Abstract
The matrix metalloproteinase MT1-MMP is a central regulator of cell invasion in both physiological and pathological settings, such as tissue surveillance by immune cells and cancer cell metastasis. MT1-MMP cleaves a plethora of intra- and extracellular proteins, including extracellular matrix proteins, matrix receptors, and also other MMPs, and thus enables modification of both the cell surface proteome and the pericellular environment. Despite its importance for cell invasion, the pathways regulating MT1-MMP exposure on the cell surface are largely unknown. Recently, our groups discovered that a specific subset of RABGTPases, most notably RAB5a, is critical for MT1-MMP trafficking in primary human macrophages and carcinoma cells. Here, we discuss and contrast our findings for both cell types, pointing out common features and differences in the RABGTPase-dependent trafficking of MT1-MMP in health and disease.
Keywords: carcinoma cells, cell invasion, intracellular trafficking, invadopodia, macrophages, MT1-MMP, podosomes, RABGTPases, vesicles
Cell Invasion and MT1-MMP–Dependent Matrix Degradation
Cell invasion is a central feature of both physiological and pathological scenarios, such as invasion of immune cells to regulate tissue homeostasis or infection,2,3 or invasion of cancer cells during metastasis.4,5 To invade into tissues, cells employ flexible, diverse invasive strategies,6 depending mostly on the properties of the surrounding extracellular matrix (ECM). First, individual cells can utilize a mesenchymal mode of invasion, which involves local degradation of dense and highly connected matrix.7-9 Using this mode, cells generate micro-channels and pores through which they extend elongated protrusions that transiently attach to ECM fibers, thus coordinating actin-driven, pushing forces and traction for locomotory propulsion. Alternatively, cells can resort to an amoeboid migration strategy, in which they utilize actomyosin-dependent elevation of hydrostatic intracellular pressure to extend short-lived blebs or elongated lobopodia (blunt cylindrical protrusions where compartmentalized hydrostatic pressure is generated by the nucleus that acts as a piston10) that squeeze through relatively sparse and loosely cross-linked matrix.
Degradation of ECM can be achieved by the localized release of a variety of matrix-lytic enzymes, including serine proteinases, ADAMs (a disintegrin and metalloproteinase) or matrix metallproteinases, either from cancer cells or the associated host cells, such as macrophages and fibroblasts.11-13 One of the best-studied and most crucial enzymes in this process is the membrane-bound matrix metalloproteinase MT1-MMP.14,15 MT1-MMP can cleave a variety of matrix components such as collagens, fibronectin and fibrin,14 and also matrix receptors such as CD44 and syndecan-1.16,17 MT1-MMP can also proteolytically activate other MMPs such as MMP-2, MMP-8, and MMP-13,15 placing it in the center stage of the cellular program for invasion. As a consequence, MT1-MMP exposure and activity on the cell surface have to be tightly controlled.
Vesicle-mediated transport of MT1-MMP has emerged as a central mechanism that regulates the cell surface pool of the proteinase, through both exocytic delivery and endocytic re-uptake.18 Accordingly, transport and surface exposure of MT1-MMP has been shown to depend on a variety of intracellular trafficking components, including the microtubule system,19 motor proteins, such as kinesin-1 and kinesin-2,20 and regulators of vesicle fusion, such as VAMP-7.21 Considering the central importance of vesicle transport for MT1-MMP regulation, it is somewhat surprising that the potential importance of RABGTPases, a major group of vesicle regulators comprising more than 70 members in mammalian cells,22 has been studied only in a limited fashion.23,24
Our labs have recently shown that RABGTPase-dependent endocytic uptake of MT1-MMP, and its recycling back to the cell surface, is a crucial regulatory loop in adjusting the amount of surface-exposed MT1-MMP, in both primary human macrophages25 and in cancer cells.26 We identified several RAB-GTPases and in particular RAB5a, as central regulators of this circuitry, which appears to be necessary for the regulated and spatially confined delivery of MT1-MMP. In principle, release or exposure of MT1-MMP and other proteinases could happen all over the cell surface. However, podosomes and invadopodia, actin-rich adhesions of monocytic cell and cancer cells, respectively,27 are now recognized as preferred sites for these processes, and transient physical contact and local release of MT1-MMP vesicles to these structures has been demonstrated.20,21,28,29 Podosomes and invadopodia are thus closely associated with ECM pericellular proteolysis and are considered to be crucial for cell invasion.30,31
MT1-MMP Trafficking in Primary Macrophages
In order to screen for RABGTPases potentially involved in MT1-MMP trafficking, we chose a number of candidates, namely RAB4, RAB5a, RAB6a, RAB8a, RAB9, RAB11, RAB14, RAB21 and RAB22a, that are known to be involved in diverse aspects of intracellular trafficking, acting as central hubs of a variety of vesicular routes. RABGTPases are molecular on/off switches, which, like all small G proteins, are only active in their GTP-bound state.32 Moreover, their activity is thought to be restricted to the vesicular compartment they are localized at.33 Therefore, we initially colocalized both overexpressed and endogenous forms of these RABGTPases with overexpressed MT1-MMP. Using this criterion, only RAB5a, RAB8a, RAB14, RAB21 and RAB22a showed pronounced colocalization with MT1-MMP on vesicles.25 This was subsequently also verified for endogenous MT1-MMP.
Notably, RAB6a, which does not localize to podosomes, had no detectable influence on podosome formation and matrix degradation, thereby validating the potential relevance of our screen. Similarly, endogenous RAB4 also failed to show distinct colocalization with endogenous MT1-MMP in macrophages. In contrast, both ectopically expressed and endogenous RAB4 were found to prominently localize with endogenous MT1-MMP in a subset of invasive breast cancer cells26 (see below). These findings likely underline the plasticity of the membrane trafficking routes, which can be differentially utilized for delivery of cargos, such as MT1-MMP, depending on the cell type. Still, some major trafficking routes of MT1-MMP are expected to be conserved between cell types. Consistently, our screen identified RAB8a, which was previously found to colocalize with MT1-MMP in MDA-M-231 breast carcinoma cells,23 and also RAB5a, which was subsequently identified by one of our labs also in cancer cells.26
In order to test the functional relevance of the identified RABGTPases, we assessed their influence on a variety of MT1-MMP-related parameters, including the amount of MT1-MMP at the cell surface. We interfered with the activity of these RABGTPases by overexpressing dominant active and negative mutants and also by depletion using specific siRNA. Strikingly, reducing RAB5a activity had a positive effect on the tested parameters, while interference with of RAB8a, RAB14 and RAB22a had negative effects. Silencing of RAB21 showed only minor effects. These findings indicate that, in macrophages, RAB5a appears to be a negative regulator of MT1-MMP surface exposure, probably by promoting the internalization, and thus the cell surface removal of this metalloproteinase.32 This specific influence of RAB5a is somewhat surprising, considering that macrophages, like most cells in our body, express 2 additional, functionally redundant and highly conserved RAB5 genes, RAB5b and RAB5c, pointing to a possible specific role of RAB5a in the control MT1-MMP-dependent matrix degradation in macrophages. In this context, it would be interesting to assess whether the apparent absolute requirement of RAB5a in controlling cell surface levels of MT1-MMP is cargo-specific or applies also to other membrane-bound receptors. It also remains to be established whether the effects of RAB5a on MT1-MMP are a direct consequence of a reduced rate of internalization or rather mediated by more global alterations of the trafficking or cellular distribution of other endocytic or non-endocytic factors, which, in turn, would be required for the proper turnover of MT1-MMP at the plasma membrane.
This notwithstanding, our data also provide evidence that, as opposed to RAB5a, the other MT1-MMP associated isoforms RAB8a, RAB14 and RAB22a act instead as positive regulators of MT1-MMP surface exposure. This latter notion is consistent with the involvement of these RABGTPases in exocytic or recycling pathways, both of which have already been shown to mediate the surface delivery of MT1-MMP from intracellular vesicular pools, ultimately impacting on the life time and the activity of this metalloproteinase.23,34-36 Our results also indicated that a partial co-localization of a RABGTPase with MT1-MMP, as in the case of RAB21, does not necessarily translate into a major impact of this RAB-GTPase on the final delivery of MT1-MMP, reflecting the existence either of functional redundancy among GTPases or of distinct microdomains formed by diverse RABs on the same endosomal vesicles that are used for cargo sorting to different destinations.37
MT1-MMP is a central regulator of matrix protein degradation,11 and MT1-MMP vesicles contact podosomes, which are the primary sites of matrix degradation in macrophages.20,27,36 We therefore analyzed the influence of the identified RABGTPases also on trafficking of MT1-MMP-containing vesicles to podosomes and on podosomal matrix degradation. Comparable to the results on MT1-MMP surface exposure, RAB5a was found to be a negative regulator of MT1-MMP trafficking to podosomes and also of gelatin matrix degradation, while RAB8a, RAB14 and RAB22a emerged as positive regulators of these processes. The respective changes in matrix degradation upon silencing of these RABGTPases correlate well with the roles of these proteins in the regulation of MT1-MMP surface exposure. However, the observed changes in vesicle contact with podosomes appear counterintuitive at first glance, as silencing of RAB5a, leading to enhanced surface exposure of MT1-MMP, also leads to enhanced contact of MT1-MMP vesicles with podosomes. A possible explanation could be that these vesicles are part of a compensatory response of the cell and may reflect internalization events by RAB5a-independent means, for example by clathrin.19 Alternatively, they may arise from increased trafficking of Golgi-derived vesicles in the biosynthetic pathway.
Matrix degradation at a 2-dimensional cell substrate interface is biologically relevant, as such interfaces also exist in the body, for example during extravasation of blood cells and breaching of the basement membrane.27 However, in many cases, cells in vivo are embedded within a 3-dimensional context. We therefore also studied MT1-MMP-dependent matrix degradation in a 3D model system of macrophages embedded in collagen I fibers. Consistent with results from the 2D matrix degradation assay, RAB5a was found to negatively regulate 3D collagen matrix degradation, while RAB8a, RAB14 and RAB22a were shown to be positive regulators. It is important to note that macrophages completely change their morphology from a “fried egg” shape in 2D to a compact cell body with numerous elongated protrusions in 3D. Classical podosomes at the ventral cell surface are thus no longer found in a 3D context. Instead, cells form several actin-rich accumulations at the end of some protrusions that are also positive for other podosome components such as Arp2/3 complex or vinculin.38 Using an antibody that detects a neo-epitope of cleaved collagen, we could show that collagen matrix degradation occurs especially at these actin-rich accumulations, and that MT1-MMP is also enriched at these sites. These results identified these structures as the bona fide 3D counterpart of classical 2D podosomes.
Consistently, we could further show that the RABGTPases regulating podosome-dependent matrix degradation in 2D, RAB5a, RAB8a, RAB14 and RAB22a, also regulate matrix degradation in 3D. However, testing their impact on actual cell invasion in 3D, RAB22a showed no discernible effect. By contrast, RAB5a emerged as an important negative regulator of macrophage invasion, whereas RAB8a and RAB14 were shown to be positive regulators. These results seem to be consistent with respective roles of these RABGTPases in regulating the surface pool of MT1-MMP, most probably through endocytosis (RAB5a), biosynthetic exocytic delivery (RAB8a) and fast recycling pathways (RAB14) (Fig. 1). Indeed, a combined knockdown of RAB5a and RAB14 showed intermediate levels of invasion, indicating that both RABGTPases counteract each other in this setup. Moreoever, addition of a specific inhibitor of MT1-MMP drastically reduced cell invasion, even under knockdown of RAB5a, indicating that the role of RAB5a in cell invasion consists mainly in regulating MT1-MMP surface exposure.
Figure 1.

RAB-dependent trafficking of MT1-MMP in macrophages. Exocytic pathways (green arrows) for newly synthesized MT1-MMP are regulated by RAB8a and can proceed through exocytic vesicles or recycling endosomes. MT1-MMP is endocytosed from the cell surface by a RAB5a-dependent pathway (red arrow). Recycling back to the cell surface, including podosomes, can proceed by fast (orange arrows) and slow (gray arrows) recycling pathways, regulated by RAB14 and RAB22a, respectively. RAB8a, RAB14 and RAB22a thus drive surface exposure of MT1-MMP and subsequent matrix degradation, whereas RAB5a controls recovery of MT1-MMP. Model modified from.25
However, it is remarkable that RAB22a failed to influence macrophage invasion, while having a clear impact on all other parameters tested, including matrix degradation in 3D. The reason for this is currently unclear, but it may be that different trafficking routes used for MT1-MMP recycling to the cell surface might affect the properties of the thus-delivered metalloproteinase, for example by affecting oligomerization or secondary modifications, as has been speculated earlier.39 Fast recycling by RAB14-governed pathways thus appears to be more relevant for MT1-MMP-dependent cell invasion, compared to RAB22a-dependent slow recycling. Moreover, these results show that effects of RABGTPases on matrix degradation in 2D or even in 3D can not be used to correctly predict their impact on cell invasion, clearly indicating the necessity for rigorous testing of specific cellular effects in a variety of appropriate setups.
MT1-MMP Trafficking in Cancer Cells
The picture emerging from the analysis in normal macrophages in vitro points to a key role of various RABGTPases in the proper regulation of MT1-MMP via primarily controlling the rate of endo/exocytic cycles of this metalloprotease. This notion is consistent with evidence supporting the relevance of vesicular trafficking, and in particular of late endosomal recycling, in mediating the delivery of MT1-MMP to podosomes and invadopodia, and MT1-MMP-dependent matrix digestion and cell invasion into 3D matrices.18,28,35,40 A notable and striking difference, however, emerged from the analysis of the expression levels of RAB5 and RAB4 in human breast cancer and their impact on cancer invasion and dissemination.26 This analysis was motivated by a set of previous findings showing that RAB5 expression is sufficient to promote a mesenchymal mode of cell invasion.41 Additionally and consistently with this earlier finding, individual ablation of the 3 human RAB5 genes (RAB5A/B/C) has been reported to impair invasion and dissemination of different types of cancer cells.42-46 In silico meta-analysis of more than 900 breast cancer tumors revealed, indeed, that among the RAB5 genes, RAB5a is overexpressed in breast cancers, its elevated expression positively correlated with poor prognosis, and its levels are predictive of increased local and distant relapse in early stage estrogen breast cancer patients. Immunohistochemical analysis of a panel of primary human breast cancers and their matched lymph node metastases further showed that RAB5a expression is significantly higher in matched lymph node metastases, with respect to their primary tumors, consistent with the notion that RAB5 overexpression is an event that is selected for during breast cancer progression, possibly because it confers a migratory advantage to tumor cells.26
This latter possibility was experimentally verified using different breast cancer cell lines and a variety of in vivo and in vitro assays of cell invasion and metastazation. Most notably, we manipulated the expression or the functional activity of RAB5 either by overexpression of RAB5a, to mimic the alteration detected in human breast cancers, or by genetic or functional interference of RAB5a and its isoforms. We primarily examined the aggressive and invasive triple negative MDA-MB-231 cells and MCF10.DCIS.com cells, a tumorigenic derivative of normal human mammary epithelial MCF10A cells that recapitulate features of a common oncogenic lesion, the comedo-type ductal carcinoma in situ (DCIS), upon subcutaneous injection into immunodeficient mice.47 Ablation of RAB5a, b and c or the expression of a RAB5a dominant negative impaired lymph node and lung dissemination of MDA-MB-231 injected into mammary fat pad without affecting the cell proliferation or survival potential of the primary tumors. In keeping with this finding, impairing RAB5 function also delayed the conversion of DCIS-to-invasive ductal carcinoma of MCF10.DCIS.com xenograft model.26 The DCIS-to-IDC conversion requires digestion of the basement membrane and local invasion into collagenous stromal tissues, thus providing evidence that RAB5a is likely critical for this initial step of the metastatic cascade.48 Conversely, the ectopic expression of RAB5a in poorly invasive HeLa cells injected heterotopically in the mammary fat pad enhanced distant lung metastatization as well as local intra-tumoral cell motility as monitored by 2-photon intravital imaging, thereby reinforcing the notion that RAB5a elevated expression may be sufficient to promote tumor dissemination.
The subsequent search for the cellular processes and molecular mechanisms underlying RAB5a pro-metastatic function was conducted using several in vivo 3D invasion assays. We used either organotypic gels formed from acid-extracted rat-tail collagen preconditioned with human macrophages, an environment that is thought to closely recapitulate that of the tumor stroma,49 or thick, native collagen matrices, which form a dense, highly cross-linked, fibrillar network that provides a formidable barrier to invasion, unless cells acquire collagenolytic activity.50 We could thus show that RAB5a was not only necessary, but also sufficient for invasion of cancer cells. Remarkably, RAB5a-expressing cells extensively remodeled the collagen fibers, generating gaps and channels through which cells could meander in a process strictly depended on metalloprotease activity.26 Importantly, the invasive ability of all cancer cell lines tested was triggered and promoted by the presence of a potent motogenic factor, Hepatocyte Growth Factor (HGF).
Consistently, we could next show that RAB5a is critical for invadopodia formation and function, particularly when these structures are induced by the addition of motogenic factors.26 Remarkably, elevation of RAB5a was sufficient to trigger the onset of invadopodia and endowed poorly degradative cells, such as HeLa cells, with the ability to digest ECM in keeping with its ability to promote invasion into 3D matrices. Interestingly, impairing RAB5 expression of squamous cell carcinoma that efficiently form degradative invadopodia in the absence of motogenic factors, had negligible effects. These observations suggest that RAB5a is essential for the formation of these structures following growth factor modulation, which also promote a polarized migratory phenotype. Under these conditions, cell signaling and trafficking pathways are activated, and membrane bound cargos undergoes polarized endo/exocytic cycles that are key to spatially restrict their activity contributing to the execution of polarized functions.51 Not surprisingly, the formation of degradative invadopodia induced either by HGF stimulation or RAB5a expression required MT1-MMP expression. Furthermore, direct monitoring of the dynamic localization and membrane trafficking of MT1-MMP revealed that it rapidly relocalizes from early endosomal vesicles to ventrally restricted, F-actin-rich structures in response to HGF stimulation in a RAB4 and RAB5-dependent fashion.26
A molecular genetic approach was next used to validate the relevant trafficking routes and dissect the essential circuitry for MT1-MMP plasma membrane delivery. Interference with the major internalization routes by inhibition of clathrin or dynamin, or with fast and slow recycling pathways, particularly those depending on ARF6 and RAB35, reduced both HGF and RAB5a-induced invadopodia. In keeping with the key relevance of the fast recycling pathway, interference with RAB4a and RAB4b, or their effector Rabenosyn-5, robustly reduced matrix degradation.26 Conversely, silencing of RAB11-dependent slow recycling, or RAB8-biosynthetic secretory pathways had no effect on the formation of HGF-induced invadopodia. RAB7-mediated lysosomal pathways only marginally affected HGF-induced matrix degradation but reduced cell invasion into thick collagen matrix. Collectively, these findings indicate that the RAB5-Rabenosyn-5-RAB4a circuitry is a major route for the fast delivery of MT1MMP to the PM in response to motogenic stimulation. Consistently, a sizable fraction of MT1-MMP localized to RAB4-positive recycling endosomes, which were found to transiently and dynamically contact invadopodia along the ventral membrane. It must be pointed out, that MT1-MMP display localization also in late endosomal vesicles, which, as shown by other labs, may also contribute to MT1-MMP cycling.28,36,52,19,21 We would argue that the RAB5a/RAB4a route is preferentially used when rapid delivery and activation of MT1-MMP is required in response to acute stimulation and possibly certain oncogenic alteration (see below), while other recycling pathways may become relevant to sustain MT1-MMP turnover in steady-state conditions or in response to physical cues, such as matrix type or rigidity.53,54 Consistent with this possibility, HGF in addition to enhancing matrix degradation also promotes the acute activation of RAB5a. This event likely favors the subsequent recruitment of the Rabenosyn-5-RAB4 complex onto early endosomal microdomains for recycling of MT1-MMP. Indeed, Rabenosyn-5 mutants impaired in binding to either RAB5 or RAB4 could no longer restore defective matrix degradation in Rabenosyn-5 silenced cells. Notably, the RAB5a-Rabenosyn-5-RAB4a pathway may also serve as trafficking route for cell-adhesion molecules that in addition to actin and MT1-MMP are required for the formation of fully functional invadopodia. It is remarkable in this respect that one of the major integrins shown to be required for invadopodia formation, integrin αVβ3, primarily utilizes the RAB4 trafficking route to recycle efficiently in response to stimulation with Platelet Derived Growth Factor.55,56 Accordingly, we found an absolute requirement for αVβ3, but not β1-containing integrins for the formation of RAB5a and HGF-induced invadopodia. We further provided evidence that, as previously shown, αVβ3 utilizes RAB4 routes, like MT1-MMP, suggesting that the 2 proteins may be co-trafficked for efficient delivery to invadopodia.
Collectively, this set of findings and previously published data41 support a model (Fig. 2) in which RAB5 couples elongated protrusions with pericellular proteolysis by controlling RAB4-dependent fast recycling of MT1-MMP and β3 integrin cargos to enable efficient invasion. The relevance of this model was verified in breast cancer lines, whose ability to invade collagen gels was impaired not only by silencing RAB5 and MT1-MMP, but also of RAB4 or β3 integrin. Additionally, RAB4 interference, and likewise RAB5 silencing, delayed the conversion of MCF10DCIS.com cells from DCIS-to-IDC in in vivo mouse models. The observations that RAB4a is amplified in more than 14% of invasive breast cancers and upregulated in breast tumors lend further support to the notion that the RAB5/RAB4a circuitry is specifically selected in human tumors and may contribute to their invasive metalloprotease-dependent phenotype.
Figure 2.
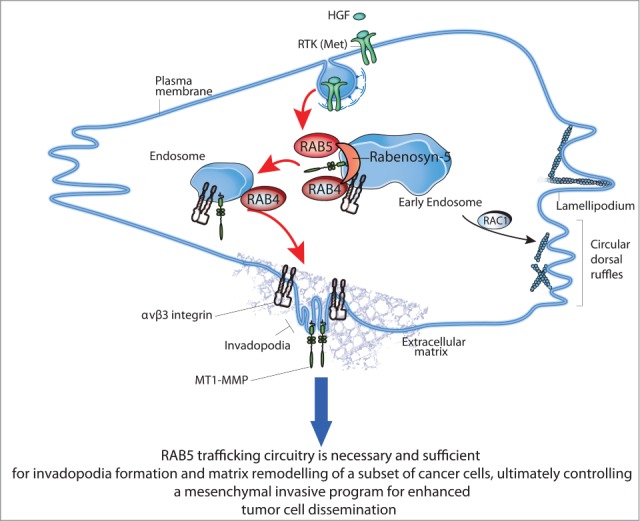
The RAB5a/RAB4a circuitries in the control of breast cancer cell invasion and dissemination. RAB5a is necessary and sufficient for the formation of invadopodia and ECM degradation, particularly in response to motogenic stimuli, such as Hepatocyte Growth Factor (HGF) of breast cancer lines. It does so by promoting a Rabenosyn-5-RAB4-dependent endo/exocytic cycles (EEC) of critical cargos (MT1-MMP and β3 integrin). This trafficking circuitry is necessary for the spatial resolution of HGF/MET signaling into polarized functions and to couple actin-driven, migratory cell protrusions (lamellipodia and circular dorsal ruffles)41- typical of mesenchymal motile tumor cells, with pericellular proteolysis, ultimately promoting extracellular matrix degradation, tissue remodeling and metastatic dissemination in vivo.
It should also be mentioned that recent work uncovered a role for cancer cell invadopodia as docking sites for multivesicular bodies (MVB), with subsequent release of MVB-derived exosomes that also contain MT1-MMP.29 Several facets of this process are controlled by RAB27a in human head and neck squamous carcinoma cells, as depletion of RAB27a leads to decreases in both invadopodia formation and also in invadopodia-associated exocytosis of MT1-MMP.29 Moreover, RAB27a also controls non-exosomal release of MMP-9 in murine mammary carcinoma cells.57 In addition to the RAB5a-controlled circuitry we describe in our studies, RAB27a is thus a further important player that influences not only MT1-MMP surface exposure, but also release of other MMPs. These results also point to the likely involvement of additional trafficking and, hence, RABGTPase-controlled pathways in MMP mobilization.
Indeed, it is well established that, for example, pro-MT1-MMP processing occurs in the trans-Golgi network and is a necessary for the delivery of mature MT1-MMP to the plasma membrane, suggesting the involvement of polarized biosynthetic/secretory pathways in confining the activity of this protease to invadopodia during directed cell migration and invasion.3 In keeping with this latter notion, the Golgi complex is frequently found near and oriented toward invadopodia, particularly in highly invasive melanoma cell lines.58 Additionally, MT1-MMP in MDA-MB-231 adenocarcinoma cells can be mobilized from biosynthetic intracellular compartments and undergo rapid RAB8-dependent polarized exocytosis when these cells migrate into collagen type I 3-dimensional matrices.23 Active exocytic transport and diverse molecular components of this transport route have also been shown to sustain ECM degradation at invadopodia.59 An exocytic machinery comprising the endosomal actin nucleation complex, WASH, cortactin, the vesicle-docking exocyst complex, and the SNARE protein vesicle-associated membrane protein 7 (VAMP7) appears to be required for MT1-MMP delivery to invadopodia and invadopodia activity in a subset of tumor cells.60,21,24,28,61-63 Within this context, the essential regulatory RABGTPase is RAB7, and was consistently shown to mediate MT1-MMP dependent matrix degradation in the highly aggressive and invasive, mesenchymal fibrosarcoma HT1080 line.24 Thus, the exocyst and the SNARE-mediated fusion machinery may also be important for invadopodia formation, suggesting that these protein complexes might be linked and cooperate to orchestrate MT1-MMP polarized delivery, at least in in certain tumors.
A plausible framework to rationalize the sum of the evidence outlined above is that different routes of MT1-MMP recycling may be differentially utilized by cancer cells to enhance their invasive potential depending on their mesenchymal or epithelial nature and type of oncogenic lesions. Indeed, key trafficking routes may be specifically targeted and rewired by diverse oncogenic stimuli. In this respect, it is remarkable that TP53 mutations found in human cancers exert part of their oncogenic potential through a P63-dependent (transcriptional-dependent) stimulation of RCP (RAB-coupling protein)-mediated recycling of integrins and EGFR, thus sustaining AKT signaling, ultimately inducing cell migration.64 Similarly, a number of RABGTPases, including RAB4a, are frequently amplified in certain human cancers, and in particular in invasive breast carcinoma,26 reinforcing the notion that MT1-MMP delivery in the context of oncogenic transformation may be achieved by exploiting a diverse set of trafficking routes.
Common and Divergent Mechanisms of RABGTPase-Regulated MT1-MMP Trafficking
Combined, our results indicate that RABGTPases play critical roles in modulating the surface-associated pool of MT1-MMP in both primary macrophages and cancer cells. We identified RABGTPase-regulated circuitries that govern MT1-MMP trafficking via endocytic uptake and subsequent recycling pathways that are used to re-expose the protease on the cell surface. These molecular circuitries have major impacts on extracellular matrix degradation by invadosomes and on cell invasion in 3D or in vivo contexts. Interestingly, the recycling pathways appear to be regulated by different master switches, namely RAB14 in macrophages and RAB4 in cancer cells. Still, both RAB-GTPases appear to fulfill similar functions, as interference with the respective isoform leads to decrease cell surface levels of MT1-MMP in both cell types.
In a curious reversal, the endocytic part of the MT1-MMP circuitry is controlled by RAB5a as a common regulator in both cell types; however, interference with RAB5a activity leads to different outcomes, namely more surface-exposed MT1-MMP in macrophages and less MT1-MMP at the surface of cancer cells. The molecular basis for this discrepancy is currently unclear. However, several differences between both cell systems could be responsible for this: i) MT1-MMP surface levels were measured in macrophages that were cultured in the constant presence of serum, while cancer cells were only stimulated with HGF before the measurements, ii) RAB5a negatively regulates contact of MT1-MMP containing vesicles with podosomes, but is not involved in podosome formation, while it is necessary for invadopodia formation, iii) in invasion of macrophages, the major effect of RAB5a in these cells is via MT1-MMP, as addition of an MT1-MMP inhibitor completely reverts the RAB5a knockdown phenotype. In cancer cells, however, RAB5a also transports integrins and key regulators of actin dynamics, such as RAC1, that are important for invadopodia formation and turnover. iv) In addition, TIMPs (tissue inhibitors of metalloproteinases), which are regulators of MMP activity, might influence the recycling and cell surface activity of MT1-MMP, as the relatively high expression of TIMP2 in cancer cells presumably leads to fast inactivation of MT1-MMP as soon as the protease reaches the surface, thus necessitating continuous delivery of MT1-MMP from internal vesicular pools.
Collectively, our results indicate that both macrophages and cancer cells use a similar strategy, namely RABGTPase-driven trafficking circuitries, to adjust their surface levels of MT1-MMP and thus drive extracellular matrix degradation and invasion. Targeting the regulators of these circuitries, the identified RAB-GTPases and their interactors, may provide alternative ways to modulate cell invasion in physiological and pathological scenarios. On a cautionary note, our results also demonstrate that interfering with these molecular circuitries may lead to different outcomes, depending on the cell type. Care should also be taken that the specific cellular response under observation, such as matrix degradation or cell invasion, is investigated in the appropriate experimental setup.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Christiane Wiesner, Emanuela Frittoli and Andrea Palamidessi for discussions and preparing figures.
Funding
The work discussed here has been supported by Wilhelm Sander Stiftung (grant number 2007.020.02 to SL), the Deutsche Forschungsgemeinschaft (grant number LI925/3–1 and LI925/2–2 to SL), the European Union´s Seventh Framework Program (FP7/2007–2013 (grant agreement number FP7–237946 (T3Net) to SL), the Associazione Italiana per la Ricerca sul Cancro (AIRC,#10168), the Italian Ministries of Education- University-Research (MIUR-PRIN- 2009X23L78), the International Association For Cancer Research (AICR-09–0582), the CARIPLO Foundation (#2010–0737); the European Research Council (Advanced-ERC#268836).
References
- 1. Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007; 7:678-89; PMID:17717539; http://dx.doi.org/ 10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- 2. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013; 229:176-85; PMID:23096265; http://dx.doi.org/ 10.1002/path.4133 [DOI] [PubMed] [Google Scholar]
- 3. Caldieri G, Ayala I, Attanasio F, Buccione R. Cell and molecular biology of invadopodia. Int Rev Cell Mol Biol 2009; 275:1-34; PMID:19491051; http://dx.doi.org/ 10.1016/S1937-6448(09)75001-4 [DOI] [PubMed] [Google Scholar]
- 4. Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 2003; 3:362-74; PMID:12724734; http://dx.doi.org/ 10.1038/nrc1075 [DOI] [PubMed] [Google Scholar]
- 5. Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol 2012; 24:277-83; PMID:22209238; http://dx.doi.org/ 10.1016/j.ceb.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol 2010; 188:11-9; PMID:19951899; http://dx.doi.org/ 10.1083/jcb.200909003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Goethem E, Poincloux R, Gauffre F, Maridonneau-Parini I, Le Cabec V. Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J Immunol 2010; 184:1049-61; PMID:20018633; http://dx.doi.org/ 10.4049/jimmunol.0902223 [DOI] [PubMed] [Google Scholar]
- 8. Wolf K, Friedl P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol 2011; 21:736-44; PMID:22036198; http://dx.doi.org/ 10.1016/j.tcb.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 9. Wiesner C, Le-Cabec V, El Azzouzi K, Maridonneau-Parini I, Linder S. Podosomes in space: macrophage migration and matrix degradation in 2D and 3D settings. Cell Adh Migr 2014; 8(3):179-91; PMID:24713854; http://dx.doi.org/ 10.4161/cam.28116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petrie RJ, Koo H, Yamada KM. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 2014; 345:1062-5; PMID:25170155; http://dx.doi.org/ 10.1126/science.1256965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol 2006; 26:716-28; PMID:16469948; http://dx.doi.org/ 10.1161/01.ATV.0000209518.58252.17 [DOI] [PubMed] [Google Scholar]
- 12. Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer 2007; 7:800-8; PMID:17851543; http://dx.doi.org/ 10.1038/nrc2228 [DOI] [PubMed] [Google Scholar]
- 13. Roycik MD, Fang X, Sang QX. A fresh prospect of extracellular matrix hydrolytic enzymes and their substrates. Curr Pharm Des 2009; 15:1295-308; PMID:19355969; http://dx.doi.org/ 10.2174/138161209787846676 [DOI] [PubMed] [Google Scholar]
- 14. Itoh Y, Seiki M. MT1-MMP: an enzyme with multidimensional regulation. Trends Biochem Sci 2004; 29:285-9; PMID:15276180; http://dx.doi.org/ 10.1016/j.tibs.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 15. Barbolina MV, Stack MS. Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis. Seminars Cell Dev Biol 2008; 19:24-33; PMID:17702616; http://dx.doi.org/ 10.1016/j.semcdb.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol 2001; 153:893-904; PMID:11381077; http://dx.doi.org/ 10.1083/jcb.153.5.893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem 2003; 278:40764-70; PMID:12904296; http://dx.doi.org/ 10.1074/jbc.M306736200 [DOI] [PubMed] [Google Scholar]
- 18. Frittoli E, Palamidessi A, Disanza A, Scita G. Secretory and endo/exocytic trafficking in invadopodia formation: the MT1-MMP paradigm. Eur J Cell Biol 2011; 90:108-14; PMID:20605060; http://dx.doi.org/ 10.1016/j.ejcb.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 19. Remacle A, Murphy G, Roghi C. Membrane type I-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J Cell Sci 2003; 116:3905-16; PMID:12915589; http://dx.doi.org/ 10.1242/jcs.00710 [DOI] [PubMed] [Google Scholar]
- 20. Wiesner C, Faix J, Himmel M, Bentzien F, Linder S. KIF5B and KIF3A/KIF3B kinesins drive MT1-MMP surface exposure, CD44 shedding, and extracellular matrix degradation in primary macrophages. Blood 2010; 116:1559-69; PMID:20505159; http://dx.doi.org/ 10.1182/blood-2009-12-257089 [DOI] [PubMed] [Google Scholar]
- 21. Steffen A, Le Dez G, Poincloux R, Recchi C, Nassoy P, Rottner K, Galli T, Chavrier P. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr Biol 2008; 18:926-31; PMID:18571410; http://dx.doi.org/ 10.1016/j.cub.2008.05.044 [DOI] [PubMed] [Google Scholar]
- 22. Hutagalung AH, Novick PJ. Role of rab GTPases in membrane traffic and cell physiology. Physiol Rev 2011; 91:119-49; PMID:21248164; http://dx.doi.org/ 10.1152/physrev.00059.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bravo-Cordero JJ, Marrero-Diaz R, Megias D, Genis L, Garcia-Grande A, Garcia MA, Arroyo AG, Montoya MC. MT1-MMP proinvasive activity is regulated by a novel rab8-dependent exocytic pathway. EMBO J 2007; 26:1499-510; PMID:17332756; http://dx.doi.org/ 10.1038/sj.emboj.7601606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams KC, Coppolino MG. Phosphorylation of membrane type 1-matrix metalloproteinase (MT1-MMP) and its vesicle-associated membrane protein 7 (VAMP7)-dependent trafficking facilitate cell invasion and migration. J Biol Chem 2011; 286:43405-16; PMID:22002060; http://dx.doi.org/ 10.1074/jbc.M111.297069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wiesner C, El Azzouzi K, Linder S. A specific subset of RabGTPases controls cell surface exposure of MT1-MMP, extracellular matrix degradation and three-dimensional invasion of macrophages. J Cell Sci 2013; 126:2820-33; PMID:23606746; http://dx.doi.org/ 10.1242/jcs.122358 [DOI] [PubMed] [Google Scholar]
- 26. Frittoli E, Palamidessi A, Marighetti P, Confalonieri S, Bianchi F, Malinverno C, Mazzarol G, Viale G, Martin-Padura I, Garre M, et al. A RAB5/RAB4 recycling circuitry induces a proteolytic invasive program and promotes tumor dissemination. J Cell Biol 2014; 206:307-28; PMID:25049275; http://dx.doi.org/ 10.1083/jcb.201403127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol 2011; 27:185-211; PMID:21801014; http://dx.doi.org/ 10.1146/annurev-cellbio-092910-154216 [DOI] [PubMed] [Google Scholar]
- 28. Monteiro P, Rosse C, Castro-Castro A, Irondelle M, Lagoutte E, Paul-Gilloteaux P, Desnos C, Formstecher E, Darchen F, Perrais D, et al. Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. J Cell Biol 2013; 203:1063-79; PMID:24344185; http://dx.doi.org/ 10.1083/jcb.201306162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep 2013; 5:1159-68; PMID:24290760; http://dx.doi.org/ 10.1016/j.celrep.2013.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol 2007; 17:107-17; PMID:17275303; http://dx.doi.org/ 10.1016/j.tcb.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 31. Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 2011; 12:413-26; PMID:21697900; http://dx.doi.org/ 10.1038/nrm3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; http://dx.doi.org/ 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- 33. Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2001; 2:107-17; PMID:11252952; http://dx.doi.org/ 10.1038/35052055 [DOI] [PubMed] [Google Scholar]
- 34. Yamamoto H, Koga H, Katoh Y, Takahashi S, Nakayama K, Shin HW. Functional cross-talk between rab14 and rab4 through a dual effector, RUFY1/Rabip4. Mol Biol Cell 2010; 21:2746-55; PMID:20534812; http://dx.doi.org/ 10.1091/mbc.E10-01-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoshino D, Koshikawa N, Suzuki T, Quaranta V, Weaver AM, Seiki M, Ichikawa K. Establishment and validation of computational model for MT1-MMP dependent ECM degradation and intervention strategies. PLoS Comput Biol 2012; 8:e1002479; PMID:22511862; http://dx.doi.org/ 10.1371/journal.pcbi.1002479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watanabe A, Hoshino D, Koshikawa N, Seiki M, Suzuki T, Ichikawa K. Critical role of transient activity of MT1-MMP for ECM degradation in invadopodia. PLoS Comput Biol 2013; 9:e1003086; PMID:23737743; http://dx.doi.org/ 10.1371/journal.pcbi.1003086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vitale G, Rybin V, Christoforidis S, Thornqvist P, McCaffrey M, Stenmark H, Zerial M. Distinct rab-binding domains mediate the interaction of rabaptin-5 with GTP-bound rab4 and rab5. EMBO J 1998; 17:1941-51; PMID:9524117; http://dx.doi.org/ 10.1093/emboj/17.7.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Goethem E, Guiet R, Balor S, Charriere GM, Poincloux R, Labrousse A, Maridonneau-Parini I, Le Cabec V. Macrophage podosomes go 3D. Eur J Cell Biol 2011; 90:224-36; PMID:20801545; http://dx.doi.org/ 10.1016/j.ejcb.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 39. Schlierf B, Fey GH, Hauber J, Hocke GM, Rosorius O. Rab11b is essential for recycling of transferrin to the plasma membrane. Exp Cell Res 2000; 259:257-65; PMID:10942597; http://dx.doi.org/ 10.1006/excr.2000.4947 [DOI] [PubMed] [Google Scholar]
- 40. Cougoule C, Le Cabec V, Poincloux R, Al Saati T, Mege JL, Tabouret G, Lowell CA, Laviolette-Malirat N, Maridonneau-Parini I. Three-dimensional migration of macrophages requires hck for podosome organization and extracellular matrix proteolysis. Blood 2010; 115:1444-52; PMID:19897576; http://dx.doi.org/ 10.1182/blood-2009-04-218735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of rac is required for its activation and for the spatial restriction of signaling in cell migration. Cell 2008; 134:135-47; PMID:18614017; http://dx.doi.org/ 10.1016/j.cell.2008.05.034 [DOI] [PubMed] [Google Scholar]
- 42. Liu SS, Chen XM, Zheng HX, Shi SL, Li Y. Knockdown of rab5a expression decreases cancer cell motility and invasion through integrin-mediated signaling pathway. J Biomed Sci 2011; 18:58; PMID:21849022; http://dx.doi.org/ 10.1186/1423-0127-18-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu L, Hui-chen F, Chen Y, Zou R, Yan S, Chun-xiang L, Wu-ru W, Li P. Differential expression of RAB5A in human lung adenocarcinoma cells with different metastasis potential. Clin Exp Metastasis 1999; 17:213-9; PMID:10432006; http://dx.doi.org/ 10.1023/A:1006617016451 [DOI] [PubMed] [Google Scholar]
- 44. Onodera Y, Nam JM, Hashimoto A, Norman JC, Shirato H, Hashimoto S, Sabe H. Rab5c promotes AMAP1-PRKD2 complex formation to enhance beta1 integrin recycling in EGF-induced cancer invasion. J Cell Biol 2012; 197:983-96; PMID:22734003; http://dx.doi.org/ 10.1083/jcb.201201065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Torres VA, Mielgo A, Barbero S, Hsiao R, Wilkins JA, Stupack DG. Rab5 mediates caspase-8-promoted cell motility and metastasis. Mol Biol Cell 2010; 21:369-76; PMID:19923319; http://dx.doi.org/ 10.1091/mbc.E09-09-0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Torres VA, Stupack DG. Rab5 in the regulation of cell motility and invasion. Curr Protein Pept Sci 2011; 12:43-51; PMID:21190523; http://dx.doi.org/ 10.2174/138920311795659461 [DOI] [PubMed] [Google Scholar]
- 47. Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst 2000; 92:1185-6; PMID:10904098; http://dx.doi.org/ 10.1093/jnci/92.14.1185A [DOI] [PubMed] [Google Scholar]
- 48. Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T, Nikolsky Y, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell 2008; 13:394-406; PMID:18455123; http://dx.doi.org/ 10.1016/j.ccr.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nystrom ML, Thomas GJ, Stone M, Mackenzie IC, Hart IR, Marshall JF. Development of a quantitative method to analyse tumour cell invasion in organotypic culture. J Pathol 2005; 205:468-75; PMID:15685705; http://dx.doi.org/ 10.1002/path.1716 [DOI] [PubMed] [Google Scholar]
- 50. Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent vs. -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol 2009; 185:11-9; PMID:19332889; http://dx.doi.org/ 10.1083/jcb.200807195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scita G, Di Fiore PP. The endocytic matrix. Nature 2010; 463:464-73; PMID:20110990; http://dx.doi.org/ 10.1038/nature08910 [DOI] [PubMed] [Google Scholar]
- 52. Yu X, Zech T, McDonald L, Gonzalez EG, Li A, Macpherson I, Schwarz JP, Spence H, Futo K, Timpson P, et al. N-WASP coordinates the delivery and F-actin-mediated capture of MT1-MMP at invasive pseudopods. J Cell Biol 2012; 199:527-44; PMID:23091069; http://dx.doi.org/ 10.1083/jcb.201203025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parekh A, Weaver AM. Regulation of cancer invasiveness by the physical extracellular matrix environment. Cell Adh Migr 2009; 3:288-92; PMID:19458499; http://dx.doi.org/ 10.4161/cam.3.3.8888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol 2008; 18:1295-9; PMID:18718759; http://dx.doi.org/ 10.1016/j.cub.2008.07.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roberts MS, Woods AJ, Shaw PE, Norman JC. ERK1 associates with α(v)β 3 integrin and regulates cell spreading on vitronectin. J Biol Chem 2003; 278:1975-85; PMID:12393886; http://dx.doi.org/ 10.1074/jbc.M208607200 [DOI] [PubMed] [Google Scholar]
- 56. Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol 2001; 11:1392-402; PMID:11566097; http://dx.doi.org/ 10.1016/S0960-9822(01)00442-0 [DOI] [PubMed] [Google Scholar]
- 57. Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Thery C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 2012; 72:4920-30; PMID:22865453; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-0925 [DOI] [PubMed] [Google Scholar]
- 58. Baldassarre M, Ayala I, Beznoussenko G, Giacchetti G, Machesky LM, Luini A, Buccione R. Actin dynamics at sites of extracellular matrix degradation. Eur J Cell Biol 2006; 85:1217-31; PMID:17010475; http://dx.doi.org/ 10.1016/j.ejcb.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 59. Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci 2009; 122:3015-24; PMID:19692588; http://dx.doi.org/ 10.1242/jcs.034561 [DOI] [PubMed] [Google Scholar]
- 60. Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res 2006; 66:3034-43; PMID:16540652; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-2177 [DOI] [PubMed] [Google Scholar]
- 61. Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res 2007; 67:4227-35; PMID:17483334; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-3928 [DOI] [PubMed] [Google Scholar]
- 62. Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D'Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of cdc42 and rhoA. J Cell Biol 2008; 181:985-98; PMID:18541705; http://dx.doi.org/ 10.1083/jcb.200709076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yu X, Zech T, McDonald L, Gonzalez EG, Li A, Macpherson I, Schwarz JP, Spence H, Futo K, Timpson P, et al. N-WASP coordinates the delivery and F-actin-mediated capture of MT1-MMP at invasive pseudopods. J Cell Biol 2012; 199:527-44; PMID:23091069; http://dx.doi.org/ 10.1083/jcb.201203025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Muller PAJ, Caswell PY, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P, et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 2009; 139:1327-41; PMID:20064378; http://dx.doi.org/ 10.1016/j.cell.2009.11.026 [DOI] [PubMed] [Google Scholar]


