Abstract
Background:
Type 2 diabetes is the fourth major public health problem worldwide. Royal Jelly (RJ) insulin-like activity and blood glucose modulating properties have been reported in animal and healthy volunteers.
Objectives:
This study aimed to investigate the effect of a single dose of fresh RJ as a complementary therapy on glycemic response in patients with type 2 diabetes.
Patients and Methods:
In this randomized clinical trial, 40 patients with type 2 diabetes were assigned into the RJ (n = 20) and placebo (n = 20) groups and received either 10 g fresh RJ or placebo after overnight fasting. Serum glucose, insulin and C-peptide concentrations were determined at 0, 60, 120 minutes after the intervention. Independent t-tests and repeated measures ANOVA were used to analyze data.
Results:
The mean serum glucose levels were significantly decreased in RJ and placebo groups; however, mean serum level was different but not statistically. (P = 0.77). One hour after RJ ingestion the mean serum insulin concentrations were increased and after 2 hours it was decreased insignificantly (P = 0.54, P = 0.20). The mean C-peptide concentrations were significantly increased after 1 and 2 hours of RJ ingestion; however, in the placebo group we observed a slight but insignificant reduction at the time of 1 and 2 hours in the mean C-peptide serum levels (P = 0.40). Moreover, there was no significant difference in none of the glycemic control parameters between both studied groups (P > 0.05).
Conclusions:
It seems that RJ does not appear to have significant immediate effects on glycemic factors in patients with type 2 diabetes. However, further studies with larger sample sizes and different doses of RJ are needed to achieve more precise results.
Keywords: Royal Jelly, Hyperglycemia, Glycemic Control
1. Background
Type 2 diabetes is one of the most prevalent chronic diseases and it is the fourth major cause of mortality worldwide (1). The world health organization (WHO) estimates that the number of people with diabetes in range of 45 - 64 years old will be more than 140 million in developing countries and more than 30 million in developed countries in 2030 (2).
Patients with type 2 diabetes are high risk for developing chronic diseases like atherosclerosis and coronary heart and renal diseases (3). The results of previous studies showed that along with routine medications, complementary medicine may improve glycemic control and insulin resistance in patients with type 2 diabetes (4-7).
Royal Jelly (RJ) is a viscous and milky substance secreted by the hypopharyngeal and mandibular glands of worker honeybees (Apis mellifera) and is an essential food for both the queen and her larvae (8). Royal jelly comprises 60% - 70% water, 10% - 12% carbohydrates, 12% - 15% proteins and 3% - 7% lipids (9). It has been suggested that RJ may have hypoglycemic functions, Munstedt et al. reported that single doses of RJ decreased blood glucose levels in healthy subjects (10). In addition, findings of in vivo and in vitro studies indicated that RJ has hypotensive, (11) anti-hypercholesterolemic, (12) anti-inflammatory (13), antitumor (14) and antioxidant effects (15). It has insulin-like activity, which may improve insulin resistance (16, 17).
We adapted with new aspects of medicine which are transferred to our country but our previous data about disorders are neglected. We have so many data in our ancient book which are subject of attention for some other scientist who use them in their researches (18); however, we seldom had such studies. So, we decided to do one of them which were never designed in this manner.
2. Objectives
To the best of our knowledge, there are no reports about the effect of a single dose of RJ administration on glycemic control in diabetic patients; therefore, the aim of this study was to determine the effects of RJ supplementation in female patients with diabetes.
3. Patients and Methods
At the baseline of the study, 200 patients with type 2 diabetes were candidate to participate in our study from the outpatient endocrine clinic of Imam Reza Teaching Hospital (a specialized, governmental and referral hospital) in Tabriz, Iran, but only 40 patients were eligible to participate in the study. This research was done between October and December 2013.
Patients with type 2 diabetes mellitus (with at least one year duration), age range of 30 - 65 years, and Body Mass Index (BMI) of 25 - 30 Kg/m2 were enrolled in this study. They were taking only glucose-lowering agents (metformine and glibenclamide). They were randomly divided into the RJ and placebo groups (n=20 in each group).
Patients who received trace elements and antioxidant supplements during 6 months ago, pregnant women and patients in a lactation period, those involved with renal disease, endocrine dysfunction and any type of allergy were excluded. Sample size was determined based on data from previous study (12,13). Considering the confidence interval of 95%, α = 0.05 and power of 80%, the following equation was used:
| (1) |
Twenty patients with diabetes were considered for each group. Forty volunteers with type 2 diabetes were randomly assigned into the RJ (n = 20) and placebo (n = 20) groups based on the random block procedure produced by random allocation software (RAS). Afterwards, the participants received either 10 g fresh RJ or placebo after overnight fasting.
A computer-generated random sequence was kept in a remote secure location and administered by an independent third party who was not involved with the clinical conduct of the study until all study data were collected and verified. Patients and those involved in enrolling participants, administering interventions and assessing outcomes were blind to group assignments. Both groups were matched in baseline characteristics (Table 1).
Table 1. Baseline Characteristics of Patients a,b.
| Variable | RJ Group (n = 20) | Placebo Group (n = 20) | P |
|---|---|---|---|
| Age, y | 50.05 ± 7.31 | 53.75 ± 6.93 | 0.10 |
| Gender c | 0.74 d | ||
| Female | 13 (65) | 12 (60) | |
| Male | 7 (35) | 8 (40) | |
| Weight, kg | 75.45 ± 14.50 | 77.95 ± 11.69 | 0.55 |
| BMI, kg/m 2 | 28.72 ± 4.66 | 29.32 ± 4.19 | 0.67 |
| Duration of diabetes, y | 7.20 ± 3.1 | 7.80 ± 2.8 | 0.87 |
| Fasting blood glucose, mmol/l | 8.62 ± 2.82 | 8.70 ± 2.25 | 0.90 |
| HbA1C, % | 8.14 ± 1.13 | 7.70 ± 0.7 | 0.29 |
| Fasting serum insulin, μU/mL | 11.35 ± 8.26 | 9.49 ± 5.65 | 0.40 |
| Fasting C-peptide, ng/mL | 2.85 ± 1.43 | 3.42 ± 0.97 | 0.15 |
a Abbreviations: BMI, body mass index; RJ, royal jelly.
b Results are expressed as mean ± SD.
c Values are presented as No. (%).
d Chi-square P value.
After 12 hours overnight fasting, 5 mL venous blood samples were collected and patients in the intervention and placebo groups received 15 g fresh RJ or 15 g placebo. The Placebo consists of nonabsorbent gum and gelatin which had identical appearance with RJ. The blood sampling repeated again after 1 and 2 hours.
The serum samples of patients were kept at -80°C until biochemical analysis. Serum fasting blood glucose (FBG), C-peptide and insulin concentration were determined by the enzymatic colorimetric method and chemiluminescence methods using Monobind kits, respectively. All devices were calibrated and we used similar type of instrumentation for all studied patients.
This parallel design randomized clinical trial was approved by the ethics committee of Tabriz University of Medical Sciences with WHO registered randomized clinical trial number: IRCT201306049626N2 (available at: http://www.who.int/trialsearch) and the written informed consent was obtained from all participants. The participants can leave the study at any time.
3.1. Statistical Analysis
Descriptive statistics were obtained for all studied variables. Results were presented as mean ± SD. The normality of variables was tested using the Kolmogorov-Smirnov test. Differences between two treatment groups were analyzed using an independent t-test. The repeated measures ANOVA was used to compare the differences within a group (RJ or placebo). All P values less than 0.05 were considered statistically significant.
4. Results
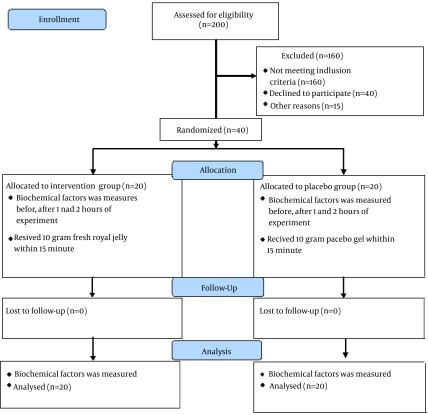
All of the patients completed the study and analyzed in last assessment. Figure 1 shows the study protocol. Baseline characteristics of patients were presented in Table 1.
Figure 1. Study Flow Diagram.
It is clear that our studied groups (RJ and placebo) were matched regarding baseline characteristics (Table 1) and glycemic control was equal statistically. Fasting blood glucose, HbA1C level, fasting insulin and C-peptide levels were similar statistically in both groups (P = 0.90, P = 0.29, P = 0.40, P = 0.15, respectively).
Glycemic control parameters of the studied groups at the onset and end of the intervention are presented in Table 2. The mean serum FBG levels were significantly decreased in both groups; however, statistically difference was found between the two groups after 1 and 2 hours of RJ ingestion (P = 0.77). One hour after the RJ ingestion the mean serum insulin concentration was increased and after 2 hours it was decreased insignificantly, but in the placebo group, the mean insulin levels were insignificantly reduced after 2 hours of placebo ingestion (P = 0.09). The mean C-peptide concentrations were increased significantly after 1 and 2 hours of RJ ingestion; however, in the placebo group we observed the slight but insignificant reduction in the mean C-peptide serum levels after 1 and 2 hours. Before and after the intervention, no significant difference was found in none of the glycemic control parameters (P = 0.77, P = 0.32, P = 0.40, respectively) (Table 2).
Table 2. Glycemic Control in Both Groups.
| Parameter | Royal Jelly (n = 20) | Placebo (n = 20) | P |
|---|---|---|---|
| Glucose, mmol/l a | |||
| Fasted | 8.62 ± 2.82 | 8.70 ± 2.25 | 0.9 |
| 1 h | 147.45 ± 48.48 | 150.35 ± 39.57 | 0.54 |
| 2 h | 133.90 ± 42.47 | 144.95 ± 37.91 | 0.2 |
| P | 0.001 | 0.001 | |
| P b | 0.77 | ||
| Insulin, μU/mL a | |||
| Fasted | 11.35 ± 8.26 | 9.49 ± 5.65 | 0.4 |
| 1 h | 11.81 ± 1.09 | 8.71 ± 6.07 | 0.39 |
| 2 h | 9.71 ± 7.99 | 7.83 ± 4.47 | 0.73 |
| P | 0.08 | 0.09 | |
| P b | 0.32 | ||
| C-peptide, ng/mL a | |||
| Fasted | 2.85 ± 1.43 | 3.42 ± 0.97 | 0.15 |
| 1 h | 3.05 ± 1.80 | 3.31 ± 0.91 | 0.11 |
| 2 h | 3.04 ± 1.49 | 3.22 ± 0.89 | 0.2 |
| P | 0.001 | 0.08 | |
| P b | 0.4 |
a Values are presented as mean ± SD.
b Parameters between the two groups.
5. Discussion
Royal jelly is a wonderful food which the results of in vivo and in vitro studies suggested several therapeutic properties for it (16, 17, 19). Kramer et al. recognized insulin-like activity of peptides in RJ (17). In another research which was done in diabetic (fructose drinking) rat, RJ administration resulted in reduction in insulin levels and insulin resistance via prevention of hypertension without any change on glucose concentration (20). To the best of our knowledge there are no studies about the effects of a single dose of RJ on glycemic control in patients with diabetes. This kind of study was not done previously and its results may alter hyperglycemic control with a non-synthetic agent, which may have lesser side effects.
In our previous report 1000 mg/day lyophilized RJ supplementation for 8 weeks, insignificantly reduced fasting blood glucose and significantly increased fasting serum insulin in type 2 diabetic females (21);. However, in the present study RJ administration significantly decreased the mean glucose levels (P = 0.001) without any significant changes in insulin concentrations (P = 0.08). Our findings support Munstedt et al. study in which the administration of 20 g of RJ significantly reduced glucose levels in 20 healthy subjects. Munstedt et al. suggested that insulin-like peptides of RJ were responsible for this action which seems it can preserve its activity even after passage through human stomach (10). However, in another report, 10 g of RJ intake did not show any statistically significant immediate effect on serum glucose, C-peptide and insulin levels in ten volunteers (22). However, the results of other studies in 2010 reveal that feeding with enteric-coated capsules containing RJ does not have any change on glycemic control factors in healthy subject. Our results confirm these reports (18).
However, in our study 10 g of placebo intake had the result similar to the RJ group, but the trend of insulin reduction and C-peptide elevation in the RJ group was slower than that in the placebo group, which can be suggested that RJ may modulate glucose-insulin response independent from its insulin-like activity.
In conclusion, our findings revealed that in comparison with the placebo group, RJ did not have immediate significant effects on glycemic factors in patients with type 2 diabetes. However, further studies with larger sample sizes and different doses of RJ are needed to achieve more precise results.
Acknowledgments
The authors are deeply indebted to all patients who participated in this study. The results of this paper are from the thesis of Dr. Gyiasvand, the resident of internal medicine, which was registered in Tabriz university of medical sciences.
Footnotes
Authors’ Contributions:Study concept and design: Majid Mobasseri. Acquisition of data: Hamid Noshad. Analysis and interpretation of data: Morteza Ghojazadeh. Drafting of the manuscript: Shahram Ghiyasvand. Critical revision of the manuscript for important intellectual content: Hamid Noshad. Statistical analysis: Morteza Ghojazadeh. Administrative, technical, and material support: Alireza Ostadrahimi. Study supervision: Samira Pourmoradian.
Funding/Support:This study was financially supported by the bone research center, Tabriz University of Medical Sciences. The funding organization had no role in the design and conduct of the study but had a partial role in data collection and management. They had no role in analysis of the data; or preparation, review, and approval of the manuscript.
References
- 1.Khuwaja AK, Khowaja LA, Cosgrove P. The economic costs of diabetes in developing countries: some concerns and recommendations. Diabetologia. 2010;53(2):389–90. doi: 10.1007/s00125-009-1581-7. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab. 2000;26(3):163–76. [PubMed] [Google Scholar]
- 4.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neri S, Signorelli SS, Torrisi B, Pulvirenti D, Mauceri B, Abate G, et al. Effects of antioxidant supplementation on postprandial oxidative stress and endothelial dysfunction: a single-blind, 15-day clinical trial in patients with untreated type 2 diabetes, subjects with impaired glucose tolerance, and healthy controls. Clin Ther. 2005;27(11):1764–73. doi: 10.1016/j.clinthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Vega-Lopez S, Devaraj S, Jialal I. Oxidative stress and antioxidant supplementation in the management of diabetic cardiovascular disease. J Investig Med. 2004;52(1):24–32. doi: 10.1136/jim-52-01-23. [DOI] [PubMed] [Google Scholar]
- 7.Lee DH, Folsom AR, Harnack L, Halliwell B, Jacobs DJ. Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am J Clin Nutr. 2004;80(5):1194–200. doi: 10.1093/ajcn/80.5.1194. [DOI] [PubMed] [Google Scholar]
- 8.Fujii A, Kobayashi S, Kuboyama N, Furukawa Y, Kaneko Y, Ishihama S, et al. Augmentation of wound healing by royal jelly (RJ) in streptozotocin-diabetic rats. Jpn J Pharmacol. 1990;53(3):331–7. doi: 10.1254/jjp.53.331. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki KM, Isohama Y, Maruyama H, Yamada Y, Narita Y, Ohta S, et al. Estrogenic activities of Fatty acids and a sterol isolated from royal jelly. Evid Based Complement Alternat Med. 2008;5(3):295–302. doi: 10.1093/ecam/nem036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munstedt K, Bargello M, Hauenschild A. Royal jelly reduces the serum glucose levels in healthy subjects. J Med Food. 2009;12(5):1170–2. doi: 10.1089/jmf.2008.0289. [DOI] [PubMed] [Google Scholar]
- 11.Tokunaga KH, Yoshida C, Suzuki KM, Maruyama H, Futamura Y, Araki Y, et al. Antihypertensive effect of peptides from royal jelly in spontaneously hypertensive rats. Biol Pharm Bull. 2004;27(2):189–92. doi: 10.1248/bpb.27.189. [DOI] [PubMed] [Google Scholar]
- 12.Guo H, Saiga A, Sato M, Miyazawa I, Shibata M, Takahata Y, et al. Royal jelly supplementation improves lipoprotein metabolism in humans. J Nutr Sci Vitaminol (Tokyo). 2007;53(4):345–8. doi: 10.3177/jnsv.53.345. [DOI] [PubMed] [Google Scholar]
- 13.Kohno K, Okamoto I, Sano O, Arai N, Iwaki K, Ikeda M, et al. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci Biotechnol Biochem. 2004;68(1):138–45. doi: 10.1271/bbb.68.138. [DOI] [PubMed] [Google Scholar]
- 14.Townsend GF, Morgan JF, Hazlett B. Activity of 10-hydroxydecenoic acid from royal jelly against experimental leukaemia and ascitic tumours. Nature. 1959;183(4670):1270–1. doi: 10.1038/1831270a0. [DOI] [PubMed] [Google Scholar]
- 15.Nagai T, Inoue R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004;84(2):181–6. doi: 10.1016/s0308-8146(03)00198-5. [DOI] [Google Scholar]
- 16.Dixit PK, Patel NG. Insulin-Like Activity in Larval Foods of the Honeybee. Nature. 1964;202:189–90. doi: 10.1038/202189a0. [DOI] [PubMed] [Google Scholar]
- 17.Kramer KJ, Tager HS, Childs CN. Insulin-like and glucagon-like peptides in insect hemolymph. Insect Biochem. 1980;10(2):179–82. doi: 10.1016/0020-1790(80)90071-2. [DOI] [Google Scholar]
- 18.Münstedt K, Böhme M, Hrgovic I, Hauenschild A. An approach to the application of Royal Jelly: Encapsulation of lyophilized Royal Jelly and its effect on glucose metabolism in humans. J Api Product Api Med Sci. 2010;2(1):29. doi: 10.3896/ibra.4.02.1.03. [DOI] [Google Scholar]
- 19.Salazar-Olivo LA, Paz-Gonzalez V. Screening of biological activities present in honeybee (Apis mellifera) royal jelly. Toxicol In Vitro. 2005;19(5):645–51. doi: 10.1016/j.tiv.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Zamami Y, Takatori S, Goda M, Koyama T, Iwatani Y, Jin X, et al. Royal jelly ameliorates insulin resistance in fructose-drinking rats. Biol Pharm Bull. 2008;31(11):2103–7. doi: 10.1248/bpb.31.2103. [DOI] [PubMed] [Google Scholar]
- 21.Pourmoradian S, Mahdavi R, Mobasseri M, Faramarzi E, Mobasseri M. Effects of royal jelly supplementation on glycemic control and oxidative stress factors in type 2 diabetic female: a randomized clinical trial. Chin J Integr Med. 2014;20(5):347–52. doi: 10.1007/s11655-014-1804-8. [DOI] [PubMed] [Google Scholar]
- 22.Münstedt K, Bargello M, Hauenschild A. Royal jelly and its influence on serum fructose and serum lipids. J Api Product Api Medical Sci. 2009;1(3):90–1. doi: 10.3896/ibra.4.01.3.05. [DOI] [Google Scholar]