Abstract
The role of polarity in cancer is an emerging research area and loss of polarity is widely considered an important event in cancer. Among the polarity regulating molecules, the small GTPase Cdc42 was extensively studied. Most attention was given to Cdc42 signaling at the plasma membrane, but whether and how Cdc42 is regulated at endomembranes remained poorly understood. Moreover, whether the endomembrane pool of Cdc42 is of any relevance to cell polarity was unknown. In our recent work, we identified a complex between the Golgi matrix protein GM130 and RasGRF and showed that it is responsible for regulating the Golgi pool of Cdc42, but had no effect on the plasma membrane pool of Cdc42. Depletion of GM130 disrupted apico-basal polarity as well as front-rear polarity, indicating that the spatial pool of Cdc42 is functionally relevant. The biomedical relevance of this finding was supported by the observation than GM130 is progressively lost in colorectal cancer. These findings support a role of the endomembrane pool of Cdc42 in cell polarity and point to a potential role of alterations of this pool in cancer.
Keywords: cancer, Cdc42, golgi apparatus, GM130, RasGRF
Establishing and maintaining cell polarity is an important characteristic of most epithelial cells of high relevance for physiologic cell function. Apico-basal polarization allows cells to create specialized plasma membrane domains that carry out different functions. Polarization in neuronal cells allows them to differentiate axons from dendrites and the formation of an immunological synapse in immune cells is also dependent on cell polarity. In migrating cells, establishing and sustaining front-rear polarity is important for directed cell movement. Therefore, the polarity machinery is essential for cell and tissue homeostasis the normal cell function. Based on this, alterations of polarity are expected to result in a loss of cell differentiation (i.e. dysplasia), which in turn promotes tumorigenesis. As such, several researchers have explored a possible connection of polarity to cancer. Epithelial-mesenchymal transition (EMT) is a process that is considered of great relevance for the formation of metastatic lesions.1,2 One of the hallmarks of EMT is the loss of cell polarity,3 but it remains currently unclear how important loss of polarity is for the pro-metastatic potential of EMT. Nevertheless, there is ample evidence that loss of polarity plays an important role in cancer. For instance, disruption of polarity by depletion of Par3, was shown to potentiate the tumorigenic and metastatic potential of breast tumors in mice and in accordance with this, human breast cancers were found to express less Par3 than the normal mammary gland.4 Another group found that knockdown of Par3 synergized with ErbB2 to induce cell invasion and metastasis in vivo.5
Given the role of polarity in cancer, it becomes important to understand its regulation, with respect to the signaling pathways that orchestrate cell polarity and the crosstalk with other cellular processes such as endocytosis and secretory membrane traffic. Signaling by small GTPases of the Rho family was shown to be of central importance for the regulation of polarity.6,7 In particular, Cdc42 was shown to play a central role for various forms of polarity in yeast, drosophila and mammals.8,9 However, most (if not all) of the data available on Cdc42 function are based on events that take place at the plasma membrane. Intracellular, endomembrane pools of Cdc42 were detected previously, but whether these are functionally relevant for polarity has not been addressed before. In our recent work, we studied the regulation of the Golgi pool of Cdc42 and investigated whether it plays a role in cell polarity.10 To study spatial regulation of Cdc42, we employed Raichu-probes that are based on fluorescence resonance energy transfer (FRET).11 We showed that depletion of the Golgi matrix protein GM130 reduced the activity of Cdc42 at the Golgi, but did not affect the plasma membrane pool of Cdc42 in the same cell (see schematic in Fig. 1A). Thus, we had a system that enabled us to study whether polarity can be established or maintained in cells with normal cell surface Cdc42, but with a defect in Golgi-localized Cdc42. Of note, no alteration of Golgi structure and ER-Golgi trafficking were observed upon GM130 depletion.10 We found that depletion of GM130 disrupts polarity, which indicates that spatial regulation of Cdc42 is relevant for cell polarization. In directionally migrating cells, we suggest a model wherein the Golgi supplies the leading edge with active Cdc42, thereby leading to an asymmetric distribution of Cdc42-GTP. We experimentally verified this asymmetry and demonstrated its dependence on post-Golgi trafficking and on GM130.10 Mechanistically, we showed that GM130 controls Cdc42 activity by sequestering the Cdc42 inhibitor RasGRF12 to the Golgi. In the absence of GM130, RasGRF is free to bind and inhibit Cdc42. At the same time, RasGRF hyperactivates Ras signaling. We hypothesize that this imbalance between Ras and Cdc42 is relevant for cancer, which is supported by the observation of GM130 is downregulated in colonic cancer.10 Whether this correlates with higher Ras activity and lower Cdc42 in tumors needs to be demonstrated in the future.
Figure 1.
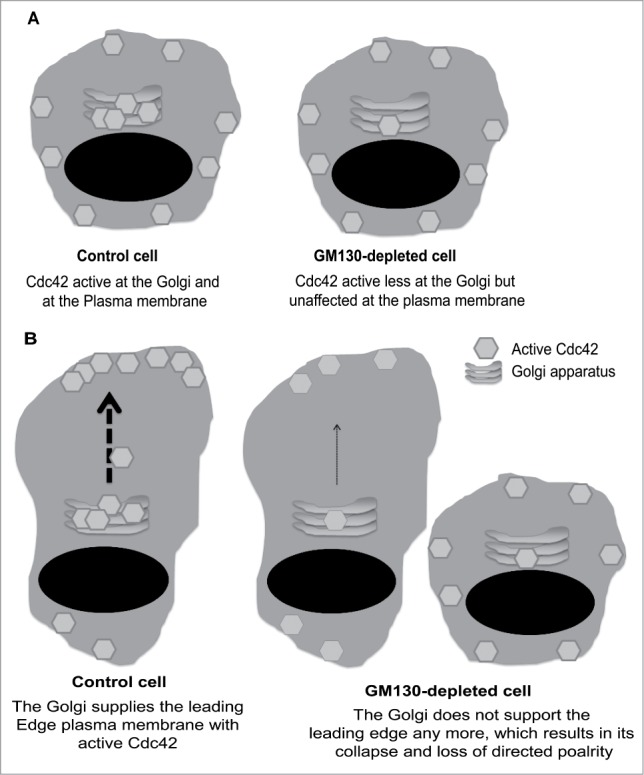
Model illustrating how the GM130-controlled Cdc42 pool at the Golgi regulates cell polarity.
Our work not only illustrates that the Golgi pool of Cdc42 is relevant for polarity, it also suggests an active role of the Golgi apparatus in the control of cell polarity. Given the widely recognized connection between Golgi and cell migration, it is somehow surprising that studies on Golgi and tumor progression are still very scarce. More than 3 decades ago, the Golgi was noted to rapidly orient toward the leading edge plasma membrane.13 Later research from several laboratories convincingly demonstrated that structural integrity of the Golgi is important for directed cell motility and for polarized secretion.14-17 However, it was never clear whether the Golgi itself controls polarity signaling, or whether it is simply a receiver of signals and itself does not shape the biologic outcome of polarity signaling. Early evidence on an active role of the Golgi came from 2 papers that described a role for GM130 in controlling polarized cell movement,18,19 which is in agreement with our recent findings.10 How GM130 controls polarity and whether polarity forms other than front-rear polarity are affected was not known. Our recent work provides a further step toward a better understanding of the role of GM130 (and the Golgi) in cell polarity.
Although there is wide agreement that polarity is important for tumorigenesis, the precise role that loss of polarity plays remains poorly understood. Therefore, we are currently only able to speculate about how alteration of spatial Cdc42 signaling could be linked to cancer. In order to do so we will break the tumorigenesis cascade into several steps and discuss the relevance of polarity for each step. The first question to ask is whether loss of polarity is sufficient to initiate cancer, or whether it is a factor that promotes growth and metastasis of an already initiated tumor.
Classically, a condition is considered as cancer-initiating if it alters cell homeostasis in way that confers growth advantage and immortality. This is typically achieved through activation of pro-proliferative pathways, and the suppression of senescence and pro-apoptotic pathways. There is currently no clear evidence that loss of polarity is a cancer-initiating event. On one hand, a lot of research is performed in transformed or immortalized cells, and in order to speak of a true cancer-initiation is it important to work with normal cells. Such an approach has already been performed by depleting the polarity regulator Par3 from primary mammary epithelial cells (MECs) and monitoring their fate after transplanting them orthotopically. This approach showed that sole Par3 depletion inhibits regrowth of the mammary gland due to increased aporptosis.4 Based on this, loss of Par3 is unlikely to be a cancer initiating event. However, loss of Par3 synergized with oncogenes like Ras or ErbB2 to promote tumorigenesis,4,5 thus placing loss of Par3 as a tumor-promoting event. Overexpression of Par6b resulted in hyperactive ERK and increased proliferation in breast cancer cell lines.20 Since ERK1/2 is a major growth-promoting pathway, this result tempts to conclude that loss of polarity induces hyperproliferation. However, since the work relied on the use of transformed breast cancer cells, it is not correct to consider polarity loss as an initiating event, considering that initiation has already occurred. Finally, a transgenic mouse expressing in epithelial breast a mutant of Scribble that localizes away from cell-cell junctions showed hyperproliferation of epithelial cells. Scribble mislocalization was described to affect subcellular localization of PTEN resulting in activation of the Akt/mTOR/S6kinase signaling pathway. Eventually, as the mice got older, tumors developed in their mammary glands. Such tumors showed a high degree of heterogeneity, thus it is likely that they developed because of mutations acquired by the cells that were proliferating abnormally fast, but it is not clear whether mislocalizaion of Scribble, which is often observed in breast cancer, can serve as a true tumor initiator event.21 Currently, it is not clear whether loss of polarity imposed by alteration of spatial Cdc42 activity is sufficient to be a tumor-initiating event. This would require depleting GM130 in MECs and determining the ability of these cells to re-grow a mammary gland and whether the newly generated organ is more prone to develop cancer. In addition, GM130 must be depleted together with oncogenes to determine whether, like in the case of Par3, it also potentiates the pro-metastatic potential of the tumor. In addition to GM130 depletion, we identified a number of Rho family exchanges factors that when depleted, reduce the activity of Cdc42 at the Golgi.10 We tested the 3 strongest candidates, ARHGEF9, 11 and12 and found that none of them interacts with GM130. Therefore, the mechanism by which they regulate the Golgi pool of Cdc42 must be a different one that still needs to be resolved. ARHGEF12 (also known as LARG) was previously reported to act as a tumor suppressor in human breast and colorectal cancer.22 Testing the role of these GEFs in cancer, in the context of spatial Cdc42 signaling represents and exciting future research area.
In summary, our data and those of others support the notion that loss of polarity is a cancer-relevant event. Other, processes of relevance to cancer such as angiogenesis, hypoxia signaling and metabolic remodeling will all have to be analyzed in the future for their connection to polarity signaling (both at the plasma membrane as well as from endomembranes). Thereby, we will gain an integrative understanding of the role of polarity in cell homeostasis and thereby be better equipped to understand how alterations of the cell polarity machinery contribute to cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by support from the German Science Foundation (DFG), by a Young Scholar Fund of the University of Konstanz, by the Swiss Science Foundation and by the Biotechnology Institute Thurgau.
References
- 1.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119:1420-8; PMID:19487818; http://dx.doi.org/ 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science 2013; 342:1234850; PMID:24202173; http://dx.doi.org/ 10.1126/science.1234850 [DOI] [PubMed] [Google Scholar]
- 3.Godde NJ, Galea RC, Elsum IA, Humbert PO. Cell polarity in motion: redefining mammary tissue organization through EMT and cell polarity transitions. J Mammary Gland Biol Neoplasia 2010; 15:149-68; PMID:20461450; http://dx.doi.org/ 10.1007/s10911-010-9180-2 [DOI] [PubMed] [Google Scholar]
- 4.McCaffrey LM, Montalbano J, Mihai C, Macara IG. Loss of the Par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer Cell 2012; 22:601-14; PMID:23153534; http://dx.doi.org/ 10.1016/j.ccr.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue B, Krishnamurthy K, Allred DC, Muthuswamy SK. Loss of Par3 promotes breast cancer metastasis by compromising cell-cell cohesion. Nat Cell Biol 2013; 15:189-200; PMID:23263278; http://dx.doi.org/ 10.1038/ncb2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mack NA, Georgiou M. The interdependence of the Rho GTPases and apicobasal cell polarity. Small GTPases 2014; 5(2):1-16; PMID:25469537; http://dx.doi.org/ 10.4161/21541248.2014.973768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu CF, Lew DJ. Beyond symmetry-breaking: competition and negative feedback in GTPase regulation. Trends Cell Biol 2013; 23:476-83; PMID:23731999; http://dx.doi.org/ 10.1016/j.tcb.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stengel K, Zheng Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signal 2011; 23:1415-23; PMID:21515363; http://dx.doi.org/ 10.1016/j.cellsig.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etienne-Manneville S. Cdc42-the centre of polarity. J Cell Sci 2004; 117:1291-300; PMID:15020669 [DOI] [PubMed] [Google Scholar]
- 10.Baschieri F, Confalonieri S, Bertalot G, Di Fiore PP, Dietmaier W, Leist M, Crespo P, Macara IG, Farhan H. Spatial control of Cdc42 signalling by a GM130-RasGRF complex regulates polarity and tumorigenesis. Nat Commun 2014; 5:4839; PMID:25208761; http://dx.doi.org/ 10.1038/ncomms5839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N, Nagashima K, Matsuda M. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J. Cell Biol 2003; 162, 223-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvo F, Sanz-Moreno V, Agudo-Ibáñez L, Wallberg F, Sahai E, Marshall CJ, Crespo P. RasGRF suppresses Cdc42-mediated tumour cell movement, cytoskeletal dynamics and transformation. Nat Cell Biol 2011; 13: 819-26; http://dx.doi.org/ 10.1038/ncb2271 [DOI] [PubMed] [Google Scholar]
- 13.Kupfer A, Louvard D, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc Natl Acad Sci U S A 1982; 79:2603-7; PMID:7045867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav S, Puri S, Linstedt AD. A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell 2009; 20:1728-36; PMID:19158377; http://dx.doi.org/ 10.1091/mbc.E08-10-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millarte V, Farhan H. The Golgi in cell migration: regulation by signal transduction and its implications for cancer cell metastasis. ScientificWorldJournal 2012; 2012:498278; PMID:22623902; http://dx.doi.org/ 10.1100/2012/498278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiterer V, Fey D, Kolch W, Kholodenko BN, Farhan H. Pseudophosphatase STYX modulates cell-fate decisions and cell migration by spatiotemporal regulation of ERK1/2. Proc Natl Acad Sci U S A 2013; 110: E2934-43; PMID:23847209; http://dx.doi.org/ 10.1073/pnas.1301985110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurtado L, Caballero C, Gavilan MP, Cardenas J, Bornens M, Rios RM. Disconnecting the Golgi ribbon from the centrosome prevents directional cell migration and ciliogenesis. J Cell Biol 2011; 193: 917-33; PMID:21606206; http://dx.doi.org/ 10.1083/jcb.201011014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preisinger C, Short B, De Corte V, Bruyneel E, Haas A, Kopajtich R, Gettemans J, Barr FA. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta. J Cell Biol 2004; 164:1009-20; PMID:15037601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodani A, Kristensen I, Huang L, Sütterlin C. GM130-dependent control of Cdc42 activity at the Golgi regulates centrosome organization. Mol Biol Cell 2009; 20:1192-200; PMID:19109421; http://dx.doi.org/ 10.1091/mbc.E08-08-0834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nolan ME, Aranda V, Lee S, Lakshmi B, Basu S, Allred DC, Muthuswamy SK. The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res 2008; 68: 8201-9; PMID:18922891; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feigin ME, Akshinthala SD, Araki K, Rosenberg AZ, Muthuswamy LB, Martin B, Lehmann BD, Berman HK, Pietenpol JA, Cardiff RD, et al.. Mislocalization of the cell polarity protein scribble promotes mammary tumorigenesis and is associated with basal breast cancer. Cancer Res 2014; 74(11):3180-94; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong DC, Ho YM, Rudduck C, Chin K, Kuo WL, Lie DK, Chua CL, Tan PH, Eu KW, Seow-Choen F, et al.. LARG at chromosome 11q23 has functional characteristics of a tumor suppressor in human breast and colorectal cancer. Oncogene 2009; 28: 4189-200; PMID:19734946; http://dx.doi.org/ 10.1038/onc.2009.266 [DOI] [PMC free article] [PubMed] [Google Scholar]


