Abstract
Until recently, epithelial cells have been a largely ignored component of host responses to microbes. However, this has been largely overturned over the last decade as an ever increasing number of studies have highlighted the key role that these cells play in many of our interactions with our microbiota and pathogens. Interactions of these cells with Candida albicans have been shown to be critical not just in host responses, but also in fungal cell responses, regulating fungal morphology and gene expression profile. In this review, we will explore the interactions between C. albicans and epithelial cells, and discuss how these interactions affect our relationship with this fungus.
Keywords: adhesion, Candida albicans, epithelial cells, fungal infection, fungal recognition, host-pathogen interaction, invasion
Introduction
With the increasing numbers of patients with compromised immune responses, the last half century has seen a dramatic rise in the incidence of fungal infections. This has come about due to the HIV pandemic, improvement and refinement of cancer therapies and transplants, as well as general improvements in life expectancy for the population as a whole. Further, the increasing use of long-term, in-dwelling medical devices that bypass innate biological barriers has contributed to this increase, as they serve as platforms for biofilm formation. As further advances are made to increase life expectancy of immunocompromised individuals (e.g. ever improving therapies for HIV+ individuals) this trend of increasing infection is likely to continue, ensuring that fungal infections become a priority for global health. The most common of the human pathogenic fungi are the Candida species – in particular Candida albicans. These fungi, although a common part of the mycobiota at mucosal surfaces of around 50% of the healthy population,1 are a significant cause of severe morbidity in millions of individuals worldwide, causing candidiasis of oral, gastrointestinal and vaginal surfaces. Further, although there is currently a lack of good epidemiological data, Candida and other pathogenic fungal species (Aspergillus, Cryptococcus and Pneumocystis) account for as many deaths each year as tuberculosis or malaria.2,3 Even non-lethal infections can carry significant morbidity. Vulvovaginal candidiasis (VVC) affects ∼75% of women at least once during their fertile years.4,5 This amounts to ∼30 million infections each year, with a further 5–10% going on to develop either recurrent or chronic candidiasis (RVVC or CVVC).6,7 Furthermore, Candida infections are also common oral infections, particularly in HIV+ (50%) and AIDS (90%) patients.8-10 Although Candida infections are mostly superficial, affecting the mucosal surface at the site of infection, their invasive nature can lead to much more serious systemic infections with a high degree of mortality. Indeed, studies have indicated that mucosal colonisation by Candida is a significant risk factor for subsequent systemic infections in patients.11,12 Given the immunocompromised state of many hospital patients, this has led to Candida infections being the 3rd or 4th most common nosocomial bloodstream infection.13,14
Given this ever-increasing prevalence of Candida infections, it is therefore important to understand the events that occur during host-Candida interaction. While much work has been done to explore these interactions at the level of host immune cells during systemic immunity, it is only recently that efforts have turned to examining the events and mechanisms involved in mucosal-Candida interactions – in particular the interactions between the fungus and host epithelial cells. The consensus view has been that the sole role of epithelial cells was to act as a point of attachment for colonisation and subsequent invasion, and as a food source for the invading fungi, with a function purely as a static barrier, rather than as dynamically active sensors. In the light of recent studies, however, this view has substantially changed,15-17 and epithelial cells are now seen as playing a more active role in commensal/pathogen discrimination, immunity and damage repair. Here, we will review the events that occur when C. albicans meets epithelial cells and explore their importance in host responses to the fungus.
Adhesion
The epithelial cells that comprise the majority of our mucosae play a crucial role in preventing fungal invasion across these surfaces. Their position at the outside surfaces of the body ensures that they are generally the first host cell to come in to contact with the overwhelming majority of fungi. Given this position, they play a critical role in initial fungal contact events – in both colonisation and invasion. The key part of these initial contact events is the adherence of fungal cells to host epithelial cells, a process whose outcome governs all subsequent interactions between fungus and host. This adhesion of fungus to epithelial cells is a complex process, involving several different factors. Determining events is made somewhat tricky by a degree of functional redundancy in different factors. These initial contact events require a variety of passive forces, including attractive (e.g., van der Waals forces, hydrophobic interactions) and repulsive (e.g. mutual electrorepulsive forces) effects. Once these initial events have allowed cell-cell contact, adhesion of C. albicans to epithelial cells is thought to involve a series of interactions between epithelial receptors and Candida adhesins, with these adhesins varying depending on the morphological status of the fungus.18 Our current knowledge of these adhesion events has relied largely on data obtained from in vitro experiments and primarily pertains to yeast interactions with epithelial cells. While this ignores the possible role of many important hyphal adhesins, it is worth noting that given the essentially sessile nature of hyphae, the majority of initial contact-mediated adhesion is likely to be between epithelial cells and yeast cells, with hyphae growing out of the yeast cells after first contacting a surface. At this point, the hyphal-expressed adhesins become of primary importance, playing a significant role in the pathogenesis of the fungus and affecting how the infection progresses. C. albicans also forms pseudohyphae during growth on epithelial cells, but little is known about the identity or role of pseudohyphal-specific genes involved in the adhesion of this morphotype. Despite the dominant role assumed for yeast cells in initial/early epithelial cell adhesion events, there may be direct adhesion of the hyphal form of Candida to epithelial cells without the involvement of yeast cells, as hyphae grow from one epithelial cell to an adjoining epithelial cell. There are a variety of adhesins present on fungal cells that interact with host cell surface receptors (Table 1). The host ligands that these cells interact with at epithelial cell surfaces include host extracellular matrix (ECM) components (including laminin, fibronectin, collagen, vitronectin etc18) and surface expressed receptors, such as integrins and cadherins. Most recently, ErbB receptor family members (particularly Her2) have also been demonstrated to interact with the Candida adhesins Agglutinin-Like Sequence 3 (Als3p) and the Heat Shock Protein, Ssa1p19 (Fig. 1).
Table 1.
Different C. albicans adhesins that mediate binding to epithelial surfaces and their target host molecules
| Candida adhesin | Cellular receptor/substrate | Reference |
|---|---|---|
| Eap1p | Polystyrene, epithelial cells | 32,33 |
| Iff4p | Plastic, epithelial cells | 34,35 |
| Hwp1p | Host cell transglutaminsae substrates | 20,21 |
| Int1p | iC3b | 83 |
| Als3p | E-Cadherin, EGFR/Her2 | 19,52 |
| GlcNAc-binding protein | N-Acetylglucosamine | 42,79 |
| Fimbrial adhesin | βGalNAc(1–4β-Gal) | 78 |
Figure 1.
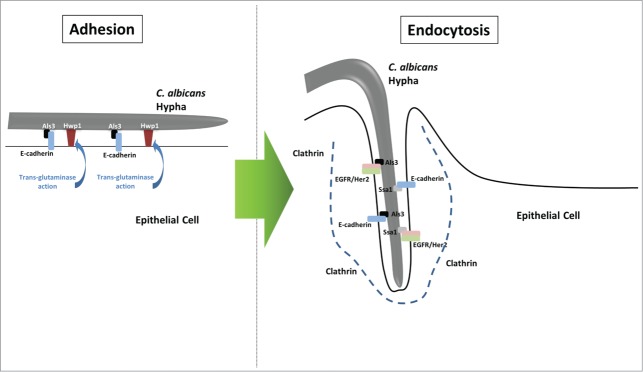
Adhesion and endocytosis of Candida albicans hyphae by epithelial cells. C. albicans induces its endocytic uptake by oral epithelial cells in 2 stages. In the first stage, adhesins such as Als3p bind to their target cellular receptors (e.g., Als3p-E-cadherin) or bind covalently to the cell surface after processing by host cell enzymes (e.g., Hwp1p and host transglutaminases). These actions ensure that the hyphal cells are securely bound to the epithelial cell surface. In the second stage, the C. albicans invasins Als3p and Ssa1p interact with target host receptors, E-cadherin and the EGFR/Her2 heterodimer, triggering activation of these receptors. This, in turn, leads to the induction of endocytosis via recruitment of clathrin and cytoskeletal reorganisation to form an invasion pocket down which the hypha invades into the host cell.
Hwp1p (Hyphal Wall Protein 1) is one of the most extensively studied adhesins. Expressed as a glycosylphosphatidylinositol-linked (GPI-linked) protein,20 this adhesin interacts with as yet unidentified host proteins, resulting in covalent attachment of Candida to host epithelial cells. N-terminal glutamines in Hwp1p are cross-linked to host proteins via host transglutaminase activity.21 The importance of this adhesion event can be seen by experiments showing that HWP1 defective C. albicans does not cause oropharyngeal candidiasis in mice.22 Interestingly, recent in vivo experiments using an hwp1Δ/Δ mutant have suggested that the role of Hwp1p in epithelial interaction/adhesion may be niche-specific, playing a role in virulence at mucosal surfaces, impacting on whether the fungus becomes disseminated to cause systemic infections, but playing no direct role in blood-stream, systemic infections.23
Perhaps the most well-known C. albicans adhesins are the ALS (Agglutinin-Like Sequence) family expressed on yeast and/or hyphae. The eight members of this gene family (ALS1–7 and ALS9) encode for large glycoproteins that are GPI-linked to the β-1,6-glucans of the fungal cell wall and share a similar 3 domain structure,24-26 with adherence being a property of the N-terminal domain.26,27 These N-terminal domains have been predicted to share structural similarities with bacterial adhesins.26 By studying the ability of deletion mutants to adhere, Als2p, Als3p and Als4p have been implicated as the primary human epithelial Als adhesins,28,29 with contradictory evidence supporting or denying a role for Als1p.28,30 Likewise, evidence for other members of the ALS family (Als5p, Als6p and Als7p) is somewhat contradictory, with both deletion mutants and S. cerevisiae over-expression mutants showing increased adherence.26,31
As well as Hwp1p and Als family proteins, other adhesins have been identified. Among these, Eap1p (Enhanced Adhesions to Polystyrene) was identified based on similarity to a Saccharomyces cerevisiae GPI-linked protein that mediates adherence to polystyrene beads32,33 and has now been shown to also mediate adhesion to epithelial cells. One of the more recently discovered adhesins is Iff4p. Overexpression of this cell surface protein increases adherence to oral epithelial cells,34 while an iff4Δ/Δ null mutant shows reduced binding to plastic.35
Recognition and Activation
Once the C. albicans cells have adhered to the epithelial cell surface, 2 key events then take place–recognition and invasion. The recognition process needs to function in a way to discriminate between commensal and pathogenic phases of C. albicans growth/colonisation. Given the number of microbes resident at epithelial surfaces, the cells that comprise these surfaces (predominantly epithelial cells) must have evolved mechanisms to be able to identify and respond only to those microbes which will cause disease and damage, while remaining non-responsive to those which are commensal. In recent years, the role that epithelial cells play in this process has become increasingly apparent–particularly in the case of C. albicans. It is well established that epithelial cells produce a variety of cytokines in response to the presence of this fungus, including G-CSF, GM-CSF, IL-1α, IL-1β and IL-6, along with the chemokines RANTES, IL-8 and MIP3α.36-40 In addition to cytokines, epithelial cells have also been shown to produce a variety of antimicrobial peptides in response to the presence of C. albicans, including β-defensins and cathelicidin (LL-37).41 These anti-microbial proteins kill C. albicans using a variety of mechanisms involving interactions with the cell wall. For example, LL-37 induces cell wall reorganisation and thinning, as well as affecting the fungal transcriptome.42 The importance of these antimicrobial peptides in host mucosal responses to this fungus is underlined by the role that the S100A8 alarmin plays in host responses in vulvovaginal candidiasis.43,44 What has not been apparent until recently is whether these secreted products are the result of a response to cellular damage or a specific response to the fungus. Now, however, it has become apparent that epithelial cells have developed a mechanism that enables them to discriminate between the colonising yeast and the invasive hyphal form of this fungus37 (Fig. 2), with an early, transient response to yeast followed by a later, stronger response to hyphae, which in turn leads to activation of epithelial cells and the production of cytokines and other inflammatory mediators. This work has identified that epithelial cells rapidly respond to the presence of any form of the fungus, activating 5 key intracellular signaling pathways–the 3 main Mitogen Activated Protein Kinase (MAPK) pathways (JNK, p38 and ERK1/2), the Phosphatidylinositide-3-kinase (PI3K) pathway and the Nuclear Factor-kappa-enhancer of B cell function (NF-κB) pathway.36,37,45,46 Strikingly, although the β-glucan receptor, dectin-1, has been reported as a major receptor in myeloid cell recognition of Candida species, there is no evidence to indicate activation of this receptor in epithelial cells when they interact with C. albicans. Tyrosine phosphorylation of Spleen Tyrosine Kinase (SYK) (an event synonymous with dectin-1 activation) is not induced when epithelial cells are infected with C. albicans, nor does stimulation of epithelial cells with β-glucan activate MAPK/MKP1/c-Fos signalling.37 This is despite dectin-1 gene expression being detected in oral gingival epithelial cells, albeit at low levels.47 What is conspicuous about the activation of these pathways is that although NF-κB and PI3K signaling show consistent up regulation during the first 3 hours of interaction, the MAPK pathways and their subsequent downstream transcription factors show a more dynamic activation profile.36,37,46 In contrast to the other 2 pathways, MAPK signaling has 2 phases. An initial, transient, morphology-independent activation of all 3 pathways leading to JNK and ERK1/2 dependent activation of the c-Jun transcription factor. This is an early response that appears by 5 minutes post-infection, but rapidly fades, having disappeared by 1 hour. This initial response is followed by a sustained reactivation of all 3 MAPK pathways at 2 hours, but now leading to activation of the c-Fos transcription factor (driven by p38) and phosphorylation of the MAPK phosphatase, MKP1 (driven by ERK1/2). Along with cell culture, these factors are found in human biopsies, suggesting that they are activated in vivo as well as in vitro. This second phase is dependent on the presence of hyphae, with only hypha producing strains of Candida capable of inducing this response.37,45 It is not simply the presence of hyphae that affect epithelial activation, but the levels of hyphal burden.37 Below a certain threshold level, even the presence of hyphae goes undetected. Importantly, different tissues (oral and vaginal) show differing sensitivities.36,37 The results of these differing sensitivities can be seen in human live challenge experiments of vulvovaginal candidiasis (VVC).48 In these experiments, women with no or little previous history of VVC who developed recurrent episodes of VVC post-challenge were found to show a higher sensitivity to Candida fungal burdens than women with one or less occurrences of post-challenge VVC. The second epithelial signaling response (c-Fos), but not the first (c-Jun) is essential for the production of the majority of cytokines secreted by epithelial cells in response to C. albicans,37 indicating that it is part of the activatory response to C. albicans. Studies indicate that this response is common to multiple epithelial sub-sets, having been found in both oral and vaginal epithelial cells.36,37 Further, in vivo experiments using hyphal or yeast mutants demonstrated a clear requirement for the hyphal form to trigger damage, pro-inflammatory cytokine release and neutrophil recruitment in a murine model of vaginitis.49 However, the net effects of the discriminatory pathway differ between epithelial cell types, with differences in the profile of cytokines/chemokines secreted.36,39,40 This may be explained by differences in the initial recognition characteristics, leading to altered activation of c-Jun and ELK-1 transcription factors.
Figure 2.
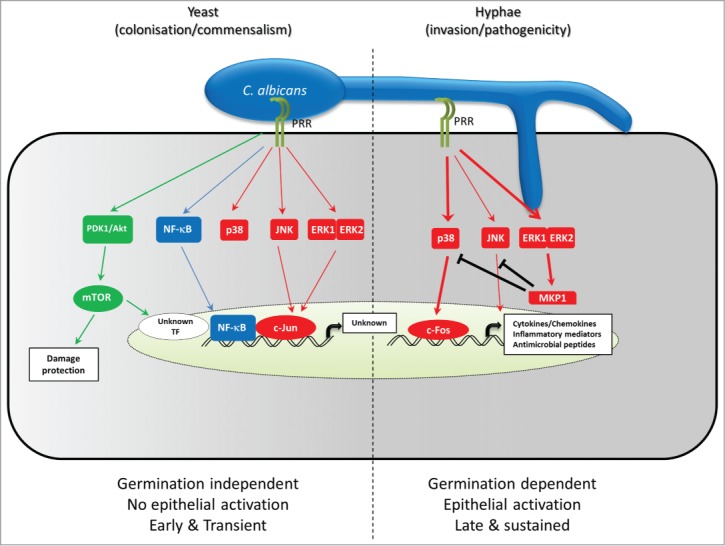
Epithelial cell recognition and discrimination of Candida albicans yeast and hyphae. Infection of Oral epithelial cells by C. albicans results in the triggering of 3 dominant pathways, each with distinct functions. There are 2 phases of signal pathway activation by C. albicans. In phase 1, C. albicans cells are recognized in a morphologically independent fashion, leading to activation of NF-κB, and mTOR via the PI3K/Akt pathway, involving activation of PDK1 and Akt. Activation of PI3K/Akt/mTor signaling leads to initiation of the cellular damage protection responses, as well as activation of an unknown transcription factor involved in growth factor transcription. There is also a low level triggering of all 3 MAPK pathways (p38, JNK and ERK1/2) leading to activation of the c-Jun transcription factor. Both JNK and ERK1/2 but not p38 play a role in this process. The role that p38 plays in this early phase is not yet known. While NF-κB and PI3K/Akt/mTOR pathways showed sustained activation, triggering of MAPK signaling in this phase is transient, and returns to resting levels after 1 hour if no further signal is received. When a burden threshold of C. albicans hyphal cells is reached, a second phase is triggered. Through an as yet unidentified receptor-ligand interaction, epithelial cells show further, stronger triggering of all 3 MAPK pathways, leading to induction of c-Fos expression and DNA binding through the p38 pathway and stabilization of the MAPK phosphatase, MKP1 via ERK1/2-mediated phosphorylation. In conjunction with NF-κB and an mTOR induced transcription factor, c-Fos then up-regulates the production of cytokines, chemokines and other inflammatory mediators. In parallel, MKP1 acts in a negative feedback loop to limit the activity of MAPK signaling by deactivating p38 and JNK.
Invasion
Induced endocytosis
Adhesion and recognition of C. albicans by epithelial cells has a wide range of effects through ligand-receptor interactions. One of the earliest effects of these interactions is to trigger host receptor signaling leading to cytoskeletal reorganisation, forming membrane processes that endocytose surface adherent hyphae in a clathrin-mediated mechanism.19,50-53 Notably, this reorganisation is not triggered by interactions with yeast cells. This ability to induce host endocytosis mechanisms is a common feature of many microbe-epithelial interactions, with many bacteria including Salmonella, Shigella and Yersinia species utilizing similar mechanisms.54,55 The key features of this induced endocytosis mechanism is that it is host-driven, requiring the host cell to take the active part in the process. In contrast, viability of the Candida cell is not important in the process.51 Importantly, the ability to induce this endocytic uptake is a function of location. Although there is significant evidence to demonstrate endocytic uptake of C. albicans by oral epithelial cells, uptake in the gut appears to be independent of these endocytic mechanisms as it has not, so far, been observed.56 The induction of endocytosis is a rapid event, occurring within the first 4 hours after initial contact57 and involves actin and other proteins associated with clathrin-mediated endocytosis (CME).50 Another potential mechanism, involving the small GTPases Cdc42, Rac1 and RhoA, and ZO-1 (Zonula Occludens) has also been identified,58 although at this time, it is unclear whether this mechanism is involved in endocytosis of fungal cells or shed/secreted fungal molecules.
A recent series of studies has begun to elucidate a model by which C. albicans induces its endocytosis by epithelial cells, involving receptor tyrosine kinases (RTKs) and E-cadherin (Fig. 1). The adhesin/invasins Als3p and Ssa1p bind to and interact with E-cadherin52,59 and the RTKs, EGFR and Her219 on epithelial cell surfaces, inducing phosphorylation of EGFR and Her2 by as yet unidentified mechanisms, leading to endocytic uptake of the C. albicans cell through CME.50 Interestingly, although EGFR and Her2 appear to operate as a heterodimer to induce these responses, they are also capable of functioning independently of each other, as cells expressing only Her2 are still able to endocytose C. albicans.19 Importantly, endocytosis can also be induced by E-cadherin, as cells expressing this surface receptor are still able to endocytose fungal cells in the absence of EGFR/Her2 expression.19 Further, Her2 and E-cadherin function as part of the same pathway, as inhibiting both leads to no further decrease in endocytosis than inhibiting each individually.19 This induction of endocytosis through RTKs is far from unique to C. albicans with both E-cadherin and the RTK, MET, having been shown to be involved in endocytosis of Listeria monocytogenes.60,61
As well as the Als3/Ssa1-E-cadherin/Her2 mechanism, other Candida proteins have been proposed to play a role in the induction of endocytosis. A null mutant of the pH regulation transcription factor Rim101p has been shown to have a reduced ability to induce its own endocytosis by oral epithelial cells.62 However, a group of cell surface proteins regulated by this transcription factor (the cell surface chitinase Cht2p, the cell surface protein Pga7p and the plasma membrane zinc transporter Zrt1p) have been identified that when overexpressed in the rim101Δ/Δ mutant restore the ability of this mutant to induce endocytosis.62 What is unclear is whether the actions of these proteins are a direct effect or whether they are acting through an indirect process – e.g., by affecting cell wall properties and activities.
Active penetration
Most of the mucosal surfaces that support Candida colonisation (e.g., oral cavity, vaginal lumen) consist of stratified, squamous epithelium. In these structures, the surface layers are terminally differentiated and thus non-proliferative. Conventional wisdom regards them as being functionally inactive and, as a result, they are unlikely to be able to support active endocytic processes to internalise fungal cells. Given this, C. albicans needs an alternative method of cell entry referred to as active penetration. Distinct from endocytic mechanisms, this process requires viable hyphae invading through or between individual epithelial cells, whether dead or alive, even with endocytic mechanisms blocked.53,56 The exact processes and proteins involved in active penetration are currently poorly understood, despite being the subject of many studies. It is known that the adhesins important in endocytic uptake, such as Als3p do not play an essential role in this active penetration.53 However, even if they play no active role in the penetrative process, they are likely to have a peripheral role via indirect mechanisms such as anchoring to epithelial cell surfaces.
Besides hyphal formation and maintenance, the only other factors that have so far been implicated as playing a role in this process are the Secreted Aspartic Protease (SAP) family. Indeed, experiments using the protease inhibitor, pepstatin A, indicate that in the presence of this inhibitor, C. albicans shows a reduced ability to damage oral epithelium.63 However, this association is debatable, as other studies, using sap deletion mutants, have not shown the same effect.64 It is probable that the main contribution of these enzymes is not in the active penetration of the epithelial cells themselves, but rather in penetrating between individual cells and epithelial layers. For example, Sap5p released by C. albicans hyphae degrades the epithelial tight junction protein, E-cadherin.65 Degradation of this host protein is, therefore, likely to lead to disruption of the epithelium and allow greater ease of hyphal penetration into the mucosal tissues. Notably, despite a plethora of other secreted enzymes such as lipases and phospholipases as well as other proteases, none have been implicated as playing a role in active penetration of epithelial cells.56
Damage
The processes of active penetration and induced endocytosis of viable, wild-type C. albicans both carry the end result of damage to the epithelial cell via a combination of 2 distinct mechanisms: necrosis and apoptosis. Most recently, a new damage mechanism called pyroptosis has been identified in the damage of macrophages by C. albicans, but it is currently unknown if this inflammasome-driven mechanism is also a factor in epithelial cell damage.66,67 Damage of epithelial cells and loss of epithelium is a key feature of mucosal candidiasis,68 and this is reflected in cell culture, where infection results in significant levels of damage.36,37,45,46 An in vivo study exploring damage caused by hyphal versus non-hyphal mutants demonstrated the latter induced significantly lower cell damage. However, some mutants that form hyphae still showed reduced damage, suggesting that specific hyphal effectors and not just hyphae are important for causing damage in vivo.49 Although it seems obvious that active penetration will cause damage, given the mechanism, process and outcomes, it is less obvious that endocytosis would cause damage. However, studies blocking the induction of Candida endocytosis51 or using mutants with defects in inducing epithelial endocytosis51,52,62,69 indicate that in these circumstances, damage is reduced. It is, however, important to note here that the endocytosis of Candida cells is not, in itself, enough to cause damage, as the endocytosed Candida cells need to be both viable and have normal damage-inducing genes to damage epithelial cells after endocytosis.37,56,62 Thus, invasion alone is not enough to cause damage – a finding strongly supported by data obtained using the adherence and invasion competent but damage incompetent eed1Δ/Δ null mutant.37,70
It is most likely that the induction of damage during induced endocytosis is via active penetration of the endocytic compartment into which the Candida cell has been taken up. Thus, damage of epithelial cells by active penetration may occur both directly at the epithelial cell surface, or from with the endocytic compartment subsequent to induced endocytosis of a fungal cell.
This picture is further complicated by the finding that epithelial cells have a damage protection/prevention strategy that activates in response to the presence of C. albicans cells.46 This mechanism is morphologically independent, and is dependent on a PI3K/Akt/mTOR epithelial cell signaling circuit. While activation of this circuit does not block Candida-induced damage, suppression results in infection driving greater levels of damage.46
A multitude of fungal factors have been implicated in damaging epithelial cells, with some, such as the SAPs having arguments both for and against (see above). Attempts with large panels of fungal deletion mutants, have been made to screen for potential fungal factors involved in the epithelial cell damage process, as well as those involved in either adhesion or invasion.53 The results of this study revealed a panel of genes involved in all 3 of these functions (RIM101, HGC1, TUP1, TEC1, TPK1, RAS1, EFG1, VPS1, ALS3, ECM33). Curiously, with the exception of ALS3, all these genes play a role in the yeast-hyphal morphological switch. This clearly demonstrates the importance of this switch in these infection processes. This study further identified 2 genes involved in glycerol biosynthesis (GPD2 and GPP1) and a further gene involved in hyphal extension (EED1) that exhibited normal adhesion and invasion, but reduced damage, implicating a role for these processes in causing necrotic damage of epithelial cells.
Apoptosis
Programmed cell death, or apoptosis, is an important host response in many diseases, and involves a series of events, usually triggered by a family of cysteine aspartic proteases called caspases.71,72 In the last few years, it has been shown that Candida can induce apoptosis in epithelial cells,57,73 as well as being able to inactivate the anti-apoptotic proteins Bcl-2 and Bcl-xL in macrophages and neutrophils.74,75 Similar studies investigating bacterial infection of epithelial cells have indicated that apoptosis is a delayed response, occurring late in the infection time line, rather than part of the initial response to microbial recognition.76 However, microarray data of cells infected with C. albicans indicate that both pro- and anti-apoptotic genes are activated even as early as 6 hours post-infection.46 Along with data from another study indicating that apoptosis is an early event and is followed by necrosis,57 these data suggest that, unlike bacterial infection, apoptosis is an early event during C. albicans infections. In this study, the authors report that the dynamics of cell death during C. albicans infection of epithelial cells involves early induction of apoptosis, followed later by necrotic death. Apoptotic events in this study were defined by increased numbers of cells showing Annexin V on their surface, along with internucleosomal degradation of nuclear chromatin. In confirmation of these findings, treatment of epithelial cells with a pan-caspase inhibitor (Z-VAD-FMK) that blocks caspase-mediated apoptosis and cell death resulted in significant reductions in the level of cell death during the first 12 hours of infection, although after this time, levels of cell death were normal, demonstrating the later role that necrosis plays in late epithelial cell death. Notably, inhibiting endocytosis of the fungus has been shown to result in a decrease in early apoptotic events,57 highlighting the potentially important role that this process may play in epithelial cell death. A subsequent study has identified that although early apoptotic events are induced in response to C. albicans in ∼50% of oral epithelial cells, only 15% of these cells move into late apoptotic events, indicating that there is a block in the apoptotic process in these cells.73 In common with our microarray findings,46 the authors of this study found a significant increase of some anti-apoptotic genes with an accompanying decrease in other pro-apoptotic genes. Thus it would appear that although C. albicans can initiate apoptosis pathways, epithelial cell responses limit these pathways to ensure that only a minority of cells progress. Given the protective effects mediated by PI3K/Akt/mTor signalling,46 it is possible that this pathway plays some role in this block.
The fungal components that induce cell death are not well known and little data is available to identify them. Currently, only 2 components have been proposed with evidence to support their role. In common with other PAMP effects, N- and O-linked glycosylation of mannoproteins has been shown to be of significant importance in apoptosis, inducing cell cycle arrest and apoptosis in oral epithelial cells.77 Interestingly, there appears to be a hierarchy of importance. Experiments using mutants in key mannosylation enzymes (och1Δ/Δ (N-mannosylation), mnt1/mnt2Δ/Δ (O-linked mannosylation) and pmr1Δ/Δ (N- and O-linked mannosylation) have identified that the loss of O-linked glycosylation alone appears to have little effect on C. albicans cell wall induction of apoptosis, while loss of N-linked glycosylation results in a large reduction. However, the combination of loss of both N- and O-linked glycosylation results in almost complete inability to induce apoptosis. It is important to note, however, that some of these mutants have been shown to be defective in morphogenesis, demonstrating aberrant abilities to form hyphae,78-80 and it is possible that this may provide partial explanation for the loss in ability to induce apoptosis. The quorum sensing molecule, farnesol, has also been proposed as a potential activator of oral epithelial cell apoptosis.81 However, there are a number of key issues with these findings as they relate to physiological conditions. Firstly, given that farnesol is a secreted molecule, it seems unlikely that it could induce apoptosis in a fungal-contact-dependent manner. It is possible that high local concentrations of farnesol are required to trigger apoptosis, although how achievable these levels would be, given the diluting and washing effects of saliva in the locality, is unknown. It is possible however, that sufficient concentrations of farnesol may be achieved at a local level by collection/secretion in the contact-dependent invasion pocket (Fig. 1B), rather than through achieving a high global concentration in the environment as a whole. This may also hold true for other fungal factors affecting epithelial cells.
Concluding Remarks
In this review we have explored how theories of the role of epithelial cells in host-C. albicans interactions have changed from that of a static barrier, to dynamically reactive sentinels that are the first responders to invasive infections. With the advances in our knowledge over the last decade we can now begin to build a picture showing that interactions between epithelial cells and C. albicans are key to host responses to this medically important fungal pathogen. The series of events outlined in this review describe a process whereby C. albicans adheres to epithelial cells, triggers a series of signaling circuits that result in i.) recognition of specific morphological types of the fungus and ii.) induction of endocytosis. Endocytosed C. albicans then initiates early apoptotic events followed by phagosomal escape through active penetration, with these events resulting in damage to the epithelial cell. These early recognition events result in the epithelial cell mitigating some of the effects of this early damage – possibly by causing the block in progression of apoptotic signaling that is observed. It is key to our understanding of mucosal immunity to this fungus to note that these events do not happen in isolation, and that other cell types have a critical effect on epithelial responses to C. albicans. Neutrophils, in particular, appear to play an important role in epithelial anti-Candida defense, secreting factors that induce epithelial cell protection against damage in a TLR4-dependent manner.82 Notably, this protection is dependent on epithelial activation of neutrophils. Thus, epithelial cells are one key component of the host response circuit to fungal infections. While the last 10 y has seen a dramatic increase in our knowledge and understanding of the important role that epithelial cells play in host responses, we have barely scratched the surface of their function, and the next decade and beyond will, without doubt, see our appreciation of the functionality of epithelial cells in host-fungal interactions advance exponentially. From the clinical standpoint, these advances promise the prospects of entirely novel therapeutic strategies that will revolutionise the treatment of not just Candida and fungal infections, but other, equally important mucosal infections.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Odds F. Candida and Candidosis. Bailliere: Tindall, 1988. [Google Scholar]
- 2. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med 2012; 4:165rv13; PMID:23253612; http://dx.doi.org/ 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- 3. Brown GD, Denning DW, Levitz SM. Tackling human fungal infections. Science 2012; 336:647; PMID:22582229; http://dx.doi.org/ 10.1126/science.1222236 [DOI] [PubMed] [Google Scholar]
- 4. Sobel JD. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann N Y Acad Sci 1988; 544:547-57; PMID:3063184; http://dx.doi.org/ 10.1111/j.1749-6632.1988.tb40450.x [DOI] [PubMed] [Google Scholar]
- 5. Sobel JD. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis 1992; 14 Suppl 1:S148-53; PMID:1562688; http://dx.doi.org/ 10.1093/clinids/14.Supplement_1.S148 [DOI] [PubMed] [Google Scholar]
- 6. Fidel PL., Jr. Immunity in vaginal candidiasis. Curr Opin Infect Dis 2005; 18:107-11; PMID:15735412; http://dx.doi.org/ 10.1097/01.qco.0000160897.74492.a3 [DOI] [PubMed] [Google Scholar]
- 7. Foxman B, Muraglia R, Dietz JP, Sobel JD, Wagner J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: results from an internet panel survey. J Low Genit Tract Dis 2013; 17:340-5; PMID:23486072; http://dx.doi.org/ 10.1097/LGT.0b013e318273e8cf [DOI] [PubMed] [Google Scholar]
- 8. Korting HC, Ollert M, Georgii A, Froschl M. In vitro susceptibilities and biotypes of Candida albicans isolates from the oral cavities of patients infected with human immunodeficiency virus. J Clin Microbiol 1988; 26:2626-31; PMID:3068254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palmer GD, Robinson PG, Challacombe SJ, Birnbaum W, Croser D, Erridge PL, Hodgson T, Lewis D, McLaren A, Zakrzewska JM. Aetiological factors for oral manifestations of HIV. Oral Dis 1996; 2:193-7; PMID:9081758; http://dx.doi.org/ 10.1111/j.1601-0825.1996.tb00223.x [DOI] [PubMed] [Google Scholar]
- 10. Phelan JA, Saltzman BR, Friedland GH, Klein RS. Oral findings in patients with acquired immunodeficiency syndrome. Oral Sur Oral Med Oral Pathol 1987; 64:50-6; PMID:3475658; http://dx.doi.org/ 10.1016/0030-4220(87)90116-2 [DOI] [PubMed] [Google Scholar]
- 11. Marr KA, Seidel K, White TC, Bowden RA. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J Infect Dis 2000; 181:309-16; PMID:10608780; http://dx.doi.org/ 10.1086/315193 [DOI] [PubMed] [Google Scholar]
- 12. Takesue Y, Kakehashi M, Ohge H, Imamura Y, Murakami Y, Sasaki M, Morifuji M, Yokoyama Y, Kouyama M, Yokoyama T, et al. Combined assessment of beta-D-glucan and degree of candida colonization before starting empiric therapy for candidiasis in surgical patients. World J Sur 2004; 28:625-30; PMID:15366757; http://dx.doi.org/ 10.1007/s00268-004-7302-y [DOI] [PubMed] [Google Scholar]
- 13. Giusiano G, Mangiaterra M, Garcia Saito V, Rojas F, Gomez V, Diaz MC. Fluconazole and itraconazole resistance of yeasts isolated from the bloodstream and catheters of hospitalized pediatric patients. Chemotherapy 2006; 52:254-9; PMID:16899974; http://dx.doi.org/ 10.1159/000094867 [DOI] [PubMed] [Google Scholar]
- 14. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309-17; PMID:15306996; http://dx.doi.org/ 10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 15. Naglik JR, Moyes D. Epithelial cell innate response to Candida albicans. Adv Dental Res 2011; 23:50-5; PMID:21441481; http://dx.doi.org/ 10.1177/0022034511399285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naglik JR, Moyes DL, Wachtler B, Hube B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect 2011; 13:963-76; PMID:21801848; http://dx.doi.org/ 10.1016/j.micinf.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu W, Filler SG. Interactions of Candida albicans with epithelial cells. Cell Microbiol 2010; 12:273-82; PMID:19919567; http://dx.doi.org/ 10.1111/j.1462-5822.2009.01412.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaffin WL. Candida albicans cell wall proteins. Microbiol Mol Biol Rev 2008; 72:495-544; PMID:18772287; http://dx.doi.org/ 10.1128/MMBR.00032-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu W, Phan QT, Boontheung P, Solis NV, Loo JA, Filler SG. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc Natl Acad Sci U S A 2012; 109:14194-9; PMID:22891338; http://dx.doi.org/ 10.1073/pnas.1117676109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Staab JF, Bahn YS, Tai CH, Cook PF, Sundstrom P. Expression of transglutaminase substrate activity on Candida albicans germ tubes through a coiled, disulfide-bonded N-terminal domain of Hwp1 requires C-terminal glycosylphosphatidylinositol modification. J Biol Chem 2004; 279:40737-47; PMID:15262971; http://dx.doi.org/ 10.1074/jbc.M406005200 [DOI] [PubMed] [Google Scholar]
- 21. Staab JF, Bradway SD, Fidel PL, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 1999; 283:1535-8; PMID:10066176; http://dx.doi.org/ 10.1126/science.283.5407.1535 [DOI] [PubMed] [Google Scholar]
- 22. Sundstrom P, Balish E, Allen CM. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J Infect Dis 2002; 185:521-30; PMID:11865405; http://dx.doi.org/ 10.1086/338836 [DOI] [PubMed] [Google Scholar]
- 23. Staab JF, Datta K, Rhee P. Niche-specific requirement for hyphal wall protein 1 in virulence of Candida albicans. PloS One 2013; 8:e80842; PMID:24260489; http://dx.doi.org/ 10.1371/journal.pone.0080842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaur NK, Klotz SA. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect Immun 1997; 65:5289-94; PMID:9393828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoyer LL. The ALS gene family of Candida albicans. Trends Microbiol 2001; 9:176-80; PMID:11286882; http://dx.doi.org/ 10.1016/S0966-842X(01)01984-9 [DOI] [PubMed] [Google Scholar]
- 26. Sheppard DC, Yeaman MR, Welch WH, Phan QT, Fu Y, Ibrahim AS, Filler SG, Zhang M, Waring AJ, Edwards JE, Jr. Functional and structural diversity in the Als protein family of Candida albicans. J Biol Chem 2004; 279:30480-9; PMID:15128742; http://dx.doi.org/ 10.1074/jbc.M401929200 [DOI] [PubMed] [Google Scholar]
- 27. Loza L, Fu Y, Ibrahim AS, Sheppard DC, Filler SG, Edwards JE, Jr. Functional analysis of the Candida albicans ALS1 gene product. Yeast 2004; 21:473-82; PMID:15116430; http://dx.doi.org/ 10.1002/yea.1111 [DOI] [PubMed] [Google Scholar]
- 28. Zhao X, Oh SH, Cheng G, Green CB, Nuessen JA, Yeater K, Leng RP, Brown AJ, Hoyer LL. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology 2004; 150:2415-28; PMID:15256583; http://dx.doi.org/ 10.1099/mic.0.26943-0 [DOI] [PubMed] [Google Scholar]
- 29. Zhao X, Oh SH, Yeater KM, Hoyer LL. Analysis of the Candida albicans Als2p and Als4p adhesins suggests the potential for compensatory function within the Als family. Microbiology 2005; 151:1619-30; PMID:15870470; http://dx.doi.org/ 10.1099/mic.0.27763-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kamai Y, Kubota M, Hosokawa T, Fukuoka T, Filler SG. Contribution of Candida albicans ALS1 to the pathogenesis of experimental oropharyngeal candidiasis. Infect Immun 2002; 70:5256-8; PMID:12183577; http://dx.doi.org/ 10.1128/IAI.70.9.5256-5258.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao X, Oh SH, Hoyer LL. Deletion of ALS5, ALS6 or ALS7 increases adhesion of Candida albicans to human vascular endothelial and buccal epithelial cells. Med Mycol 2007; 45:429-34; PMID:17654269; http://dx.doi.org/ 10.1080/13693780701377162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li F, Palecek SP. EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryot Cell 2003; 2:1266-73; PMID:14665461; http://dx.doi.org/ 10.1128/EC.2.6.1266-1273.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li F, Palecek SP. Distinct domains of the Candida albicans adhesin Eap1p mediate cell-cell and cell-substrate interactions. Microbiology 2008; 154:1193-203; PMID:18375812; http://dx.doi.org/ 10.1099/mic.0.2007/013789-0 [DOI] [PubMed] [Google Scholar]
- 34. Fu Y, Luo G, Spellberg BJ, Edwards JE, Jr., Ibrahim AS. Gene overexpression/suppression analysis of candidate virulence factors of Candida albicans. Eukaryot Cell 2008; 7:483-92; PMID:18178776; http://dx.doi.org/ 10.1128/EC.00445-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kempf M, Apaire-Marchais V, Saulnier P, Licznar P, Lefrancois C, Robert R, Cottin J. Disruption of Candida albicans IFF4 gene involves modifications of the cell electrical surface properties. Colloids Surf B Biointerfaces 2007; 58:250-5; PMID:17481864; http://dx.doi.org/ 10.1016/j.colsurfb.2007.03.017 [DOI] [PubMed] [Google Scholar]
- 36. Moyes DL, Murciano C, Runglall M, Islam A, Thavaraj S, Naglik JR. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PloS One 2011; 6:e26580; PMID:22087232; http://dx.doi.org/ 10.1371/journal.pone.0026580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 2010; 8:225-35; PMID:20833374; http://dx.doi.org/ 10.1016/j.chom.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schaller M, Mailhammer R, Grassl G, Sander CA, Hube B, Korting HC. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J Invest Dermatol 2002; 118:652-7; PMID:11918712; http://dx.doi.org/ 10.1046/j.1523-1747.2002.01699.x [DOI] [PubMed] [Google Scholar]
- 39. Steele C, Fidel PL, Jr. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect Immun 2002; 70:577-83; PMID:11796585; http://dx.doi.org/ 10.1128/IAI.70.2.577-583.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dongari-Bagtzoglou A, Fidel PL, Jr. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J Dental Res 2005; 84:966-77; PMID:16246925; http://dx.doi.org/ 10.1177/154405910508401101 [DOI] [PubMed] [Google Scholar]
- 41. Diamond G, Beckloff N, Ryan LK. Host defense peptides in the oral cavity and the lung: similarities and differences. J Dental Res 2008; 87:915-27; PMID:18809744; http://dx.doi.org/ 10.1177/154405910808701011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Critchley IA, Douglas LJ. Isolation and partial characterization of an adhesin from Candida albicans. J Gen Microbiol 1987; 133:629-36; PMID:3309163 [DOI] [PubMed] [Google Scholar]
- 43. Yano J, Lilly E, Barousse M, Fidel PL., Jr. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun 2010; 78:5126-37; PMID:20823201; http://dx.doi.org/ 10.1128/IAI.00388-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yano J, Palmer GE, Eberle KE, Peters BM, Vogl T, McKenzie AN, Fidel PL, Jr. Vaginal epithelial cell-derived S100 alarmins induced by Candida albicans via pattern recognition receptor interactions are sufficient but not necessary for the acute neutrophil response during experimental vaginal candidiasis. Infect Immun 2014; 82:783-92; PMID:24478092; http://dx.doi.org/ 10.1128/IAI.00861-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moyes DL, Murciano C, Runglall M, Kohli A, Islam A, Naglik JR. Activation of MAPK/c-Fos induced responses in oral epithelial cells is specific to Candida albicans and Candida dubliniensis hyphae. Med Microbiol Immunol 2012; 201:93-101; PMID:21706283; http://dx.doi.org/ 10.1007/s00430-011-0209-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moyes DL, Shen C, Murciano C, Runglall M, Richardson JP, Arno M, Aldecoa-Otalora E, Naglik JR. Protection against epithelial damage during candida albicans infection is mediated by PI3K/Akt and mammalian target of rapamycin signaling. J Infect Dis 2014; 209:1816-26; PMID:24357630; http://dx.doi.org/ 10.1093/infdis/jit824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weindl G, Wagener J, Schaller M. Epithelial cells and innate antifungal defense. J Dental Res 2010; 89:666-75; PMID:20395411; http://dx.doi.org/ 10.1177/0022034510368784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fidel PL, Jr., Barousse M, Espinosa T, Ficarra M, Sturtevant J, Martin DH, Quayle AJ, Dunlap K. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun 2004; 72:2939-46; PMID:15102806; http://dx.doi.org/ 10.1128/IAI.72.5.2939-2946.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peters BM, Palmer GE, Nash AK, Lilly EA, Fidel PL, Jr., Noverr MC. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect Immun 2014; 82:532-43; PMID:24478069; http://dx.doi.org/ 10.1128/IAI.01417-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moreno-Ruiz E, Galan-Diez M, Zhu W, Fernandez-Ruiz E, d'Enfert C, Filler SG, Cossart P, Veiga E. Candida albicans internalization by host cells is mediated by a clathrin-dependent mechanism. Cell Microbiol 2009; 11:1179-89; PMID:19416270; http://dx.doi.org/ 10.1111/j.1462-5822.2009.01319.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Park H, Myers CL, Sheppard DC, Phan QT, Sanchez AA, E Edwards J, Filler SG. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol 2005; 7:499-510; PMID:15760450; http://dx.doi.org/ 10.1111/j.1462-5822.2004.00476.x [DOI] [PubMed] [Google Scholar]
- 52. Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE, Jr, Filler SG. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol 2007; 5:e64; PMID:17311474; http://dx.doi.org/ 10.1371/journal.pbio.0050064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wachtler B, Wilson D, Haedicke K, Dalle F, Hube B. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PloS One 2011; 6:e17046; PMID:21407800; http://dx.doi.org/ 10.1371/journal.pone.0017046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goosney DL, Knoechel DG, Finlay BB. Enteropathogenic E. coli, Salmonella, and Shigella: masters of host cell cytoskeletal exploitation. Emerg Infect Dis 1999; 5:216-23; PMID:10221873; http://dx.doi.org/ 10.3201/eid0502.990205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Isberg RR. Uptake of enteropathogenic Yersinia by mammalian cells. Curr Top Microbiol Immunol 1996; 209:1-24; PMID:8742243 [DOI] [PubMed] [Google Scholar]
- 56. Dalle F, Wachtler B, L'Ollivier C, Holland G, Bannert N, Wilson D, Labruère C, Bonnin A, Hube B. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol 2010; 12:248-71; PMID:19863559; http://dx.doi.org/ 10.1111/j.1462-5822.2009.01394.x [DOI] [PubMed] [Google Scholar]
- 57. Villar CC, Zhao XR. Candida albicans induces early apoptosis followed by secondary necrosis in oral epithelial cells. Mol Oral Microbiol 2010; 25:215-25; PMID:20536749; http://dx.doi.org/ 10.1111/j.2041-1014.2010.00577.x [DOI] [PubMed] [Google Scholar]
- 58. Atre AN, Surve SV, Shouche YS, Joseph J, Patole MS, Deopurkar RL. Association of small Rho GTPases and actin ring formation in epithelial cells during the invasion by Candida albicans. FEMS Immunol Med Microbiol 2009; 55:74-84; PMID:19077030; http://dx.doi.org/ 10.1111/j.1574-695X.2008.00504.x [DOI] [PubMed] [Google Scholar]
- 59. Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, Liu Y, Dongari-Bagtzoglou A, Edgerton M, Filler SG. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog 2010; 6:e1001181; PMID:21085601; http://dx.doi.org/ 10.1371/journal.ppat.1001181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shen Y, Naujokas M, Park M, Ireton K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 2000; 103:501-10; PMID:11081636; http://dx.doi.org/ 10.1016/S0092-8674(00)00141-0 [DOI] [PubMed] [Google Scholar]
- 61. Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol 2005; 7:894-900; PMID:16113677; http://dx.doi.org/ 10.1038/ncb1292 [DOI] [PubMed] [Google Scholar]
- 62. Nobile CJ, Solis N, Myers CL, Fay AJ, Deneault JS, Nantel A, Mitchell AP, Filler SG. Candida albicans transcription factor Rim101 mediates pathogenic interactions through cell wall functions. Cell Microbiol 2008; 10:2180-96; PMID:18627379; http://dx.doi.org/ 10.1111/j.1462-5822.2008.01198.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Naglik JR, Moyes D, Makwana J, Kanzaria P, Tsichlaki E, Weindl G, Tappuni AR, Rodgers CA, Woodman AJ, Challacombe SJ, et al. Quantitative expression of the Candida albicans secreted aspartyl proteinase gene family in human oral and vaginal candidiasis. Microbiology 2008; 154:3266-80; PMID:18957581; http://dx.doi.org/ 10.1099/mic.0.2008/022293-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lermann U, Morschhauser J. Secreted aspartic proteases are not required for invasion of reconstituted human epithelia by Candida albicans. Microbiology 2008; 154:3281-95; PMID:18957582; http://dx.doi.org/ 10.1099/mic.0.2008/022525-0 [DOI] [PubMed] [Google Scholar]
- 65. Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun 2007; 75:2126-35; PMID:17339363; http://dx.doi.org/ 10.1128/IAI.00054-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Krysan DJ, Sutterwala FS, Wellington M. Catching fire: candida albicans, macrophages, and pyroptosis. PLoS Pathog 2014; 10:e1004139; PMID:24967821; http://dx.doi.org/ 10.1371/journal.ppat.1004139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wellington M, Koselny K, Sutterwala FS, Krysan DJ. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell 2014; 13:329-40; PMID:24376002; http://dx.doi.org/ 10.1128/EC.00336-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Farah CS, Ashman RB, Challacombe SJ. Oral candidosis. Clin Dermatol 2000; 18:553-62; PMID:11134850; http://dx.doi.org/ 10.1016/S0738-081X(00)00145-0 [DOI] [PubMed] [Google Scholar]
- 69. Martinez-Lopez R, Park H, Myers CL, Gil C, Filler SG. Candida albicans Ecm33p is important for normal cell wall architecture and interactions with host cells. Eukaryot Cell 2006; 5:140-7; PMID:16400176; http://dx.doi.org/ 10.1128/EC.5.1.140-147.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zakikhany K, Naglik JR, Schmidt-Westhausen A, Holland G, Schaller M, Hube B. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol 2007; 9:2938-54; PMID:17645752; http://dx.doi.org/ 10.1111/j.1462-5822.2007.01009.x [DOI] [PubMed] [Google Scholar]
- 71. Green D. Means to an End: Apoptosis and other Cell Death Mechanisms. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2011 [Google Scholar]
- 72. Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ 2007; 14:44-55; PMID:17053807; http://dx.doi.org/ 10.1038/sj.cdd.4402047 [DOI] [PubMed] [Google Scholar]
- 73. Villar CC, Chukwuedum Aniemeke J, Zhao XR, Huynh-Ba G. Induction of apoptosis in oral epithelial cells by Candida albicans. Mol Oral Microbiol 2012; 27:436-48; PMID:23134609; http://dx.doi.org/ 10.1111/j.2041-1014.2012.00648.x [DOI] [PubMed] [Google Scholar]
- 74. Ibata-Ombetta S, Idziorek T, Trinel PA, Poulain D, Jouault T. Candida albicans phospholipomannan promotes survival of phagocytosed yeasts through modulation of bad phosphorylation and macrophage apoptosis. J Biol Chem 2003; 278:13086-93; PMID:12551950; http://dx.doi.org/ 10.1074/jbc.M210680200 [DOI] [PubMed] [Google Scholar]
- 75. Rotstein D, Parodo J, Taneja R, Marshall JC. Phagocytosis of Candida albicans induces apoptosis of human neutrophils. Shock 2000; 14:278-83; PMID:11028543; http://dx.doi.org/ 10.1097/00024382-200014030-00006 [DOI] [PubMed] [Google Scholar]
- 76. Kim JM, Eckmann L, Savidge TC, Lowe DC, Witthoft T, Kagnoff MF. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest 1998; 102:1815-23; PMID:9819367; http://dx.doi.org/ 10.1172/JCI2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wagener J, Weindl G, de Groot PW, de Boer AD, Kaesler S, Thavaraj S, Bader O, Mailänder-Sanchez D, Borelli C, Weig M, et al. Glycosylation of Candida albicans cell wall proteins is critical for induction of innate immune responses and apoptosis of epithelial cells. PloS one 2012; 7:e50518; PMID:23226301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yu L, Lee KK, Sheth HB, Lane-Bell P, Srivastava G, Hindsgaul O, Paranchych W, Hodges RS, Irvin RT. Fimbria-mediated adherence of Candida albicans to glycosphingolipid receptors on human buccal epithelial cells. Infect Immun 1994; 62:2843-8; PMID:8005674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Critchley IA, Douglas LJ. Role of glycosides as epithelial cell receptors for Candida albicans. J Gen Microbiol 1987; 133:637-43; PMID:3309164 [DOI] [PubMed] [Google Scholar]
- 80. Munro CA, Bates S, Buurman ET, Hughes HB, Maccallum DM, Bertram G, Atrih A, Ferguson MA, Bain JM, Brand A, et al. Mnt1p and Mnt2p of Candida albicans are partially redundant alpha-1,2-mannosyltransferases that participate in O-linked mannosylation and are required for adhesion and virulence. J Biol Chem 2005; 280:1051-60; PMID:15519997; http://dx.doi.org/ 10.1074/jbc.M411413200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Scheper MA, Shirtliff ME, Meiller TF, Peters BM, Jabra-Rizk MA. Farnesol, a fungal quorum-sensing molecule triggers apoptosis in human oral squamous carcinoma cells. Neoplasia 2008; 10:954-63; PMID:18714396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weindl G, Naglik JR, Kaesler S, Biedermann T, Hube B, Korting HC, Schaller M. Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling. J Clin Invest 2007; 117:3664-72; PMID:17992260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hostetter MK. Adhesins and ligands involved in the interaction of Candida spp. with epithelial and endothelial surfaces. Clin Microbiol Rev 1994; 7:29-42; PMID:8118789 [DOI] [PMC free article] [PubMed] [Google Scholar]


