Abstract
The present study developed Galleria mellonella and murine infection models for the study of Trichosporon infections. The utility of the developed animal models was demonstrated through the assessment of virulence and antifungal efficacy for 7 clinical isolates of Trichosporon asahii, T. asteroides and T. inkin. The susceptibility of the Trichosporon isolates to several common antifungal drugs was tested in vitro using the broth microdilution and the E-test methods. The E-test method depicted a lower minimal inhibitory concentration (MIC) for amphotericin and a slightly higher MIC for caspofungin, while MICs observed for the azoles were different but comparable between both methods. All three Trichosporon species established infection in both the G. mellonella and immunosuppressed murine models. Species and strain dependent differences were observed in both the G. mellonella and murine models. T. asahii was demonstrated to be more virulent than the other 2 species in both animal hosts. Significant differences in virulence were observed between strains for T. asteroides in the murine model. In both animal models, fluconazole and voriconazole were able to improve the survival of the animals compared to the untreated control groups infected with any of the 3 Trichosporon species. In G. mellonella, amphotericin was not able to reduce mortality in any of the 3 species. In contrast, amphotericin was able to reduce murine mortality in the T. asahii or T. inkin models, respectively. Hence, the developed animal infection models can be directly applicable to the future deeper investigation of the molecular determinants of Trichosporon virulence and antifungal resistance.
Keywords: antifungal, experimental infection, Galleria mellonella, murine model, Trichosporon
Abbreviations
- AMB
Amphotericin B
- CFG
Caspofungin
- CLSI
Clinical Laboratory Standards Institute
- FLC
Fluconazole
- GMS
Gomori methenamine silver
- ITZ
Itraconazole
- MIC
Minimal Inhibitory Concentration
- PBS
Phosphate Buffered Saline
- PSC
Posaconazole
- SDA
Sabouraud Dextrose Agar
- VRC
Voriconazole
Introduction
Trichosporon species are anamorphous yeast-like basidiomycetes that have a worldwide distribution and can cause a range of opportunistic infections, including superficial infections such as white piedra in immunocompetent patients, plus mucosa-associated and systemic infections in immunocompromised patients.1 Trichosporon spp. can be found in soil, decomposing wood, air, rivers, lakes, seawater, cheese, scarab beetles, bird droppings, bats, pigeons, and cattle.2 They belong to the human microbiome, as colonizers of the gastrointestinal and oral cavities, male perigenital skin (scrotal, perianal, and inguinal sites of the body) and temporarily inhabiting the respiratory tract and skin.3-12 It is not clear how Trichosporonosis can be acquired, but the following possibilities can be envisaged: (i) poor hygiene, (ii) bathing in contaminated water, (iii) sexual transmission, (iv) hair humidity and the length of the scalp hair (more specifically in the case of acquiring white piedra), (v) gastrointestinal colonization and further translocation throughout the gut (deep-seated infections), and (vi) exogenously acquired through a percutaneously inserted intravascular catheter via colonized skin.13-18
In spite of the fact that Trichosporon spp are probably the second or third most common non-Candida yeast infections causing invasive disease in patients with hematological cancer, there are few reports related to virulence factors of this genus.2,19-21 The main causes of infection by invasive Trichosporon spp. are central and vesical venous catheters, and peritoneal catheter-related devices. Since these organisms are skilled at adhering to these devices and apt at forming biofilms, they are able to escape from antifungal drugs and host immune responses.2,22 It has been speculated that the secretion of proteases and phospholipases are important virulence factors for scavenging nutrient inside the host.23 These pathogens are also able to produce glucuronoxylomannan (GXM) in their cell walls, similarly to Cryptococcus neoformans24,25 and it is possible this polysaccharide may help to attenuate phagocytosis by host immune cells.25 However, there is no genetic evidence about the functionality of these virulence factors.
In recent years the number of cases of Trichosporon infections has increased, and in the cases of systemic infections the prognosis is very poor.26 Three of the most common species isolated from a clinical setting are T. asahii, T. asteroides and T. inkin. Fatal disseminated infections are predominately associated with T. asahii,27,28 while superficial infections are more frequently associated with T. asteroides, which can also occasionally establish disseminated infections.26,28 T. inkin is commonly isolated from cases of white piedra, while rarely being reported to cause deep-seated infections.29,30 Traditional antifungal drugs commonly used to treat other fungal infections, such as amphotericin B and fluconazole, usually are not very efficient against members of the genus Trichosporon, including the 3 aforementioned species.31,32 Resistance to current antifungal therapies therefore contributes to the poor survival rate of immunocompromised patients with Trichosporonosis. Subsequently, a better understanding of the virulence mechanisms deployed by Trichosporon species, and molecular determinants for antifungal resistance, is urgently required to improve the efficacy of current treatments. In order to develop more effective antifungal therapies, new in vitro and in vivo assays that permit large scale molecular studies are required.
The present study evaluated the in vitro activity of multiple antifungal agents with differing modes of action, including azoles fluconazole (FLC), itraconazole (ITZ), posaconazole (PSC) and voriconazole (VRC), one echinocandin, caspofungin (CFG) and the polyene amphotericin B (AMB). Two different in vitro antifungal efficacy assays were used against 7 clinical isolates of T. asahii, T. asteroides and T. inkin. Previous to our study, there were few studies reporting animal models for Trichosporonosis.33-39 We extended these studies by developing Galleria mellonella and murine models of Trichosporon infection and used them to further assess the efficacy of the 3 antifungal therapies, AMB, FLC, and VRC, in vivo. The combination of these in vitro and in vivo assays represents the framework for use in large scale investigations that study the molecular determinants of virulence and antifungal resistance.
Results
In vitro antifungal efficacy evaluation
The CLSI broth microdilution and the E-test methods for determining the minimum inhibitory concentration (MIC) were used to assess the efficacy of FLC, ITZ, PSC, VRC, CFG and AMB against the 7 Trichosporon isolates (Tables 1 and 2). The MICs obtained with the CLSI method were in agreement with those obtained by Chagas-Neto et al.,26 which tested the same T. asahii and T. asteroides strains. The E-test method depicted a lower MIC for AMB and a slightly higher MIC for CFG, while MICs observed for the azoles were different but comparable between both methods. These variations between methods are in accordance with the results obtained by Metin et al.40 which also tested the susceptibility of Trichosporon isolates.
Table 1.
In vitro antifungal activity of FLC, ITZ, PSC, VRC, AMB and CFG against all Trichosporon strains using the CLSI broth microdilution method
| Fluconazole | Itraconazole | Posaconazole | Voriconazole | Amphotericin B | Caspofungin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) |
MIC (μg/ml) |
MIC (μg/ml) |
MIC (μg/ml) |
MICa (μg/ml) |
MIC (μg/ml) |
|||||||
| Strain | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h |
| T. asahii 04 | 1.0 | 1.0 | 0.06 | 0.06 | 0.25 | 0.25 | <0.03 | 0.06 | 1 | 8 | 8 | 16 |
| T. asahii05 | 0.5 | 1.0 | 0.06 | 0.06 | 0.125 | 0.125 | <0.03 | <0.03 | 1 | 8 | 16 | 16 |
| T. asahii07 | 1.0 | 1.0 | 0.06 | 0.06 | 0.125 | 0.125 | <0.03 | <0.03 | 1 | 1 | 16 | 16 |
| T. asteroides01 | 0.5 | 0.5 | 0.06 | 0.06 | 0.125 | 0.125 | <0.03 | <0.03 | 1 | 2 | 4 | 8 |
| T. asteroides06 | 0.5 | 0.5 | 0.06 | 0.06 | 0.125 | 0.25 | <0.03 | <0.03 | 1 | 8 | 16 | 16 |
| T. asteroides13 | 0.5 | 0.5 | 0.06 | 0.06 | 0.125 | 0.125 | <0.03 | <0.03 | 1 | 4 | 16 | 16 |
| T. inkin | 1.0 | 2.0 | <0.03 | 0.125 | 1.0 | 1.0 | <0.03 | 0.06 | 0.5 | 2 | 8 | 8 |
For amphotericin B the MIC corresponded to the concentration that produced a complete inhibition of growth (100%). For the rest of the drugs, the MIC corresponded to a growth inhibition of 50%.
Table 2.
In vitro antifungal activity of, FLC, ITZ, PSC, VRC, AMB and CFG against the 7 strains of Trichosporon using the E-test method
| Fluconazole | Itraconazole | Posaconazole | Voriconazole | Amphotericin B | Caspofungin | |
|---|---|---|---|---|---|---|
| Strain | MIC (μg/ml) | MIC (μg/ml) | MIC (μg/ml) | MIC (μg/ml) | MICa (μg/ml) | MIC (μg/ml) |
| T. asahii04 | 4.0 | 0.38 | 0.25 | 0.064 | 0.38 | >32 |
| T. asahii05 | 0.75 | 0.064 | 0.47 | 0.032 | 2.0 | >32 |
| T. asahii07 | 0.75 | 2.0 | 0.38 | 0.064 | 0.38 | >32 |
| T. asteroides01 | 1.5 | 0.047 | 0.47 | 0.032 | 0.25 | >32 |
| T. asteroides 06 | 0.75 | 1.5 | 0.25 | 0.047 | 1.0 | >32 |
| T. asteroides 13 | 0.5 | 0.5 | 0.064 | 0.023 | 0.19 | >32 |
| T. inkin | 0.38 | 1.0 | 0.19 | 0.064 | 0.064 | >32 |
For amphotericin B the MIC corresponded to the concentration that produced a complete inhibition of growth (100%). For the rest of the drugs, the MIC corresponded to an eminent growth inhibition (80%).
Galleria mellonella virulence model
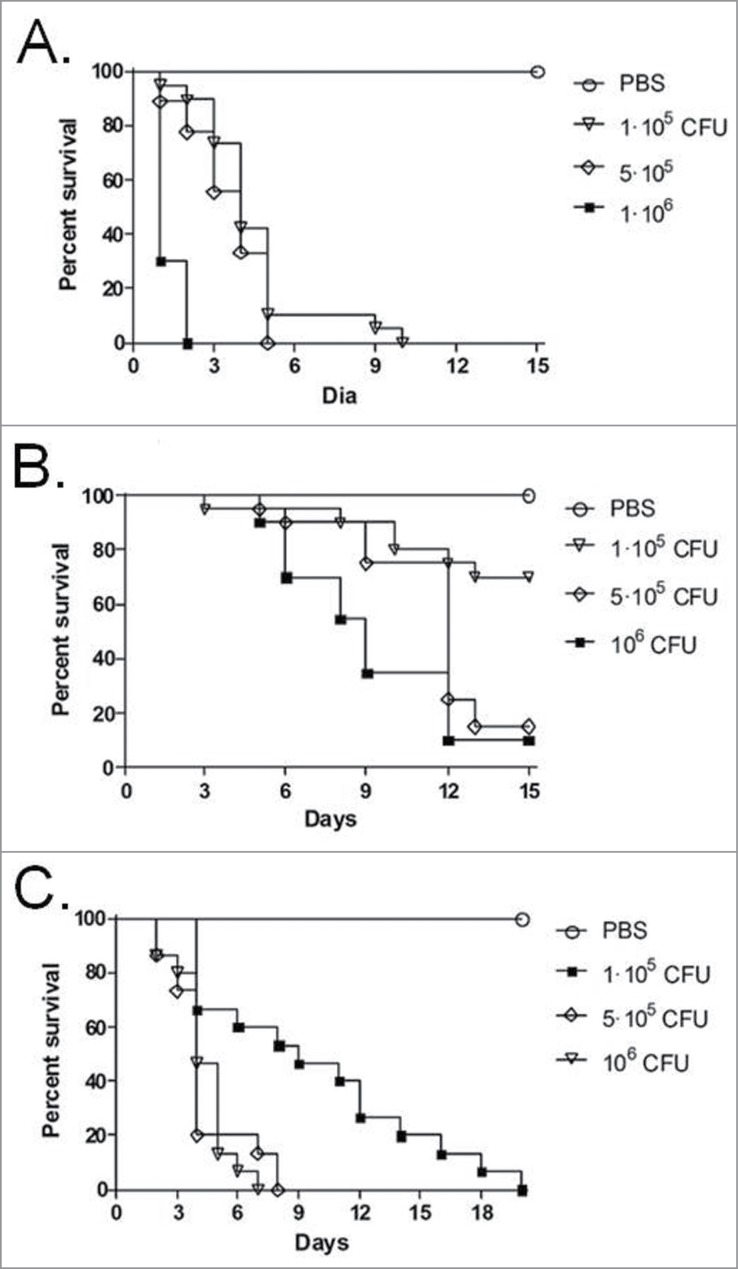
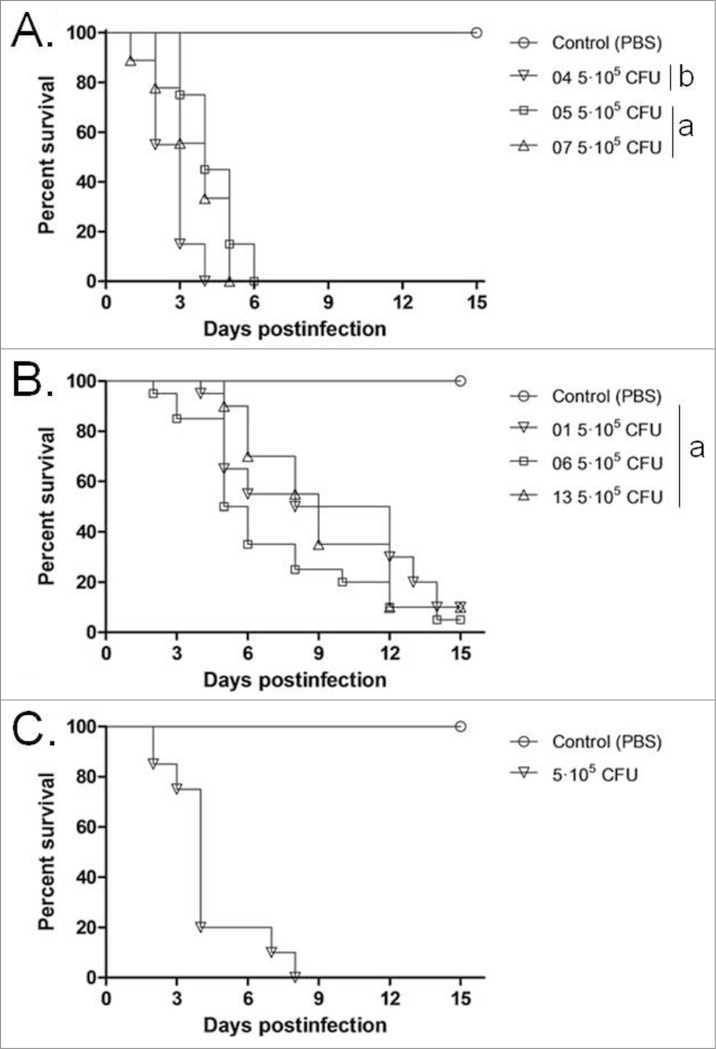
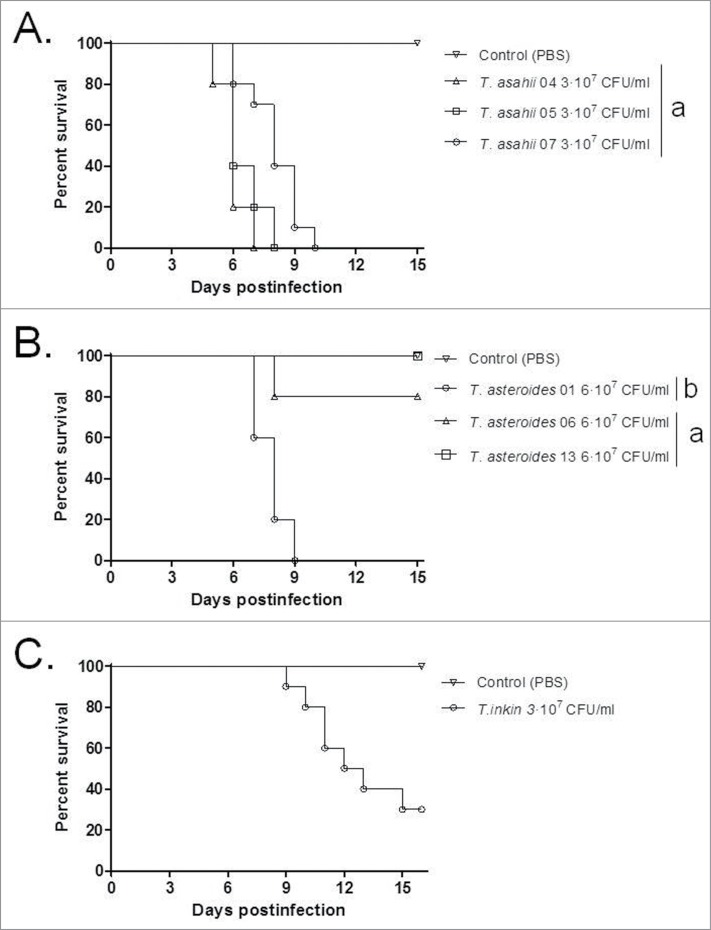
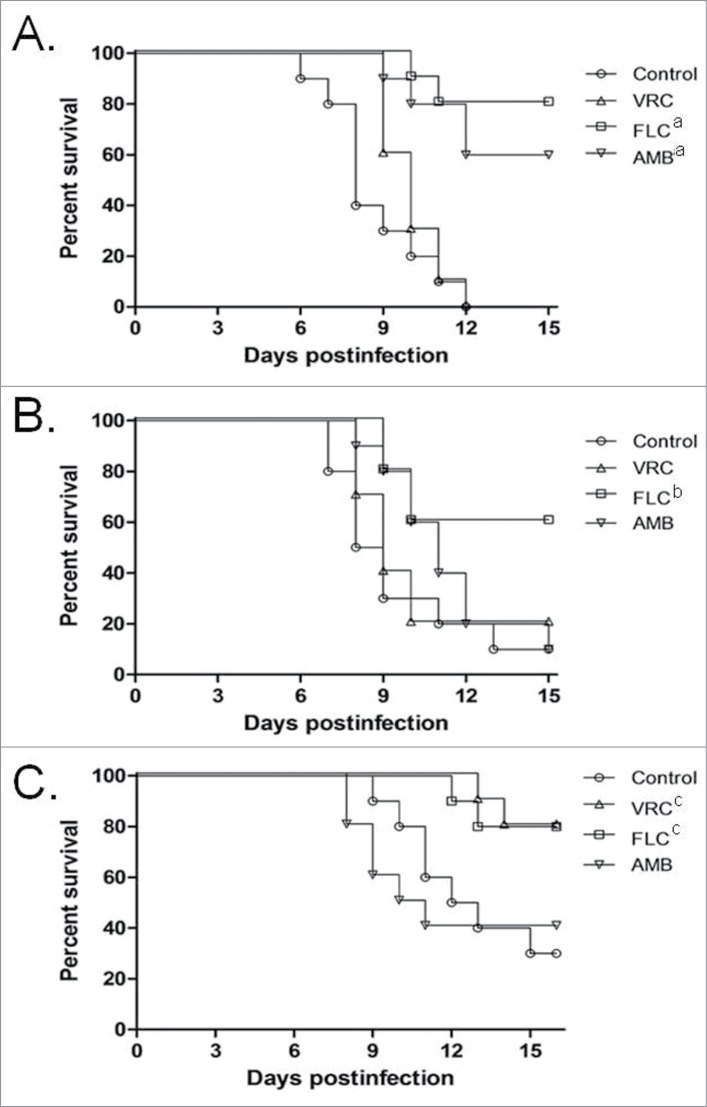
Inocula from one strain of each species, ranging in concentration between 1 × 105 and 1 × 106 CFU/larva, were used to investigate the virulence of Trichosporon against G. mellonella (Fig. 1). An inoculum of 5 × 105 CFU/larva was considered to be the optimal dose for producing acute infection, with 80 to 100% of larvae dying within 15 days post-infection and was subsequently used to compare all the 7 strains (Fig. 2). In this model, T. asahii was demonstrated to be the most virulent species, with the 3 strains killing all the larvae within 6 days. Increasing the inoculum of the T. asahii 07 strain to 1 × 106 CFU killed all larvae within 3 d. T. inkin was also able to kill all the infected larvae within 8 days after infection, but no significant increase in mortality rate was observed with the larger inoculum. T. asteroides proved to be the least virulent species in the insect virulence model, with none of the tested strains being able to attain a mortality rate of 100% within 15 days post-infection, even when applying a larger inoculum of 1 × 106 CFU for the 01 strain (data not shown). Histopathological sections of the larvae show the high amount of fungal elements present in the larvae 2 days after infection for all 3 species (Fig. 3).
Figure 1.
Determination of the cell concentrations for mortality experiments of G. mellonella larvae infected with T. asahii strain 07 (A), T. asteroides strain 13 (B) or T. inkin (C).
Figure 2.
Cumulative mortality of G. mellonella larvae infected with T. asahii (A), T. asteroides (B) or T.inkin (C). The mean survival time was estimated by the Kaplan-Meier method and compared among groups using the log-rank test (a, P > 0.05, andb, P < 0.05). All groups are different from the control (PBS).
Figure 3.
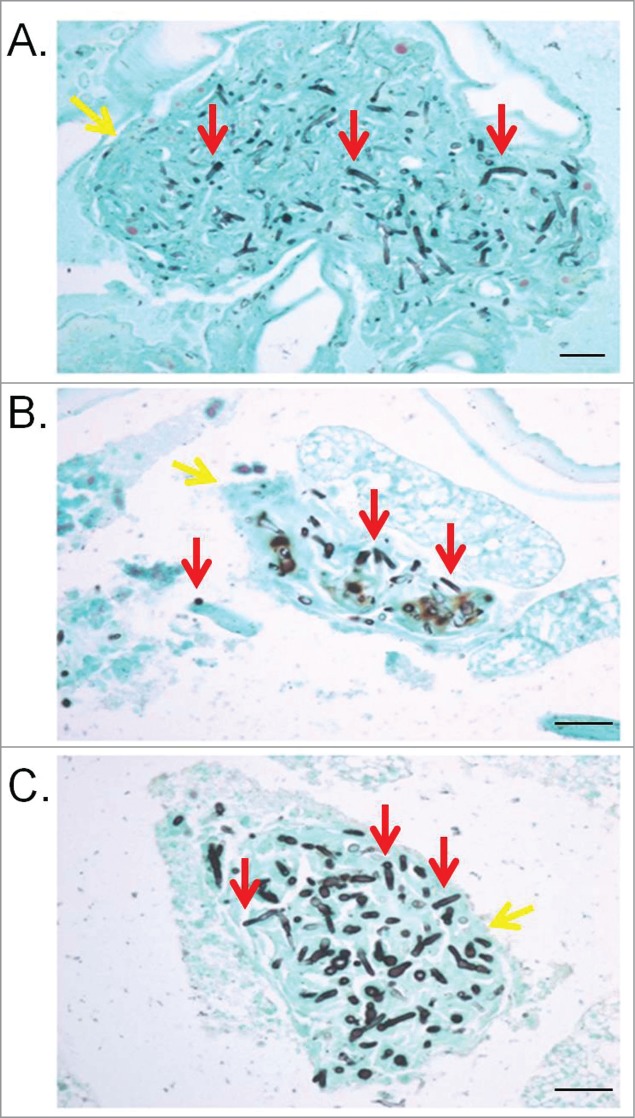
G. mellonella internal structures affected by trichorporonosis caused by T. asahii 07 (A), T. asteroides 01 (B) or T. inkin (C) 2 days postinfection. The red arrows point the fungal cells and the yellow arrows point G. mellonella tissues. GMS stain. Bars, 20 μm. Magnification, x400.
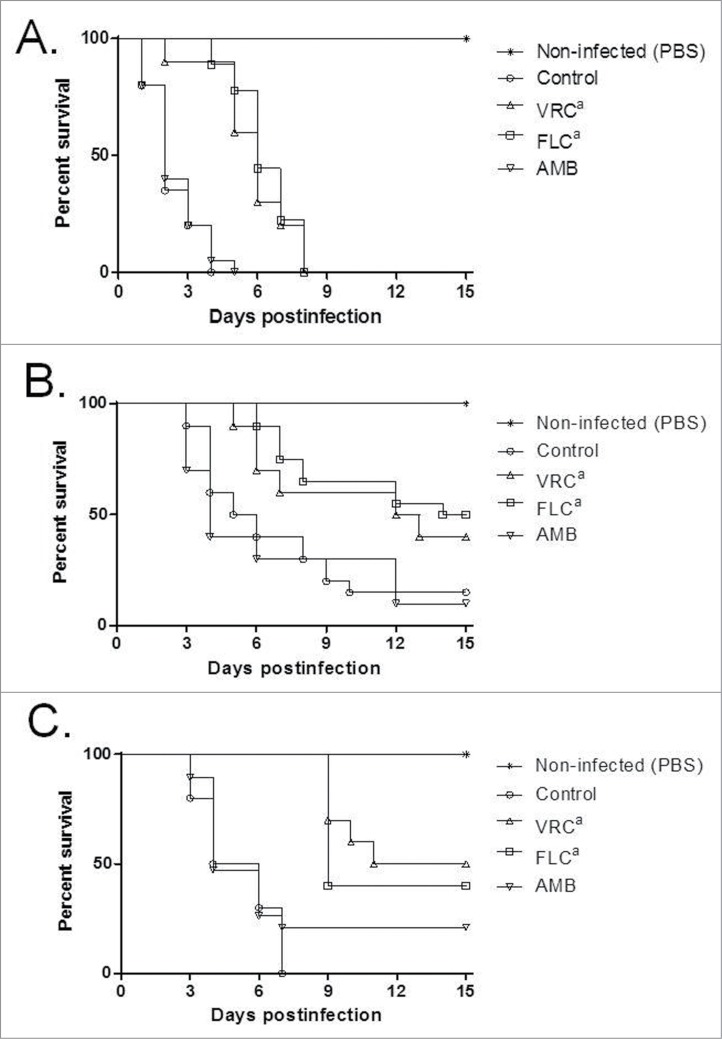
The effect of the AMB, FLC and VRC on G. mellonella mortality rate was assessed by using previously defined drug concentrations.41,42 A high dose of FLC or VRC was able to improve the survival of the animals compared to the untreated control groups infected with any of the 3 Trichosporon species, while AMB was not able to reduce mortality in any of the 3 species (Fig. 4).
Figure 4.
Effects of antifungal treatments on cumulative mortality of G. mellonella larvae infected with T. asahii strain 07 (A), T. asteroides strain 01 (B) or T. inkin (C). Single doses of antifungal drugs were administered 4 hours after infection in a volume of 10 μl. Control groups received 10 μl of PBS 4 hours postinfection. Drug concentrations were: AMB, amphotericin B deoxycholate at 0.5 mg/kg; FLC, Fluconazole at 20 mg/kg; and VRC, voriconazole at 15 mg/kg (a, P < 0.05 versus control and amphotericin B).
Murine virulence model
The different Trichosporon strains and species were evaluated in a murine virulence model. T. asahii once again showed greater virulence compared to the other 2 species, which both required a larger inoculum or stronger immunosuppression to attain a significant mortality rate (Fig. 5). The three T. asteroides strains demonstrated a range of virulence in the murine model, when using an inoculum concentration double that of T. asahii, including T. asteroides strain 13 which was unable to kill the immunosuppressed mice. The single T. inkin strain tested was unable to kill immunosuppressed mice when using the same inoculum concentrations as T. asahii and was only able to attain a 60% mortality rate when using the same inoculum as the one used for T. asteroides strains (6 × 107 CFU/ml, data not shown). A larger inoculum of T. inkin (1 × 108 CFU/ml) caused the death of all the mice within hours after infection, possibly due to a physical obstruction of capillaries.43 To avoid the immediate death of the animals, a stronger immunosuppression program and the same inoculum as T. asahii were used (3 × 107 CFU/ml), attaining a mortality rate of approximately 70%, and in turn providing a suitable model for evaluating the efficacy of antifungal agents in vivo. One strain of each species was subsequently selected to evaluate the efficacy of antifungal agents in vivo, T. asahii strain 07, T. asteroides strain 01 and the single T. inkin strain. Histological studies of the 3 selected strains revealed conidial and hyphal elements for all 3 Trichosporon species diffusely infiltrated the kidneys of the animals (Fig. 6).
Figure 5.
Cumulative mortality of mice infected with different strains of T. asahii, T. asteroides and the single one of T. inkin. Mice infected with T. inkin received an additional dose of 5-fluorouracil on day 5 after infection. The mean survival time was estimated by the Kaplan-Meier method and compared among groups using the log-rank test (a, P > 0.05, andb, P < 0.05).
Figure 6.
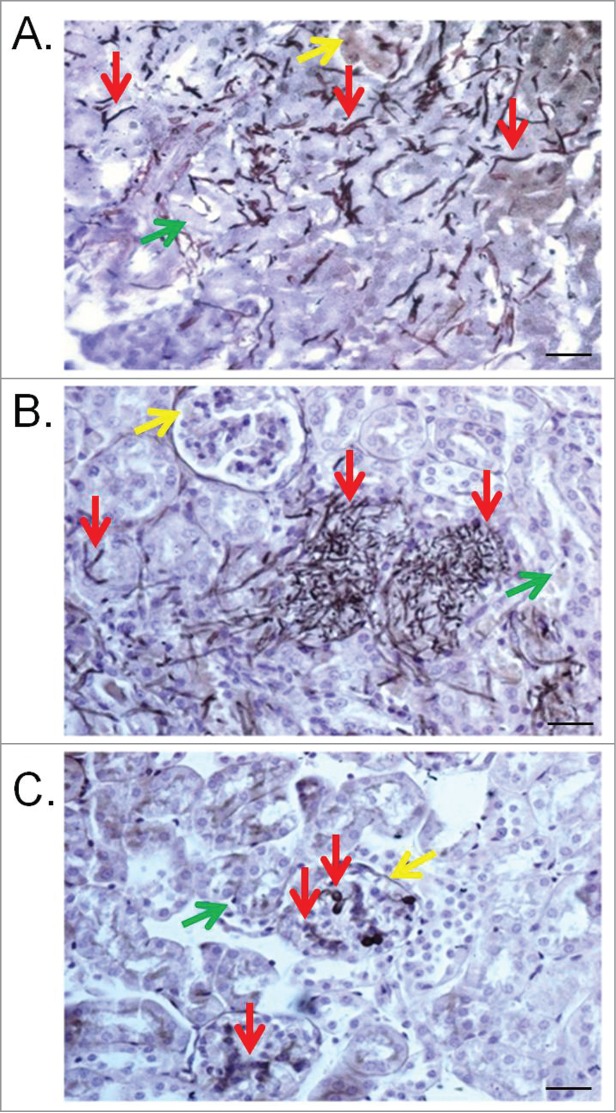
Kidney lesions in the murine model caused by T. asahii 07 (A), T. asteroides 01 (B) or T. inkin (C) infections 6 days after challenge. Kidney transversal sections showing hyphae, blastoconidia and arthroconidia (red arrows) in renal tubules (green arrows) and some of them in the glomerular structure (yellow arrows). GMS and hematoxylin stain. Bars, 20 μm. Magnification, x400.
The effect of AMB, FLC and VRC on murine mortality rate was assessed (Fig. 7). A high dose of FLC was able to improve the survival of the animals compared to the untreated control groups infected with any of the 3 Trichosporon species, while AMB and VRC were able to reduce mortality in the T. asahii or T. inkin models, respectively.
Figure 7.
Effects of antifungal treatments on cumulative mortality of mice infected with T. asahii 07 3 × 107 CFU/ml (A), T. asteroides 01 6 × 107 CFU/ml (B) or T. inkin 3 × 107 CFU/ml (C). AMB, amphotericin B deoxycholate at 1.5 mg/kg/day. FLC, fluconazole at 80 mg/kg/day. VRC, voriconazole at 60 mg/kg/day. a, P < 0.05 vs. control. The mean survival time was estimated by the Kaplan-Meier method and compared among groups using the log-rank test (a, P < 0.05, compared to the control and VRC;b, P < 0.05, compared to the control and VRC;c, P < 0.05, compared to the control and AMB).
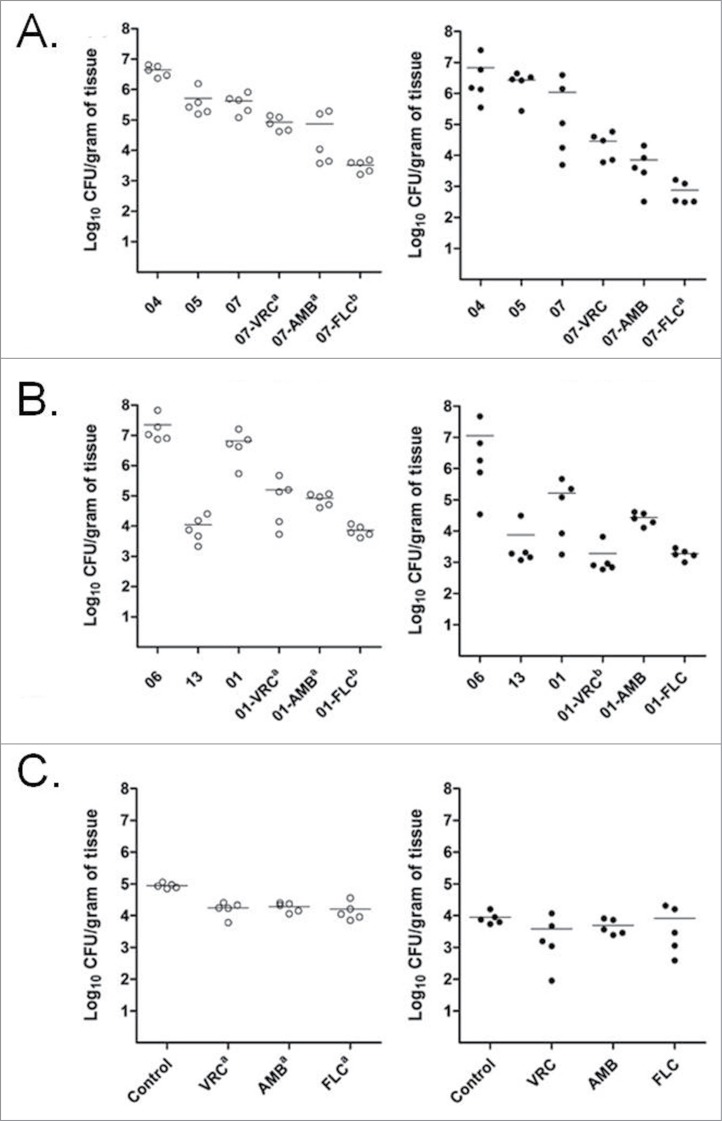
Subsequently, the fungal burden within the kidneys and spleen of the infected mice was determined. All the 3 antifungal treatments showed some degree of reduction in fungal burden against the 3 Trichosporon species. These reductions were statistically significant mostly on kidneys (Fig. 8). All three antifungal treatments reduced the fungal burden in the kidneys of mice infected with any of the 3 species studied, but only FLC and VRC reduced the fungal burned in the spleens infected with T. asahii and T. asteroides respectively. FLC was more effective than VRC at reducing T. asahii (Fig. 8A) and T. asteroides (Fig. 8B) burden in the kidneys, while VRC was more effective than AMB in the case of T. asteroides infected spleens (Fig. 8B). Fungal burden reductions were more modest in the spleens of infected animals. None of 3 antifungals significantly reduced the fungal burden in spleens of mice infected with T. inkin.
Figure 8.
Effects of the antifungal treatments on tissue burden of T. asahii (A), T. asteroides (B) or T. inkin (C) in kidneys (○) and spleen () of mice. AMB, amphotericin B deoxycholate at 1.5 mg/kg/day. FLC, fluconazole at 80 mg/kg/day. VRC, voriconazole at 60 mg/kg/day. a, P < 0.05 versus control. b, P < 0.05 vs. control and versus one of the other treatments [i.e., FLC vs. VRC (A), FLC versus AMB and VRC vs. AMB (B)].
Discussion
Prior to the taxonomic rearrangement of the genus in the nineties,44 reports of the clinical importance of Trichosporon were scarce and inconsistent. Only recently, larger studies which utilized more accurate molecular approaches for species identification, started to shed light on the species-specific distribution, the importance of clinical manifestations and the antifungal susceptibilities, of Trichosporon.26,45 The established Candida breakpoints have been used as a reference to describe the susceptibility results obtained with Trichosporon,28,46,47 but unfortunately the availability of clinical and in vitro susceptibly data is not sufficient enough to clearly define guidelines for Trichosporon treatments or even susceptibility breakpoints. However, a common trend exists for the use of azoles, and specially VRC, rather than AMB or the echinocandins,2 although some clinical reports have described the efficacy of CAS against T. inkin when used solely or in combination with AMB.48,49
Over recent years several non-vertebrate animal models have emerged as an alternative to the mammalian models of fungal infection. These models represent a powerful tool to study fungal pathogenesis, the efficacy of antifungal compounds, and innate antifungal immunity.50 The advantages of these non-vertebrate models with respect to the classic mammalian models include the lower logistical and ethical constraints, plus the chance to design larger scale studies at low cost. The presented study developed a novel G. mellonella infection model for Trichosporon species, which can be used to assess the efficacy of new antifungal treatments or screening for new virulence factors using genetically modified strains. The results obtained with the G. mellonella model can be used to complement those obtained with the murine models. Hence, large scale non-vertebrate investigations can subsequently be used to refine the use of mammalian systems.
Few mammalian models have been developed to determine antifungal efficacies or virulence for Trichosporon species. Older studies that used the nomenclature T. beigelii do not give an insight on the differences between the redefined species.51 The only animal models currently developed are for T. asahii infections.52-54 The present study, developed murine models of infection for T. asteroides and T. inkin, comparable to the already established one for T. asahii. The combined use of the G. mellonella and murine Trichosporon infection models will prove valuable for the evaluation of new antifungal treatments, assisting in the development of more specific therapies for the treatment of trichosporonosis.
The delayed killing rate of G. mellonella larvae by T. asteroides and T. inkin compared to T. asahii correlated with the lower virulence of these 2 species in the murine model. A remarkable observation was the strain dependent virulence of T. asteroides, were 2 of the 3 strains were unable to yield a high mortality rate, which was in contrast to the high fungal burden in kidneys that was similar to that of T. asahii. These results are in agreement with several studies where clinical data suggest that T. asahii is more prone to cause deep-seated infections and in the case of disseminated infections results in more fatalities than T. asteroides or T. inkin.2,55 Interestingly, T. asteroides strain 06 and 13 are less virulent than T. asteroides strain 01. Recently, it was shown that T. asteroides strains 06 and 13 produce less biofilm than T. asteroides strain 01, and these differences could be related to the differences in virulence observed between these strains.22 We are currently sequencing the genome of these strains and comparison among them associated to functional studies can help to reveal why they have different virulence abilities.
The antifungal susceptibility results obtained in the present study were within the range previously described for these Trichosporon species.26,28 All strains were susceptible to the azole drugs and similar results were obtained using both the E-test and the microdilution methods. The in vitro antifungal sensitivity results correlate with the obtained reduction in mortality in the murine model, where both FLC and VRC demonstrated some degree of efficacy in the treatment of the disseminated Trichosporon infection. However, none of the tested antifungals completely cured the infection despite the usage of high drug doses. VRC presented lower MICs than FLC, but was not as effective at improving the survival of mice against 2 of the 3 Trichosporon species. This can be attributable to the inherent problems of testing VRC in the murine model where VRC levels in the serum decay rapidly even when administering grapefruit juice to the animals, which has been shown to increase the serum amounts of this drug up to therapeutic levels.56 Accordingly, in an alternative mammalian model, the guinea pig, VRC performed better against T. asahii infections even at a lower dose.57 In contrast, both VRC and FLC improved the G. mellonella survival infected with all 3 Trichosporon species. In the present study, AMB in general showed higher MICs than the azoles against all the stains tested, while overall its performance in the murine model was similar to that of VRC and slightly lower to that of FLC.
Similar to previous reports, the echinocandin CAS had almost no effect on any of the Trichosporon strains in vitro and its usage in the murine model was expected to be poorly informative. However, the usage of CAS or micafungin has yielded good results in both an experimental model and in clinical settings when used in combination with other drugs such as AMB and FLC.49,52 In this sense, and taking in account the modest results obtained with monotherapies in our study, and their scarce efficacy in clinical setting, the usage of combined therapies appears to be the most fitting strategy to improve the outcomes of disseminated Trichosporon infections.2
The recent sequencing of T. asahii genome and the soon to become available genome sequences of other Trichosporon species (Goldman et al., forthcoming) will provide a powerful tool for the dissection of the molecular virulence mechanisms deployed by Trichosporon species, while facilitating the identification of novel fungal targets for antifungal therapies to treat this kind of infection. Subsequently, the combination G. mellonella and murine assays developed in the presented study will be useful for the study of Trichosporon virulence and the future improvement of antifungal strategies for controlling trichosporonosis.
Materials and Methods
Strains
The three T. asahii strains (04,05 and 07) and 3 T. asteroides strains (01, 06 and 13) used in the study were all isolated from blood samples.26 The single T. inkin strain used was obtained from a case of white piedra. The isolates were stored at −80°C in Yeast extract-Peptone-Dextrose with glycerol, and prior to testing they were sub-cultured twice on Sabouraud dextrose agar (SDA) at 37°C.
In vitro susceptibility testing
The in vitro susceptibility of the Trichosporon strains to VRC, PSC, ITZ, FLC, AMB and CFG (Sigma-Aldrich Brasil Ltda., PZ0005, 32103, I6657, F8929, Y0000005, SML0425) was determined using a microdilution method following the CLSI guidelines for yeasts.25,51 The MICs of the echinocandins and azoles corresponded to prominent growth inhibition (approximately 50% inhibition relative to control growth) or a complete growth inhibition in the case of amphotericin B. All the strains were also tested using the E-test susceptibility method, according to the manufacturer's instructions for VRC, PSC, ITZ, FLC, AMB and CFG (bioMérieux SA, 532800, 532100, 525808, 510800, 526300, 532400).
Animal models
Experimental infection models were established for G. mellonella and mice. All experiments were developed in accordance with the guidelines established by the Animal Care Committee of the Universidade de São Paulo, Campus Ribeirão Preto, São Paulo, Brazil. To prepare the inocula, 24 h SDA cultures were suspended in sterile saline and filtered through sterile gauze to remove clumps of cells or hyphae. The resulting suspensions, containing ≥95% of conidial forms (arthroconidia and blastoconidia), were counted and adjusted to the desired inoculum concentration. Serial dilutions of the original suspension were cultured on SDA plates to confirm the accuracy of the counts.
Galleria model
Animals
G. mellonella larvae were obtained by cultivating crossing adult moths.26,59,60 G. mellonella larvae of a similar size were selected (approximately 275–330 mg) and kept without food in petri dishes, at 37°C, in darkness for 24 h prior to use.
Drugs
AMB deoxycholate was purchased as Unianf (União Química Farmacêutica Nacional S/A, MS 1.0497.0223.003-9), VRC was purchased as Vfend (i.v.) (Pfizer Ltda., MS 1.0216.0090.003-5), and FLC (PHR1160 Sigma).
Infection
Fungal inocula of differing concentrations were tested in order to determine the virulence of the respective strains. An inoculum of 5 × 105 CFU/animal yielded a mortality rate of 100% within 10 days after infection for T. asahii and T. inkin and >80% within 15 days for T. asteroides. The inoculum was prepared as described above and delivered via a Hamilton syringe to the injection site, the last left pro-leg in a volume of 5 μl.26 After injection and recovery lapse, they were transferred to a new Petri dish and incubated at 37°C in the dark for the duration of the experiment.
Experimental design
Groups of 20 larvae were randomly chosen for each strain, while the non-infected control group was challenged with PBS. The different groups were treated as follows: AMB deoxycholate at 0.5 mg/kg of body weight; VRC 15 mg/kg and FLC at 20 mg/kg were administered in 10 μl by using a Hamilton syringe once 4 hours post-infection.41 Control animals received 10 μl of PBS. The animals were checked daily for survival up to 15 days after infection. For survival studies, larvae were checked daily for 15 days. Larvae were sectioned in half and fixed for 24 hours in 3.7% formaldehyde–PBS. After dehydration in a series of alcohol solutions, the samples were diaphanized in xylol and embedded in paraffin. 5-μm-thick sections were collected on glass slides and stained with Gomori methenamine silver (GMS) stain following standard protocols. Briefly, sections were deparaffinized, oxidized with 4% chromic acid, stained with methenamine silver solution, and counterstained with light green. Microscopic analyses were performed using an Axioplan 2 imaging microscope (Carl Zeiss) at the stated magnifications under bright-field conditions.
Murine model
Animals
All the mice were obtained from the main bioterium from the Campus Ribeirão Preto from the Universidade de São Paulo, Ribeirão Preto, São Paulo, Brazil. Male Swiss mice with a mean weight of 30 g were used. The animals were housed in standard boxes with wood bedding and free access to food and water. From 2 days prior to infection, the mice that received VRC were given diluted (50%) grapefruit juice (Ceres Fruit Juices Pty Ltd.) instead of water.
Drugs
AMB deoxycholate and VRC were acquired as above described and FLC as Fluconazole 150 (Prati-Donaduzzi, 7030).
Immunosuppression
For survival and tissue burden studies, mice were immunosuppressed by a single intraperitoneal (i.p.) injection of 200 mg/kg of cyclophosphamide (Genuxal, Baxter Hospitalar Ltda., MS 1.0683.0168.002-1) plus an intravenous injection of 150 mg/kg of 5-fluorouracil (Fauldfluor, Libbs Farmacêutica Ltda., MS 1.0033.0139.004-5) on the day of infection. In the case of T. inkin mice were immunosuppressed one day before infection and received an additional dose of 5-fluorouracil (75 mg/kg) 5 days after infection.
Infection
For survival and tissue burden studies mice were challenged with 6 × 106 CFU (T. asahii and T. inkin) or 1.2 × 107 CFU (T. asteroides) in 0.2 ml into the lateral tail vein. These inocula yielded a mortality rate of 90–100% within 12 days after infection, but allowing a treatment course of 5 days after infection for the strains T. asahii 07 and T. asteroides 01. For T. inkin, a mortality rate of 70% was attained 15 days after infection.
Experimental design
Groups of 10 mice were randomly established for survival, and groups of 5 for tissue burden studies. The different groups were treated as follows: AMB deoxycholate at 1.5 mg/kg of body weight/dose given i.p. once daily; VRC 60 mg/kg and FLC at 80 mg/kg were given orally once daily.60,61 Control animals received no treatment. All treatments began 24 h after challenge, and the therapy lasted for 5 days. For survival studies, mice were checked daily for 15 days. For tissue burden and histopathology studies mice were killed one day after the completion of treatment. Spleens and kidneys were aseptically removed, and the entire organs were homogenized in 1 ml of sterile saline. Serial 10-fold dilutions of the homogenates were plated on SDA, incubated at 37°C and examined daily for 3 days. For the histopathology studies, portions of kidneys were placed in 3.7% formaldehyde–PBS and dehydrated in a series of alcohol solutions. The organs were embedded in paraffin blocks, sectioned and stained with GMS as described above. In this case the samples were counterstained with hematoxylin.
Statistics
Mean survival time was estimated by the Kaplan-Meier method and compared among groups using the log-rank test. Colony counts in tissue burden studies were analyzed using the Mann-Whitney U test. Calculations were made using Graph Pad Prism version 5.0. A P value of ≤ 0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Ana Carolina Padovan and Arnaldo Lopes Colombo from the Universidade Federal de São Paulo (UNIFESP), Brazil, who kindly provided the clinical strains. We also would like to thank the editor and the 4 reviewers for their comments and suggestions.
Funding
We would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico for the financial support.
References
- 1. Araujo Ribeiro M, Alastruey-Izquierdo A, Gomez-Lopez A, Rodriguez-Tudela JL, Cuenca-Estrella M. Molecular identification and susceptibility testing of Trichosporon isolates from a Brazilian hospital. Rev Iberoam Micol 2008; 25:221-5; PMID:19071890; http://dx.doi.org/ 10.1016/S1130-1406(08)70053-6 [DOI] [PubMed] [Google Scholar]
- 2. Colombo AL, Padovan AC, Chaves GM. Current knowledge of Trichosporon spp and Trichosporonosis. Clin Microbiol Rev 2011; 24:682-700; PMID:21976604; http://dx.doi.org/ 10.1128/CMR.00003-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cafarchia C, Romito D, Coccioli C, Camarda A, Otranto D. Phospholipase activity of yeasts from wild birds and possible implications for human disease. Med Mycol 2008; 46:429-34; PMID:18608940; http://dx.doi.org/ 10.1080/13693780701885636 [DOI] [PubMed] [Google Scholar]
- 4. Fuentefria AM, Suh SO, Landell MF, Faganello J, Schrank A, Vainstein MH, Blackwell M, Valente P. Trichosporon insectorum sp. nov., a new anamorphic basidiomycetous killer yeast. Mycol Res 2008; 112:93-9; PMID:18222677; http://dx.doi.org/ 10.1016/j.mycres.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 5. Gueho E, Smith MT, de Hoog GS. Trichosporon Behrend, p. 854-72. In Kurtzman C. P. and Fell J. W. (ed.), The Yeasts, a Taxonomic Study, 1998, 4th ed Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 6. Haupt HM, Merz WG, Beschorner WE, Vaughan WP, Saral R. Colonization and infection with Trichosporon species in the immunosuppressed host. J Infect Dis 1983; 147:199-203; PMID:6827136; http://dx.doi.org/ 10.1093/infdis/147.2.199 [DOI] [PubMed] [Google Scholar]
- 7. Kwon-Chung KJ, Bennett JE. Medical Mycology, 1992. Lea & Febiger, Philadelphia, PA. [Google Scholar]
- 8. Lussier N, Laverdiere M, Delorme J, Weiss K, Dandavino R. Trichosporon beigelii funguria in renal transplant recipients. Clin Infect Dis 2000; 31:1299-301; PMID:11073770; http://dx.doi.org/ 10.1086/317463 [DOI] [PubMed] [Google Scholar]
- 9. Silvestre AM, Jr, Miranda MAR, Camargo ZP. Trichosporon species isolated from the perigenital region, urine and catheters of a Brazilian population. Braz J Microbiol 2010; 41:628-34; PMID:24031538; http://dx.doi.org/ 10.1590/S1517-83822010000300013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sugita T, Kikuchi K, Makimura K, Urata K, Someya T, Kamei K, Niimi M, Uehara Y. Trichosporon species isolated from guano samples obtained from bat-inhabited caves in Japan. Appl Environ Microbiol 2005; 71:7626-9; PMID:16269819; http://dx.doi.org/ 10.1128/AEM.71.11.7626-7629.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walsh TJ. Role of surveillance cultures in prevention and treatment of fungal infections. NCI Monogr 1990; 9:43-5; PMID:2342594 [PubMed] [Google Scholar]
- 12. Walsh TJ, Groll A, Hiemenz J, Fleming R, Roilides E, Anaissie E. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect 2004; 10 Suppl 1:48-66; PMID:14748802; http://dx.doi.org/ 10.1111/j.1470-9465.2004.00839.x [DOI] [PubMed] [Google Scholar]
- 13. Benson PM, Lapins NA, Odom RB. White piedra. Arch Dermatol 1983; 119:602-4; PMID:6859904; http://dx.doi.org/ 10.1001/archderm.1983.01650310064013 [DOI] [PubMed] [Google Scholar]
- 14. Kiken DA, Sekaran A, Antaya RJ, Davis A, Imaeda S, Silverberg NB. White piedra in children. J Am Acad Dermatol 2006; 55:956-61; PMID:17097391; http://dx.doi.org/ 10.1016/j.jaad.2005.11.1033 [DOI] [PubMed] [Google Scholar]
- 15. Kontoyiannis DP, Torres HA, Chagua M, Hachem R, Tarrand JJ, Bodey GP, Raad II. Trichosporonosis in a tertiary care cancer center: risk factors, changing spectrum and determinants of outcome. Scand J Infect Dis 2004; 36:564-9; PMID:15370667; http://dx.doi.org/ 10.1080/00365540410017563 [DOI] [PubMed] [Google Scholar]
- 16. Roshan AS, Janaki C, Parveen B. White piedra in a mother and daughter. Int J Trichol 2009; 1:140-1; PMID:20927238; http://dx.doi.org/ 10.4103/0974-7753.58559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sano M, Sugitani M, Ishige T, Homma T, Kikuchi K, Sunagawa K, Obana Y, Uehara Y, Kumasaka K, Uenogawa K, et al. Supplemental utility of nested PCR for the pathological diagnosis of disseminated trichosporonosis. Virchows Arch 2007; 451:929-35; PMID:17786472; http://dx.doi.org/ 10.1007/s00428-007-0484-6 [DOI] [PubMed] [Google Scholar]
- 18. Youker SR, Andreozzi RJ. White piedra: further evidence of a synergistic infection. J Am Acad Dermatol 2003; 49:746-9. [DOI] [PubMed] [Google Scholar]
- 19. Chagas-Neto TC, Chaves GM, Melo AS, Colombo AL. Bloodstream infections due to Trichosporon spp.: species distribution, Trichosporon asahii genotypes determined on the basis of ribosomal DNA intergenic spacer 1 sequencing, and antifungal susceptibility testing. J Clin Microbiol 2009; 47:1074-81; PMID:19225102; http://dx.doi.org/ 10.1128/JCM.01614-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Bonaventura G, Pompilio A, Picciani C, Iezzi M, D'Antonio D, Piccolomini R. Biofilm formation by the emerging fungal pathogen Trichosporon asahii: development, architecture, and antifungal resistance. Antimicrob Agents Chemother 2006; 50:3269-76; PMID:17005804; http://dx.doi.org/ 10.1128/AAC.00556-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, Pastore D, Picardi M, Bonini A, Chierichini A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 2006; 91:1068-75; PMID:16885047 [PubMed] [Google Scholar]
- 22. Iturrieta-González IA, Padovan AC, Bizerra FC, Hahn RC, Colombo AL. Multiple species of trichosporon produce biofilms highly resistant to triazoles and amphotericin B. PLoS One 2014; 9:e109553; http://dx.doi.org/ 10.1371/journal.pone.0109553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev 2000; 13:122-43; PMID:10627494; http://dx.doi.org/ 10.1128/CMR.13.1.122-143.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mizobe T, Ando M, Yamasaki H, Onoue K, Misaki A. Purification and characterization of the serotype-specific polysaccharide antigen of Trichosporon cutaneum serotype II: a disease-related antigen of Japanese summer-type hypersensitivity pneumonitis. Clin Exp Allergy 1995; 25:265-72; PMID:7540499; http://dx.doi.org/ 10.1111/j.1365-2222.1995.tb01039.x [DOI] [PubMed] [Google Scholar]
- 25. Rodrigues ML, Fonseca FL, Frases S, Casadevall A, Nimrichter L. The still obscure attributes of cryptococcal glucuronoxylomannan. Med Mycol 2009; 47:783-8; PMID:19343609; http://dx.doi.org/ 10.3109/13693780902788621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chagas-Neto TC, Chaves GM, Melo ASA, Colombo AL. Bloodstream infections due to Trichosporon spp.: Species distribution, Trichosporon asahii genotypes determined on the basis of ribosomal DNA intergenic spacer 1 sequencing, and antifungal susceptibility testing. J Clin Microbiol 2009; 47:1074-81; PMID:19225102; http://dx.doi.org/ 10.1128/JCM.01614-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Itoh T, Hosokawa H, Kohdera U, Toyazaki N, Asada Y. Disseminated infection with Trichosporon asahii. Mycoses 1996; 39:195-9; PMID:8909029; http://dx.doi.org/ 10.1111/j.1439-0507.1996.tb00124.x [DOI] [PubMed] [Google Scholar]
- 28. Ruan SY, Chien JY, Hsueh PR. Invasive trichosporonosis caused by Trichosporon asahii and other unusual Trichosporon species at a medical center in Taiwan. Clin Infect Dis 2009; 49:e11-e17; PMID:19489711; http://dx.doi.org/ 10.1086/599614 [DOI] [PubMed] [Google Scholar]
- 29. Chaumentin G, Boibieux A, Piens MA, Douchet C, Buttard P, Bertrand JL, Peyramond D. Trichosporon inkin endocarditis: short-term evolution and clinical report. Clin Infect Dis 1996; 23:396-7; PMID:8842284; http://dx.doi.org/ 10.1093/clinids/23.2.396 [DOI] [PubMed] [Google Scholar]
- 30. Lopes JO, Alves SH, Klock C, Oliveira LT, Dal Forno NR. Trichosporon inkin peritonitis during continuous ambulatory peritoneal dialysis with bibliography review. Mycopathologia 1997; 139:15-8; PMID:9511232; http://dx.doi.org/ 10.1023/A:1006870017725 [DOI] [PubMed] [Google Scholar]
- 31. Steinbach WJ, Perfect JR. Newer antifungal therapy for emerging fungal pathogens. Int J Infect Dis 2003; 7:525; http://dx.doi.org/ 10.1016/S1201-9712(03)90037-3 [DOI] [PubMed] [Google Scholar]
- 32. Tokimatsu I, Kushima H, Hashinaga K, Umeki K, Ohama M, Ishii H, Kishi K, Hiramatsu K, Kadota J. The prophylactic effectiveness of various antifungal agents against the progression of trichosporonosis fungemia to disseminated disease in a neutropenic mouse model. Int J Antimicrob Agents 2007; 29:84-8; PMID:17189098; http://dx.doi.org/ 10.1016/j.ijantimicag.2006.09.013 [DOI] [PubMed] [Google Scholar]
- 33. Yang RY, Wang WL, Ao JH, Hao ZF, Zhang J, Wang CM. Pathogenicity of Trichosporon asahii in a murine model of disseminated trichosporonosis. Chin Med J (Engl) 2008; 121:2557-60; PMID:19187595 [PubMed] [Google Scholar]
- 34. Serena C, Gilgado F, Mariné M, Pastor FJ, Guarro J. Efficacy of voriconazole in a guinea pig model of invasive trichosporonosis. Antimicrob Agents Chemother 2006; 50:2240-3; PMID:16723595; http://dx.doi.org/ 10.1128/AAC.00045-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lacy SH, Gardner DJ, Olson LC, Ding L, Holland SM, Bryant MA. Disseminated trichosporonosis in a murine model of chronic granulomatous disease. Comp Med 2003; 53:303-8; PMID:12868577 [PubMed] [Google Scholar]
- 36. Tokimatsu I, Kushima H, Hashinaga K, Umeki K, Ohama M, Ishii H, Kishi K, Hiramatsu K, Kadota J. The prophylactic effectiveness of various antifungal agents against the progression of trichosporonosis fungemia to disseminated disease in a neutropenic mouse model. Int J Antimicrob Agents 2007; 29:84-8; PMID:17189098; http://dx.doi.org/ 10.1016/j.ijantimicag.2006.09.013 [DOI] [PubMed] [Google Scholar]
- 37. Dörlemann A, Listemann H, Iglauer F. Invasive Trichosporon beigelii infection in immunosuppressed rats. Mycoses 1994; 37:109-16; PMID:7845415; http://dx.doi.org/ 10.1111/j.1439-0507.1994.tb00785.x [DOI] [PubMed] [Google Scholar]
- 38. Yamamoto K, Makimura K, Sudo T, Shibuya K, Uchida K, Yamaguchi H. Experimental disseminated trichosporonosis in mice: tissue distribution and therapy with antifungal agents. J Med Vet Mycol 1997; 35:411-8; PMID:9467108; http://dx.doi.org/ 10.1080/02681219780001511 [DOI] [PubMed] [Google Scholar]
- 39. Hospenthal D, Belay T, Lappin P, Rogers A, Kennedy M. Disseminated trichosporonosis in a neutropenic murine model. Mycopathologia 1993; 122:115-22; PMID:8327000; http://dx.doi.org/ 10.1007/BF01103609 [DOI] [PubMed] [Google Scholar]
- 40. Metin DY, Hilmioglu-Polat S, Hakim F, Inci R, Tumbay E. Evaluation of the microdilution, Etest and disk diffusion methods for antifungal susceptibility testing of clinical strains of Trichosporon spp. J Chemother 2005; 17:404-8; PMID:16167520; http://dx.doi.org/ 10.1179/joc.2005.17.4.404 [DOI] [PubMed] [Google Scholar]
- 41. Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, Diener A. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun 2005; 73:3842-50; PMID:15972469; http://dx.doi.org/ 10.1128/IAI.73.7.3842-3850.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li DD, Deng L, Hu GH, Zhao LX, Hu DD, Jiang YY, Wang Y. Using Galleria mellonella-Candida albicans infection model to evaluate antifungal agents. Biol Pharm Bull 2013; 36:1482-7; PMID:23995660; http://dx.doi.org/ 10.1248/bpb.b13-00270 [DOI] [PubMed] [Google Scholar]
- 43. Ortoneda M, Guarro J, Madrid MP, Caracuel Z, Roncero MI, Mayayo E, Di Pietro A. Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infect Immun 2004; 72:1760-6; PMID:14977985; http://dx.doi.org/ 10.1128/IAI.72.3.1760-1766.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gueho E, Improvisi L, de Hoog GS, Dupont B. Trichosporon on humans: a practical account. Mycoses 1994; 37:3-10; PMID:7935589; http://dx.doi.org/ 10.1111/j.1439-0507.1994.tb00277.x [DOI] [PubMed] [Google Scholar]
- 45. Girmenia C, Pagano L, Martino B, D’Antonio D, Fanci R, Specchia G, Melillo L, Buelli M, Pizzarelli G, Venditti M, et al. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J Clin Microbiol 2005; 43:1818-28; PMID:15815003; http://dx.doi.org/ 10.1128/JCM.43.4.1818-1828.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. CLSI Reference method for broth dilution antifungal susceptibility testing of yeasts, fourth informational supplement.CLSI document M27-S4. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 47. Guinea J, Recio S, Escribano P, Peláez T, Gama B, Bouza E. In vitro antifungal activities of isavuconazole and comparators against rare yeast pathogens. Antimicrob Agents Chemother 2010; 54:4012-5; PMID:20566770; http://dx.doi.org/ 10.1128/AAC.00685-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Madariaga MG, Tenorio A, Proia L. Trichosporon inkin peritonitis treated with caspofungin. J Clin Microbiol 2003; 41:5827-9; PMID:14662994; http://dx.doi.org/ 10.1128/JCM.41.12.5827-5829.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bassetti M, Bisio F, Di Biagio A, Pierri I, Balocco M, Soro O, Cruciani M, Bassetti D. Trichosporon asahii infection treated with caspofungin combined with liposomal amphotericin B. J Antimicrob Chemother 2004; 54:575-7; PMID:15231763; http://dx.doi.org/ 10.1093/jac/dkh337 [DOI] [PubMed] [Google Scholar]
- 50. Lionakis MS. Drosophila and Galleria insect model hosts: new tools for the study of fungal virulence, pharmacology and immunology. Virulence 2011; 2:521-7; PMID:22186764; http://dx.doi.org/ 10.4161/viru.2.6.18520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Anaissie EJ, Hachem R, Karyotakis NC, Gokaslan A, Dignani MC, Stephens LC, Tin-U CK. Comparative efficacies of amphotericin B, triazoles, and combination of both as experimental therapy for murine trichosporonosis. Antimicrob Agents Chemother 1994; 38:2541-4; PMID:7872744; http://dx.doi.org/ 10.1128/AAC.38.11.2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Serena C, Pastor FJ, Gilgado F, Mayayo E, Guarro J. Efficacy of micafungin in combination with other drugs in a murine model of disseminated trichosporonosis. Antimicrob Agents Chemother 2005; 49:497-02; PMID:15673724; http://dx.doi.org/ 10.1128/AAC.49.2.497-502.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Serena C, Gilgado F, Mariné M, Pastor FJ, Guarro J. Efficacy of voriconazole in a guinea pig model of invasive trichosporonosis. Antimicrob Agents Chemother 2006; 50:2240-3; PMID:16723595; http://dx.doi.org/ 10.1128/AAC.00045-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Treviño-Rangel RD, López LJ, Palma-Nicolás JP, Hernández-Bello R, González JG, González GM. Therapeutic efficacy of posaconazole in a murine model of disseminated trichosporonosis. J Antimicrob Chemother 2014; 69:1075-8; http://dx.doi.org/ 10.1093/jac/dkt466 [DOI] [PubMed] [Google Scholar]
- 55. Silvestre AM, Jr, Miranda MAR, Camargo ZP. Trichosporon species isolated from the perigenital region, urine and catheters of a Brazilian population. Braz J Microbiol 2010; 41:628-34; PMID:24031538; http://dx.doi.org/ 10.1590/S1517-83822010000300013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sugar AM, Liu XP. Effect of grapefruit juice on serum voriconazole concentrations in the mouse. Med Mycol 2000; 38:209-12; PMID:10892988; http://dx.doi.org/ 10.1080/mmy.38.3.209.212 [DOI] [PubMed] [Google Scholar]
- 57. CLSI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard. CLSI Document M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 58. Fuchs BB, O’Brien E, Khoury JB, Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 2010; 1:475-82; PMID:21178491; http://dx.doi.org/ 10.4161/viru.1.6.12985 [DOI] [PubMed] [Google Scholar]
- 59. Fallon J, Kelly J, Kavanagh K. Galleria mellonella as a model for fungal pathogenicity testing. Methods Mol Biol 2012; 845:469–85; PMID:22328396; http://dx.doi.org/ 10.1007/978-1-61779-539-8_33 [DOI] [PubMed] [Google Scholar]
- 60. Arvanitis M, Fuchs BB, Mylonakis E. Nonmammalian model systems to investigate fungal biofilms. Methods Mol Biol 2014;1147:159-72; PMID:24664832; http://dx.doi.org/ 10.1007/978-1-4939-0467-9_11 [DOI] [PubMed] [Google Scholar]
- 61. Serena C, Pastor FJ, Mariné M, Rodríguez MM, Guarro J. Efficacy of voriconazole in a murine model of cryptococcal central nervous system infection. J Antimicrob Chemother 2007; 61:877-9; http://dx.doi.org/ 10.1093/jac/dkn012 [DOI] [PubMed] [Google Scholar]
- 62. Rodríguez MM, Calvo E, Serena C, Mariné M, Pastor FJ, Guarro J. Effects of double and triple combinations of antifungal drugs in a murine model of disseminated infection by Scedosporium prolificans. Antimicrob Agents Chemother 2009; 53:2153-5; http://dx.doi.org/ 10.1128/AAC.01477-08 [DOI] [PMC free article] [PubMed] [Google Scholar]