Abstract
CONTEXT:
Ovulation induction in patients with hypogonadotropic hypogonadism (HH) is a challenge to the treating physician. The threshold for ovarian response in HH may differ substantially from that of normal patients. To reach that threshold levels of follicle stimulating hormone, in a step-up protocol longer duration of stimulation is required in some cases so as to prevent multiple pregnancy and to eliminate the risk of ovarian hyperstimulation syndrome.
AIM:
To evaluate the duration of stimulation, quality of oocytes, and embryo, and the pregnancy outcome in the assisted reproductive technology (ART) cycles in patients with HH.
MATERIALS AND METHODS:
Over the period of 4 years, we had 14 patients with HH in whom 21 cycles of ovulation induction were done. Of these 7 patients underwent oocyte retrieval and intracytoplasmic sperm injection (ICSI). We present a retrospective study of these 7 patients who underwent ART to evaluate the duration of stimulation, quality of oocytes and embryo, and the pregnancy outcome.
RESULTS:
In the study group on ovulation induction with gonadotropins, only one patient had the duration of stimulation of the standard 12 days, the remaining 6 patients took ≥12 days to respond to stimulation (maxium being 54 days). Mean ET in these patients was 8.9 mm. Six patients had >70% good quality MII oocytes. One patient responded poorly and had only 2 good quality MII oocytes (50%). After ICSI procedure, resultant embryos were of grade 1 and 2 in all the patients irrespective of the duration of stimulation. Fertilization rate in these patients was 85% (except in one 50% fertilization rate), and the cumulative pregnancy rate was 68.6%.
CONCLUSION:
In the patients with HH the quality of oocytes and embryos, and the pregnancy rate is not affected even if the duration of stimulation is prolonged.
KEY WORDS: Embryo, gonadotropins, hypogonadotropic hypogonadism, intrauterine insemination, oocytes, ovulation induction
INTRODUCTION
Hypogonadotropic hypogonadism (HH) is the 2nd most frequent cause of ovarian failure in young women in whom pulsatile GnRh therapy or replacement of gonadotropin can restore ovulation. The threshold for ovarian response in HH may differ substantially from that established for normal patients. These patients require a combination of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) for inducing ovulation. The success of ovulation induction is reported to be as high 60–80% with a high multiple pregnancy rate (20–50%).[1] In order to avoid higher order pregnancies these patients may require a longer duration of stimulation[2] with gradually stepping up the dose in order to reach the threshold of FSH and LH. Assisted reproductive technology (ART) is beneficial in this group of patients to prevent higher order multiple pregnancy or to increase the chance of pregnancy whenever there is poor response on ovarian stimulation. We present 7 cases of HH, who underwent ART to evaluate the duration of ovarian stimulation, quality of oocytes and embryo, and the pregnancy outcome in the ART cycle in patients with HH.
MATERIALS AND METHODS
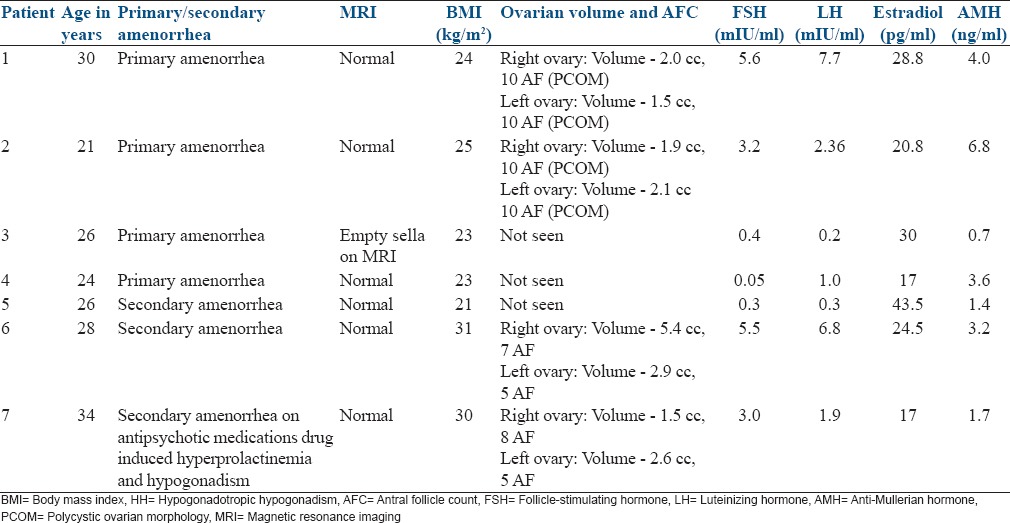
Over the period of 4 years, we had 14 patients with HH in whom 21 cycles of ovulation induction were done. 7 patients underwent ART. 3 of the 7 patients were initially planned for ovulation induction and intrauterine insemination (IUI) but later converted to ART cycle while 4 patients were planned directly for controlled ovarian hyperstimulation and intracytoplasmic sperm injection (ICSI). All these patients had amenorrhea, and they were evaluated for it. Serum prolactin and thyroid function test were normal in all the patients. Serum FSH, LH, estradiol, anti-mullerian hormone (AMH), ultrasonography (USG) pelvis, and magnetic resonance imaging (MRI) were done for all patients and the findings are tabulated in Table 1.
Table 1.
Base line profile of the HH patients who underwent oocyte retrieval
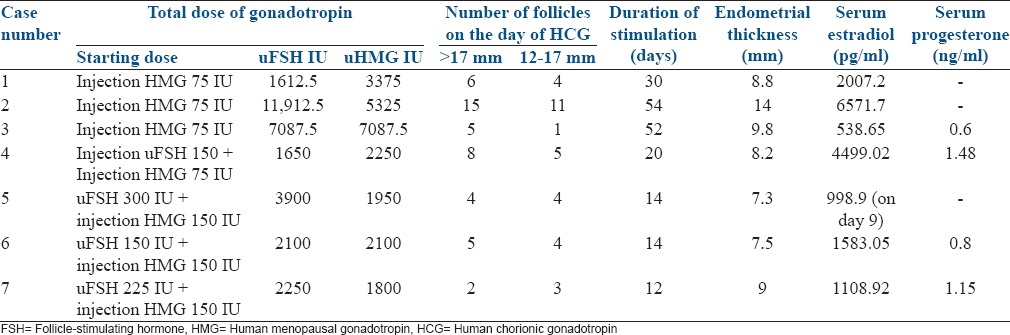
Before ovulation induction, adequate size of the uterus (at least >5 cm) was obtained by giving cyclical estrogen and progesterone therapy for a minimal of 3 months. Tubal patency was established by doing HSG. Semen analysis of husband was done for all the patients and was found to be normal in all of them. Patients planned for ovulation induction and IUI were started on injection human menopausal gonadotropins (HMG) 75 IU for 7 days, later the dose was gradually stepped up with injection HMG with or without injection urinary FSH (uFSH) depending upon the response with the aim to obtain 2 or 3 follicles. Whenever there was hyper response or poor response during ovulation induction for IUI these patients were counseled for conversion to an ART cycle. In patients planned for ART higher dose of injection HMG 150 IU in combination with injection uFSH 150–225 IU was started depending upon the age and AMH of the patient. When 3 follicles had reached ≥18 mm urinary human chorionic gonadotropin 10,000, IU IM trigger was given, and oocyte retrieval was done 36 h later using 17 g needle, under short GA anesthesia under TVS guidance. ICSI was done for all patients and grading of embryos was done by Veeck's scoring system. Embryo transfer was done using Labotect catheter on day 3 after retrieval. When there was a high risk of ovarian hyperstimulation syndrome (OHSS), fresh embryo transfer was avoided, and embryos were frozen. In such patients later frozen embryo transfer was done after hormone replacement therapy. In the fresh cycle, luteal phase was supplemented for all patients with estradiol valerate 2 mg twice daily orally and micronized progesterone 200 mg thrice daily vaginally. Serum beta human chorionic gonadotropin was estimated 14 days after embryo transfer.
The stimulation details as in Table 2.
Table 2.
Ovarian stimulation details
RESULTS
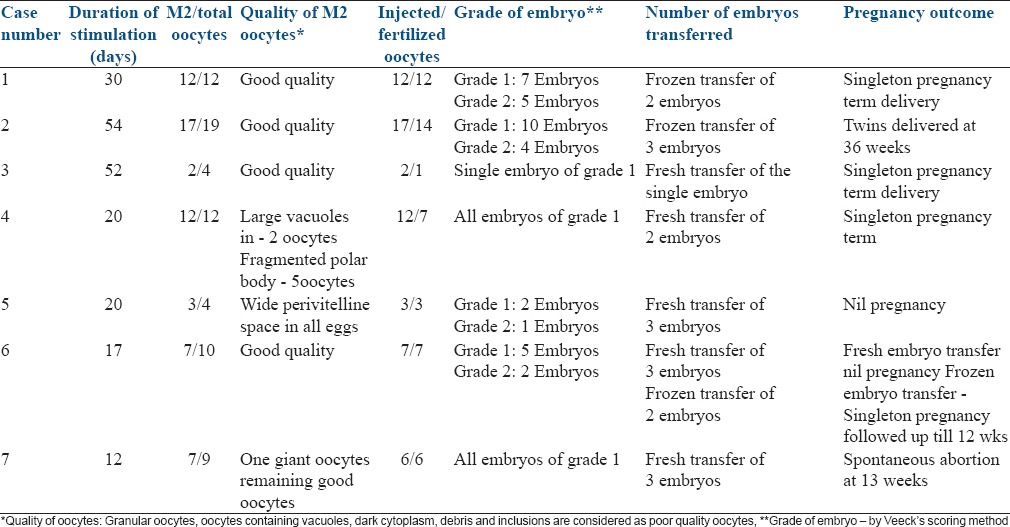
In the study group, 4 patients had primary amenorrhea remaining 3 had presented as a case of secondary amenorrhea. Ovaries were visualized in 4 patients, and two of these showed polycystic ovarian morphology (PCOM) features, but volume was <12 cc. Only in two patients the exact cause was identified (one case was having features of empty sella on MRI and the other had secondary hypogonadism due to anti psychiatric medications). FSH and LH were low in 4 patients and in the remaining 3 patients the levels were near normal values. In the later three patients, the history of amenorrhea and serum estradiol levels of <30 pg/ml helped us to make the diagnosis of hypogonadism. Only one patient had the duration of stimulation of the standard 12 days, the remaining 6 patients took >12 days to respond to stimulation (maximum being 54 days). Mean ET in these patients was 8.9 mm. Six patients had >70% good quality MII oocytes (One patient who had poor response had only 2 MII good quality oocytes). After ICSI, resultant embryos were of grade 1 and 2 in all patients. Fertilization rate in these patients was 85% (except in one who had 50% fertilization rate), and the cumulative pregnancy rate was 68.6%. The details of the quality of oocyte and embryos and pregnancy outcome are tabulated in Table 3.
Table 3.
Quality of oocyte and embryos
DISCUSSION
HH is a rare disorder of reproductive function characterized by the absence of normal hypothalamic – pituitary synchronous activity. These patients belong to WHO Group 1 anovulation which account for 10% of cases for anovulation and present with the history of amenorrhea, hypogonadism (<30 pg/ml of estradiol)[3] and the gonadotropin levels low or in the low normal range. In the present study 5 pts had low FSH & LH levels while the remaining 2 patients had normal to near normal FSH & LH levels. In the later 2 patients serum estradiol levels were <30 pg/ml which pointed to the diagnosis of HH.
Prediction of an individual patient to respond to gonadotropin treatment in HH cannot be based on baseline LH and FSH estimation, which we usually use for other patients. AMH may be useful to some extent.[4] Ovaries may or may not be seen on USG in these patients. Only after a patient is subjected to gonadotropin stimulation, we can estimate her pattern of response. The threshold for ovarian response in HH may differ substantially from that established for normal patients.
Gonadotropin treatment in patients with HH is the accepted method of ovulation induction. In such patients, optimal clinical results are achieved by using FSH combined with LH,[5] which is accomplished by administration of HMG, recombinant LH or low dose human chorionic gonadotropin. Multifetal pregnancy is the most frequent complication of ovulation induction with gonadotropin in these patients. In order to decrease the incidence of multiple pregnancies, if step-up protocol is used it results in a longer duration of stimulation. In two patients (case 1 and 2) in spite of step-up hyper response could not be avoided though they took a longer time to respond (30 days and 54 days) details in Table 2. In both of these patients, ovaries were seen and showed PCOM with a low volume of the ovary. These patients underwent rescue in vitro fertilization (IVF) with freezing of the embryos due to the risk of OHSS. Later frozen embryo transfer was done and both the patients conceived. The hyper response might have been due to underlying PCOS, which may get unmasked after giving gonadotropin as shown by Wang and Lobo.[6] AMH in both these patients was >3.7 ng/ml suggesting high risk for hyper response. The second patient had moderate OHSS and was managed conservatively. GnRh agonist trigger to avoid OHSS cannot be used in these patients due to endogenous deficiency of gonadotropin.
The third patient was stimulated by step-up protocol but when she did not show any response till the 36th day, serum AMH was done which was 0.7 ng/ml. Tran et al.[7] mentioned in the clinical case seminar “The role of AMH in Assessing Ovarian Reserve” that AMH has limitations because it only reflects the growing follicular pool that is responsive to gonadotropins. Hence, conditions that cause a permanent or sustained interruption of gonadotropin release may lead to a decrease AMH levels and therefore an underestimation of the true ovarian reserve suggesting that AMH may not be a very good predictor of ovarian reserve in patients with HH. They reported a case with HH in which during the 16-week course of treatment with HMG, follicular development was observed. Both AMH and antral follicle count (AFC) gradually increased during treatment from the initial AMH and AFC of 0.20 ng/ml and zero, and peaked to 1.26 ng/ml and 6, respectively. Considering this we convinced the patient as she was young (26 years) and decided to continue stimulation until the maximum dose of gonadotropin 600 IU/day was used or till response was noted whichever was earlier. The couple agreed to continue the gonadotropins injections and on the 47th day of stimulation with uFSH 225 IU and HMG 225 IU ovaries were seen and the same dose was continued. On the 52nd day patient developed 5 follicles of >17mm. The couple was counseled for oocyte retrieval as she was a poor responder and had responded after a long course of gonadotropin, details in Tables 2 and 3. This patient required higher dose to respond, and she formed relatively less number of follicles compared to patients who had higher AMH.[6]
Last four cases (4th to 7th) were planned for ART hence these patients were stimulated with a higher dose gonadotropin, and they responded relatively faster.
The quality of oocytes and embryo in all these 7 patients were good irrespective of the duration of stimulation as shown in Table 3 proving that long duration of stimulation in HH did not affect the quality of oocytes or embryo. Lewit and Kol[8] has also reported successful stimulation after 29 days of gonadotropin in a patient in whom twice stimulation was cancelled after 20 days due to nonresponse to gonadotropin. They concluded that patients with HH undergoing ovarian stimulation for IVF should be carefully assessed on a trial and error basis for ovarian response before we give up on obtaining fertilizable oocytes. Our observation also helps us to confidently continue ovulation induction in patients with HH in step-up protocol till follicular response is seen irrespective of duration of stimulation. Juan Balasch has suggested to use step-down and step-up approach where patients receive 2 or 3 ampoules of HMG daily (depending upon the patient's body mass index) for 2 days later one ampoule from day 3 to 7. From day 8 onward, HMG is administered on the principles of step-up regimen as per the requirement of the patient.[8] This might reduce the duration and the cost of the ovarian stimulation.
CONCLUSIONS
Ovulation induction in a patient with HH is a challenge to the treating physician. Ovarian reserve is difficult to assess in these patients as AMH levels in these patients have to be interpreted cautiously. When planned for ovulation induction we must be prepared for longer duration of stimulation which does not affect the quality of oocytes and embryo, and the pregnancy rate. The treating physician and the patient need a lot of patience and motivation to continue the treatment for longer duration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This work was supported by the staff and the Reproductive Medicine DM postgraduates in the Department of Reproductive Medicine, Sri Ramachandra Medical College and Research Institute, Sri Ramachandra University.
REFERENCES
- 1.Allahbadia G. 1st ed. Rotunda Medical Technologies PVT. LTD; 2001. Manual of Ovulation Induction; pp. 93–4. [Google Scholar]
- 2.Kumbak B, Kahraman S. Women with hypogonadotropic hypogonadism: Cycle characteristics and results of assisted reproductive techniques. Acta Obstet Gynecol Scand. 2006;85:1453–7. doi: 10.1080/00016340600839619. [DOI] [PubMed] [Google Scholar]
- 3.Aboulghar M, Rizk B. 1st ed. Cambridge University Press; 2011. Ovarian Stimulation; pp. 162–8. [Google Scholar]
- 4.Sönmezer M, Özmen B, Atabekoglu CS, Papuccu EG, Ozkavukcu S, Berker B, et al. Serum anti-mullerian hormone levels correlate with ovarian response in idiopathic hypogonadotropic hypogonadism. J Assist Reprod Genet. 2012;29:597–602. doi: 10.1007/s10815-012-9759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgués S. Spanish Collaborative Group on Female Hypogonadotrophic Hypogonadism. The effectiveness and safety of recombinant human LH to support follicular development induced by recombinant human FSH in WHO group I anovulation: Evidence from a multicentre study in Spain. Hum Reprod. 2001;16:2525–32. doi: 10.1093/humrep/16.12.2525. [DOI] [PubMed] [Google Scholar]
- 6.Wang JG, Lobo RA. The complex relationship between hypothalamic amenorrhea and polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:1394–7. doi: 10.1210/jc.2007-1716. [DOI] [PubMed] [Google Scholar]
- 7.Tran ND, Cedars MI, Rosen MP. The role of anti-müllerian hormone (AMH) in assessing ovarian reserve. J Clin Endocrinol Metab. 2011;96:3609–14. doi: 10.1210/jc.2011-0368. [DOI] [PubMed] [Google Scholar]
- 8.Lewit N, Kol S. The low responder female IVF patient with hypogonadotropic hypogonadism: Do not give up! Fertil Steril. 2000;74:401–2. doi: 10.1016/s0015-0282(00)00599-9. [DOI] [PubMed] [Google Scholar]


