Abstract
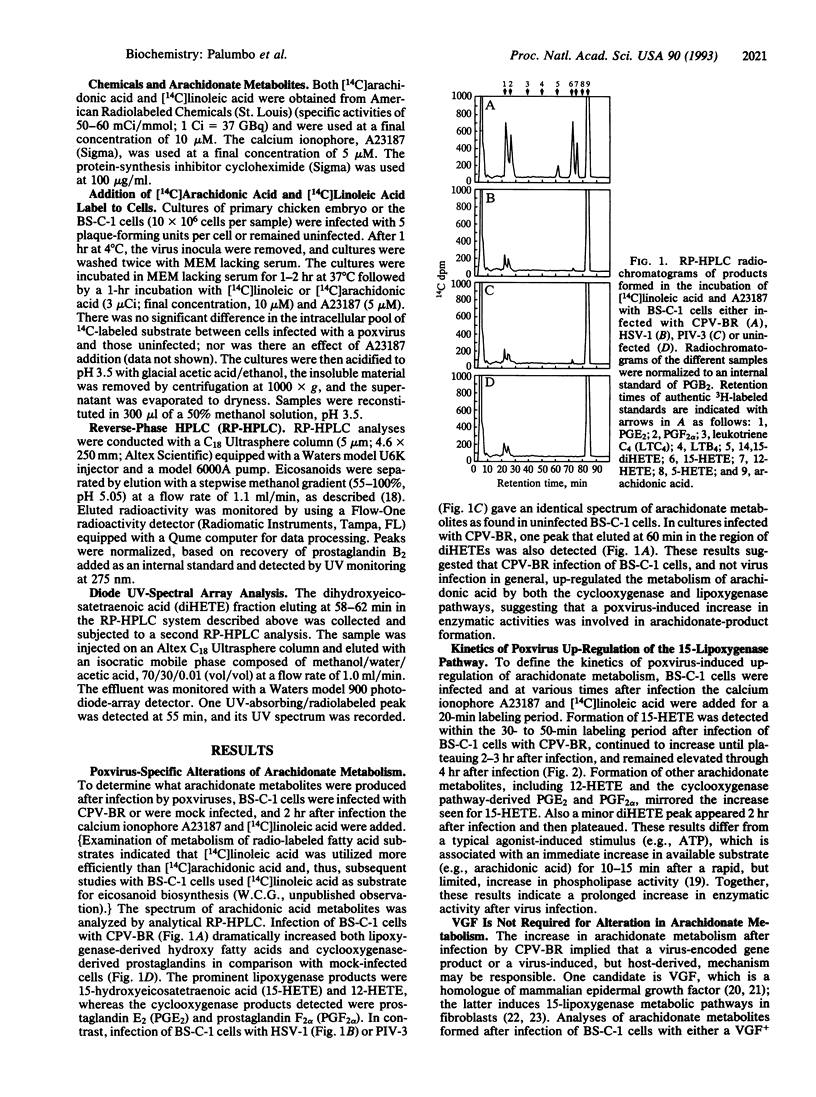
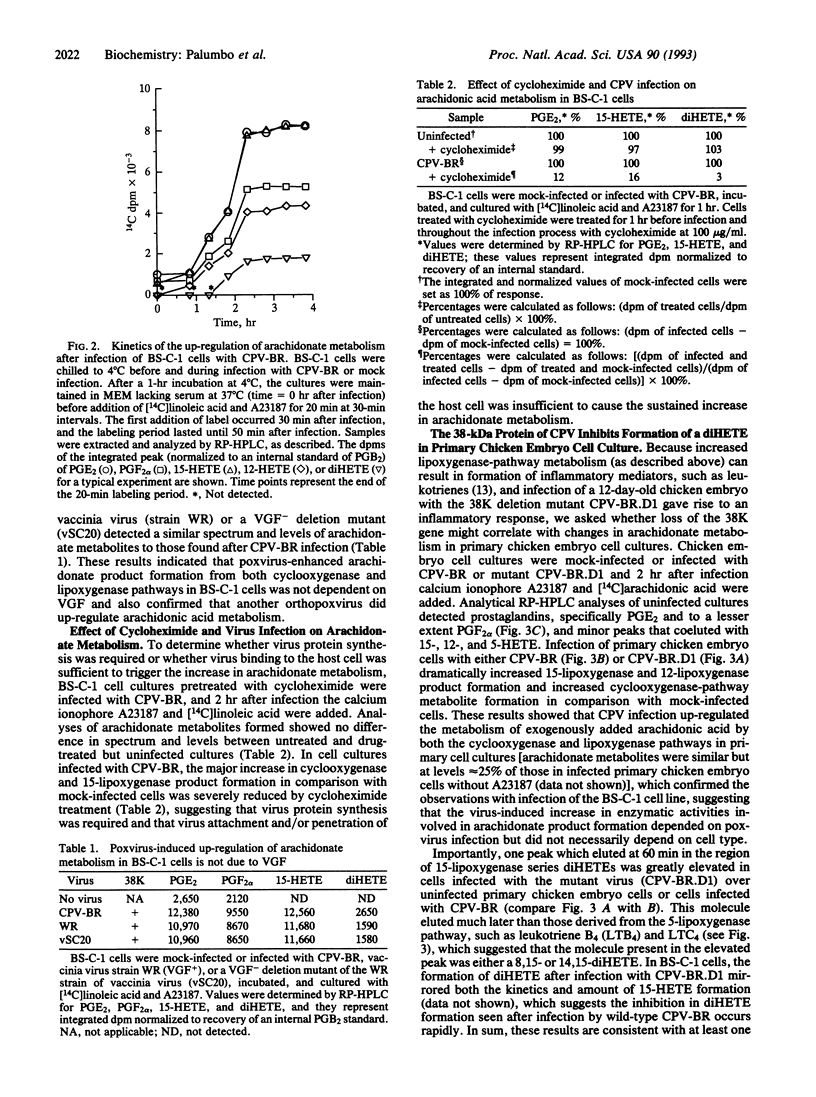
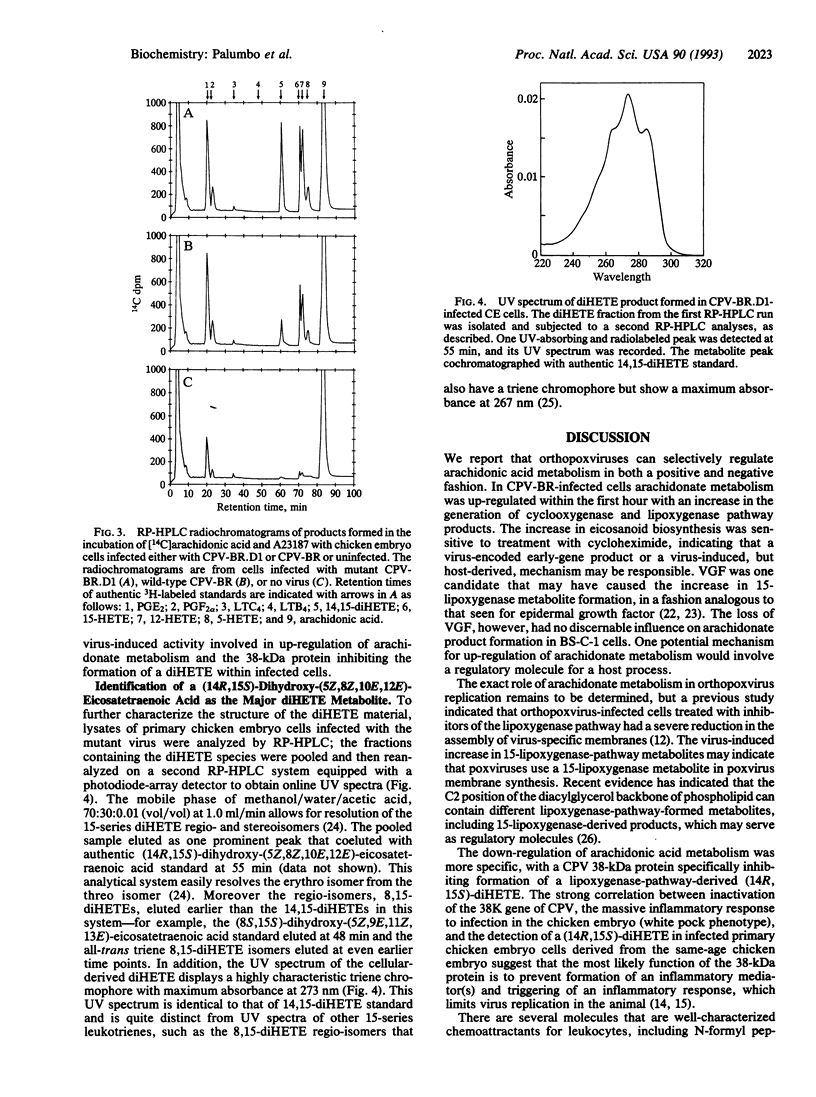
Recent evidence suggests that orthopoxviruses have an obligate requirement for arachidonic acid metabolites during replication in vivo and in vitro. Our report indicates that a virus family (Poxviridae) possesses multiple genes that function to regulate arachidonate metabolism. Analyses of BS-C-1 cells infected with cowpox virus or vaccinia virus detected enhanced arachidonate product formation from both the cyclooxygenase (specifically prostaglandins E2 and F2 alpha) and lipoxygenase (specifically 15-hydroxyeicosatetraenoic acid and 12-hydroxyeicosatetraenoic acid) pathways. In contrast, human parainfluenza type 3 or herpes simplex virus type 1 infections did not increase arachidonate metabolism. Results were consistent with a virus early-gene product either directly mediating or inducing a host factor that mediated the up-regulation of arachidonate metabolism, although vaccinia growth factor was not responsible. In addition, the cowpox virus 38-kDa protein-encoding gene, which is associated with inhibition of an inflammatory response, correlated with inhibition of formation of a product biochemically characteristic of (14R,15S)-dihydroxyeicosatetraenoic acid. We propose that orthopoxvirus-induced up-regulation of arachidonic acid metabolism during infection renders the infected cells susceptible to generation of inflammatory mediators from both the cyclooxygenase and the lipoxygenase pathways, and poxviruses, therefore, possess at least one gene (38K) that can alter the lipoxygenase-metabolite spectrum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AbuBakar S., Boldogh I., Albrecht T. Human cytomegalovirus stimulates arachidonic acid metabolism through pathways that are affected by inhibitors of phospholipase A2 and protein kinase C. Biochem Biophys Res Commun. 1990 Jan 30;166(2):953–959. doi: 10.1016/0006-291x(90)90903-z. [DOI] [PubMed] [Google Scholar]
- AbuBakar S., Boldogh I., Albrecht T. Human cytomegalovirus. Stimulation of [3H] release from [3H]-arachidonic acid prelabelled cells. Arch Virol. 1990;113(3-4):255–266. doi: 10.1007/BF01316678. [DOI] [PubMed] [Google Scholar]
- Betz M., Fox B. S. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991 Jan 1;146(1):108–113. [PubMed] [Google Scholar]
- Brezinski M. E., Serhan C. N. Selective incorporation of (15S)-hydroxyeicosatetraenoic acid in phosphatidylinositol of human neutrophils: agonist-induced deacylation and transformation of stored hydroxyeicosanoids. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6248–6252. doi: 10.1073/pnas.87.16.6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Cooper J. A., Twardzik D. R., Moss B. Deletion of the vaccinia virus growth factor gene reduces virus virulence. J Virol. 1988 Mar;62(3):866–874. doi: 10.1128/jvi.62.3.866-874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick F. A., Stringfellow D. A. Virus and interferon effects on cellular prostaglandin biosynthesis. J Immunol. 1980 Jul;125(1):431–437. [PubMed] [Google Scholar]
- Fredrickson T. N., Sechler J. M., Palumbo G. J., Albert J., Khairallah L. H., Buller R. M. Acute inflammatory response to cowpox virus infection of the chorioallantoic membrane of the chick embryo. Virology. 1992 Apr;187(2):693–704. doi: 10.1016/0042-6822(92)90472-2. [DOI] [PubMed] [Google Scholar]
- Glasgow W. C., Afshari C. A., Barrett J. C., Eling T. E. Modulation of the epidermal growth factor mitogenic response by metabolites of linoleic and arachidonic acid in Syrian hamster embryo fibroblasts. Differential effects in tumor suppressor gene (+) and (-) phenotypes. J Biol Chem. 1992 May 25;267(15):10771–10779. [PubMed] [Google Scholar]
- Glasgow W. C., Eling T. E. Epidermal growth factor stimulates linoleic acid metabolism in BALB/c 3T3 fibroblasts. Mol Pharmacol. 1990 Oct;38(4):503–510. [PubMed] [Google Scholar]
- Goetzl E. J., Brash A. R., Tauber A. I., Oates J. A., Hubbard W. C. Modulation of human neutrophil function by monohydroxy-eicosatetraenoic acids. Immunology. 1980 Apr;39(4):491–501. [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J. Selective feed-back inhibition of the 5-lipoxygenation of arachidonic acid in human T-lymphocytes. Biochem Biophys Res Commun. 1981 Jul 30;101(2):344–350. doi: 10.1016/0006-291x(81)91266-3. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Guinea R., López-Rivas A., Carrasco L. Modification of phospholipase C and phospholipase A2 activities during poliovirus infection. J Biol Chem. 1989 Dec 25;264(36):21923–21927. [PubMed] [Google Scholar]
- Hagmann W., Steffan A. M., Kirn A., Keppler D. Leukotrienes as mediators in frog virus 3-induced hepatitis in rats. Hepatology. 1987 Jul-Aug;7(4):732–736. doi: 10.1002/hep.1840070419. [DOI] [PubMed] [Google Scholar]
- Hartshorn K. L., Collamer M., Auerbach M., Myers J. B., Pavlotsky N., Tauber A. I. Effects of influenza A virus on human neutrophil calcium metabolism. J Immunol. 1988 Aug 15;141(4):1295–1301. [PubMed] [Google Scholar]
- Harvath L. Neutrophil chemotactic factors. EXS. 1991;59:35–52. doi: 10.1007/978-3-0348-7494-6_3. [DOI] [PubMed] [Google Scholar]
- Henke D. C., Kouzan S., Eling T. E. Analysis of leukotrienes, prostaglandins, and other oxygenated metabolites of arachidonic acid by high-performance liquid chromatography. Anal Biochem. 1984 Jul;140(1):87–94. doi: 10.1016/0003-2697(84)90137-4. [DOI] [PubMed] [Google Scholar]
- Laegreid W. W., Taylor S. M., Leid R. W., Silflow R. M., Evermann J. R., Breeze R. G., Liggitt H. D. Virus-induced enhancement of arachidonate metabolism by bovine alveolar macrophages in vitro. J Leukoc Biol. 1989 Apr;45(4):283–292. doi: 10.1002/jlb.45.4.283. [DOI] [PubMed] [Google Scholar]
- Maas R. L., Brash A. R., Oates J. A. A second pathway of leukotriene biosynthesis in porcine leukocytes. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5523–5527. doi: 10.1073/pnas.78.9.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo G. J., Buller R. M. Inhibitors of the lipoxygenase pathway specifically block orthopoxvirus replication. Virology. 1991 Jan;180(1):457–463. doi: 10.1016/0042-6822(91)90058-j. [DOI] [PubMed] [Google Scholar]
- Palumbo G. J., Pickup D. J., Fredrickson T. N., McIntyre L. J., Buller R. M. Inhibition of an inflammatory response is mediated by a 38-kDa protein of cowpox virus. Virology. 1989 Sep;172(1):262–273. doi: 10.1016/0042-6822(89)90128-1. [DOI] [PubMed] [Google Scholar]
- Ray C. A., Black R. A., Kronheim S. R., Greenstreet T. A., Sleath P. R., Salvesen G. S., Pickup D. J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992 May 15;69(4):597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- Raz A., Wyche A., Siegel N., Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Setty B. N., Graeber J. E., Stuart M. J. The mitogenic effect of 15- and 12-hydroxyeicosatetraenoic acid on endothelial cells may be mediated via diacylglycerol kinase inhibition. J Biol Chem. 1987 Dec 25;262(36):17613–17622. [PubMed] [Google Scholar]
- Shak S., Perez H. D., Goldstein I. M. A novel dioxygenation product of arachidonic acid possesses potent chemotactic activity for human polymorphonuclear leukocytes. J Biol Chem. 1983 Dec 25;258(24):14948–14953. [PubMed] [Google Scholar]
- Shukla M. I., Chaturvedi U. C. Dengue virus-induced suppressor factor stimulates production of prostaglandin to mediate suppression. J Gen Virol. 1981 Oct;56(Pt 2):241–249. doi: 10.1099/0022-1317-56-2-241. [DOI] [PubMed] [Google Scholar]
- Strayer D. S., Korber K., Dombrowski J. Immunosuppression during viral oncogenesis. IV. Generation of soluble virus-induced immunologic suppressor molecules. J Immunol. 1988 Mar 15;140(6):2051–2059. [PubMed] [Google Scholar]
- Stroobant P., Rice A. P., Gullick W. J., Cheng D. J., Kerr I. M., Waterfield M. D. Purification and characterization of vaccinia virus growth factor. Cell. 1985 Aug;42(1):383–393. doi: 10.1016/s0092-8674(85)80133-1. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Brown J. P., Ranchalis J. E., Todaro G. J., Moss B. Vaccinia virus-infected cells release a novel polypeptide functionally related to transforming and epidermal growth factors. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5300–5304. doi: 10.1073/pnas.82.16.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N., Yamamoto S., Oates J. A., Brash A. R. Stereoselective hydrogen abstraction in leukotriene A4 synthesis by purified 5-lipoxygenase of porcine leukocytes. Prostaglandins. 1986 Jul;32(1):43–48. doi: 10.1016/0090-6980(86)90141-3. [DOI] [PubMed] [Google Scholar]
- Villani A., Cirino N. M., Baldi E., Kester M., McFadden E. R., Jr, Panuska J. R. Respiratory syncytial virus infection of human mononuclear phagocytes stimulates synthesis of platelet-activating factor. J Biol Chem. 1991 Mar 25;266(9):5472–5479. [PubMed] [Google Scholar]
- Wahl L. M., Corcoran M. L., Pyle S. W., Arthur L. O., Harel-Bellan A., Farrar W. L. Human immunodeficiency virus glycoprotein (gp120) induction of monocyte arachidonic acid metabolites and interleukin 1. Proc Natl Acad Sci U S A. 1989 Jan;86(2):621–625. doi: 10.1073/pnas.86.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. J., Huang N. N., Gonzalez F. A., Heppel L. A. Multiple signal transduction pathways lead to extracellular ATP-stimulated mitogenesis in mammalian cells: I. Involvement of protein kinase C-dependent and -independent pathways. J Cell Physiol. 1991 Mar;146(3):473–482. doi: 10.1002/jcp.1041460319. [DOI] [PubMed] [Google Scholar]
- Yokoyama C., Shinjo F., Yoshimoto T., Yamamoto S., Oates J. A., Brash A. R. Arachidonate 12-lipoxygenase purified from porcine leukocytes by immunoaffinity chromatography and its reactivity with hydroperoxyeicosatetraenoic acids. J Biol Chem. 1986 Dec 15;261(35):16714–16721. [PubMed] [Google Scholar]



