Abstract
Pathogens have evolved highly specialized mechanisms to infect hosts. Several microorganisms modulate the eukaryotic cell surface to facilitate their engulfment. Once internalized, they hijack the molecular machinery of the infected cell for their own benefit. At different stages of phagocytosis, particularly during invasion, certain pathogens manipulate pathways governed by small GTPases. In this review, we focus on the role of Rho proteins on curable, sexually transmitted infections caused by Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis and Treponema pallidum. Despite the high, worldwide frequencies of these sexually-transmitted diseases, very little is known about the strategies developed by these microorganisms to usurp key eukaryotic proteins that control intracellular signaling and actin dynamics. Improved knowledge of these molecular mechanisms will contribute to the elucidation of how these clinically important pathogens manipulate intracellular processes and parasitize their hosts.
Keywords: chlamydia trachomatis, Cdc42, neisseria gonorrhoeae, pathogens, pathogen-host cell interaction, Rac1, Rho, small GTPases, trichomonas vaginalis, treponema pallidum
Introduction
Rho GTPases constitute one of the 5 distinct families, Ras, Arf, Ran, Rab and Rho, of the Ras superfamily of small GTP-binding proteins.1 These GTPases act as molecular switches, cycling between a GDP-bound form (inactive state) and a GTP-bound form (active state). This cycle is carefully regulated by more than 60 activators (guanine nucleotide exchange factors, GEFs) and over 70 inhibitors (GTPase-activating proteins, GAPs). Multiple and diverse processes of eukaryotic cells are regulated by these GTPases, and more than 100 target/effector proteins have been described.2 Ras (rat sarcoma) oncoproteins are mainly involved in the control of gene expression and cell proliferation; Arf (ADP-ribosylation factor) members participate in cargo sorting and in the formation of vesicle coats along different transport pathways; Ran (Ras-related nuclear) GTPase functions in the nucleo-cytoplasmic transport of both RNA and proteins and regulates mitotic spindle and nuclear envelope assemblies; Rab (Ras-related proteins in brain) proteins are master controllers of intracellular vesicular transport; and the Rho (Ras homologous) GTPases serve as key regulators of extracellular stimulus-mediated signaling networks that regulate actin organization, cell cycle progression and gene expression.3,4
After the discovery of Rho as a Ras-related protein in 1985, 20 genes were subsequently identified as Rho family members, RhoA, Rac1 and Cdc42 being the best characterized.5,6 Rho GTPases participate in many essential cellular processes; the most important ones are the regulation of the assembly and organization of the actin cytoskeleton. Additionally, they regulate cell polarity, microtubule dynamics, vesicular transport pathways, G1 cell cycle progression and a multiplicity of enzymatic activities.7 Interestingly, Rho GTPases also play critical roles in the interaction between pathogens and host cells by controlling innate and adaptive immune responses.8,9
Pathogens have evolved different strategies to alter host cell functions. By modifying eukaryotic GTPases, microorganisms can manipulate a variety of cellular pathways to favor microbial colonization and/or proliferation, thereby parasitizing and even killing the host cell. Rho GTPases are involved in actin reprogramming at the underlying site of adhesion of microbes to the cell surface, in the entry or invasion of host cells, in the intracellular survival of microbes, or in the polymerization of actin to propel microorganisms into adjacent cells. A large number of bacterial virulence factors target Rho GTPases. These virulence factors can be classified into 2 distinct categories: the first group includes injected effectors, in which bacteria must be in contact with their host cells to deploy their effectors via secretion apparatuses that are similar to molecular syringes;10 and the second group includes exotoxins secreted by bacteria into their environs, which does not require direct contact with the target cell.8,11 Once the toxin reaches the cell surface, it binds to specific receptors and enters the cell by endocytosis. Both injected effectors and exotoxins alter GTPase function through direct chemical modifications of the molecule or by interfering with regulatory elements of the GTPase cycle.
This review focuses on the relationship between Rho GTPases and 4 sexually transmitted pathogens, 3 bacteria (Chlamydia trachomatis, Neisseria gonorrhoeae, and Treponema pallidum) and the parasite Trichomonas vaginalis. A recent World Health Organization (WHO) study (http://www.who.int/reproductivehealth/publications/rtis/stisestimates/en/) estimates that 498 million adults, aged between 15 and 49, are infected with at least one of these microorganisms. Therefore, knowledge of the interplay between these pathogens and human cells is of great importance.
Chlamydia trachomatis
C. trachomatis is the major causative agent of bacterial sexually transmitted diseases and preventable blindness worldwide. Infections can result in urethritis, cervicitis, epididimitis to trachoma, lymphogranuloma venereum, pelvic inflammatory disease, tubal obstruction, ectopic pregnancy and infertility.12 Persistent infections have recently been linked to severe chronic inflammatory diseases and cancer.13
C. trachomatis is a Gram-negative, obligate intracellular bacterium that is restricted to humans. It has developed diverse strategies to invade, survive and multiply within eukaryotic cells.14,15 Chlamydia resides in a vacuole, called “inclusion,” where it avoids intracellular degradation and acquires nutrients and structural molecules from host cells.16–19 Chlamydia displays a unique biphasic lifecycle that starts when the infectious bacterial form, the elementary body (EB), enters the cell. Then, the EB differentiates into a larger, metabolically active, but non-infectious form, the reticulate body (RB), which multiplies by binary fission. After numerous rounds of replication, RBs undergo transformation back into infectious EBs to disseminate to adjacent cells.20–22
Rho GTPases in Chlamydia Entry
Invasion starts with the attachment of EBs to the plasma membrane of host cells. This binding is highly specific and efficient, and has been termed parasite-specific phagocytosis.23 Despite the importance of this early event in chlamydial pathogenesis, the specific receptor-ligand interaction involved in bacterial entry remains elusive. The diversity of chlamydial strains and eukaryotic cells used in different studies, the variability in the experimental setups, and the difficulty of genetically manipulating these bacteria are the main reasons for this lack of consensus regarding the adhesins and ligands involved in Chlamydia entry.24–28 Independently of host cell types or bacterial serovars, the unifying feature of chlamydial entry is actin remodelling at attachment sites, an event controlled by Rho GTPases.
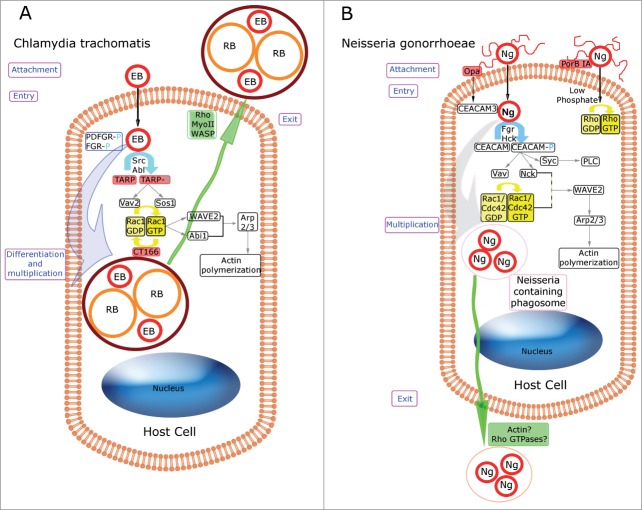
Rho GTPases involved in the internalization step appear to be species-specific; only Rac1 is involved in C. trachomatis entry,29,30 whereas both Cdc42 and Rac1 are activated during C. caviae invasion.31 Basically, Rac1, which is recruited to the entry sites where actin polymerizes,30 is rapidly and transiently activated after the binding of C. trachomatis to host cells. It is likely that C. trachomatis activates a cascade involving both bacterial and host proteins, which results in the rearrangement of the actin cytoskeleton and leads to successful colonization of the host cell. In fact, after bacterial attachment, EBs secrete a protein called Translocated Actin Recruiting Protein (TARP), which is injected into the host cell cytosol through a type III secretion system (a multiprotein needle-like delivery system).32,33 Once on the cytosolic face of the plasma membrane, TARP is phosphorylated on its N-terminal, tyrosine-rich tandem repeats by host Src34 and Abelson (Abl) kinases.29 This phosphorylation allows TARP to recruit the GEFs Sos1 and Vav2, which in turn activate Rac1.35 Subsequently, Rac1 recruits WAVE2 and Abl interactor 1 (Abi-1), leading to actin-related protein (Arp2/3) complex activation and actin recruitment and polymerization at the bacterial binding site.36 It has been proposed that a synergistic action between both bacterial and host cell proteins promotes invasion. In addition, other host tyrosine kinases, such as platelet derived growth factor receptor (PDFGR) and feline Gardner-Rasheed sarcoma viral oncogene homolog (FGR), are phosphorylated upon infection and recruited to the Chlamydia attachment site. It is possible that these kinases might function redundantly in the internalization step.29,37 The last stage in the cascade is probably regulated by another bacterial protein, CT166, which inactivates Rac1 (but not Rho A) via glucosylation,38 thus completing the activation/inactivation cycle of Rac1. In summation, this is a complex and tightly regulated process in which diverse bacterial and host proteins play essential roles in the attachment and entry of Chlamydia, in which Rac1 plays an important function throughout the process (Fig. 1A).
Figure 1.
Schematic comparison of Rho GTPases involved in Chlamydia trachomatis and Neisseria gonorrhoeae-host cell interplay. Bacterial proteins are shown in red boxes. Yellow arrows indicate Rho GTPase cycling. Phosphorylation events are shown in light blue. Putative roles of Rho GTPases are indicated in purple boxes. Bacterial exit processes are represented in green. (A) Rho GTPases and Chlamydia trachomatis. The bacterial infective form (Elementary Body, EB), through the injection of the bacterial factor TARP (Translocated Actin Recruitment Protein), triggers phosphorylation events and the recruitment of the guanine-nucleotide exchange factors (GEFs), Sos1 and Vav2, leading to Rac1 activation. Then, Rac1-GTP recruits WAVE2 and Abi-1, promoting Arp2/3 complex activation and actin polymerization at the bacterial entry site. After chlamydial differentiation into the replicative form (Reticulate Body, RB) and numerous rounds of multiplication, the Chlamydia-containing vacuole is extruded from the infected host cell by a mechanism that involves WASP, myosin II and RhoA. (B) Rho GTPases and Neisseria gonorrhoeae. OpaCEA-expressing Neisseria gonorrhoeae (Ng), through Opa-CEACAM3 recognition, triggers Rac1 and Cdc42 activation, and the downstream recruitment of WAVE2 and the actin recruiting protein Arp2/3, which ends in actin cytoskeleton rearrangements and bacterial internalization. In contrast, Rho-mediated entry triggered by the bacterial porin PorBIA drives actin polymerization that promotes the internalization of piliated gonococci into non-phagocytic cells. The release of Neisseria-containing phagosomes might involve Rho GTPases and actin reorganization.
Rho GTPases in Chlamydia Exit
Paradoxically, another member of the Rho family is involved in the release of the bacteria from infected cells. After their intracellular multiplication, Chlamydia disseminate and colonize neighboring cells. Two different mutually exclusive mechanisms have been described. The first one involves the lysis of the infected cells in an ordered sequence of membrane ruptures, beginning with the inclusion membrane, followed by the nuclear envelope and finally the plasma membrane. The second mechanism, known as extrusion, consists of pinching off the inclusion, protrusion out of the cell within a membrane compartment, and ultimately detachment from the host cell. The extrusion mechanism requires actin polymerization, neuronal Wiskott–Aldrich syndrome protein (WASP), myosin II and the GTPase Rho A39 (Fig. 1A).
Rho GTPases at Other Stages of Chlamydial Infection
Infection-triggered arthritis
The triggering of joint inflammation by infectious agents has been well established in the case of reactive arthritis.40 Infection with the obligate intracellular pathogen C. trachomatis is a common antecedent for reactive arthritis, as the synocyte is a suitable host cell for these bacteria.41 In this regard, Zhang et al. demonstrated that Rac proteins have a dual role in the outcome of Chlamydia-triggered arthritis.42 In the acute phase, Rac functions to exacerbate joint inflammation by promoting neutrophil migration, whereas during the chronic phase, Rac expression serves to alleviate arthritis, probably aiding the host clearance of Chlamydia and coordinating the appropriate expression of Toll-like receptor 4 (TLR-4) in neutrophils.43
Neisseria gonorrhoeae
Neisseria gonorrhoeae (the gonococcus) is the causative agent of gonorrhoeae, which is exclusively a human disease. These bacteria colonize the mucosae of the urethra, endocervix, fallopian tube, rectum, conjunctiva and pharynx. Neisserial infections range in severity from acute infections with good prognoses to severe diseases, such as purulent arthritis, pelvic inflammatory disease, endocarditis and meningitis.44 In addition, chronic infections can lead to ectopic pregnancies and infertility.45
N. gonorrhoeae is a Gram-negative bacterium with a typical signature: a high capacity to modulate its antigenic surface via phase variations that result from changes in gene expression. Additionally, it rapidly accumulates point mutations, acquiring antibiotic resistance, which are the basis of its ability to colonize host cells and evade immune system surveillance.46,47 Gonococci can reside inside several human cell types (neutrophils, monocytes, mucosal epithelial cells, endothelial cells) or act as extracellular pathogens.
Rho GTPases in Neisseria Entry
The infection begins with the attachment of the bacteria to the apical surface of mucosal cells via the gonococcal pilus.48,49 N. gonorrhoeae approach the cell host by extending their pili, and this is followed by their retraction or disassembly. Pilus retraction50 triggers a tight association between the bacterial opacity-associated (Opa) outer membrane proteins and the host cell receptors, which promotes invasion.48,50–53 A single gonococcal strain can harbour 11 opa genes that can be switched on and off independently. These genes contain 5′ tandem repeats [CTCTT]n that cause high-frequency, phase-variable expression,54 in addition to intra- and inter-genomic recombination events.55,56 Opa adhesins are classified into 2 main groups, OpaHS or OpaCEA, according to their host cell receptor. The heparan-sulfate proteoglycan (HSPG) receptors present in epithelial cells interact with one particular OpaHS variant, Opa50,57 while the carcinoembryonic antigen cell adhesion molecules (CEACAM) expressed by a variety of cell types recognize OpaCEA.58–60 The CEACAM family belongs to the immunoglobulin superfamily of adhesion molecules,61 and 4 members are receptors for Opa proteins: CEA, CEACAM1, CEACAM3 and CEACAM6.62,63 Even when the first cell-cell contact prior to host-pathogen adherence is carried out by pili, the specificity of the binding is controlled by Opa proteins. In addition to proteoglycan-mediated adherence that involves Opa50, several CEACAM receptors are differentially recognized by gonococcal OpaCEA variants. The strategies targeting these receptors might constitute an interesting alternative for drug design against gonorrhoea, especially to overcome the antigenic variability of Neisseria and its high capacity to generate antibiotic resistance.
Rho GTPases in CEACAM-mediated neisseria entry
The involvement of Rho GTPases varies depending on the receptor used for internalization. Invasion mediated by CEACAM1 and CEACAM6 does not require actin cytoskeleton reorganization; however, entry involving CEACAM3 triggers a high and localized reorganization of the host cell surface that is regulated by Rac1 and Cdc42, but not Rho A.64 The most distinctive feature of CEACAM3 is the presence of a sequence in its cytoplasmic domain that is reminiscent of an immunoreceptor tyrosine-based activation motif (ITAM) characterized by 2 precisely spaced tyrosine residues in a particular sequence context. Of note, CEACAM3 is exclusively expressed in neutrophils, and the majority of Opa-expressing Neisseria interact with neutrophils, leading to non-opsonic phagocytosis.59,65,66 Recent studies indicate that the CEACAM3-ITAM sequence associates directly with Vav, a Rac GEF, thus promoting the phagocytosis and elimination of CEACAM-bound bacteria.67 Interestingly, Vav directly associates via its Src homology 2 (SH2) domain with a phosphorylated tyrosine residue within the ITAM-like sequence of the receptor. Homologous SH2 domains are also found in the adaptor molecules Nck1 and Nck2, and mediate their interaction with CEACAM3 in a complex with the Rac effector WAVE2. Finally, these steps lead to F-actin polymerization during Neisseria uptake68 (Fig. 1B).
Rac1 and Cdc42 also participate in the activation of proinflammatory cytokines via a cascade of cellular stress response kinases, leading to the activation of JNK/AP1 via p21-activated kinase1 (PAK),69 in addition to their roles in bacteria internalization.70–74
Rho GTPases in porin-mediated neisseria entry
Porins are the major component of the outer membrane of pathogenic Neisseria species. In primary cervical epithelial cells, bacterial entry involves the binding of PorB, pili and lipooligosacharide to complement receptor type 3.75,76 In non-professional phagocytes, bacterial internalization is mediated by the PorB porin subtype A (PorBIA).77 PorBIA is a bacterial GTP binding protein that forms a voltage-gated channel that translocates into mammalian cell membranes and modulates host cell signaling events. This porin-mediated mechanism of entry is independent of the Opa proteins.77 In addition, this invasion process is not dependent on host microtubules, PI3K, or Src kinases, but requires low intracellular phosphate levels.78 It is noteworthy that the signaling cascades triggered by PorBIA in epithelial cells involve Rho GTPases and actin (Fig. 1B).
Rho GTPases in caveolin-mediated impairment of neisseria entry
Interestingly, type IV piliated N. gonorrhoeae induce the recruitment of host cell caveolin-1 (Cav1) and trigger Cav1 phosphorylation and downstream phosphotyrosine signaling. These events lead to cytoskeletal rearrangements that impair bacterial internalization. In brief, Cav1 interacts directly with Vav2, a Rho-family GEF, and both Vav2 and its substrate, RhoA, play a major role in the Cav1-mediated prevention of bacterial uptake.79
Rho GTPases in Neisseria Exit
After invading the host cell through the apical region, N. gonorrhoeae traverses the cell by transcytosis, exiting through the basal region.80 Host dynein and kinesin play a role in microtubule-mediated Neisseria transit across the cell. Conversely, myosin I is involved in the movement along the actin filament network.81 At present, the mechanisms used by this bacterium to disrupt the plasma membrane and disseminate into surrounding cells are not clear. This process may require actin reorganization, and it could be orchestrated by the activity of Rho GTPases (Fig. 1B). The exit process requires further study to unravel the molecular participants and their functions.
Rho GTPases at Other Stages of Gonococcal Infection
Rho GTPases and apoptosis
The PorB porin may induce apoptosis in epithelial cells and phagocytes.82 It is the only neisserial factor identified thus far that induces apoptosis in infected cells. Two host pro-apoptotic proteins, Bim and Bmf, act as crucial regulators of neisserial-induced apoptosis. Both eukaryotic proteins belong to the Bcl-2 family, and they function synergistically. Bim and Bmf are associated with the cytoskeleton and are released in a Rac-1-dependent manner after gonococcal infection.83
Trichomonas vaginalis
Trichomonas vaginalis, an extracellular protozoan parasite, is the etiological causative agent of trichomonosis, a widely disseminated, non-viral, sexually transmitted disease in humans.84 The symptoms associated with infection in women include abdominal pain, vulvar itching, purulence, the presence of a frothy malodorous discharge with leucorrhoea, signs of colpitis macularis, and vulvar and vaginal erythema.84 In men, it is usually asymptomatic; however, occasionally it can cause pruritus, urethritis with or without purulent secretions, epididymitis and prostatitis.85 Additionally, T. vaginalis can cause infertility in both men and women,84,86 and has been associated with an increased risk of acquiring HIV, cervical cancer and aggressive prostate cancer.84,87 Despite the high incidence of trichomonosis, the exact mechanism of infection used by T. vaginalis is not well established.
Trichomonas Attachment: Participation of Rho-Related GTPases in Actin Remodeling
Despite its extracellular nature, this protozoan must adhere to epithelial cells in the urogenital tract to survive. Several adhesins have been implicated, including multifunctional metabolic proteins, lipophosphoglycans and membrane proteins.88–92 Recent studies proposed a novel strategy for the adherence of T. vaginalis to host cells that involves the secretion of exosomes that have similar physical characteristics and protein compositions to mammalian exosomes. These exosomes promote both host-parasite and parasite-parasite interactions, and they play a role in the attachment of these protozoa to host epithelial cells.93
Actually, the binding to host cells causes the transition of T. vaginalis from an ovoid, free-swimming parasite into an amoeboid, highly adherent form.94 This process involves a transcriptomic response in the parasite that is triggered by contact with the host cell surface, which increases the expression of genes related to protein synthesis, phenotypic plasticity and host cell degradation. In fact, a major up-regulation of actin and actin-associated genes is evident, suggesting a role for the cytoskeleton in the amoeboid transition.95 Interestingly, TvRsp, a Rac1 homolog gene, has been found in T. vaginalis.96 The relationship between the Rac-related genes of this protozoan and actin cytoskeleton rearrangements remains to be further explored (Fig. 2).
Figure 2.
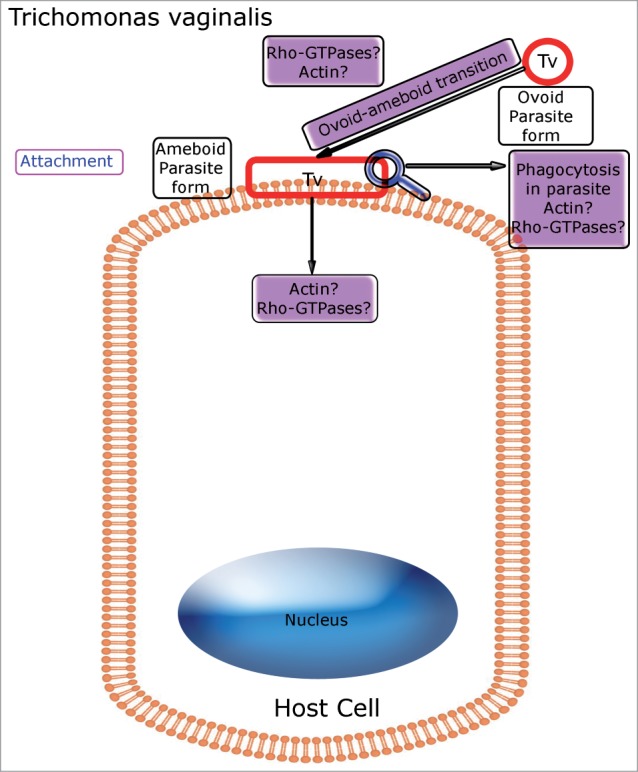
Schema depicting Rho GTPases involved in Trichomonas vaginalis-host cell interplay. Putative roles for Rho GTPases are indicated in purple boxes. Rho GTPase homologues are probably involved in the actin reorganization that occurs inside the parasite during the transition from the ovoid, free-swimming form to the amoeboid form, after its attachment to the host cell. In addition, Rho-related GTPases and actin polymerization apparently play a role in phagocytic processes that take place within the parasite.
Putative Role for Rho-Related GTPases in Nutrient Uptake by T. Vaginalis
T. vaginalis attachment to the host is important not only for establishing infection, but also for nutrient acquisition. However, a crucial source of nutrients comes from phagocytic processes occurring within these parasites. It is now clear that T. vaginalis is a phagocytic cell, able to efficiently ingest particles, bacteria and even mammalian cells.97 In immune cells, phagocytosis involves the reorganization of the cytoskeleton and relies on the control of actin dynamics by Rho GTPases.97,98 In contrast, the precise functions of actin and Rho-related GTPases during the phagocytic processes occurring in T. vaginalis remain elusive (Fig. 2).
Treponema pallidum
Treponema pallidum is the causative agent of syphilis, a chronic and multi-stage disease, with a diverse and wide range of clinical manifestations.99 The lack of treatment can lead to the development of primary, secondary and tertiary syphilis. The first stage is characterized by the appearance of a single, painless lesion (the chancre) at the site of inoculation. This pathogen is sexually transmitted through microabrasions in mucosal membranes where T. pallidum adheres to epithelial cells and extracellular matrix components of the skin and mucosa. In the second stage, characterized by a disseminated maculopapular rash, T. pallidum trespasses intercellular junctions of the endothelium, resulting in haematogenous dissemination and the seeding of the central nervous system and the remainder of the body.100–102 This pathogen may also use transcytosis to spread through the endothelium.100 In the tertiary and last stage, the disease may progress to cardiovascular disease, lesions of the skin, bones or viscera (gummata), or neurosyphilis.103
T. pallidum belongs to the Spirochaetaceae family of spiral-shaped bacteria. It is exclusively a human pathogen, able to survive for decades within the host, but extremely fragile ex vivo. This fragility represents one of the main challenges for its study. At present, it can only be transiently cultured in rabbit testes.104 Analysis of the T. pallidum genome led to the identification of numerous transport proteins and a limited number of genes encoding metabolic and biosynthetic enzymes, indicating that Treponema relies heavily on scavenging compounds from the human host.105
Treponema pallidum attachment to host cells
Infection starts with the attachment of T. pallidum to a wide variety of cell types, including epithelial, fibroblast-like and endothelial cells.106 After binding to the epithelial surface, this microorganism traverses the tissue barrier and enters the circulation by passing through the tight junctions between endothelial cells.100 T. pallidum invasion results in widespread dissemination.
The host cell components involved in Treponema attachment include host serum, cell membrane compounds, and the extracellular matrix.107 In particular, host cell fibronectin has been identified as the main target for bacterial adhesion. Actually, several proteins expressed in T. pallidum have been described as adhesins. Among them, Tp0155 binds to matrix fibronectin, whereas Tp0483 binds to both the soluble and matrix forms of fibronectin of host cells.108 To enter the circulatory system and penetrate cells, Tp0155 is required for attachment in the bloodstream, and Tp0483 is involved in tissue attachment; in both cases, attachment is promoted via binding to fibronectin. Tp0136 is another bacterial adhesin that binds to fibronectin and laminin, 2 host extracellular matrix glycoproteins.109 In addition, Tp0750 has a dual function, as it binds specifically to a variety of laminin isoforms,110 and it is able to degrade host molecules, suggesting that it plays a role in the proteolytic mechanisms involved in tissue damage, destruction and dissemination of T. pallidum during infection111 (Fig. 3).
Figure 3.
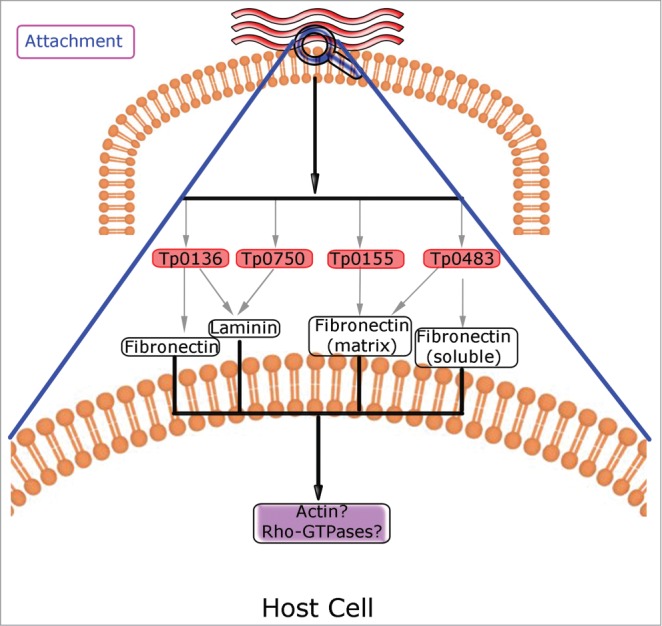
Schema depicting Rho GTPases involved in Treponema pallidum-host cell interplay. Putative roles for Rho GTPases are indicated in purple boxes. The bacterial adhesins Tp0136, Tp0155, Tp0483 and Tp0750, which binds to host fibronectin and/or laminin, are involved in the attachment to host cells. Treponema binding to cell surface might trigger actin polymerization that is regulated by Rho GTPases. The details of the events occurring at the plasma membrane are enlarged in the lower part of the panel.
Fibronectin and laminin may act in concert with Rho GTPases in a variety of physiological and pathological processes in eukaryotic cells.112–115 In non-infected cells, Rac1, RhoA and Cdc42, in connection with fibronectin and laminin, are involved in polarization, cell cycle progression117 and cell-cell adhesion.118 Interestingly, some microorganisms, through the release of adhesion molecules, bind to fibronectin and activate Rho GTPases to promote host cell invasion.119 For instance, the bacterial fibronectin-binding protein of Campylobacter jejuni, CadF, and the intact flagellum are involved in eukaryotic Rac1 GTPase activation and host cell invasion.116,120 It has been postulated that CadF binding to fibronectin results in integrin clustering and activation. These events lead to the activation of signaling cascades that involves epidermal growth factor receptor (EGFR) and focal adhesion kinase (FAK), resulting in association of c-Src and phosphorylation of paxillin. Finally, the recruitment of Dock180, a Rac1-specific guanine nucleotide exchange factor, provokes the activation of Rac1, leading to a local restructuring of the actin cytoskeleton and the engulfment of the bacteria.116,120 Similarly, in infections caused by T. pallidum, the bacterial adhesins might target fibronectin and laminin not only to enhance the binding to host cells108–110 but also to trigger intracellular signaling pathways that might promote Rac1 activation and consequently actin remodelling at the attachment site, facilitating the invasion of host tissues. Nevertheless, the role of Rho GTPases and the actin cytoskeleton in treponemal infections remains almost unexplored.
Conclusion
In pathogen-host cell interactions, the cytoskeleton plays a central role. It is necessary for epithelial and endothelial barrier integrity, and for preventing and limiting the invasion and dissemination of the pathogen to other tissues.121 It also participates actively in several processes in immune cells: migration,122 phagocytosis,9 immune synapsis and cell signaling.123,124 Actin dynamics are critical for phagocytosis and endocytosis in a wide variety of cell types. Conveniently, several pathogens, through diverse mechanisms, manipulate the cytoskeleton to infect host cells.125
Rho GTPases participate in multiple and essential cellular functions, acting as the most important controllers of the assembly and organization of the actin cytoskeleton. Therefore, it is not surprising that pathogens target Rho GTPases to promote their entry into host cells and to manipulate actin-dependent processes of infected cells. Here, we update the actual knowledge about the role played by Rho GTPases in infections caused by 4 different pathogens that cause the most important (non-viral), curable, sexually transmitted diseases, and the main data are summarized in Table 1.
Table 1.
Rho GTPases in sexually-transmitted infections. The table summarizes the main characteristics of certain sexually-transmitted pathogens, plus the role of Rho GTPases in several stages of the infectious processes. In dark gray are indicated the proteins belonging to the pathogen. nd: not determined.
| Pathogen | Chlamydia trachomatis | Neisseria gonorrhoeae | Trichomonas vaginalis | Treponema pallidum | ||
|---|---|---|---|---|---|---|
| Pathogen type | Bacteria Gram (-) | Bacteria Gram (-) (diplococci) | Flagellated protozoan | Bacteria Gram (-) (spirochetae) | ||
| Niche | Intracellular obligate | Intracellular facultative | Extracellular | Extracellular | ||
| Host cell type | Epithelial cells | Epithelial cells | Epithelial cells | Epithelial, fibroblast-like and endotelial cells | ||
| Step |
Entry |
Exit |
CEACAM-mediated entry |
PorinA-mediated entry |
Ovoid-ameboid transition |
Attachment to host cell |
| Member of the Rho family | Rac1 | RhoA | Rac1 – Cdc42 | RhoA | nd | nd |
| Rho activity regulators | Vav2- Sos1 (GEFs) | nd | Vav (GEF) - Nck | nd | nd | nd |
| Rho effectors | WAVE2- Abi1 | MyoII- WASP | WAVE2-Arp2/3 | nd | nd | nd |
| Pathogen molecules | TARP (actin recruiting protein) | CT 166 (Rac1 inactivator) | nd | PorBIA (GTP-binding protein) | TvRSP (Rac1 homolog) | nd |
C. trachomatis, a Gram-negative, obligate intracellular bacterium, utilizes Rac1 to facilitate its entry into host cells, and it uses RhoA to exit host cells and to disseminate to neighboring tissues.
N. gonorrhoeae, a Gram-negative, facultative intracellular bacterium, utilizes Rac1 and Cdc42 to promote Opa-CEACAM3 mediated entry into phagocytic host cells, and Rho for PorBIA-mediated entry for piliated gonococci in non-phagocytic host cells. In both cases, a signaling cascade involving phosphorylation events, kinases, GTPases and GEFs leads to the final actin cytoskeleton rearrangement at the sites of entry of the bacteria.
T. vaginalis, an extracellular protozoan, does not provoke significant cytoskeletal rearrangements in host cells. Instead, actin reorganization occurs in the parasite itself. The role of Rho GTPases is not fully unravelled, but the parasite has genes that encode Rho homologues. Further investigation of T. vaginalis Rho-like GTPases and their relationship with the actin cytoskeleton of the parasite is necessary.
Finally, in the case of T. pallidum, a spiral-shaped bacterium, the role of the actin cytoskeleton and Rho GTPases during infection remains unexplored. This is largely due to the difficulties in the culturing and manipulation of Treponema, which hinder a full description of the infection process.
Knowledge of the mechanisms of invasion, proliferation and dissemination of these pathogens should contribute to more effective treatments of these sexually transmitted diseases. Rho GTPases, which are likely involved in many stages of the infection, such as the attachment, engulfment and spread of these pathogens, are molecular targets that require further studies to determine their usefulness in the control of these sexually transmitted infectious diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors apologize for not mentioning all published data due to space restrictions. We thank Andrew Lindsay (Molecular Cell Biology Laboratory, School of Biochemistry and Cell Biology, Biosciences Institute, University College Cork, Cork, Ireland) and Stephanie Miserey-Lenkei (Laboratory of Molecular mechanisms of intracellular transport, CNRS UMR 144, Curie Institute, Paris, France) for critical reading of this manuscript and useful suggestions.
Funding
Our work on pathogens-host cells interaction is supported by the Bunge&Born Foundation, Sectyp-UNCuyo, Foncyt (PICT 2116 grant) and the Argentine Council of Research (CONICET).
References
- 1. Rojas AM, Fuentes G, Rausell A, Valencia A. The ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol 2012; 196:189-201; PMID:22270915; http://dx.doi.org/ 10.1083/jcb.201103008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 2000; 348 Pt 2:241-55; PMID:10816416; http://dx.doi.org/ 10.1042/0264-6021:3480241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thumkeo D, Watanabe S, Narumiya S. Physiological roles of rho and rho effectors in mammals. Eur J Cell Biol 2013; 92(10-11):303-15; PMID:24183240; http://dx.doi.org/ 10.1016/j.ejcb.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 4. Wennerberg K, Rossman KL, Der CJ. The ras superfamily at a glance. J Cell Sci 2005; 118:843-6; PMID:15731001; http://dx.doi.org/ 10.1242/jcs.01660 [DOI] [PubMed] [Google Scholar]
- 5. Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279:509-14; PMID:9438836; http://dx.doi.org/ 10.1126/science.279.5350.509 [DOI] [PubMed] [Google Scholar]
- 6. Madaule P, Axel R. A novel ras-related gene family. Cell 1985; 41:31-40; PMID:3888408; http://dx.doi.org/ 10.1016/0092-8674(85)90058-3 [DOI] [PubMed] [Google Scholar]
- 7. Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629-35; PMID:12478284; http://dx.doi.org/ 10.1038/nature01148 [DOI] [PubMed] [Google Scholar]
- 8. Boquet P, Lemichez E. Bacterial virulence factors targeting rho GTPases: parasitism or symbiosis? Trends Cell Biol 2003; 13:238-46; PMID:12742167; http://dx.doi.org/ 10.1016/S0962-8924(03)00037-0 [DOI] [PubMed] [Google Scholar]
- 9. Bokoch GM. Regulation of innate immunity by rho GTPases. Trends Cell Biol 2005; 15:163-71; PMID:15752980; http://dx.doi.org/ 10.1016/j.tcb.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 10. Galan JE, Cossart P. Host-pathogen interactions: a diversity of themes, a variety of molecular machines. Curr Opin Microbiol 2005; 8:1-3; PMID:15694849; http://dx.doi.org/ 10.1016/j.mib.2004.12.015 [DOI] [PubMed] [Google Scholar]
- 11. Aktories K, Barbieri JT. Bacterial cytotoxins: targeting eukaryotic switches. Nat Rev Microbiol 2005; 3:397-410; PMID:15821726; http://dx.doi.org/ 10.1038/nrmicro1150 [DOI] [PubMed] [Google Scholar]
- 12. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol 2010; 63:576-86; PMID:20192953; http://dx.doi.org/ 10.1111/j.1600-0897.2010.00819.x [DOI] [PubMed] [Google Scholar]
- 13. Knowlton AE, Brown HM, Richards TS, Andreolas LA, Patel RK, Grieshaber SS. Chlamydia trachomatis infection causes mitotic spindle pole defects independently from its effects on centrosome amplification. Traffic 2011; 12:854-66; PMID:21477082; http://dx.doi.org/ 10.1111/j.1600-0854.2011.01204.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scidmore MA. Recent advances in chlamydia subversion of host cytoskeletal and membrane trafficking pathways. Microbes Infect 2011; 13:527-35; PMID:21334451; http://dx.doi.org/ 10.1016/j.micinf.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fields KA, Hackstadt T. The chlamydial inclusion: escape from the endocytic pathway. Annu Rev Cell Dev Biol 2002; 18:221-45; PMID:12142274; http://dx.doi.org/ 10.1146/annurev.cellbio.18.012502.105845 [DOI] [PubMed] [Google Scholar]
- 16. Robertson DK, Gu L, Rowe RK, Beatty WL. Inclusion biogenesis and reactivation of persistent chlamydia trachomatis requires host cell sphingolipid biosynthesis. PLoS Pathog 2009; 5:e1000664; PMID:19936056; http://dx.doi.org/ 10.1371/journal.ppat.1000664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cocchiaro JL, Valdivia RH. New insights into chlamydia intracellular survival mechanisms. Cell Microbiol 2009; 11:1571-8; PMID:19673891; http://dx.doi.org/ 10.1111/j.1462-5822.2009.01364.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leiva N, Capmany A, Damiani MT. Rab11-family of interacting protein 2 associates with chlamydial inclusions through its rab-binding domain and promotes bacterial multiplication. Cell Microbiol 2013; 15:114-29; PMID:23006599; http://dx.doi.org/ 10.1111/cmi.12035 [DOI] [PubMed] [Google Scholar]
- 19. Capmany A, Damiani MT. Chlamydia trachomatis intercepts golgi-derived sphingolipids through a rab14-mediated transport required for bacterial development and replication. PLoS One 2010; 5:e14084; PMID:21124879; http://dx.doi.org/ 10.1371/journal.pone.0014084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valdivia RH. Chlamydia effector proteins and new insights into chlamydial cellular microbiology. Curr Opin Microbiol 2008; 11:53-9; PMID:18299248; http://dx.doi.org/ 10.1016/j.mib.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 21. Capmany A, Leiva N, Damiani MT. Golgi-associated rab14, a new regulator for chlamydia trachomatis infection outcome. Commun Integr Biol 2011; 4:590-3; PMID:22046472; http://dx.doi.org/ 10.4161/cib.16594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Damiani MT, Gambarte Tudela J, Capmany A. Targeting eukaryotic rab proteins: a smart strategy for chlamydial survival and replication. Cell Microbiol 2014; 16(9):1329-38; PMID:24948448. [DOI] [PubMed] [Google Scholar]
- 23. Byrne GI, Moulder JW. Parasite-specified phagocytosis of chlamydia psittaci and chlamydia trachomatis by L and HeLa cells. Infect Immun 1978; 19:598-606; PMID:344217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Su H, Raymond L, Rockey DD, Fischer E, Hackstadt T, Caldwell HD. A recombinant chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc Natl Acad Sci U S A 1996; 93:11143-8; PMID:8855323; http://dx.doi.org/ 10.1073/pnas.93.20.11143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang JP, Stephens RS. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell 1992; 69:861-9; PMID:1591780; http://dx.doi.org/ 10.1016/0092-8674(92)90296-O [DOI] [PubMed] [Google Scholar]
- 26. Abromaitis S, Stephens RS. Attachment and entry of chlamydia have distinct requirements for host protein disulfide isomerase. PLoS Pathog 2009; 5:e1000357; PMID:19343202; http://dx.doi.org/ 10.1371/journal.ppat.1000357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Conant CG, Stephens RS. Chlamydia attachment to mammalian cells requires protein disulfide isomerase. Cell Microbiol 2007; 9:222-32; PMID:16925789; http://dx.doi.org/ 10.1111/j.1462-5822.2006.00783.x [DOI] [PubMed] [Google Scholar]
- 28. Puolakkainen M, Kuo CC, Campbell LA. Chlamydia pneumoniae uses the mannose 6-phosphate/insulin-like growth factor 2 receptor for infection of endothelial cells. Infect Immun 2005; 73:4620-5; PMID:16040974; http://dx.doi.org/ 10.1128/IAI.73.8.4620-4625.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elwell CA, Ceesay A, Kim JH, Kalman D, Engel JN. RNA interference screen identifies abl kinase and PDGFR signaling in Chlamydia trachomatis entry. PLoS Pathog 2008; 4:e1000021; PMID:18369471; http://dx.doi.org/ 10.1371/journal.ppat.1000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carabeo RA, Grieshaber SS, Hasenkrug A, Dooley C, Hackstadt T. Requirement for the rac GTPase in chlamydia trachomatis invasion of non-phagocytic cells. Traffic 2004; 5:418-25; PMID:15117316; http://dx.doi.org/ 10.1111/j.1398-9219.2004.00184.x [DOI] [PubMed] [Google Scholar]
- 31. Subtil A, Wyplosz B, Balana ME, Dautry-Varsat A. Analysis of chlamydia caviae entry sites and involvement of cdc42 and rac activity. J Cell Sci 2004; 117:3923-33; PMID:15265988; http://dx.doi.org/ 10.1242/jcs.01247 [DOI] [PubMed] [Google Scholar]
- 32. Beeckman DS, Vanrompay DC. Bacterial secretion systems with an emphasis on the chlamydial type III secretion system. Curr Issues Mol Biol 2010; 12:17-41; PMID:19605938. [PubMed] [Google Scholar]
- 33. Clifton DR, Fields KA, Grieshaber SS, Dooley CA, Fischer ER, Mead DJ, Carabeo RA, Hackstadt T. A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proc Natl Acad Sci U S A 2004; 101:10166-71; PMID:15199184; http://dx.doi.org/ 10.1073/pnas.0402829101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jewett TJ, Dooley CA, Mead DJ, Hackstadt T. Chlamydia trachomatis tarp is phosphorylated by src family tyrosine kinases. Biochem Biophys Res Commun 2008; 371:339-44; PMID:18442471; http://dx.doi.org/ 10.1016/j.bbrc.2008.04.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lane BJ, Mutchler C, Al Khodor S, Grieshaber SS, Carabeo RA. Chlamydial entry involves TARP binding of guanine nucleotide exchange factors. PLoS Pathog 2008; 4:e1000014; PMID:18383626; http://dx.doi.org/ 10.1371/journal.ppat.1000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carabeo RA, Dooley CA, Grieshaber SS, Hackstadt T. Rac interacts with abi-1 and WAVE2 to promote an arp2/3-dependent actin recruitment during chlamydial invasion. Cell Microbiol 2007; 9:2278-88; PMID:17501982; http://dx.doi.org/ 10.1111/j.1462-5822.2007.00958.x [DOI] [PubMed] [Google Scholar]
- 37. Kim JH, Jiang S, Elwell CA, Engel JN. Chlamydia trachomatis co-opts the FGF2 signaling pathway to enhance infection. PLoS Pathog 2011; 7:e1002285; PMID:21998584; http://dx.doi.org/ 10.1371/journal.ppat.1002285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thalmann J, Janik K, May M, Sommer K, Ebeling J, Hofmann F, Genth H, Klos A. Actin re-organization induced by chlamydia trachomatis serovar D–evidence for a critical role of the effector protein CT166 targeting rac. PLoS One 2010; 5:e9887; PMID:20360858; http://dx.doi.org/ 10.1371/journal.pone.0009887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hybiske K, Stephens RS. Mechanisms of host cell exit by the intracellular bacterium chlamydia. Proc Natl Acad Sci U S A 2007; 104:11430-5; PMID:17592133; http://dx.doi.org/ 10.1073/pnas.0703218104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hyrich KL, Inman RD. Infectious agents in chronic rheumatic diseases. Curr Opin Rheumatol 2001; 13:300-4; PMID:11555732; http://dx.doi.org/ 10.1097/00002281-200107000-00010 [DOI] [PubMed] [Google Scholar]
- 41. Inman RD, Chiu B. Synoviocyte-packaged chlamydia trachomatis induces a chronic aseptic arthritis. J Clin Invest 1998; 102:1776-82; PMID:9819362; http://dx.doi.org/ 10.1172/JCI2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang X, Glogauer M, Zhu F, Kim T, Chiu B, Inman RD. Innate immunity and arthritis neutrophil rac and toll-like receptor 4 expression define outcomes in infection-triggered arthritis. Arthritis Rheum 2005; 52:1297-304; PMID:15818670; http://dx.doi.org/ 10.1002/art.20984 [DOI] [PubMed] [Google Scholar]
- 43. Zhang X, Glogauer M, Zhu F, Kim TH, Chiu B, Inman RD. Innate immunity and arthritis: neutrophil rac and toll-like receptor 4 expression define outcomes in infection-triggered arthritis. Arthritis Rheum 2005; 52:1297-304; PMID:15818670; http://dx.doi.org/ 10.1002/art.20984 [DOI] [PubMed] [Google Scholar]
- 44. Skerlev M, Čulav-Košćak I. Gonorrhea: new challenges. Clin Dermatol 2014; 32:275-81; PMID:24559563; http://dx.doi.org/ 10.1016/j.clindermatol.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 45. Nolan GH, Osborne N. Gonococcal infections in the female. Obstet Gynecol 1973; 42:156-64; PMID:4198268. [PubMed] [Google Scholar]
- 46. Segal E, Hagblom P, Seifert HS, So M. Antigenic variation of gonococcal pilus involves assembly of separated silent gene segments. Proc Natl Acad Sci U S A 1986; 83:2177-81; PMID:2870495; http://dx.doi.org/ 10.1073/pnas.83.7.2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stern A, Brown M, Nickel P, Meyer TF. Opacity genes in neisseria gonorrhoeae: control of phase and antigenic variation. Cell 1986; 47:61-71; PMID:3093085; http://dx.doi.org/ 10.1016/0092-8674(86)90366-1 [DOI] [PubMed] [Google Scholar]
- 48. Merz AJ, Rifenbery DB, Arvidson CG, So M. Traversal of a polarized epithelium by pathogenic neisseriae: facilitation by type IV pili and maintenance of epithelial barrier function. Mol Med 1996; 2:745-54; PMID:8972489. [PMC free article] [PubMed] [Google Scholar]
- 49. Virji M, Heckels JE. The role of common and type-specific pilus antigenic domains in adhesion and virulence of gonococci for human epithelial cells. J Gen Microbiol 1984; 130:1089-95; PMID:6147388. [DOI] [PubMed] [Google Scholar]
- 50. Merz AJ, So M, Sheetz MP. Pilus retraction powers bacterial twitching motility. Nature 2000; 407:98-102; PMID:10993081; http://dx.doi.org/ 10.1038/35024105 [DOI] [PubMed] [Google Scholar]
- 51. Meyer TF, Pohlner J, van Putten JP. Biology of the pathogenic neisseriae. Curr Top Microbiol Immunol 1994; 192:283-317; PMID:7859510. [DOI] [PubMed] [Google Scholar]
- 52. Swanson J. Studies on gonococcus infection. IV. pili: their role in attachment of gonococci to tissue culture cells. J Exp Med 1973; 137:571-89; PMID:4631989; http://dx.doi.org/ 10.1084/jem.137.3.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Swanson J. Colony opacity and protein II compositions of gonococci. Infect Immun 1982; 37:359-68; PMID:6809633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stern A, Brown M, Nickel P, Meyer TF. Opacity genes in neisseria gonorrhoeae: control of phase and antigenic variation. Cell 1986; 47:61-71; PMID:3093085; http://dx.doi.org/ 10.1016/0092-8674(86)90366-1 [DOI] [PubMed] [Google Scholar]
- 55. Bilek N, Ison CA, Spratt BG. Relative contributions of recombination and mutation to the diversification of the opa gene repertoire of Neisseria gonorrhoeae. J Bacteriol 2009; 191:1878-90; PMID:19114493; http://dx.doi.org/ 10.1128/JB.01518-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Blake MS, Gotschlich EC. Purification and partial characterization of the opacity-associated proteins of neisseria gonorrhoeae. J Exp Med 1984; 159:452-62; PMID:6420502; http://dx.doi.org/ 10.1084/jem.159.2.452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Van Putten JP, Paul SM. Binding of syndecan-like cell surface proteoglycan receptors is required for neisseria gonorrhoeae entry into human mucosal cells. EMBO J 1995; 14:2144-54; PMID:7774572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF. CD66 carcinoembryonic antigens mediate interactions between opa-expressing neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J 1997; 16:3435-45; PMID:9218786; http://dx.doi.org/ 10.1093/emboj/16.12.3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gray-Owen SD, Lorenzen DR, Haude A, Meyer TF, Dehio C. Differential opa specificities for CD66 receptors influence tissue interactions and cellular response to neisseria gonorrhoeae. Mol Microbiol 1997; 26:971-80; PMID:9426134; http://dx.doi.org/ 10.1046/j.1365-2958.1997.6342006.x [DOI] [PubMed] [Google Scholar]
- 60. Virji M, Makepeace K, Ferguson DJ, Watt SM. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for opa proteins of pathogenic neisseriae. Mol Microbiol 1996; 22:941-50; PMID:8971715; http://dx.doi.org/ 10.1046/j.1365-2958.1996.01551.x [DOI] [PubMed] [Google Scholar]
- 61. Obrink B. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol 1997; 9:616-26; PMID:9330864; http://dx.doi.org/ 10.1016/S0955-0674(97)80114-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol 2006; 18:565-71; PMID:16919437; http://dx.doi.org/ 10.1016/j.ceb.2006.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sadarangani M, Pollard AJ, Gray-Owen SD. Opa proteins and CEACAMs: pathways of immune engagement for pathogenic neisseria. FEMS Microbiol Rev 2011; 35:498-514; PMID:21204865; http://dx.doi.org/ 10.1111/j.1574-6976.2010.00260.x [DOI] [PubMed] [Google Scholar]
- 64. Billker O, Popp A, Brinkmann V, Wenig G, Schneider J, Caron E, Meyer TF. Distinct mechanisms of internalization of neisseria gonorrhoeae by members of the CEACAM receptor family involving rac1- and cdc42-dependent and -independent pathways. EMBO J 2002; 21:560-71; PMID:11847104; http://dx.doi.org/ 10.1093/emboj/21.4.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Belland RJ, Chen T, Swanson J, Fischer SH. Human neutrophil response to recombinant neisserial opa proteins. Mol Microbiol 1992; 6:1729-37; PMID:1630313; http://dx.doi.org/ 10.1111/j.1365-2958.1992.tb01345.x [DOI] [PubMed] [Google Scholar]
- 66. Virji M, Heckels JE. The effect of protein II and pili on the interaction of neisseria gonorrhoeae with human polymorphonuclear leucocytes. J Gen Microbiol 1986; 132:503-12; PMID:2872268. [DOI] [PubMed] [Google Scholar]
- 67. Schmitter T, Pils S, Sakk V, Frank R, Fischer KD, Hauck CR. The granulocyte receptor carcinoembryonic antigen-related cell adhesion molecule 3 (CEACAM3) directly associates with vav to promote phagocytosis of human pathogens. J Immunol 2007; 178:3797-805; PMID:17339478; http://dx.doi.org/ 10.4049/jimmunol.178.6.3797 [DOI] [PubMed] [Google Scholar]
- 68. Pils S, Kopp K, Peterson L, Delgado Tascon J, Nyffenegger-Jann NJ, Hauck CR. The adaptor molecule nck localizes the WAVE complex to promote actin polymerization during CEACAM3-mediated phagocytosis of bacteria. PLoS One 2012; 7:e32808; PMID:22448228; http://dx.doi.org/ 10.1371/journal.pone.0032808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Naumann M, Rudel T, Wieland B, Bartsch C, Meyer TF. Coordinate activation of activator protein 1 and inflammatory cytokines in response to neisseria gonorrhoeae epithelial cell contact involves stress response kinases. J Exp Med 1998; 188:1277-86; PMID:9763607; http://dx.doi.org/ 10.1084/jem.188.7.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nagel G, Grunert F, Kuijpers TW, Watt SM, Thompson J, Zimmermann W. Genomic organization, splice variants and expression of CGM1, a CD66-related member of the carcinoembryonic antigen gene family. Eur J Biochem 1993; 214:27-35; PMID:8508798; http://dx.doi.org/ 10.1111/j.1432-1033.1993.tb17892.x [DOI] [PubMed] [Google Scholar]
- 71. Hauck CR, Gulbins E, Lang F, Meyer TF. Tyrosine phosphatase SHP-1 is involved in CD66-mediated phagocytosis of opa52-expressing neisseria gonorrhoeae. Infect Immun 1999; 67:5490-4; PMID:10496937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sarantis H, Gray-Owen SD. The specific innate immune receptor CEACAM3 triggers neutrophil bactericidal activities via a syk kinase-dependent pathway. Cell Microbiol 2007; 9:2167-80; PMID:17506820; http://dx.doi.org/ 10.1111/j.1462-5822.2007.00947.x [DOI] [PubMed] [Google Scholar]
- 73. Buntru A, Kopp K, Voges M, Frank R, Bachmann V, Hauck CR. Phosphatidylinositol 3’-kinase activity is critical for initiating the oxidative burst and bacterial destruction during CEACAM3-mediated phagocytosis. J Biol Chem 2011; 286:9555-66; PMID:21216968; http://dx.doi.org/ 10.1074/jbc.M110.216085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen T, Bolland S, Chen I, Parker J, Pantelic M, Grunert F, Zimmermann W. The CGM1a (CEACAM3/CD66d)-mediated phagocytic pathway of neisseria gonorrhoeae expressing opacity proteins is also the pathway to cell death. J Biol Chem 2001; 276:17413-9; PMID:11278708; http://dx.doi.org/ 10.1074/jbc.M010609200 [DOI] [PubMed] [Google Scholar]
- 75. Edwards JL, Brown EJ, Uk-Nham S, Cannon JG, Blake MS, Apicella MA. A co-operative interaction between neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell Microbiol 2002; 4:571-84; PMID:12390350; http://dx.doi.org/ 10.1046/j.1462-5822.2002.t01-1-00215.x [DOI] [PubMed] [Google Scholar]
- 76. Edwards JL, Apicella MA. The role of lipooligosaccharide in neisseria gonorrhoeae pathogenesis of cervical epithelia: lipid a serves as a C3 acceptor molecule. Cell Microbiol 2002; 4:585-98; PMID:12390351; http://dx.doi.org/ 10.1046/j.1462-5822.2002.00212.x [DOI] [PubMed] [Google Scholar]
- 77. Van Putten JP, Duensing TD, Carlson J. Gonococcal invasion of epithelial cells driven by P.IA, a bacterial ion channel with GTP binding properties. J Exp Med 1998; 188:941-52; PMID:9730895; http://dx.doi.org/ 10.1084/jem.188.5.941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kuhlewein C, Rechner C, Meyer TF, Rudel T. Low-phosphate-dependent invasion resembles a general way for neisseria gonorrhoeae to enter host cells. Infect Immun 2006; 74:4266-73; PMID:16790801; http://dx.doi.org/ 10.1128/IAI.00215-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Boettcher JP, Kirchner M, Churin Y, Kaushansky A, Pompaiah M, Thorn H, Brinkmann V, Macbeath G, Meyer TF. Tyrosine-phosphorylated caveolin-1 blocks bacterial uptake by inducing vav2-rhoa-mediated cytoskeletal rearrangements. PLoS Biol 2010; 8(8): e1000457. PMID:20808760; http://dx.doi.org/ 10.1371/journal.pbio.1000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Criss AK, Seifert HS. Gonococci exit apically and basally from polarized epithelial cells and exhibit dynamic changes in type IV pili. Cell Microbiol 2006; 8:1430-43; PMID:16922862; http://dx.doi.org/ 10.1111/j.1462-5822.2006.00722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang J, Meyer TF, Rudel T. Cytoskeleton and motor proteins are required for the transcytosis of neisseria gonorrhoeae through polarized epithelial cells. Int J Med Microbiol 2008; 298:209-21; PMID:17683982; http://dx.doi.org/ 10.1016/j.ijmm.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 82. Muller A, Gunther D, Dux F, Naumann M, Meyer TF, Rudel T. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J 1999; 18:339-52; PMID:9889191; http://dx.doi.org/ 10.1093/emboj/18.2.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kepp O, Gottschalk K, Churin Y, Rajalingam K, Brinkmann V, Machuy N, Kroemer G, Rudel T. Bim and bmf synergize to induce apoptosis in neisseria gonorrhoeae infection. PLoS Pathog. 2009;5(3):e1000348; PMID:19300516; http://dx.doi.org/ 10.1371/journal.ppat.1000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of trichomonas vaginalis. Clin Microbiol Rev 1998; 11:300-17; PMID:9564565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Krieger JN. Trichomoniasis in men: old issues and new data. Sex Transm Dis 1995; 22:83-96; PMID:7624817; http://dx.doi.org/ 10.1097/00007435-199503000-00003 [DOI] [PubMed] [Google Scholar]
- 86. Lloyd G, Case JR, De Frias D, Brannigan RE. Trichomonas vaginalis orchitis with associated severe oligoasthenoteratospermia and hypogonadism. J Urol 2003; 170:924; PMID:12913736; http://dx.doi.org/ 10.1097/01.ju.0000080375.18547.cc [DOI] [PubMed] [Google Scholar]
- 87. Sutcliffe S, Giovannucci E, Alderete JF, Chang TH, Gaydos CA, Zenilman JM, De Marzo AM, Willett WC, Platz EA. Plasma antibodies against trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006; 15:939-45; PMID:16702374; http://dx.doi.org/ 10.1158/1055-9965.EPI-05-0781 [DOI] [PubMed] [Google Scholar]
- 88. Lehker MW, Sweeney D. Trichomonad invasion of the mucous layer requires adhesins, mucinases, and motility. Sex Transm Infect 1999; 75:231-8; PMID:10615308; http://dx.doi.org/ 10.1136/sti.75.4.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hirt RP, Noel CJ, Sicheritz-Ponten T, Tachezy J, Fiori PL. Trichomonas vaginalis surface proteins: a view from the genome. Trends Parasitol 2007; 23:540-7; PMID:17962075; http://dx.doi.org/ 10.1016/j.pt.2007.08.020 [DOI] [PubMed] [Google Scholar]
- 90. Lama A, Kucknoor A, Mundodi V, Alderete JF. Glyceraldehyde-3-phosphate dehydrogenase is a surface-associated, fibronectin-binding protein of trichomonas vaginalis. Infect Immun 2009; 77:2703-11; PMID:19380472; http://dx.doi.org/ 10.1128/IAI.00157-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fichorova RN, Trifonova RT, Gilbert RO, Costello CE, Hayes GR, Lucas JJ, Singh BN. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect Immun 2006; 74:5773-9; PMID:16988255; http://dx.doi.org/ 10.1128/IAI.00631-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Okumura CY, Baum LG, Johnson PJ. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite trichomonas vaginalis. Cell Microbiol 2008; 10:2078-90; PMID:18637021; http://dx.doi.org/ 10.1111/j.1462-5822.2008.01190.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Twu O, de Miguel N, Lustig G, Stevens GC, Vashisht AA, Wohlschlegel JA, Johnson PJ. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host : parasite interactions. PLoS Pathog. 2013; 9(7):e1003482; PMID:23853596; http://dx.doi.org/ 10.1371/journal.ppat.1003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Arroyo R, Gonzalez-Robles A, Martinez-Palomo A, Alderete JF. Signalling of trichomonas vaginalis for amoeboid transformation and adhesion synthesis follows cytoadherence. Mol Microbiol 1993; 7:299-309; PMID:8446032; http://dx.doi.org/ 10.1111/j.1365-2958.1993.tb01121.x [DOI] [PubMed] [Google Scholar]
- 95. De Miguel N, Lustig G, Twu O, Chattopadhyay A, Wohlschlegel JA, Johnson PJ. Proteome analysis of the surface of trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol Cell Proteomics 2010; 9:1554-66; PMID:20467041; http://dx.doi.org/ 10.1074/mcp.M000022-MCP201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xu M-Y, Liu J-L, Zhang R-L, Fu Y. Isolation of a novel ras gene from trichomonas vaginalis: a possible evolutionary ancestor of the ras and rap genes of higher eukaryotes. Biochem Cell Biol 2007; 85:239-45; PMID:17534405; http://dx.doi.org/ 10.1139/O07-008 [DOI] [PubMed] [Google Scholar]
- 97. Rendon-Maldonado JG, Espinosa-Cantellano M, Gonzalez-Robles A, Martinez-Palomo A. Trichomonas vaginalis: in vitro phagocytosis of lactobacilli, vaginal epithelial cells, leukocytes, and erythrocytes. Exp Parasitol 1998; 89:241-50; PMID:9635448; http://dx.doi.org/ 10.1006/expr.1998.4297 [DOI] [PubMed] [Google Scholar]
- 98. Twu O, de Miguel N, Lustig G, Stevens GC, Vashisht AA, Wohlschlegel JA, Johnson PJ. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate hostratioparasite interactions. PLoS Pathog 2013; 9:e1003482; PMID:23853596; http://dx.doi.org/ 10.1371/journal.ppat.1003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Farhi D, Dupin N. Origins of syphilis and management in the immunocompetent patient: facts and controversies. Clin Dermatol 2010; 28:533-8; PMID:20797514; http://dx.doi.org/ 10.1016/j.clindermatol.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 100. Thomas DD, Navab M, Haake DA, Fogelman AM, Miller JN, Lovett MA. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci U S A 1988; 85:3608-12; PMID:3285346; http://dx.doi.org/ 10.1073/pnas.85.10.3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Juanpere-Rodero N, Martin-Ezquerra G, Fernandez-Casado A, Magan-Perea L, Garcia-Alguacil MA, Barranco-Sanz C, Serrano-Figueras S, Pujol-Vallverdu RM, Lloreta-Trull J. Cell and tissue interactions of treponema pallidum in primary and secondary syphilitic skin lesions: an ultrastructural study of serial sections. Ultrastruct Pathol 2013; 37:36-42; PMID:21736426; http://dx.doi.org/ 10.3109/01913123.2011.584498 [DOI] [PubMed] [Google Scholar]
- 102. Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis 1980; 7:161-4; PMID:7455863; http://dx.doi.org/ 10.1097/00007435-198010000-00002 [DOI] [PubMed] [Google Scholar]
- 103. Kent ME, Romanelli F. Reexamining syphilis: an update on epidemiology, clinical manifestations, and management. Ann Pharmacother 2008; 42(2):226-36; PMID:18212261; http://dx.doi.org/ 10.1345/aph.1K086 [DOI] [PubMed] [Google Scholar]
- 104. Tight RR, Perkins RL. Treponema pallidum infection in subcutaneous polyethylene chambers in rabbits. Infect Immun 1976; 13:1606-12; PMID:786879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GG, Dodson R, Gwinn M, Hickey EK, Clayton R, Ketchum KA, et al. . Complete genome sequence of treponema pallidum, the syphilis spirochete. Science 1998; 281:375-88; PMID:9665876; http://dx.doi.org/ 10.1126/science.281.5375.375 [DOI] [PubMed] [Google Scholar]
- 106. Fitzgerald TJ, Johnson RC, Miller JN, Sykes JA. Characterization of the attachment of treponema pallidum (nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun 1977; 18:467-78; PMID:336548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hayes NS, Muse KE, Collier AM, Baseman JB. Parasitism by virulent treponema pallidum of host cell surfaces. Infect Immun 1977; 17:174-86; PMID:328394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cameron CE, Brown EL, Kuroiwa JM, Schnapp LM, Brouwer NL. Treponema pallidum fibronectin-binding proteins. J Bacteriol 2004; 186:7019-22; PMID:15466055; http://dx.doi.org/ 10.1128/JB.186.20.7019-7022.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Brinkman MB, McGill MA, Pettersson J, Rogers A, Matejkova P, Smajs D, Weinstock GM, Norris SJ, Palzkill T. A novel treponema pallidum antigen, TP0136, is an outer membrane protein that binds human fibronectin. Infect Immun 2008; 76:1848-57; PMID:18332212; http://dx.doi.org/ 10.1128/IAI.01424-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cameron CE. Identification of a treponema pallidum laminin-binding protein. Infect Immun 2003; 71:2525-33; PMID:12704124; http://dx.doi.org/ 10.1128/IAI.71.5.2525-2533.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Houston S, Hof R, Francescutti T, Hawkes A, Boulanger MJ, Cameron CE. Bifunctional role of the treponema pallidum extracellular matrix binding adhesin Tp0751. Infect Immun 2011; 79:1386-98; PMID:21149586; http://dx.doi.org/ 10.1128/IAI.01083-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Watanabe T, Takahashi Y. Tissue morphogenesis coupled with cell shape changes. Curr Opin Genet Dev 2010; 20:443-7; PMID:20677359; http://dx.doi.org/ 10.1016/j.gde.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 113. Calì G, Mazzarella C, Chiacchio M, Negri R, Retta SF, Zannini M, Gentile F, Tarone G, Nitsch L, Garbi C. RhoA activity is required for fibronectin assembly and counteracts beta1B integrin inhibitory effect in FRT epithelial cells. J Cell Sci 1999; 112 (Pt 6):957-65; PMID:10036245. [DOI] [PubMed] [Google Scholar]
- 114. Dubash AD, Wennerberg K, García-Mata R, Menold MM, Arthur WT, Burridge K. A novel role for lsc/p115 rhoGEF and LARG in regulating rhoa activity downstream of adhesion to fibronectin. J Cell Sci 2007; 120:3989-98; PMID:17971419; http://dx.doi.org/ 10.1242/jcs.003806 [DOI] [PubMed] [Google Scholar]
- 115. O’Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol 2001; 3:831-8; PMID:11533663; http://dx.doi.org/ 10.1038/ncb0901-831 [DOI] [PubMed] [Google Scholar]
- 116. Eucker TP, Konkel ME. The cooperative action of bacterial fibronectin-binding proteins and secreted proteins promote maximal campylobacter jejuni invasion of host cells by stimulating membrane ruffling. Cell Microbiol. 2012; 14(2):226-38; PMID:21999233; http://dx.doi.org/ 10.1111/j.1462-5822.2011.01714.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bourdoulous S, Orend G, MacKenna DA, Pasqualini R, Ruoslahti E. Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J Cell Biol 1998; 143:267-76; PMID:9763437; http://dx.doi.org/ 10.1083/jcb.143.1.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Arthur WT, Noren NK, Burridge K. Regulation of rho family GTPases by cell-cell and cell-matrix adhesion. Biol Res 2002; 35:239-46; PMID:12415742; http://dx.doi.org/ 10.4067/S0716-97602002000200016 [DOI] [PubMed] [Google Scholar]
- 119. Chung JW, Hong SJ, Kim KJ, Goti D, Stins MF, Shin S, Dawson VL, Dawson TM, Kim KS. 37-kda laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated rhoa activation and bacterial uptake. J Biol Chem 2003; 278:16857-62; PMID:12615923; http://dx.doi.org/ 10.1074/jbc.M301028200 [DOI] [PubMed] [Google Scholar]
- 120. Boehm M, Krause-Gruszczynska M, Rohde M, Tegtmeyer N, Takahashi S, Oyarzabal OA, Backert S. Major host factors involved in epithelial cell invasion of campylobacter jejuni: role of fibronectin, integrin beta1, FAK, tiam-1, and DOCK180 in activating rho GTPase rac1. Front Cell Infect Microbiol 2011; 1:17; PMID: 22919583; http://dx.doi.org/ 10.3389/fcimb.2011.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lemichez E, Lecuit M, Nassif X, Bourdoulous S. Breaking the wall: targeting of the endothelium by pathogenic bacteria. Nat Rev Microbiol 2010; 8:93-104; PMID:20040916. [DOI] [PubMed] [Google Scholar]
- 122. Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell 2009; 17:310-22; PMID:19758556; http://dx.doi.org/ 10.1016/j.devcel.2009.08.012 [DOI] [PubMed] [Google Scholar]
- 123. Beemiller P, Krummel MF. Mediation of T-cell activation by actin meshworks. Cold Spring Harb Perspect Biol 2010; 2:a002444; PMID:20702599; http://dx.doi.org/ 10.1101/cshperspect.a002444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Harwood NE, Batista FD. The cytoskeleton coordinates the early events of B-cell activation. Cold Spring Harb Perspect Biol 2011; 3:a002360. PMID:21047917; http://dx.doi.org/ 10.1101/cshperspect.a002360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Frischknecht F, Way M. Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol 2001; 11:30-8; PMID:11146296; http://dx.doi.org/ 10.1016/S0962-8924(00)01871-7 [DOI] [PubMed] [Google Scholar]