the RIF group improved after pET with values similar to those seen in our fresh non-RIF IVF cycles. In the sub-group analysis done on 33 RIF patients wherein 1 FET with the transfer of 2 good quality blastocysts had been done at our center, we found that one-third of the patients had a NR endometrium. Personalized ET improved the reproductive outcome in these patients as also patients with 1 previous IVF failure.
Another difficult group is the women with thin endometrium as thin endometrium is associated with poor implantation. It was reassuring to know that the proportion of nonreceptive endometrium was not more than our 1 IVF failure group and that after pET the PR and IRs were also similar to that group.
Though we saw an improvement of results, the numbers in our study are not high enough to draw definite conclusions.
What then is the place of ERA in the treatment of infertile patients given the existing knowledge? In the current setting, ERA is the only test available that can determine ER with accuracy. Since it is reproducible and does not change over a long period of time (1–2 years), it need not be repeated in the event of a delay in treatment. In the clinical setting, ERA definitely has a place in RIF where endometrial factor could be the contributory cause in a quarter of the patients. In women with even 1 IVF-OD failure with the transfer of 2 good quality embryos, it is advisable to rule out an altered WOI. Defining a receptive window would avoid embryo wastage and emotional, physical, and financial distress. Its use in patients with adenomyosis, endometriosis, and chronic endometritis can prove beneficial, as these conditions are associated with an altered ER. Persistent thin or thick endometrium is also an indication for carrying out ERA. ERA is a valuable addition to our diagnostic armamentarium. The invasive nature of the test, the need for embryo vitrification and cost are some of its limitations.
Much of the implantation process still remains to be unraveled. It has to be remembered that the embryo remains a major player in this equation and genetic testing of the embryo with array comparative genomic hybridization has shown improved IRs.[37] However, there are no reports suggesting a 100% success even after doing a pET with a euploid embryo. Maternal factors especially the immune system involvement needs to be understood.
CONCLUSION
ERA is the most objective and accurate test available today for diagnosing ER. It has been used to define an altered WOI, and thus establish a personalized WOI for each patient. It has shown benefit in improving reproductive performance in patients with RIF. However, more studies are required to confirm these initial findings. It is limited by its invasive nature and associated costs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Dr. Shalu Gupta and Ms. Nitu for their invaluable help with data collection and analysis. Dr. Puneet R Arora for inputs on study design. Dr. Kumkum Rani, Dr. Shilpa Sharma, Dr. Padmaja Naidu for help with sample collection.
REFERENCES
- 1.Ruiz-Alonso M, Blesa D, Simón C. The genomics of the human endometrium. Biochim Biophys Acta. 2012;1822:1931–42. doi: 10.1016/j.bbadis.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Lessey BA. Assessment of endometrial receptivity. Fertil Steril. 2011;96:522–9. doi: 10.1016/j.fertnstert.2011.07.1095. [DOI] [PubMed] [Google Scholar]
- 3.Dickey RP, Olar TT, Curole DN, Taylor SN, Rye PH. Endometrial pattern and thickness associated with pregnancy outcome after assisted reproduction technologies. Hum Reprod. 1992;7:418–21. doi: 10.1093/oxfordjournals.humrep.a137661. [DOI] [PubMed] [Google Scholar]
- 4.Bonilla-Musoles F, Raga F, Osborne NG, Castillo JC, Bonilla F., Jr Endometrial receptivity: Evaluation with ultrasound. Ultrasound Q. 2013;29:3–20. doi: 10.1097/RUQ.0b013e318281b60a. [DOI] [PubMed] [Google Scholar]
- 5.Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 6.Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, et al. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264–72. doi: 10.1016/j.fertnstert.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 7.Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, et al. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81:1333–43. doi: 10.1016/j.fertnstert.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 8.American Society for Reproductive Medicine. A Practice Committee report: Optimal evaluation of the infertile female. ASRM. Fertil Steril. 2012;98:302–7. [Google Scholar]
- 9.Nikas G. Cell-surface morphological events relevant to human implantation. Hum Reprod. 1999;14(Suppl 2):37–44. doi: 10.1093/humrep/14.suppl_2.37. [DOI] [PubMed] [Google Scholar]
- 10.Quinn CE, Casper RF. Pinopodes: A questionable role in endometrial receptivity. Hum Reprod Update. 2009;15:229–36. doi: 10.1093/humupd/dmn052. [DOI] [PubMed] [Google Scholar]
- 11.Cavagna M, Mantese JC. Biomarkers of endometrial receptivity – A review. Placenta. 2003;24(Suppl B):S39–47. doi: 10.1016/s0143-4004(03)00184-x. [DOI] [PubMed] [Google Scholar]
- 12.Nevins JR, Potti A. Mining gene expression profiles: Expression signatures as cancer phenotypes. Nat Rev Genet. 2007;8:601–9. doi: 10.1038/nrg2137. [DOI] [PubMed] [Google Scholar]
- 13.Horcajadas JA, Mínguez P, Dopazo J, Esteban FJ, Domínguez F, Giudice LC, et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab. 2008;93:4500–10. doi: 10.1210/jc.2008-0588. [DOI] [PubMed] [Google Scholar]
- 14.Díaz-Gimeno P, Ruíz-Alonso M, Blesa D, Simón C. Transcriptomics of the human endometrium. Int J Dev Biol. 2014;58:127–37. doi: 10.1387/ijdb.130340pd. [DOI] [PubMed] [Google Scholar]
- 15.Díaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martínez-Conejero JA, Alamá P, et al. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril. 2013;99:508–17. doi: 10.1016/j.fertnstert.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 16.Ponnampalam AP, Weston GC, Trajstman AC, Susil B, Rogers PA. Molecular classification of human endometrial cycle stages by transcriptional profiling. Mol Hum Reprod. 2004;10:879–93. doi: 10.1093/molehr/gah121. [DOI] [PubMed] [Google Scholar]
- 17.Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki S. DNA microarray analysis in endometriosis for development of more effective targeted therapies. Front Biosci (Elite Ed) 2011;3:1139–53. doi: 10.2741/e317. [DOI] [PubMed] [Google Scholar]
- 19.Habermann JK, Bündgen NK, Gemoll T, Hautaniemi S, Lundgren C, Wangsa D, et al. Genomic instability influences the transcriptome and proteome in endometrial cancer subtypes. Mol Cancer. 2011;10:132. doi: 10.1186/1476-4598-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirkin S, Nikas G, Hsiu JG, Díaz J, Oehninger S. Gene expression profiles and structural/functional features of the peri-implantation endometrium in natural and gonadotropin-stimulated cycles. J Clin Endocrinol Metab. 2004;89:5742–52. doi: 10.1210/jc.2004-0605. [DOI] [PubMed] [Google Scholar]
- 21.Altmäe S, Martínez-Conejero JA, Salumets A, Simón C, Horcajadas JA, Stavreus-Evers A. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod. 2010;16:178–87. doi: 10.1093/molehr/gap102. [DOI] [PubMed] [Google Scholar]
- 22.Koler M, Achache H, Tsafrir A, Smith Y, Revel A, Reich R. Disrupted gene pattern in patients with repeated in vitro fertilization (IVF) failure. Hum Reprod. 2009;24:2541–8. doi: 10.1093/humrep/dep193. [DOI] [PubMed] [Google Scholar]
- 23.Horcajadas JA, Riesewijk A, Polman J, van Os R, Pellicer A, Mosselman S, et al. Effect of controlled ovarian hyperstimulation in IVF on endometrial gene expression profiles. Mol Hum Reprod. 2005;11:195–205. doi: 10.1093/molehr/gah150. [DOI] [PubMed] [Google Scholar]
- 24.Simon C, Oberyé J, Bellver J, Vidal C, Bosch E, Horcajadas JA, et al. Similar endometrial development in oocyte donors treated with either high- or standard-dose GnRH antagonist compared to treatment with a GnRH agonist or in natural cycles. Hum Reprod. 2005;20:3318–27. doi: 10.1093/humrep/dei243. [DOI] [PubMed] [Google Scholar]
- 25.Haouzi D, Assou S, Mahmoud K, Tondeur S, Rème T, Hedon B, et al. Gene expression profile of human endometrial receptivity: Comparison between natural and stimulated cycles for the same patients. Hum Reprod. 2009;24:1436–45. doi: 10.1093/humrep/dep039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Critchley HO, Kelly RW, Brenner RM, Baird DT. The endocrinology of menstruation – A role for the immune system. Clin Endocrinol (Oxf) 2001;55:701–10. doi: 10.1046/j.1365-2265.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- 27.Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Alamá P, Pellicer A, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. (60.e1-15).Fertil Steril. 2011;95:50–60. doi: 10.1016/j.fertnstert.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 28.Garrido-Gómez T, Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Vilella F, Simón C. Profiling the gene signature of endometrial receptivity: Cinical results. Fertil Steril. 2013;99:1078–85. doi: 10.1016/j.fertnstert.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Horcajadas JA, Pellicer A, Simón C. Wide genomic analysis of human endometrial receptivity: New times, new opportunities. Hum Reprod Update. 2007;13:77–86. doi: 10.1093/humupd/dml046. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: A prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344–8. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818–24. doi: 10.1016/j.fertnstert.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Raziel A, Schachter M, Strassburger D, Bern O, Ron-El R, Friedler S. Favorable influence of local injury to the endometrium in intracytoplasmic sperm injection patients with high-order implantation failure. Fertil Steril. 2007;87:198–201. doi: 10.1016/j.fertnstert.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Alonso M, Galindo N, Pellicer A, Simón C. What a difference two days make: Personalized embryo transfer (pET) paradigm: A case report and pilot study. Hum Reprod. 2014;29:1244–7. doi: 10.1093/humrep/deu070. [DOI] [PubMed] [Google Scholar]
- 34.Polanski LT, Baumgarten MN, Quenby S, Brosens J, Campbell BK, Raine-Fenning NJ. What exactly do we mean by ‘recurrent implantation failure’?. A systematic review and opinion. Reprod Biomed Online. 2014;28:409–23. doi: 10.1016/j.rbmo.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Simon R. Using DNA microarrays for diagnostic and prognostic prediction. Expert Rev Mol Diagn. 2003;3:587–95. doi: 10.1586/14737159.3.5.587. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Lee KF, Ng EH, Yeung WS, Ho PC. Gene expression profiling of human peri-implantation endometria between natural and stimulated cycles. Fertil Steril. 2008;90:2152–64. doi: 10.1016/j.fertnstert.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Hellani A, Abu-Amero K, Azouri J, El-Akoum S. Successful pregnancies after application of array-comparative genomic hybridization in PGS-aneuploidy screening. Reprod Biomed Online. 2008;17:841–7. doi: 10.1016/s1472-6483(10)60413-0. [DOI] [PubMed] [Google Scholar]


