Abstract
Background and Purpose:
Antibiotic-loaded cement spacers are typically manufactured by surgeons in the operating room. However, if the infecting organism is known preoperatively, the cement spacer can be fabricated (Spacer-G® or the InterSpace® Hip) in advance. It is unclear if preformed hip spacers are superior to surgeon-made hip spacers in the treatment of periprosthetic joint infection following primary THA.
Methods:
A literature review of the peer-reviewed literature indexed by MEDLINE and Embase was performed to identify studies reporting the outcomes of preformed and surgeon-made hip spacers in the treatment of infection following primary total hip arthroplasty (THA). A total of 43 articles met the inclusion criteria and were included in the analysis to compare the reinfection rate, Harris Hip Score (HHS) and spacer complication rates between surgeon-made and preformed hip spacers.
Results and Interpretation:
The analyzed studies included a total number of 1631 infected THA cases (n=1027 surgeonmade; n=604 preformed spacers). We found similar reinfection rates (6.0% surgeon-made, and 5.5% preformed spacers) and similar mean HHS at latest follow-up after reimplantation (HHS=84.3 surgeon-made, and HHS=81.8 preformed spacers) between both groups. However, patients treated with a surgeon-made articulating spacer had a higher spacer fracture rate compared to preformed articulating spacer. The use of preformed articulating spacers in the treatment of infected THA is not superior to surgeon-made articulating spacers regarding infection control and functional outcomes. However, the use of surgeon-made antibiotic spacers increased the risk of spacer fracture.
Keywords: Articulating spacer, preformed spacer, periprosthetic infection, surgeon-made spacer.
INTRODUCTION
Periprosthetic joint infection (PJI) after total hip arthroplasty (THA) is a devastating complication with infection rates varying from 0.5%-1.4% [1]. The standard of care for treatment of PJI has been two-stage revision arthroplasty with implantation of an antibiotic-impregnated cement spacer to eradicate the infection prior reimplantation. The cement spacer in the interim period can be classified as either a static/ non-articulating spacers (e.g. traditionally simple cement blocks) or a mobile/articulating spacer. The main goal of the spacer is to provide a local delivery of a high dose of antibiotics directly to the infection site. In addition, an articulating spacer has the advantage over a static spacer of allowing more comfortable motion between spaces, maintaning limb length, preventing contracture and increasing patient comfort during the interval prior to reimplantation.
In the recent years, the use of articulating cement spacers has become increasingly popular in both the hip and the knee following resection. Traditionally, antibiotic-loaded cement spacers have been performed by surgeons intraoperatively using a variety of technique ranging from hand-made techniques using a bulb syringe to fashion a femoral head, to molded systems with or wihtout a metal endo-skeleton to prevent fracture, to a sophisticated multi-size and multi-length spacer system with a large endo-skeleton and a constrained metal on polyethylene articulation to prevent both dislocation and fracture (PROSTALAC, DePuy, Warsaw, IN). However, in cases in which the infecting organisms are known preoperatively, prefabricated cement spacers (Spacer.G® or InterSpace® Hip) may be utilized.
In 2005, D´Angelo and colleagues performed a study comparing preformed and custom-made spacers with regards to functional outcomes and infection control in 20 patients [2]. The authors concluded, that preformed spacers were superior to custom-made spacer regarding functional outcome and complication rate, while the eradication rate was similar between both groups [2]. Since then, several authors have reported on the outcomes of preformed hip spacers (Spacer-G and InterSpace® Hip) in the interim period of two-stage revision arthroplasty [3-2]. Many of these studies are small case series and it remains unclear if preformed hip spacers are superior to surgeon-made hip spacers in the treatment of periprosthetic joint infection following primary THA. Therefore, we performed this literature review to analyze the outcomes between surgeon-made and preformed articulating hip spacers in the treatment of infected total hip arthroplasty. The goal of the study is to find possible differences in the treatment of periprosthetic hip infection using preformed and surgeon-made spacers.
MATERIALS AND METHODS
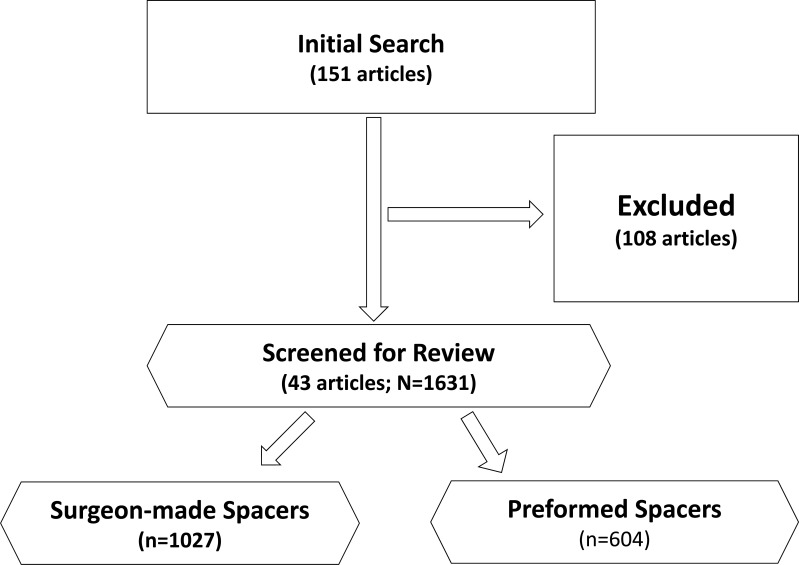
We systematically reviewed the literature for potentially relevant articles addressing two-stage revision of an infected total hip arthroplasty using the MEDLINE computerized literature databases. Following keyword terms „total hip arthroplasty or total hip replacement“ and „spacer or spacers“ were used for initial search. The literature search was performed on May 1st 2013 considering all published articles prior to May 1st 2013. The initial search yielded 151 articles. Studies were inlcuded if they (1) described patients treated with surgeon-made and prefabricated/preformed articulating spacers following primary total hip arthroplasty; (2) reported reinfection rates; (3) reported Harris Hip Score after reimplantation and/or complication rates; and (4) follow-up examination of minimum 12 months. Review articles, case reports, letter to the editors or technical notes were excluded from the study. Studies using non-human subjects or in vitro studies including biomechanical studies and studies without the use of antibiotic spacers for two-stage revision for infected total hip arthroplasty were also excluded. A total of 43 articles met the inclusion and exclusion criteria and were included for the analyzes (Fig. 1). We excluded 35 case reports, 9 case series (n<7), 7 technical notes, 12 review articles, 10 experimental studies and one brief communication. Furthermore, 15 studies reported on different functional outcomes (Merle d’Aubigné and Postel hip score, JOA hip score and WOMAC score) than HHS were also excluded. The remaining 17 articles were excluded due to follow-up examination of less than 12 months, reported outcomes in patients with fungal or multi-resistant periprothetic joint infection and patients treated without the use of antibiotic spacers for two-stage revision for infected total hip arthroplasty as well as reinfection cases after two-stage revision for infected THA.
Fig. (1).
Flow diagram outlining the inclusion and exclusion of retrieved studies utilized for the analysis.
The authors assessed the integrity of the study design and the research methods for each study. The level of evidence (level I-IV) was noted in every study according to the criteria set forth by the Oxford 2011 guidelines for level of evidence [13]. According to these guidelines, there were 3 level II, 4 level III and 36 Level IV studies (Tables1a and 1b) [2-2, 14-46].
Table 1a.
Included studies with surgeon-made articulating hip spacers in the treatment of PJI.
| Author | Year of Publication | Number of Cases | Follow-Up | Number of Reinfections | Eradication Rate(%) |
|---|---|---|---|---|---|
| Borowski | 2012 | 29 | not reported | 3 | 89,7 |
| Ben-Lulu | 2012 | 11 | 15 | 1 | 90,9 |
| Fleck | 2011 | 14 | 46,3 | 1 | 92,9 |
| Zou | 2011 | 15 | 25 | 0 | 100,0 |
| Fei | 2011 | 51 | 61,4 | 0 | 100,0 |
| Peng | 2011 | 34 | not reported | 0 | 100,0 |
| Liu | 2010 | 107 | 34,3 | 6 | 94,4 |
| Peng | 2010 | 15 | 31,2 | 0 | 100,0 |
| Dairaku K | 2009 | 10 | 18 | 1 | 90,0 |
| Biring | 2009 | 99 | 144 | 11 | 88,9 |
| Jung | 2009 | 88 | 54 | 10 | 88,6 |
| Kent | 2010 | 15 | 38 | 0 | 100,0 |
| Whittaker JP | 2009 | 41 | 49 | 3 | 92,7 |
| Fink | 2009 | 36 | 35 | 0 | 100,0 |
| Toulson | 2009 | 84 | 64,8 | 4 | 95,2 |
| McKenna | 2009 | 31 | 35 | 0 | 100,0 |
| lncavo | 2009 | 12 | 24 | 2 | 83,3 |
| Diwanji | 2008 | 9 | 42 | 2 | 77,8 |
| Masri | 2007 | 29 | 47 | 3 | 89,7 |
| D'Angelo | 2005 | 8 | 40 | 1 | 87,5 |
| Hoffmann | 2005 | 27 | 76 | 1 | 96,3 |
| Morshed | 2005 | 13 | 42,6 | 3 | 76,9 |
| Durbhakula | 2004 | 20 | 38 | 0 | 100,0 |
| Hsieh | 2004 | 58 | not reported | 2 | 96,6 |
| Hsieh | 2004 | 42 | 55,2 | 2 | 95,2 |
| Jahoda | 2004 | 11 | not reported | 0 | 100,0 |
| Yamamoto | 2003 | 17 | 38 | 0 | 100,0 |
| Takahira | 2003 | 9 | 35,7 | 1 | 88,9 |
| Koo | 2001 | 22 | 41 | 1 | 95,5 |
| Lsiklar | 1999 | 10 | 23,4 | 0 | 100,0 |
| Leunig | 1998 | 12 | 27 | 1 | 91,7 |
| Younger | 1997 | 48 | 43 | 3 | 93,8 |
Table 1b.
Included studies with preformed articulating hip spacers.
| Author | Year of Publication | Number of Cases | Follow-Up (Months) | Number of Reinfections | Eradication Rate(%) |
|---|---|---|---|---|---|
| Romano | 2012 | 183 | 60 | 10 | 94,5 |
| Degen | 2012 | 33 | 43 | 5 | 84,8 |
| Neumann | 2012 | 44 | 67 | 1 | 97,7 |
| Romano | 2011 | 20 | 56,6 | 1 | 95,0 |
| D'Angelo | 2011 | 28 | 53 | 1 | 96,4 |
| Pattyn | 2011 | 61 | 36 | 2 | 96,7 |
| Romano | 2010 | 40 | 52,1 | 1 | 97,5 |
| Pignatti | 2010 | 41 | 63,6 | 0 | 100,0 |
| Gil Gonzalez | 2010 | 35 | 32 | 5 | 85,7 |
| Romano | 2010 | 102 | 48 | 4 | 96,1 |
| D'Angelo | 2005 | 12 | 40 | 1 | 91,7 |
| Magnan | 2001 | 10 | 35 | 2 | 80,0 |
Data Analysis
All data regarding the methods and results of each of the articles included in the study were entered into a database and were analyzed. As available literature was heterogeneous and did not provide complete and quantitative information, a qualitative and descriptive summary of the results was compiled with regard to infection outcome, complication rate and functional outcome using the Harris Hip Score between preformed and surgeon-made hip spacers. Premade spacers are antibiotic-loaded preformed hip spacers. The inner part of the spacer usually contains a stainless steel rod, which is covered by antibiotic loaded cement. The cement is preloaded by the manufacturer most often with 1.9 g gentamicin sulfate [3-12]. Surgeon-made spacers are intraoperatively preformed spacers ranging from hand-made techniques using a bulb syringe to fashion a femoral head, to molded systems with or wihtout a metal endo-skeleton, while in the majority of cases, metal endoskeleton was not used.
RESULTS
The analyzed studies included a total number of 1631 infected THA cases, 1027 treated with a surgeon-made spacers and 604 treated with preformed spacers (Fig. 1, Table 1). The mean follow-up for the surgeon-made and preformed groups was at 43.7 months (range, 15 to 144 months) and 48.9 months (range, 32 to 67 months), respectively (p=0.49). Likewise, the mean patient age was similar at 63.9 years (range, 52.6 to 73.9 years; SD=5.1) and 64.7 years (range, 55.7 to 71.8 years; SD=5.6) (p=0.58), respectively (Table 1). The mean time at follow-up examination (p=0.49) and the mean spacer duration between stages were also not significantly different (p=0.15) (Table 1).
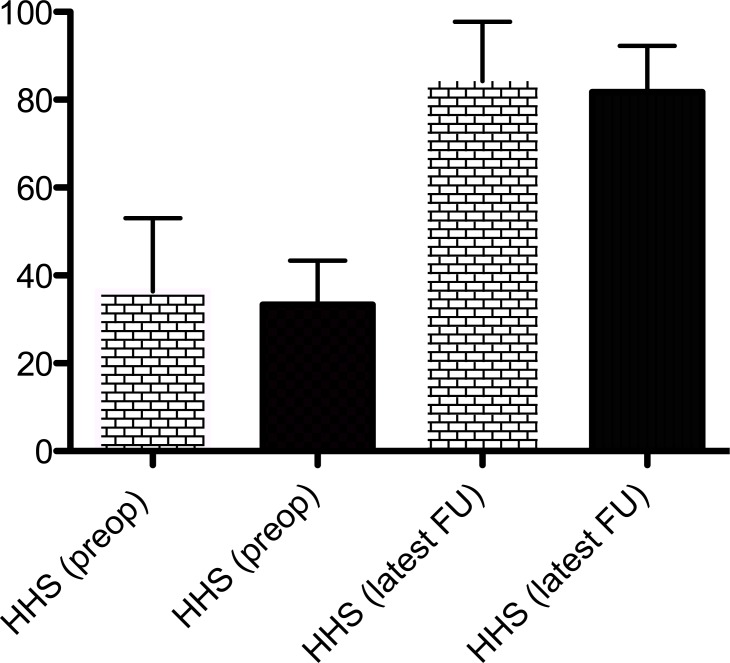
Regarding functional outcome, we analyzed the preoperative Harris Hip Score and Harris Hip Scores at latest follow-up in the two groups. Similar improvement in Harris Hip Scores was observed in the two groups (Fig. 2 and Table 2). Preoperatively, there was no difference in the Harris Hip Score between the surgeon-made group and the premade group at 36.4 (range, 11.5 to 53) and 33.4 (range, 15 to 43.4), respectively (p=0.53). At lastest follow-up, the mean HHS was 84.3 (range, 68 to 97.8) for the surgeon-made spacers and 81.8 (range, 71.2 to 92.3) (p=0.5) for the preformed spacers. (Fig. 2 and Table 2). We are unable to comment on the HHS between stages because the majority of the studies did not report on this.
Fig. (2).
reveals the mean pre- and postoperative Harris Hip scores for patients treated with either surgeon-made (yellow bars) or preformed hip spacers (black bars).
Table 2.
Descriptive information of surgeon-made and preformed hip spacer studies. *NC = not calculated. †Significant (p < 0.05).
| Variable | Surgeon Made Spacers (Range) | Preformed Spacers (Range) | p Value |
|---|---|---|---|
| No. of cases | 1027 | 604 | NC |
| Mean Age (years) | 63.9 (52.6-73.9) | 64.7 (55.7-71.8) | 0.58 |
| Mean spacer duration (months) | 5,6 (3-9.4) | 4.4 (2.9-5.6) | 0.15 |
Looking at infection control, preformed hip spacers were also not superior to surgeon-made hip spacers in the treatment of infected THA (p=0.76). The eradication rate at latest follow-up after reimplantation was 94% using surgeon-made hip spacers and 94.5% using preformed hip spacers (Table 2).
Complications
The overall spacer complication rate was similar between both groups (Tables 3 and 4). However, the spacer fracture rate was significantly higher using surgeon-made hip spacers compared to the use of preformed hip spacers (Tables 3 and 4) (p<0.05).
Table 3.
Clinical data reported in the analyzed studies. †Significant (p < 0.05).
| Variable | Surgeon Made Spacers (Range) | Preformed Spacers (Range) | p Value |
|---|---|---|---|
| Mean follow-up (months) | 43.7 (15-144) | 48.9 (32-67) | 0.49 |
| Reinfection rate | 6.0% | 5.5% | 0.76 |
| Preoperative HSS score | 36.4 (11.5-53) | 33.4 (15-43.4) | 0.53 |
| Postoperative HSS score | 84.3 (68-97.8) | 81.8 (71.2-92.3) | 0.50 |
| Spacer complication rate | 12.9% | 12.4% | >0.05 |
Table 4.
Detailed information about the complications related to the surgeon-made and preformed cement hip spacers. †Significant (p < 0.05).
| Variable | Surgeon Made Spacers | Preformed Spacers | p Value |
|---|---|---|---|
| Spacer dislocation | 7.0% | 12.4% | 0.13 |
| Spacer fracture | 5.9% | 0% | >0.05 |
DISCUSSION
Two-stage revision arthroplasty for the treatment of chronic periprosthetic joint infection of the knee or hip joint infection is currently considered the universal gold standard. The type of spacer utilized during the interim period can be either an articulating or a static/non-articulating spacer. Articulating spacer has become increasingly more popular as it maintains limb length and patient function/comfort between stages following resection and facilitates the ease of reimplantation.
Historically, spacers have been surgeon made at the time of the operation. This allows for the selection of bacteria-specific antibiotics to be added to the spacer. In the recent years; however, the use of preformed or prefabricated articulating hip or knee spacers in the treatment of PJI has been increasing in some hospitals. These preformed spacers are preloaded with a set amount of antibiotics and preformed into the shape of the hip spacer. This has the potential to save time in the operating room and facilitates ease of reimplantation. However, Concerns have always remained regarding the amount of antibiotics in these preformed spacers as well as the cost associated with their use.
D´Angelo et al. reported superior outcome of preformed articulating spacers compared to custom-made spacers regarding functional outcome and complication rate, while the eradication rate was similar between both groups [2]. Since then, several authors reported on the outcomes using those preformed hip spacers (Spacer-G and InterSpace® Hip) in the interim period of two-stage revision arthroplasty [3-12]. Most of the studies are limited due to a small number of included cases or due to a relatively short follow-up time. We therefore performed this analysis to compare the results using preformed and surgeon-made articulating hip spacers in the treatment of PJI.
In contrast to the study by D´Angelo and colleagues, our analysis of large series of both prefabricated and surgeon made spacers showed no differences regarding functional outcome. The analyzed HHS (preoperative HHS and HHS at latest follow-up) were comparable in both groups and as also reported by D´Angelo et al., the eradication rate was similar between both types of spacers. It must be noted that, we are unable to comment on the HHS between stages because the majority of the studies did not report on this.
In addition, our analysis showed a significantly higher spacer fracture rate in the surgeon made fracture group compared to the preformed spacer group. It seems that surgeon made spacers tend to fail in a more “agressive“ way than preformed ones. This might be based on the “ratherinhomogenous“ way of cement mixing and delivery during spacer preparation and application into the situs. In addition, the combination of high dose antibiotics mixing might counteract the mechanical stabilty. The higher fracture rate might be due to the absence of a metal endo-skeleton of sufficient strength.
The exception is the PROSTALAC system, which has not had a single report of implant fracture due to the robust nature of its metal endoskleton and its sophisticated molding system. It should also be noted that while not statistically significant, the preformed spacers had a higher dislocations rate. These preformed spacers may limit the ability of the surgeon to control leg length and offset thus potentially putting patients at risk for instability in the interim stage.
Our study has important limitations. First of all, as with all systematic reviews and/or meta-analyses, the study is limited by the inherent weaknesses of the component studies. Most of the included studies are Level-IV studies characterized by retrospective study designs, limited population sizes, and short- to medium-term follow-up time intervals. Interestingly, none of the included studies were level I or level II studies. Possible reasons of not existing of studies with high level of evidence could be that the technique is often based on surgeons preference and the general availability of pre-formed spacers in each country/region and the definition of clear contraindication for articulating spacers.
It must also be noted that, we did not control for confounding variables such as comorbidities, body mass index and the infecting organism. Although our study showed a significantly higher rate of fracture for the surgeon made spacer, it should be noted that there can be significant variation in the way the spacers are fabricated among surgeons and this study could not account for those technical differences. For example, some surgeons may choose to utilize the metal endoskeleton to reinforce the cement spacer, and this will minimize the fracture risk.
Despite the drawbacks, our study makes important comp-arisons between preformed and surgeon-made articulating hip spacers regarding infection control, functional outcomes following reimplantation and complication rates.
In conclusion, our results present no differences regarding infection control and functional outcome at latest follow-up using preformed or surgeon-made articulating hip spacers in the treatment of PJI. However, it must be noted that the spacer fracture rate was higher using intraoperative surgeon-made spacers. Nevertheless, the use of more costly preformed articulating hip spacers should be scrutinized.
Furthermore, our study shows the necessity of larger prospective, randomized controlled trials to further elucidate the superiority for periprosthetic joint infection following total hip arthroplasty.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
- 1.Haaker R., Senge A., Krämer J., Rubenthaler F. [Osteomyelitis after endoprostheses]. Orthopade. 2004;33(4):431–438. doi: 10.1007/s00132-003-0624-x. [DOI] [PubMed] [Google Scholar]
- 2.D’Angelo F., Negri L., Zatti G., Grassi F.A. Two-stage revision surgery to treat an infected hip implant. A comparison between a custom-made spacer and a pre-formed one. Chir. Organi Mov. 2005;90(3):271–279. [PubMed] [Google Scholar]
- 3.D’Angelo F., Negri L., Binda T., Zatti G., Cherubino P. The use of a preformed spacer in two-stage revision of infected hip arthroplasties. Musculoskelet. Surg. 2011;95(2):115–120. doi: 10.1007/s12306-011-0128-5. [DOI] [PubMed] [Google Scholar]
- 4.Degen R.M., Davey J.R., Davey J.R., Howard J.L., McCalden R.W., Naudie D.D. Does a prefabricated gentamicin-impregnated, load-bearing spacer control periprosthetic hip infection? Clin. Orthop. Relat. Res. 2012;470(10):2724–2729. doi: 10.1007/s11999-012-2350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gil Gonzalez S., Marqués López F., Rigol Ramon P., Mestre Cortadellas C., Cáceres Palou E., León García A. Two-stage revision of hip prosthesis infection using a hip spacer with stabilising proximal cementation. Hip Int. 2010;20(Suppl. 7):S128–S134. doi: 10.5301/HIP.2010.1374. [DOI] [PubMed] [Google Scholar]
- 6.Magnan B., Regis D., Biscaglia R., Bartolozzi P. Preformed acrylic bone cement spacer loaded with antibiotics: use of two-stage procedure in 10 patients because of infected hips after total replacement. Acta Orthop. Scand. 2001;72(6):591–594. doi: 10.1080/000164701317269003. [DOI] [PubMed] [Google Scholar]
- 7.Neumann D.R., Hofstaedter T., List C., Dorn U. Two-stage cementless revision of late total hip arthroplasty infection using a premanufactured spacer. J. Arthroplasty. 2012;27(7):1397–1401. doi: 10.1016/j.arth.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Pattyn C., De Geest T., Ackerman P., Audenaert E. Preformed gentamicin spacers in two-stage revision hip arthroplasty: functional results and complications. Int. Orthop. 2011;35(10):1471–1476. doi: 10.1007/s00264-010-1172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pignatti G., Nitta S., Rani N., Dallari D., Sabbioni G., Stagni C., Giunti A. Two stage hip revision in periprosthetic infection: results of 41 cases. Open Orthop. J. 2010;4:193–200. doi: 10.2174/1874325001004010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romanò C.L., Romanò D., Albisetti A., Meani E. Preformed antibiotic-loaded cement spacers for two-stage revision of infected total hip arthroplasty. Long-term results. Hip Int. 2012;22(Suppl. 8):S46–S53. doi: 10.5301/HIP.2012.9570. [DOI] [PubMed] [Google Scholar]
- 11.Romanò C.L., Romanò D., Logoluso N., Meani E. Septic versus aseptic hip revision: how different? J. Orthop. Traumatol. 2010;11(3):167–174. doi: 10.1007/s10195-010-0106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romanò C.L., Romanò D., Meani E., Logoluso N., Drago L. Two-stage revision surgery with preformed spacers and cementless implants for septic hip arthritis: a prospective, non-randomized cohort study. BMC Infect. Dis. 2011;11:129. doi: 10.1186/1471-2334-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howick J., Chalmers I., Glasziou P., et al. The Oxford 2011 levels of evidence. Available from: http://wwwcebmnet/indexaspx? o=5653 . 2011.
- 14.Ben-Lulu O., Farno A., Gross A.E., Backstein D.J., Kosashvili Y., Safir O.A. A modified cement spacer technique for infected total hip arthroplasties with significant bone loss. J. Arthroplasty. 2012;27(4):613–619. doi: 10.1016/j.arth.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Biring G.S., Kostamo T., Garbuz D.S., Masri B.A., Duncan C.P. Two-stage revision arthroplasty of the hip for infection using an interim articulated Prostalac hip spacer: a 10- to 15-year follow-up study. J. Bone Joint Surg. Br. 2009;91(11):1431–1437. doi: 10.1302/0301-620X.91B11.22026. [DOI] [PubMed] [Google Scholar]
- 16.Borowski M., Kusz D., Wojciechowski P., Cieliński Ł. Treatment for periprosthetic infection with two-stage revision arthroplasty with a gentamicin loaded spacer. The clinical outcomes. Ortop. Traumatol. Rehabil. 2012;14(1):41–54. doi: 10.5604/15093492.976900. [DOI] [PubMed] [Google Scholar]
- 17.Dairaku K., Takagi M., Kawaji H., Sasaki K., Ishii M., Ogino T. Antibiotics-impregnated cement spacers in the first step of two-stage revision for infected totally replaced hip joints: report of ten trial cases. J. Orthop. Sci. 2009;14(6):704–710. doi: 10.1007/s00776-009-1406-z. [DOI] [PubMed] [Google Scholar]
- 18.Diwanji S.R., Kong I.K., Park Y.H., Cho S.G., Song E.K., Yoon T.R. Two-stage reconstruction of infected hip joints. J. Arthroplasty. 2008;23(5):656–661. doi: 10.1016/j.arth.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Durbhakula S.M., Czajka J., Fuchs M.D., Uhl R.L. Spacer endoprosthesis for the treatment of infected total hip arthroplasty. J. Arthroplasty. 2004;19(6):760–767. doi: 10.1016/j.arth.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 20.Fei J., Liu G.D., Yu H.J., Zhou Y.G., Wang Y. Antibiotic-impregnated cement spacer versus antibiotic irrigating metal spacer for infection management after THA. Orthopedics. 2011;34(3):172. doi: 10.3928/01477447-20110124-04. [DOI] [PubMed] [Google Scholar]
- 21.Fink B., Grossmann A., Fuerst M., Schäfer P., Frommelt L. Two-stage cementless revision of infected hip endoprostheses. Clin. Orthop. Relat. Res. 2009;467(7):1848–1858. doi: 10.1007/s11999-008-0611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleck E.E., Spangehl M.J., Rapuri V.R., Beauchamp C.P. An articulating antibiotic spacer controls infection and improves pain and function in a degenerative septic hip. Clin. Orthop. Relat. Res. 2011;469(11):3055–3064. doi: 10.1007/s11999-011-1903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann A.A., Goldberg T.D., Tanner A.M., Cook T.M. Ten-year experience using an articulating antibiotic cement hip spacer for the treatment of chronically infected total hip. J. Arthroplasty. 2005;20(7):874–879. doi: 10.1016/j.arth.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh P.H., Chen L.H., Chen C.H., Lee M.S., Yang W.E., Shih C.H. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J. Trauma. 2004;56(6):1247–1252. doi: 10.1097/01.TA.0000130757.53559.BF. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh P.H., Shih C.H., Chang Y.H., Lee M.S., Shih H.N., Yang W.E. Two-stage revision hip arthroplasty for infection: comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J. Bone Joint Surg. Am. 2004;86-A(9):1989–1997. [PubMed] [Google Scholar]
- 26.Incavo S.J., Russell R.D., Mathis K.B., Adams H. Initial results of managing severe bone loss in infected total joint arthroplasty using customized articulating spacers. J. Arthroplasty. 2009;24(4):607–613. doi: 10.1016/j.arth.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Isiklar Z.U., Demirörs H., Akpinar S., Tandogan R.N., Alparslan M. Two-stage treatment of chronic staphylococcal orthopaedic implant-related infections using vancomycin impregnated PMMA spacer and rifampin containing antibiotic protocol. Bull. Hosp. Jt. Dis. 1999;58(2):79–85. [PubMed] [Google Scholar]
- 28.Jahoda D., Sosna A., Landor I., Vavrík P., Pokorný D. [A cannulated articulating spacer--a functional implant for treatment of infected hip joint prostheses]. Acta Chir. Orthop. Traumatol. Cech. 2004;71(2):73–79. [PubMed] [Google Scholar]
- 29.Jung J., Schmid N.V., Kelm J., Schmitt E., Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int. J. Med. Sci. 2009;6(5):265–273. doi: 10.7150/ijms.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kent M., Rachha R., Sood M. A technique for the fabrication of a reinforced moulded articulating cement spacer in two-stage revision total hip arthroplasty. Int. Orthop. 2010;34(7):949–953. doi: 10.1007/s00264-009-0847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koo K.H., Yang J.W., Cho S.H., Song H.R., Park H.B., Ha Y.C., Chang J.D., Kim S.Y., Kim Y.H. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J. Arthroplasty. 2001;16(7):882–892. doi: 10.1054/arth.2001.24444. [DOI] [PubMed] [Google Scholar]
- 32.Leunig M., Chosa E., Speck M., Ganz R. A cement spacer for two-stage revision of infected implants of the hip joint. Int. Orthop. 1998;22(4):209–214. doi: 10.1007/s002640050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X.C., Zhou Y.G., Wang Y., Chen J.Y., Hao L.B., Li J.D., Dong J.Y., Lin F. [Antibiotic-loaded cement articulating spacer made by a self-made mold system in the treatment of the infected hip replacement]. Zhonghua Wai Ke Za Zhi. 2010;48(14):1050–1054. [PubMed] [Google Scholar]
- 34.Masri B.A., Panagiotopoulos K.P., Greidanus N.V., Garbuz D.S., Duncan C.P. Cementless two-stage exchange arthroplasty for infection after total hip arthroplasty. J. Arthroplasty. 2007;22(1):72–78. doi: 10.1016/j.arth.2006.02.156. [DOI] [PubMed] [Google Scholar]
- 35.McKenna P.B., O’Shea K., Masterson E.L. Two-stage revision of infected hip arthroplasty using a shortened post-operative course of antibiotics. Arch. Orthop. Trauma Surg. 2009;129(4):489–494. doi: 10.1007/s00402-008-0683-x. [DOI] [PubMed] [Google Scholar]
- 36.Morshed S., Huffman G.R., Ries M.D. Extended trochanteric osteotomy for 2-stage revision of infected total hip arthroplasty. J. Arthroplasty. 2005;20(3):294–301. doi: 10.1016/j.arth.2004.09.060. [DOI] [PubMed] [Google Scholar]
- 37.Peng K.T., Hsu W.H., Hsu R.W. Improved antibiotic impregnated cement prosthesis for treating deep hip infection: a novel design using hip compression screw. J. Arthroplasty. 2010;25(8):1304–1306. doi: 10.1016/j.arth.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Peng K.T., Kuo L.T., Hsu W.H., Huang T.W., Tsai Y.H. The effect of endoskeleton on antibiotic impregnated cement spacer for treating deep hip infection. BMC Musculoskelet. Disord. 2011;12:10. doi: 10.1186/1471-2474-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romanò C.L., Romanò D., Logoluso N., Meani E. Long-stem versus short-stem preformed antibiotic-loaded cement spacers for two-stage revision of infected total hip arthroplasty. Hip Int. 2010;20(1):26–33. doi: 10.1177/112070001002000104. [DOI] [PubMed] [Google Scholar]
- 40.Takahira N., Itoman M., Higashi K., Uchiyama K., Miyabe M., Naruse K. Treatment outcome of two-stage revision total hip arthroplasty for infected hip arthroplasty using antibiotic-impregnated cement spacer. J. Orthop. Sci. 2003;8(1):26–31. doi: 10.1007/s007760300004. [DOI] [PubMed] [Google Scholar]
- 41.Toulson C., Walcott-Sapp S., Hur J., Salvati E., Bostrom M., Brause B., Westrich G.H. Treatment of infected total hip arthroplasty with a 2-stage reimplantation protocol: update on “our institution’s” experience from 1989 to 2003. J. Arthroplasty. 2009;24(7):1051–1060. doi: 10.1016/j.arth.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Whittaker J.P., Warren R.E., Jones R.S., Gregson P.A. Is prolonged systemic antibiotic treatment essential in two-stage revision hip replacement for chronic Gram-positive infection? J. Bone Joint Surg. Br. 2009;91(1):44–51. doi: 10.1302/0301-620X.91B1.20930. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto K., Miyagawa N., Masaoka T., Katori Y., Shishido T., Imakiire A. Clinical effectiveness of antibiotic-impregnated cement spacers for the treatment of infected implants of the hip joint. J. Orthop. Sci. 2003;8(6):823–828. doi: 10.1007/s00776-003-0722-y. [DOI] [PubMed] [Google Scholar]
- 44.Younger A.S., Duncan C.P., Masri B.A., McGraw R.W. The outcome of two-stage arthroplasty using a custom-made interval spacer to treat the infected hip. J. Arthroplasty. 1997;12(6):615–623. doi: 10.1016/S0883-5403(97)90133-9. [DOI] [PubMed] [Google Scholar]
- 45.Zou Y.G., Feng Z.Q., Xing J.S., Peng Z.H., Luo X. [Two-stage revision for treatment of periprosthetic infection following hip arthroplasty]. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31(4):690–693. [PubMed] [Google Scholar]
- 46.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]