Abstract
Phagocytosis is defined as a cellular uptake pathway for particles of greater than 0.5 μm in diameter. Particle clearance by phagocytosis is of critical importance for tissue health and homeostasis. The ultimate goal of anti-pathogen phagocytosis is to destroy engulfed bacteria or fungi and to stimulate cell-cell signaling that mount an efficient immune defense. In contrast, clearance phagocytosis of apoptotic cells and cell debris is anti-inflammatory. High capacity clearance phagocytosis pathways are available to professional phagocytes of the immune system and the retina. Additionally, a low capacity, so-called bystander phagocytic pathway is available to most other cell types. Different phagocytic pathways are stimulated by particle ligation of distinct surface receptors but all forms of phagocytosis require F-actin recruitment beneath tethered particles and F-actin re-arrangement promoting engulfment, which are controlled by Rho family GTPases. The specificity of Rho GTPase activity during the different forms of phagocytosis by mammalian cells is the subject of this review.
Keywords: clearance, digestion, engulfment, f-actin recruitment, internalization, membrane receptors, phagocytic cup, phagosome
Introduction
Phagocytosis is a cellular clearance process that processes solid particles of usually at least 0.5 μm in diameter. Phagocytosis is initiated when cells employ a specific receptor protein to recognize and bind particles. Phagocytic targets usually have complex topology. Receptor choice is determined by the availability of active but idle surface receptors of the phagocytic cell, by recognition patterns, often referred to as “eat me” signals,1 exposed on phagocytic particles (defined as particles that trigger their own engulfment by phagocytic pathways, e.g. pathogens), and by availability of appropriate levels of opsonins, soluble multi-domain proteins that bridge particle and phagocytic cell by binding to both particle “eat-me” signals and phagocyte receptors. Subsequent engulfment of surface-tethered particles may employ additional cell surface proteins and relies on a complex of cytosolic proteins recruited beneath bound particles, the specificity of which depends on the nature of recognition/binding receptors.
Differences in phagocytic processes depending on the phagocytic particle
There are 2 principle types of phagocytosis:
Anti-pathogen phagocytosis serves the dual purpose of removing and degrading disease-causing microbes and, through complex cytokine and chemokine secretion, recruiting activities of numerous immune cells to mount an effective inflammatory defense. This form of phagocytosis is often referred to as “inflammatory phagocytosis” but this term does not reflect the fact that cells may mount anti-inflammatory responses following pathogen uptake, e.g., in persistent infections. We shall therefore use the term anti-pathogen phagocytosis. Anti-pathogen phagocytosis is most commonly performed by professional phagocytes of the myeloid lineage, such as immature dendritic cells (DCs) and macrophages but also by tissue-resident immune cells. Several microorganisms have evolved mechanisms to block the recognition and engulfment by immune cells, therefore evade the attack from innate immune system (for a detailed review see ref. 2).
Phagocytosis of apoptotic cells or cell debris, also known as clearance phagocytosis or efferocytosis,3 is non-inflammatory or anti-inflammatory and non-immunogenic.4 There are numerous developmental events that involve pruning large numbers of cells by induction of apoptosis. Following an inflammatory reaction, activated immune cells also die by apoptosis. In such instance, immune cells engulfing apoptotic cells switch their cytokine secretion program from a pro-inflammatory to an anti-inflammatory repertoire.5 The same cell type, e.g. macrophages and DCs, may thus phagocytose via both anti-pathogen and apoptotic debris phagocytosis pathways. Furthermore, billions of cells commit to die by apoptosis every day during normal tissue turnover silently and without activating either pro- or anti-inflammatory pathways. Finally, there are specialized tissue renewal pathways that involve release of apoptotic cell-like particles from non-apoptotic cells and subsequent clearance phagocytosis. These include the consumption of residual cytoplasm during spermatogenesis by Sertoli cells and the diurnal phagocytosis of spent, shed photoreceptor outer segment tips by retinal pigment epithelial (RPE) cells in the eye. Swift and complete clearance phagocytosis is essential to prevent unwarranted inflammation auto-immune responses. Incomplete or delayed clearance of apoptotic cells therefore contributes to autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus.6 Failure of efficient clearance of photoreceptor outer segment tips causes blindness,7 while phagocytic deficiency of Sertoli cells blocks spermatogenesis and may result in sterility.8
Universal dependence of all forms of phagocytosis on dynamic F-actin
Although the 2 types of phagocytosis, anti-pathogen and apoptotic cell clearance phagocytosis, process different particles and have different systemic consequences, their mechanisms share many molecular characteristics. In general, phagocytosis is a step-wise process in which particles are first recognized and tethered to the cell surface, followed by internalization and formation of an intracellular membrane enclosed organelle, a phagosome. The initial contact between the target particle and phagocytes is crucial for determining which receptors will be involved in engulfment, how that information will be processed, and whether or not to trigger a pro-inflammatory or anti-inflammatory response.
All forms of phagocytosis have in common that F-actin is recruited beneath surface bound particles into defined 3-dimensional structures and that these F-actin structures must then resolve to allow membrane extension resulting in particle internalization. Local shape changes of the plasma membrane to wrap around the phagocytic target are guided by receptor-target interactions and often mediated by opsonins, soluble multi-domain proteins that bridge target particle and phagocytes receptor proteins. The structure formed from a network of branched F-actin beneath bound particles during engulfment by most forms of phagocytosis has been termed a phagocytic cup.9 F-actin polymerization is crucial for phagocytic cup formation, and F-actin depolymerizing drugs, cytochalasins and latrunculin, block engulfment via all types of phagocytosis.10,11 The closure of the phagocytic cup, which involves fusion of protruded membranes and disassembly of F-actin, leads to particle internalization and phagosome formation. Phagosomes mature through series of fusion and trafficking events to phagolysosomes and then lysosomes.
The extensive F-actin re-organization required for all forms of phagocytosis is controlled by the Rho GTPase family. Rho GTPases form the branch of the Ras small GTPase superfamily that is responsible for F-actin regulation in all cell types. Rho GTPases share a highly conserved GTPase domain, and possess short, divergent N- and C-termini (for a review focusing on protein domain structure and sequence comparison of Rho GTPases, please see ref. 12). An insertion region (CAAX box) at the C-terminus of Rho GTPases may be modified by cells post-translationally to form specific lipid esters, which can target Rho GTPases to membranes of different subcellular compartments (for a review specifically of Rho GTPase lipid modification, please see ref. 13). Their innate GTPase activity allows them in principle to switch autonomously between an inactive, GDP-bound state and an active, GTP-bound state. However, activation of Rho GTPases in cells, like that of other GTPases, is subject to control by large numbers of regulatory proteins of 3 functionally distinct categories: guanine nucleotide exchange factors (GEFs) promote GTP load; GDP dissociation inhibitors (RhoGDIs) sequester and stabilize cytosolic GDP-bound Rho GTPases 14; and GTPase activation proteins (GAPs) accelerate GTP hydrolysis (for a recent review focusing on these 3 classes of GTPase regulators, please see ref. 15).
The human genome contains 22 genes encoding 25 human Rho GTPase proteins.12 Among them, the specific roles of RhoA, Rac1, and Cdc42 in shaping and regulating the dynamics of F-actin are best understood.16 They are ubiquitously expressed, prototypical Rho GTPase family members and activation of each of them promotes characteristic and distinct F-actin cytoskeletal morphology.16 Activation of RhoA (or its isoforms RhoB and RhoC) stimulates acto-myosin contraction and F-actin stabilization and is required for the formation of stress fibers. Activation of Rac1 (or its isoforms Rac2, Rac3, and RhoG) promotes assembly of branched F-actin networks and is required for the formation of lamellipodia. Activation of Cdc42 promotes formation of short actin filaments and is required for formation of filopodia. These Rho family GTPases induce distinct effects on F-actin by non-covalently and transiently interacting with different cytosolic downstream effector proteins. These effectors are allosteric switches that function to engage further regulatory proteins that in turn directly interact with G- or F-actin (reviewed in ref.17). Here, we will discuss the regulation, activities, and effectors of Rho GTPases in phagocytosis pathways by mammalian cells.
Rho GTPase Activity During Anti-Pathogen Phagocytosis
Anti-pathogen phagocytosis is performed by professional phagocytes of the immune system. Macrophages especially co-express numerous surface receptor proteins whose recognition of pathogen patterns or specific motifs induces pathogen binding and engulfment, which requires extensive F-actin dynamics. Here, we will discuss phagocytosis via 3 different macrophage phagocytic receptors, for which Rho GTPase involvement in anti-pathogen phagocytosis has been investigated extensively.
Phagocytosis mediated by Fc receptors
Single span transmembrane proteins recognizing the constant Fc domain of immunoglobulins (Igs) form the Fc receptor (FcR) family. In mammals, there is one specific FcR for IgA, IgE, and IgM each, a common receptor for both IgA and IgM, and there are 4 FcγR receptors for IgG.18 The cytosolic tyrosine kinase Syk plays a key role in downstream signaling pathways of FcRs that are otherwise complex and diverse.19 Interaction with Igs is sufficient to stimulate both binding and engulfment of Ig-opsonized particles.
Because professional phagocytes of the immune system co-express a variety of phagocytic receptors connected to different or overlapping signaling and engulfment pathways, crosstalk between phagocytic pathways is common. Studying molecular pathways downstream of individual phagocytic receptors has long relied on cell culture experiments in which individual phagocytic receptors are exogenously expressed in cell types, especially fibroblasts, which lack endogenous professional and anti-pathogen phagocytic capacity. Using this approach, Caron and Hall in a classic set of experiments found that antibody crosslinking of cell-surface FcγRIIA in Swiss 3T3 fibroblasts is sufficient to induce formation of Cdc42-dependent filopodia and Rac1-dependent membrane ruffles.16 Challenge with IgG-opsonized particles of these FcγRIIA-expressing cells recruited all 3 GTPases, RhoA, Rac1, and Cdc42, into F-actin phagocytic cups. Blocking the activity of Cdc42 or Rac1 by expression of dominant-negative mutant forms prevented formation of filopodia or membrane ruffles even when FcγRIIA was engaged by bound particles. Such inhibition of either Rac1 or Cdc42 also resulted in failure of the cells to take up IgG-opsonized red blood cells (IgG-RBCs) by FcγRIIA-transfected Cos fibroblasts.16 A similar result was obtained during phagocytosis via endogenous FcεRI by basophil leukemia mast cells in culture. Uptake of IgE-coated zymosan particles was inhibited by expression of either Rac1 or Cdc42 dominant-negative mutant proteins.20 In these experiments, F-actin assembled at least to some extent beneath bound particles but phagocytic cups did not close to permit internalization. More recently, Hoppe and Swanson observed microscopically in live cells the interaction of Rho GTPases with the p21 GTPase-binding domain of PAK1, which is a known downstream effector of Rac1, Rac2, and Cdc42.21 Using fluorescence resonance energy transfer (FRET) imaging, these investigators tracked activation and localization of both total and active Rho GTPases in real time during FcγR-mediated phagocytosis in RAW 264.7 macrophages. Their results confirmed the relocalization of Rac1 and Cdc42 to phagocytic particles and showed that Rac2 was also recruited. Active Cdc42 completely colocalized with F-actin in phagocytic cups to promote pseudopods formation during membrane extension. Rac1 also participated in the initial step of pseudopod formation, probably to promote local contractile activity. Additionally, active Rac1 and Rac2 associated with nascent phagosomes immediately before their closure possibly to regulate reactive oxygen species production and/or phagosome pH.22 An RNAi screen suggested that Rac2 may be more relevant to FcR-dependent phagocytosis by J774 macrophages than Rac1.23 The same study also suggested that yet another Rho GTPase family member of the Rac subfamily, RhoG, may contribute to FcR-mediated phagocytosis. Depletion of RhoG abolished F-actin recruitment to phagocytic cups, thereby attenuating the phagocytic capacity of J774 macrophages. RhoG has been linked to the activation of Rac1 through its interaction with the scaffolding protein ELMO, together with which Rac-GEF DOCK-180 regulates Rac1 activation during cell migration24 and apoptotic cell phagocytosis (discussed below).25 However, the signaling pathway from RhoG to Rac1 has not yet been reported to contribute to FcR-mediated phagocytosis.
The role of RhoA in FcR-dependent phagocytosis may be distinct from the roles of Rac1 and Cdc42. Hackam and Grinstein in 1997 reported a requirement for RhoA in FcγRIIA-dependent phagocytosis of J774 macrophages or transfected Cos fibroblasts by imaging cells challenged with IgG-opsonized latex beads after microinjection of the RhoA inhibiting bacterial toxin C3 transferase. Their experiments showed dramatic effects of RhoA inhibition on both total uptake as well as numbers of acidified bead-containing phagosomes suggesting that both particle binding and engulfment was affected. Yet, they also reported dramatic overall reduction in F-actin in microinjected cells. In contrast, Caron and Hall studying the same FcR and model cell types did not find decreased phagocytosis of IgG-RBC upon RhoA inactivation and in their experiments the cellular F-actin cytoskeleton was largely preserved.16 Later on, Olazabal and colleagues showed that inhibition of key RhoA downstream effectors, Rho kinases (ROCKs), with the pharmacological agent Y-27632 did not affect F-actin recruitment or FcγR-dependent IgG-RBC phagocytosis by J744 macrophages.26 In contrast, Hall and colleagues observed decreased IgG-RBC phagocytosis after introducing TAT-C3 transferase into bone marrow-derived macrophages (BMMs) under experimental conditions that maintained largely normal F-actin recruitment.27 Thus, all evidence indicates that RhoA has no role in phagocytic cup formation and it remains controversial whether or not RhoA contributes to a late stage of FcR dependent phagocytosis. Altogether, RhoA, Rac1, Rac2 and Cdc42 differ in precise localization and time of activation at phagocytic cups suggesting that they play different and specific roles in regulating progression of FcγR-dependent phagocytosis (Fig. 1A).
Figure 1.
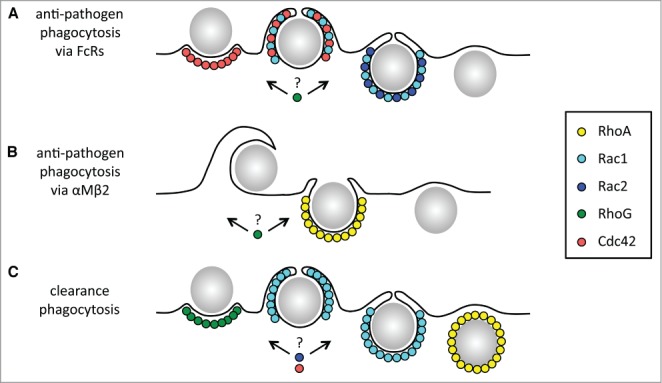
Distinct roles and recruitment of Rho GTPases to phagocytic particles at different steps during anti-pathogen phagocytosis via Fc receptors or αMβ2 integrin/CR3 and during clearance phagocytosis mediated by αv integrins and TAM receptors. The time course and distribution of Rho GTPases activation during phagocytosis differs depending on phagocyte receptors as illustrated for FcR-dependent phagocytosis (A), αMβ2-mediated phagocytosis (B), and clearance phagocytosis (C). (B) Note phagocytic particle capture in αMβ2-mediated phagocytosis involves F-actin-rich membrane ruffles that require Rap1-talin signaling, which is also responsible for αMβ2 activation. There are no published studies indicating a role for Rho GTPases in this process. (A-C) Rho GTPases that contribute to the different forms of phagocytosis but with yet unknown localization or specific role are also indicated. Note that most cytoplasmic proteins also involved in phagocytic signaling but not directly implicated in RhoGTPase activity have been omitted from this scheme.
Upstream pathways of Rho GTPases during FcR-dependent particle engulfment have been the focus of numerous investigations. Inactive Rho GTPases are distributed in the cytosol in association with Rho-GDIs.28 Activation and function of Rho GTPases requires dissociation from Rho-GDI and re-association with the plasma membrane. This cycle of cytosol-membrane relocalization of Rho GTPases remains poorly understood and has yet to be studied specifically during phagocytosis. In brief, cells may allosterically regulate Rho-GDI affinity for Rho GTPases. For instance, Rho-GDI phosphorylation by Src-family kinases decreases its affinity for RhoA, Rac1, and Cdc42.29 Moreover, anionic membrane phospholipids such as PI(3,4,5)P3 compete with the negatively charged N-terminus of Rho-GDI for binding to the polybasic C-terminus of Rac1.30,31 Thus, altering abundance of specific membrane lipids may facilitate Rac1 release from Rho-GDI, promoting in turn its membrane insertion and activation. Interestingly, some pathogens, e.g., select Yersinia strains, secrete proteins with Rho-GDI function to block activation of Rac1, causing phagocytic deficiency in infected macrophages.32
The link between surface FcγR and Rac1 activation was first thought to be the Rac-GEF, Vav, as microinjection of a mutant, non-functional SH2-domain of Vav proposed to act as dominant-negative mutant decreased IgG-opsonized RBCs uptake in J774 macrophages. This reduction in phagocytic capacity was accompanied by decrease of Rac1 activation during phagocytosis.33 However, genetic depletion of all 3 Vav genes had no effect on FcγR-mediated phagocytosis of IgG-RBCs by BMMs.27 This suggests that the SH2-domain mutant of Vav may interact with and affect functions of proteins other than Vavs that are required for phagocytosis. Testing a different Rac-GEF, Lee and colleagues found that the adaptor protein CrkII and the Rac-GEF DOCK-180 both reside in a complex with Fc receptors during phagocytosis.34 Either overexpression of a dominant-negative mutant of CrkII or siRNA-mediated silencing of CrkII or DOCK-180 was sufficient to decrease phagocytosis of IgG-opsonized beads. This decrease of phagocytosis is probably caused by a failure of cells to recruit Rac1 to the phagocytic site when either protein is absent.
The molecular links of FcRs and Cdc42 remain under investigation. The work by Patel and colleagues suggests that activation of Cdc42 downstream of FcγR during phagocytosis is independent of Vav.33 However, overexpression of Vav induced Cdc42-dependent filopodia formation.35 A very large number of candidate GEFs for Cdc42, often co-expressed and only some of them specific for Cdc42,36 continue to make it difficult to pinpoint GEFs required for Cdc42-dependent phagocytosis.
Notably, the RhoA GEF p115-Rho-GEF (but not RhoA) has been detected in a complex with ligand-bound FcγR in RAW 264.7 macrophages.37 Moreover, expression of a dominant-negative mutant form of p115 or siRNA-mediated silencing decreased phagocytosis of IgG-opsonized beads by these cells. Like with Rho-GDI function, pathogenic bacteria may interfere with p115 Rho-GEF function to attenuate their FcγR-dependent phagocytosis and destruction by macrophages.38 It is however not yet clear whether this attenuation is specifically dependent on p115 Rho-GEF as bacteria also reduce function of another DH-PH domain containing RhoGEF, Dbl, decreasing Cdc42 activity.38
Little is known to date about Rho GTPase inactivation following their contributions to FcR-dependent phagocytosis. Immediately following phagocytic cup formation, Cdc42 activity must decrease to allow phagocytic cup closure: persistent activation of Cdc42 by a constitutively active mutant form of Cdc42 decreases phagocytosis.39 In contrast, persistent activation of Rac1/2 has no such inhibitory effect. Increased local concentration of PtdIns(3,4,5)P3 in the membrane extensions around the phagocytosed particle is responsible for Cdc42 inactivation, which will subsequently promote disassembly of phagocytic cups.39 The brief duration of Rho GTPase activity and localization to phagocytic sites during phagocytic processes suggests that GTPases inactivating GAPs are very likely important. Most cell types co-express several GAPs and their contributions to phagocytosis have not yet been tested systematically. However, the Rac-GAP, ARH25-GAP, has recently been identified as a negative regulator of FcR-dependent phagocytosis.40
Phagocytosis mediated by dectin-1
The single span transmembrane protein dectin-1 is involved in innate immunity against fungal infection.41 Recognition by its extracellular C-type lectin-like domain of cell wall components, such as β-1,3-glucans stimulates internalization and degradation of fungal pathogens accompanied by activation of pro-inflammatory pathways.42 Of note, we are discussing only dectin-1 here but other pattern recognition receptors are also important in phagocytic processes. For instance, the related dectin-2 also plays important roles in pro-inflammatory phagocytosis of fungal particles.43 However, we will focus on dectin-1 here as the molecular pathways regulating F-actin in response to dectin-2 engagement has not yet been reported. Its F-actin phagocytic cup formation is morphologically similar to phagocytic cup formation in FcR-mediated phagocytosis. In a subset of macrophages, dectin-1-mediated phagocytosis activates the tyrosine kinase Syk that is also downstream of FcRs although this activation is not required for engulfment.44 Upon dectin-1 ligation, F-actin structures protrude from the phagocyte and promote tight wrapping of plasma membrane around the bound particle involving activation of Rac1 and Cdc42, but not RhoA.45,46 However, unlike in FcR-dependent phagocytosis, Rac1 and Cdc42 activity may not be strictly required for dectin-1-mediated phagocytosis: Toxin B treatment of macrophages reduced but did not abrogate their uptake of zymosan particles made from the insoluble fraction of yeast cell walls, but completely blocked uptake of IgG-RBCs.46 This reduced requirement for Rho GTPases in internalization could be a specific characteristic of dectin-1-dependent phagocytosis or it could be due to the smaller size of individual zymosan particles as compared to IgG-RBCs. It is well established that the F-actin and membrane dynamics and thus requirement for Rho GTPase activity during phagocytosis can vary merely depending on particle size.39,47 Whether increasing zymosan particle size renders Rac1 and Cdc42 activities essential for phagocytosis via dectin-1 remains to be directly tested experimentally.
There are no reports to date on upstream regulators linking dectin-1 to Rho GTPases. Dectin-1 shares signaling motifs in its cytosolic domain with FcRs.48 As a result, the downstream signaling events during dectin-1-dependent particle uptake are highly similar to FcR signaling 45 and may thus involve the same or equivalent GDIs, GEFs, and GAPs.
Phagocytosis mediated by the complement receptor αMβ2 integrin/CR3/CD11b-CD18
The integrin heterodimer composed of the single-span transmembrane integrin subunits αM (CD11b) and β2 (CD18) is a pattern recognition receptor that can bind to a variety of surface epitopes exposed by pathogenic bacteria to induce a pro-inflammatory form of phagocytosis. As αMβ2 also binds to particles opsonized by the processed, soluble complement component C3bi, it is also called complement receptor, CR3. Unlike Fc family receptors and dectin-1, αMβ2 is not constitutively active. Like other integrin family receptors αMβ2 is regulated by “inside-out” signals. Activation prior to challenge with complement-opsonized particles is required for αMβ2-mediated phagocytosis. In cell culture models of phagocytosis, αMβ2 may be activated by inflammatory mediators such as lipopolysaccharide 49 and manipulation of intracellular signaling pathways with phorbol esters 50 or of the small GTPase Rap-150 (not a Rho family GTPase), which regulates the integrin-binding protein talin via intermediates RGS14 51 and/or RIAM.52,53
The two forms of anti-pathogen phagocytosis discussed above and non-inflammatory clearance phagocytosis (to be discussed below) share the general F-actin-dependent steps that lead to particle internalization: dramatic F-actin stabilized plasma membrane extensions reach out from the plasma membrane to first form a bona fide phagocytic cup, which then matures to first completely surround the tethered particle and to eventually fuse, which completes engulfment. This mechanism is best known as the “zipper model” for phagocytosis.54 The engulfment process induced by engagement of particles or their opsonins with the complement receptor αMβ2 integrin/CR3 is different: Surface-tethering of particles induce an invagination in the phagocyte plasma membrane into which the particle sinks, drawn by F-actin cytoskeletal forces.55,56 Although Patel and Harrison50 observed formation of obvious membrane ruffles during CR3-mediated phagocytosis after CR3 activation by phorbol ester, these membrane ruffles were distinct from the membrane extensions of the zipper mechanism: They extend over bound phagocytic particles (C3bi-opsonized sheep RBC) only from one side, while cell membrane associates tightly around the entire surface of the particle in FcR dependent zipper phagocytosis. Furthermore, the membrane ruffles formed facilitate capture and binding but not internalization of particles. These observations further confirm that the engulfment processes in FcR and CR3 mediated phagocytosis are driven by different forms of F-actin regulation (Fig. 1A and B).
In contrast to FcR mediated phagocytosis, αMβ2 integrin/CR3-dependent phagocytosis requires activity of RhoA and RhoG, but not of Rac1 or Cdc42.16,23 Antibody crosslinking of αMβ2 integrin/CR3 is sufficient to cause RhoA-dependent cell contraction and stress fiber formation.16 When challenged with complement-opsonized particles, RhoA but not Rac1 or Cdc42 was recruited beneath tethered particles in J774 macrophages. Overexpressing a dominant-negative mutant form of RhoA (but not of Rac1 or Cdc42) was sufficient to inhibit αMβ2 integrin/CR3-mediated phagocytosis. RhoA inhibition by C3 transferase or inhibition of Rho effector ROCKs decrease particle uptake and F-actin recruitment beneath CR3-bound particles.26
The link of αMβ2 integrin/CR3 ligation to RhoA activation has recently been elucidated by Wiedemann and colleagues.57 Recruitment of RhoA to αMβ2 integrin/CR3-tethered phagocytic particles in Cos-7 cells is controlled independently of its activation. Activation of RhoA after integrin ligation requires a 16-amino acid region in the cytoplasmic domain of the β2 integrin subunit, but is independent of αM subunit. Recruitment of RhoA requires a triple-threonine motif in the β2 integrin cytosolic domain but activation of RhoA was observed without its recruitment when expressing β2 mutant lacking this motif. While signaling from αMβ2 integrin/CR3 to RhoA activation remains poorly understood these data suggest a mobile intermediate for RhoA activation during αMβ2 integrin/CR3-dependent phagocytosis. The Vav GEF family is likely involved as BMMs lacking both Vav1 and Vav3 can neither recruit F-actin to nor phagocytose C3bi-opsonized beads.27 However, changes in RhoA activity in Vav-depleted phagocytes have yet to be shown.
Taken together, the engulfment step of anti-pathogen phagocytosis requires rapid and complex activation and inactivation of Rho GTPases beneath receptor-bound particles to promote formation of F-actin structures and membrane remodeling, followed by local acto-myosin contraction,58 phagosome closure and phagosomal F-actin disassembly.
Rho GTPase Activity During Clearance Phagocytosis
Clearance phagocytosis mediated by αv integrins and TAM receptor tyrosine kinases
Clearance phagocytosis is triggered by exposure of “eat-me” signals on cells undergoing apoptosis and on cell debris. A number of “eat me” signals have been described in specific tissue or experimental systems (for a recent review see ref. 59), but surface exposure of the inner leaflet lipid phosphatidylserine (PS) appears to be universally relevant to all forms of clearance phagocytosis.60-62 Clearance phagocytosis is more common in tissues than inflammatory phagocytosis with billions of apoptotic cells removed daily in the human body as part of normal tissue renewal plus additional removal of significant numbers of apoptotic cells that are spent for instance at the end of an inflammatory reaction. Most cell types possess a low capacity clearance phagocytosis pathway for apoptotic cells, a non-professional activity called “bystander phagocytosis”. Furthermore, professional tissue phagocytes, such as macrophages, dendritic cells, and neutrophils, may phagocytose apoptotic cells with high capacity. Finally, retinal pigment epithelial (RPE) cells support photoreceptor neurons in the vertebrate eye with a special form of high capacity clearance phagocytosis: once a day, synchronized by the circadian rhythm and/or dark-light cycle, RPE cells first contribute to PS exposure precisely of distal tips of light-sensitive outer segment portions of photoreceptor. A brief and vigorous burst of phagocytic activity immediately follows through which RPE cells clear spent photoreceptor outer segment particles (POS) from the retina.62-64 RPE cells are post-mitotic in the mammalian eye and each cell engulfs and digests dozens of spent POS daily for life. RPE cells thus likely phagocytose more material than any other cell type.
Most forms of clearance phagocytosis have in common that they employ 2 surface receptors, αvβ3/β5 integrins and TAM sub-family receptor tyrosine kinases (RTKs) for particle recognition/tethering and particle internalization, respectively. Receptor specificity differs among phagocytes: Non-professional phagocytes may use either αvβ3 or αvβ5 integrin for bystander phagocytosis, depending on receptor expression and activation; the same is true for macrophages,65-67 αvβ5 is used by immature DCs and RPE cells in vivo and in culture.68-70 The three TAM RTKs, Tyro3, Axl, and MerTK are co-expressed by macrophages and DCs and thus function redundantly to some extent in clearance phagocytosis by these cell types.71 Lack of MerTK activity alone impairs but does not abrogate clearance phagocytosis by macrophages.72 Inactivation of both Axl and Tyro3 was needed to abolish apoptotic cell uptake by DCs.73 RPE cells express Tyro3 and MerTK but strictly require MerTK for diurnal clearance phagocytosis of spent photoreceptor outer segment tips.74 Exposed PS is opsonized in tissues by secreted glycoproteins MFG-E8 (a physiological ligand for αvβ3/5 integrins.75,76) and Gas6 or ProteinS (physiological ligands for TAM RTKs77,78). Professional phagocytes may express additional surface receptors (such as BAI-1 and TIM-4) that also respond to PS exposure and that may provide redundant clearance pathways (for a recent review see ref. 59).
Clearance phagocytosis proceeds upon receptor ligation with the type of F-actin dynamics characteristic of bona fide phagocytic cup extension.7,79 In transfected, non-professional HEK 293T cells particle engulfment during clearance phagocytosis requires Rac1 activity.80,81 The same is true for NIH 3T3 fibroblasts.82 Rac1 silencing or expressing dominant-negative mutant form of Rac1 is also sufficient to inhibit phagocytic cup formation and POS engulfment by RPE cells in culture.83 Moreover, taking advantage of the strict diurnal rhythm of RPE clearance phagocytosis in the mammalian eye, it was for the first time possible to detect Rac1 activation associated with a phagocytic process in situ.83 In FRET experiments, Rac1 dynamics during apoptotic cell clearance via the integrin pathway was observed at the single cell level.84 Rac1 activation was observed at lamellipodia of NIH 3T3/integrin and Swiss 3T3/integrin fibroblasts, and primarily at these dynamic structures cells bound and engulfed apoptotic cells. Active Rac1 was next concentrated at phagocytic cups surrounding apoptotic cells before rapid inactivation immediately before phagosome closure. These results were also confirmed in the macrophage cell line BAM3. This spatiotemporal profile of Rac1 activity agrees well with the finding that expressing a constitutively active mutant form of Rac1 decreased phagocytic efficiency. These results suggest that activation of Rac1 must be followed by prompt inactivation to allow phagocytic cup closure and apoptotic cell engulfment. In NIH 3T3 fibroblasts, a dominant-negative mutant form of RhoG, a close relative of Rac1, also showed a moderate inhibitory effect on apoptotic cell engulfment.82 Moreover, RhoG, as well as its GEF Trio, has been shown to promote Rac1-dependent phagocytosis of carboxylated beads in the LR73 cells, a cell line derived from CHO fibroblasts.25 Double knockout of RhoG and Trio homolog in C. elegans significantly increased the number of unengulfed corpses confirming their roles in apoptotic cell clearance in vivo. Co-immunoprecipitation experiments further showed that RhoG interacts with ELMO1 and possibly targets it to the cell membrane. Elmo in turn recruits and activates the Rac1-GEF, DOCK-180. Altogether, there is ample evidence that RhoG function in apoptotic cell phagocytosis is linked to Rac1 activation.
RhoA activation is not only not required for the engulfment step of clearance phagocytosis but has an inhibitory effect: expression of a constitutively-active mutant form of RhoA inhibited membrane ruffle-based phagocytic cups during clearance phagocytosis and decreases engulfment of apoptotic cells, while inhibition of RhoA or downstream effector ROCKs enhanced clearance phagocytosis by BMMs and J774 macrophage cell lines, respectively.82,85 This inhibitory effect appears to be mediated only via ROCKs and not mDia formin, since only mutant forms of active RhoA with ROCK-binding, but not mDia-binding, effector domain mutant caused inhibition of apoptotic cell uptake in macrophage cell lines.85 Finally, RhoA activity may increase subsequent to engulfment of apoptotic cells and contribute to phagolysosomal maturation as inhibition of RhoA or ROCKs delays phagosome acidification during clearance phagocytosis by J774 and BMMs.86 RhoA recruitment to phagocytic cups may thus occur in advance of RhoA function on internalized phagosomes containing apoptotic cells.
Functions of Cdc42 during clearance phagocytosis remain somewhat unclear. Expression of a dominant-negative mutant form of Cdc42 expression prevented F-actin recruitment to phagocytic cups and decreased phagocytosis of apoptotic cells by BMMs.87 Lack of the Cdc42 downstream effector, WASP, had a similar effect on clearance phagocytosis in this experimental system.88 These findings were supported later by Nakaya and colleagues who also showed that a dominant-negative form of Cdc42 decreased phagocytosis of apoptotic cells by BMMs and NIH3T3 cells.82 However, overexpression of Cdc42 did not affect phagocytosis by either cell type, unlike overexpression of Rac1, which significantly increased phagocytosis. In RPE cells, Cdc42 is not activated during clearance phagocytosis and expressing the same dominant-negative construct used for the earlier experiments had no effect on uptake of POS.83 Taken together, all forms of clearance phagocytosis tested to date strictly require Rac1 for engulfment. Cdc42 may contribute depending on the particular type of phagocytic cell. One can speculate that the contribution of Cdc42 to phagocytosis may differ dependent on phagocytic particle size.47 Cdc42 might only be required for phagocytosis of apoptotic cells, given that early apoptotic, whole thymocytes or neutrophils commonly used in phagocytosis assays are generally somewhat larger than photoreceptor outer segment fragments. A summary view of what is known to date on the distribution of Rho GTPases during clearance phagocytosis is illustrated in Figure 1C.
The pathways upstream of Rho GTPase activity in clearance phagocytosis have been addressed in numerous studies. A complex consisting of adaptor proteins and Rac1-GEFs has been shown to function directly upstream of Rac1 GTPase activation in several cell types during clearance phagocytosis. Expression of the DOCK family member DOCK-180 in dendritic cells correlates with their phagocytic activity toward apoptotic cells.81 In HEK 293T cells, antisense reduction of DOCK-180 inhibited engulfment of apoptotic cells.81 The adaptor protein CrkII was first identified as part of the Rac1 activation complex in response to αvβ5 integrin ligation during phagocytosis, as CrkII was recruited beneath phagocytic particles in HEK 293T cells and coimmunoprecipitated with DOCK-180.80 A later study showed that the adaptor protein ELMO cooperated with DOCK-180 and CrkII to enhance uptake of carboxylate-modified latex beads by LR73 cells.89 ELMO enhanced Rac1 activation, but did not act as Rac-GEF alone. Rather, a DOCK-180/ELMO complex formed a bipartite Rac-GEF.89-91 CrkII has been suggested not to contribute directly to GEF activity and not to be strictly required for DOCK-180/ELMO localization to membrane ruffles.92 Yet, overexpression of wild-type CrkII increased apoptotic cell phagocytosis,92 and overexpression of CrkII-mutants that do not bind DOCK-180 decreased apoptotic cell phagocytosis.80 It is thus possible that additional CrkII binding partners remain to be identified that are relevant to clearance phagocytosis and that may include Rac-GEFs other than DOCK-180. The precise combination of adaptor proteins and GEFs employed for clearance phagocytosis may vary dependent on the phagocytic cell.
Which phagocyte surface receptors mediate Rac1 activation has been the subject of intense research. αvβ5 integrin ligation in HEK 293T cells over-expressing β5 integrin has been shown to be sufficient to increase Rac1 activity and complex formation of CrkII with DOCK-180.80 However, this study did not test expression or activation of TAM receptors. A later set of experiments relying on overexpression of both MerTK and β5 integrin suggested an additional role for MerTK in Rac1 activation in CS-1 melanoma cells, another cell line used as model for non-professional clearance phagocytosis.93 This study suggested a possible synergistic effect of MerTK and αvβ5 integrin on Rac1 activation and a signaling pathway during clearance phagocytosis by non-professional phagocytes, in which cytosolic focal adhesion kinase (FAK) was downstream of surface MerTK.
In contrast, mutant RPE cells lacking MerTK but with normal levels of αvβ5 integrin respond to POS challenge with FAK activation.94 Mutant RPE cells in cell culture or in vivo lacking αvβ5 integrin but with normal levels of MerTK fail to activate either FAK or MerTK upon POS stimulation.70 Thus, αvβ5 integrin ligation is sufficient to induce FAK activation and FAK activity is upstream of MerTK during clearance phagocytosis by RPE cells. Strikingly, activation of Rac1 in RPE cells requires αvβ5 integrin, but is independent of either FAK or MerTK activity.83 RPE cells lacking MerTK activate Rac1 both in vitro upon POS challenge and in vivo at the peak of POS phagocytosis.83 Moreover, expressing a dominant-negative mutant form of FAK or inhibiting tyrosine kinases with non-specific tyrosine kinase inhibitors had no effect on Rac1 activation upon POS challenge, while inhibiting POS engulfment. These observations suggest a bifurcation of phagocytic signaling downstream of αvβ5 integrin toward FAK and MerTK and toward Rac1. The pathway toward Rac1 may involve similar adaptor proteins and Rac-GEFs as other forms of clearance phagocytosis but these proteins remain yet to be directly studied in clearance phagocytosis by RPE cells.
Downstream Effects of Rho GTPase Activity During Phagocytosis
Effects of Rho GTPases on F-actin dynamics during phagocytosis
The de novo formation of branched F-actin by Arp2/3 complexes plays a critical role in F-actin dynamics during phagocytic processes.95,96 Arp2/3 complex proteins localize to F-actin beneath surface-bound particles during FcγR- and αMβ2 integrin/CR3-mediated phagocytosis and are essential for both forms of phagocytosis. Expression of a C-terminal fragment of the Arp2/3-binding protein Scar1 decreases internalization via both FcγR and αMβ2 integrin/CR3 by preventing the recruitment of the complex by activated proteins of the Wiskott Aldrich Syndrome protein family (WASPs).96 Like other proteins directly upstream of F-actin regulators, WASPs contain an effector domain and an auto-inhibitory domain that acts to block the effector domain until its release through binding to the auto-inhibitory domain of active Rho GTPases and/or membrane phospholipids, such as, PI(4,5)P2 for N-WASP.95
The recruitment of the Arp2/3 complex is dependent on Rac1 and Cdc42 activity in FcγR-mediated phagocytosis, but on RhoA activity during CR3-mediated phagocytosis.96 Rac1 in principle may interact with the WAVE members of the WASP family.97 Rac1 interacting proteins during phagocytosis remain to be specified but WAVE2 has recently been determined to be irrelevant to Rac1-mediated FcR-dependent phagocytosis by RAW/LR5 cells and BMMs.98 Cdc42 activation at the early stage of phagocytosis promotes pseudopod formation by cooperating with cytosolic adaptor proteins to specifically target WASP and N-WASP in Arp2/3 activation.99-101 WASP was also found in formation of phagocytic cups for apoptotic cell phagocytosis.88
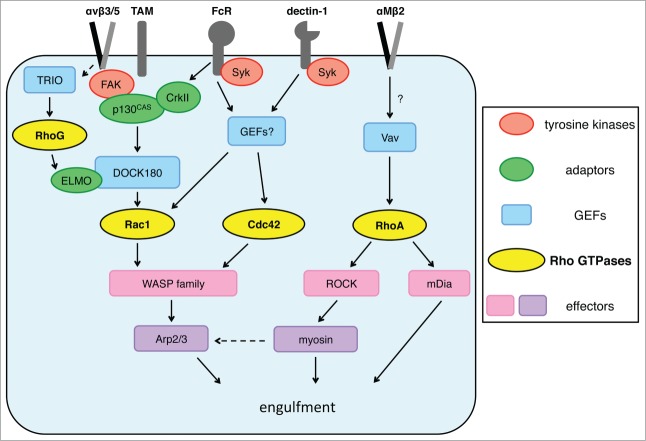
A role for specific WASPs in αMβ2 integrin/CR3-dependent phagocytosis has thus far not been identified. RhoA-dependent regulation of Arp2/3 may rather involve ROCKs. Pharmacological agents inhibiting either ROCKs or their direct substrate myosin light chain kinase abolished Arp2/3 complex and F-actin recruitment to phagocytic particles.26 Additionally, Mammalian Diaphanous-related (mDia) formins, which promote de novo formation of unbranched F-actin, colocalize with polymerizing F-actin beneath bound particles at the early stage of αMβ2 integrin/CR3-dependent phagocytosis in RAW 264.7 cells and BMMs.102 Inactivating mDia formin alone by expressing dominant-negative mutant of mDia decreased F-actin recruitment and particle engulfment via αMβ2 integrin/CR3 but not via FcRs. Inhibition of both mDia formin and ROCK decreased engulfment of C3bi-opsonized RBCs by macrophages more dramatically than inhibiting either protein individually. These results suggest that ROCKs and mDia formins may make distinct contributions to F-actin assembly in αMβ2 integrin/CR3-dependent phagocytosis.56 The signaling pathways upstream and downstream of Rho GTPases are illustrated in Figure 2
Figure 2.
Summary diagram of Rho GTPases and their upstream regulators and downstream effectors during clearance phagocytosis mediated by αv integrins and TAM receptors, or during anti-pathogen phagocytosis via Fc receptors, dectin-1, or αMβ2 integrin/CR3. Phagocytosis triggered by engagement of different surface receptors uses complex and overlapping cytosolic proteins upstream and downstream of Rho GTPases. For details and references please consult the main text.
Effects of Rho GTPases on membrane dynamics during phagocytosis
Phagocytosis is an orchestrated process of step-wise F-actin rearrangement and membrane remodeling. The protrusion of the plasma membrane during phagocytic cup formation is driven by cortical F-actin rearrangement, but at the same time requires expansion of membrane area. Upon PI3-kinase/Rac1 activation early during FcR-dependent phagocytosis, F-actin-based pseudopods form and extend along the particle surface making use of extra available surface membrane folds.103 Once the surface membrane reservoir is depleted during this extension, for instance in “frustrated” phagocytosis (where the phagocytic particle is simply too large or too immobile to be engulfed), membrane tension increases, which stimulates focal exocytosis at sites of phagocytosis. Although there is no direct evidence yet of exocytosis during αMβ2 integrin/CR3-mediated phagocytosis, its characteristic dramatic invagination of the plasma membrane very likely also requires bulk membrane transport to the cell surface.
Rho GTPases play a key role in regulation of focal exocytosis in general.104 Specifically in phagocytosis, inhibition of Cdc42 results in defects in integrin-dependent internalization of large but not small particles by transfected fibroblasts.47 Particle-size dependent differences in phagocytosis were only observed in Cdc42-depleted cells but not in cells in which Rac1 was inhibited. During phagocytosis of large particles Cdc42 interacted transiently with exocyst proteins and these were essential for uptake. Overexpression of Rab11, a docking protein for exocytosis, was sufficient to restore phagocytic activity toward large particles in Cdc42-depleted cells. Further studies are needed to fully elucidate the contributions of Rho GTPases to membrane dynamics during phagocytosis.
Perspective
The activities of Rho GTPases are critical to all forms of phagocytosis, regardless of type of particle and phagocytic cell. Much progress has been made in deciphering specific molecular links and dependencies. Given the complexity of mechanisms involved exploring phagocytosis by mammalian cells in culture has been a necessary and appropriate experimental strategy that has been proven highly productive. However, ultimately studies of phagocytosis in situ will be needed to fully understand the relevance of molecules and mechanisms to phagocytosis in complex tissues and organs.
In particular, the initiation of phagocytosis by particle sensing, recognition, and binding is only poorly mimicked in cell culture experiments. Consequently, our insight into the molecules governing these processes is very limited. Gravity induced contact with particle targets in tissue culture-type media and solutions bears little if any resemblance to phagocytosis in intact tissues. Yet the attraction and initial contact of particle and phagocyte are complex, active processes preceded by phagocytes constantly probing their environment for exogenous antigens or apoptotic cells. Some of these processes involve changes in F-actin and Rho GTPase activity. Even macrophages in culture may probe and engage distant particles by extending filopodia or membrane ruffles for extended distances. Rac1 but not Cdc42 activity is required for this “probing” behavior preceding particle binding.105 Finally, F-actin stabilization by the compound jasplakinolide decreases binding of IgG-opsonized particles by RAW 264.7 cells and BMMs.105,106 The same is true when BMMs were treated with F-actin destabilization agent, latrunculin B, at a relatively higher concentration,106 suggesting that a specific level of actin polymerization, that is, a dynamic actin cytoskeleton, is required for probing. These intriguing findings suggest that F-actin dynamics also contribute to phagocytosis during phases other than particle engulfment. Altogether, much remains to be learned about F-actin dynamics in phagocytic pathways and their regulation in vivo and further contributions of Rho GTPases will surely be discovered.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH grant R01-EY13295 and by the Empire State Stem Cell Fund through New York State Department of Health Contract # C028505. Opinions expressed here are solely those of the authors and do not necessarily reflect those of the Empire State Stem Cell Board, the New York State Department of Health, or the State of New York.
References
- 1. Depraetere V. “Eat me” signals of apoptotic bodies. Nat Cell Biol 2000; 2:E104; PMID:10854338 [DOI] [PubMed] [Google Scholar]
- 2. Reddick LE, Alto NM. Bacteria fighting back: how pathogens target and subvert the host innate immune system. Mol Cell 2014; 54:321-8; PMID:24766896; http://dx.doi.org/ 10.1016/j.molcel.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. deCathelineau AM,Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem 2003; 39:105-17; PMID:14585077 [DOI] [PubMed] [Google Scholar]
- 4. Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 2007; 131:1124-36; PMID:18083102; http://dx.doi.org/ 10.1016/j.cell.2007.10.034 [DOI] [PubMed] [Google Scholar]
- 5. Henson PM. Dampening inflammation. Nat Immunol 2005; 6:1179-81; PMID:16369556 [DOI] [PubMed] [Google Scholar]
- 6. Clemens MJ, van Venrooij WJ, van de Putte LB. Apoptosis and autoimmunity. Cell Death Differ 2000; 7:131-3; PMID:10858073; http://dx.doi.org/ 10.1038/sj.cdd.4400633 [DOI] [PubMed] [Google Scholar]
- 7. Chaitin MH, Hall MO. The distribution of actin in cultured normal and dystrophic rat pigment epithelial cells during the phagocytosis of rod outer segments. Invest Ophthalmol Vis Sci 1983; 24:821-31; PMID:6345446 [PubMed] [Google Scholar]
- 8. Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, et al. . Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 1999; 398:723-8; PMID:10227296; http://dx.doi.org/ 10.1038/19554 [DOI] [PubMed] [Google Scholar]
- 9. Swanson JA. Shaping cups into phagosomes and macropinosomes. Nature reviews Mol Cell Biol 2008; 9:639-49; PMID:18612320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaplan G. Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand J Immunol 1977; 6:797-807; PMID:561436 [DOI] [PubMed] [Google Scholar]
- 11. de Oliveira CA, Mantovani B. Latrunculin A is a potent inhibitor of phagocytosis by macrophages. Life Sci 1988; 43:1825-30; PMID:3200109; http://dx.doi.org/ 10.1016/0024-3205(88)90282-2 [DOI] [PubMed] [Google Scholar]
- 12. Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho (and I like it). J Cell Sci 2004; 117:1301-12; PMID:15020670; http://dx.doi.org/ 10.1242/jcs.01118 [DOI] [PubMed] [Google Scholar]
- 13. Mitin N, Roberts PJ, Chenette EJ, Der CJ. Posttranslational lipid modification of Rho family small GTPases. Meth Mol Biol 2012; 827:87-95; PMID:22144269; http://dx.doi.org/ 10.1007/978-1-61779-442-1_6 [DOI] [PubMed] [Google Scholar]
- 14. Isomura M, Kikuchi A, Ohga N, Takai Y. Regulation of binding of rhoB p20 to membranes by its specific regulatory protein, GDP dissociation inhibitor. Oncogene 1991; 6:119-24; PMID:1899476 [PubMed] [Google Scholar]
- 15. Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev 2013; 93:269-309; PMID:23303910; http://dx.doi.org/ 10.1152/physrev.00003.2012 [DOI] [PubMed] [Google Scholar]
- 16. Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 1998; 282:1717-21; PMID:9831565; http://dx.doi.org/ 10.1126/science.282.5394.1717 [DOI] [PubMed] [Google Scholar]
- 17. Burridge K, Wennerberg K. Rho and Rac take center stage. Cell 2004; 116:167-79; PMID:14744429; http://dx.doi.org/ 10.1016/S0092-8674(04)00003-0 [DOI] [PubMed] [Google Scholar]
- 18. Akula S, Mohammadamin S, Hellman L. Fc receptors for immunoglobulins and their appearance during vertebrate evolution. PloS one 2014; 9:e96903; PMID:24816777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcγ receptors on macrophages. J Exp Med 1997; 186:1027-39; PMID:9314552; http://dx.doi.org/ 10.1084/jem.186.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Massol P, Montcourrier P, Guillemot JC, Chavrier P. Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J 1998; 17:6219-29; PMID:9799231; http://dx.doi.org/ 10.1093/emboj/17.21.6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoppe AD, Swanson JA. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell 2004; 15:3509-19; PMID:15169870; http://dx.doi.org/ 10.1091/mbc.E03-11-0847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savina A, Peres A, Cebrian I, Carmo N, Moita C, Hacohen N, Moita LF, Amigorena S. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity 2009; 30:544-55; PMID:19328020; http://dx.doi.org/ 10.1016/j.immuni.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 23. Tzircotis G, Braga VM, Caron E. RhoG is required for both FcγR- and CR3-mediated phagocytosis. J Cell Sci 2011; 124:2897-902; PMID:21878497; http://dx.doi.org/ 10.1242/jcs.084269 [DOI] [PubMed] [Google Scholar]
- 24. Katoh H, Yasui H, Yamaguchi Y, Aoki J, Fujita H, Mori K, Negishi M. Small GTPase RhoG is a key regulator for neurite outgrowth in PC12 cells. Mol Cell Biol 2000; 20:7378-87; PMID:10982854; http://dx.doi.org/ 10.1128/MCB.20.19.7378-7387.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. deBakker CD, Haney LB, Kinchen JM, Grimsley C, Lu M, Klingele D, Hsu PK, Chou BK, Cheng LC, Blangy A, et al. . Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr Biol 2004; 14:2208-16; PMID:15620647; http://dx.doi.org/ 10.1016/j.cub.2004.12.029 [DOI] [PubMed] [Google Scholar]
- 26. Olazabal IM, Caron E, May RC, Schilling K, Knecht DA, Machesky LM. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcγR, phagocytosis. Curr Biol 2002; 12:1413-18; PMID:12194823; http://dx.doi.org/ 10.1016/S0960-9822(02)01069-2 [DOI] [PubMed] [Google Scholar]
- 27. Hall AB, Gakidis MA, Glogauer M, Wilsbacher JL, Gao S, Swat W, Brugge JS. Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcγR- and complement-mediated phagocytosis. Immunity 2006; 24:305-16; PMID:16546099; http://dx.doi.org/ 10.1016/j.immuni.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 28. Garcia-Mata R, Boulter E, Burridge K. The 'invisible hand': regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol 2011; 12:493-504; PMID:21779026; http://dx.doi.org/ 10.1038/nrm3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DerMardirossian C, Rocklin G, Seo JY, Bokoch GM. Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol Biol Cell 2006; 17:4760-8; PMID:16943322; http://dx.doi.org/ 10.1091/mbc.E06-06-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ugolev Y, Berdichevsky Y, Weinbaum C, Pick E. Dissociation of Rac1(GDP).RhoGDI complexes by the cooperative action of anionic liposomes containing phosphatidylinositol 3,4,5-trisphosphate, Rac guanine nucleotide exchange factor, and GTP. J Biol Chem 2008; 283:22257-71; PMID:18505730; http://dx.doi.org/ 10.1074/jbc.M800734200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ueyama T, Son J, Kobayashi T, Hamada T, Nakamura T, Sakaguchi H, Shirafuji T, Saito N. Negative Charges in the Flexible N-Terminal Domain of Rho GDP-Dissociation Inhibitors (RhoGDIs) Regulate the Targeting of the RhoGDI-Rac1 Complex to Membranes. J Immun 2013; 191:2560-9; PMID:23918979; http://dx.doi.org/ 10.4049/jimmunol.1300209 [DOI] [PubMed] [Google Scholar]
- 32. Groves E, Rittinger K, Amstutz M, Berry S, Holden DW, Cornelis GR, Caron E. Sequestering of Rac by the Yersinia effector YopO blocks Fcγ receptor-mediated phagocytosis. J Bio Chem 2010; 285:4087-98; PMID:19926792; http://dx.doi.org/ 10.1074/jbc.M109.071035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel JC, Hall A, Caron E. Vav regulates activation of Rac but not Cdc42 during FcγR-mediated phagocytosis. Mol Biol Cell 2002; 13:1215-26; PMID:11950933; http://dx.doi.org/ 10.1091/mbc.02-01-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee WL, Cosio G, Ireton K, Grinstein S. Role of CrkII in Fcγ receptor-mediated phagocytosis. J Biol Chem 2007; 282:11135-43; PMID:17308335; http://dx.doi.org/ 10.1074/jbc.M700823200 [DOI] [PubMed] [Google Scholar]
- 35. Olson MF, Pasteris NG, Gorski JL, Hall A. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryonic development, are upstream regulators of Rho GTPases. Curr Biol 1996; 6:1628-33; PMID:8994827; http://dx.doi.org/ 10.1016/S0960-9822(02)70786-0 [DOI] [PubMed] [Google Scholar]
- 36. Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev 2002; 16:1587-609; PMID:12101119; http://dx.doi.org/ 10.1101/gad.1003302 [DOI] [PubMed] [Google Scholar]
- 37. Jankowski A, Zhu P, Marshall JG. Capture of an activated receptor complex from the surface of live cells by affinity receptor chromatography. Analyt Biochem 2008; 380:235-48; PMID:18601892; http://dx.doi.org/ 10.1016/j.ab.2008.05.047 [DOI] [PubMed] [Google Scholar]
- 38. Dong N, Liu L, Shao F. A bacterial effector targets host DH-PH domain RhoGEFs and antagonizes macrophage phagocytosis. EMBO J 2010; 29:1363-76; PMID:20300064; http://dx.doi.org/ 10.1038/emboj.2010.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beemiller P, Zhang Y, Mohan S, Levinsohn E, Gaeta I, Hoppe AD, Swanson JA. A Cdc42 activation cycle coordinated by PI 3-kinase during Fc receptor-mediated phagocytosis. Mol Biol Cell 2010; 21:470-80; PMID:19955216; http://dx.doi.org/ 10.1091/mbc.E08-05-0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Csepanyi-Komi R, Sirokmany G, Geiszt M, Ligeti E. ARHGAP25, a novel Rac GTPase-activating protein, regulates phagocytosis in human neutrophilic granulocytes. Blood 2012; 119:573-82; PMID:22096251; http://dx.doi.org/ 10.1182/blood-2010-12-324053 [DOI] [PubMed] [Google Scholar]
- 41. Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, et al. . Dectin-1 is required for β-glucan recognition and control of fungal infection. Nature Immunol 2007; 8:31-8; PMID:17159984; http://dx.doi.org/ 10.1038/ni1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown GD, Gordon S. Immune recognition. A new receptor for β-glucans. Nature 2001; 413:36-7; PMID:11544516; http://dx.doi.org/ 10.1038/35092620 [DOI] [PubMed] [Google Scholar]
- 43. Ifrim DC, Bain JM, Reid DM, Oosting M, Verschueren I, Gow NA, van Krieken JH, Brown GD, Kullberg BJ, Joosten LA, et al. . Role of Dectin-2 for host defense against systemic infection with Candida glabrata. Infect Immun 2014; 82:1064-73; PMID:24343653; http://dx.doi.org/ 10.1128/IAI.01189-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005; 106:2543-50; PMID:15956283; http://dx.doi.org/ 10.1182/blood-2005-03-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan AS, Magee AS, Danielson ME, et al. . Activation of the innate immune receptor Dectin-1 upon formation of a 'phagocytic synapse'. Nature 2011; 472:471-5; PMID:21525931; http://dx.doi.org/ 10.1038/nature10071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herre J, Marshall AS, Caron E, Edwards AD, Williams DL, Schweighoffer E, Tybulewicz V, Reis e Sousa C, Gordon S, Brown GD. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 2004; 104:4038-45; PMID:15304394; http://dx.doi.org/ 10.1182/blood-2004-03-1140 [DOI] [PubMed] [Google Scholar]
- 47. Mohammadi S, Isberg RR. Cdc42 interacts with the exocyst complex to promote phagocytosis. J Cell Biol 2013; 200:81-93; PMID:23295348; http://dx.doi.org/ 10.1083/jcb.201204090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ariizumi K, Shen GL, Shikano S, Ritter R, 3rd, Zukas P, Edelbaum D, Morita A, Takashima A. Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J Biol Chem 2000; 275:11957-63; PMID:10766825; http://dx.doi.org/ 10.1074/jbc.275.16.11957 [DOI] [PubMed] [Google Scholar]
- 49. Caron E, Self AJ, Hall A. The GTPase Rap1 controls functional activation of macrophage integrin αMβ2 by LPS and other inflammatory mediators. Curr Biol 2000; 10:974-8; PMID:10985384; http://dx.doi.org/ 10.1016/S0960-9822(00)00641-2 [DOI] [PubMed] [Google Scholar]
- 50. Patel PC, Harrison RE. Membrane ruffles capture C3bi-opsonized particles in activated macrophages. Mol Biol Cell 2008; 19:4628-39; PMID:18768756; http://dx.doi.org/ 10.1091/mbc.E08-02-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lim J, Thompson J, May RC, Hotchin NA, Caron E. Regulator of G-Protein Signalling-14 (RGS14) regulates the activation of αMβ2 integrin during phagocytosis. PLoS One 2013; 8:e69163; PMID:23805333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lim J, Dupuy AG, Critchley DR, Caron E. Rap1 controls activation of the αMβ2 integrin in a talin-dependent manner. J Cell Biochem 2010; 111:999-1009; PMID:20665668; http://dx.doi.org/ 10.1002/jcb.22788 [DOI] [PubMed] [Google Scholar]
- 53. Medrano-Fernandez I, Reyes R, Olazabal I, Rodriguez E, Sanchez-Madrid F, Boussiotis VA, Reche PA, Cabanas C, Lafuente EM. RIAM (Rap1-interacting adaptor molecule) regulates complement-dependent phagocytosis. Cell Mol Life Sci 2013; 70:2395-410; PMID:23420480; http://dx.doi.org/ 10.1007/s00018-013-1268-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Griffin FM, Jr., Griffin JA, Leider JE, Silverstein SC. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med 1975; 142:1263-82; PMID:1194852; http://dx.doi.org/ 10.1084/jem.142.5.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Allen LA, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med 1996; 184:627-37; PMID:8760816; http://dx.doi.org/ 10.1084/jem.184.2.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee CY, Herant M, Heinrich V. Target-specific mechanics of phagocytosis: protrusive neutrophil response to zymosan differs from the uptake of antibody-tagged pathogens. J Cell Sci 2011; 124:1106-14; PMID:21385838; http://dx.doi.org/ 10.1242/jcs.078592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wiedemann A, Patel JC, Lim J, Tsun A, van Kooyk Y, Caron E. Two distinct cytoplasmic regions of the β2 integrin chain regulate RhoA function during phagocytosis. J Cell Biol 2006; 172:1069-79; PMID:16567504; http://dx.doi.org/ 10.1083/jcb.200508075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Swanson JA, Johnson MT, Beningo K, Post P, Mooseker M, Araki N. A contractile activity that closes phagosomes in macrophages. J Cell Sci 1999; 112(Pt 3):307-16; PMID:9885284 [DOI] [PubMed] [Google Scholar]
- 59. Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol 2013; 5:a008748; PMID:23284042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 1992; 148:2207-16; PMID:1545126 [PubMed] [Google Scholar]
- 61. McEvoy L, Williamson P, Schlegel RA. Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc Natl Acad Sci U S A 1986; 83:3311-5; PMID:3458184; http://dx.doi.org/ 10.1073/pnas.83.10.3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ruggiero L, Connor MP, Chen J, Langen R, Finnemann SC. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proc Natl Acad Sci U S A 2012; 109:8145-8; PMID:22566632; http://dx.doi.org/ 10.1073/pnas.1121101109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science 1976; 194:1071-4; PMID:982063; http://dx.doi.org/ 10.1126/science.982063 [DOI] [PubMed] [Google Scholar]
- 64. Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 1969; 42:392-403; PMID:5792328; http://dx.doi.org/ 10.1083/jcb.42.2.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Finnemann SC, Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for αvβ3 and αvβ5 integrins, and protein kinase C regulates αvβ5 binding and cytoskeletal linkage. J Exp Med 1999; 190:861-74; PMID:10499924; http://dx.doi.org/ 10.1084/jem.190.6.861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid αv integrins. Proc Natl Acad Sci U S A 2007; 104:15823-8; PMID:17895374; http://dx.doi.org/ 10.1073/pnas.0707421104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature 1990; 343:170-3; PMID:1688647; http://dx.doi.org/ 10.1038/343170a0 [DOI] [PubMed] [Google Scholar]
- 68. Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med 1998; 188:1359-68; PMID:9763615; http://dx.doi.org/ 10.1084/jem.188.7.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc Natl Acad Sci U S A 1997; 94:12932-7; PMID:9371778; http://dx.doi.org/ 10.1073/pnas.94.24.12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J Exp Med 2004; 200:1539-45; PMID:15596525; http://dx.doi.org/ 10.1084/jem.20041447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science 2001; 293:306-11; PMID:11452127; http://dx.doi.org/ 10.1126/science.1061663 [DOI] [PubMed] [Google Scholar]
- 72. Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 2001; 411:207-11; PMID:11346799; http://dx.doi.org/ 10.1038/35075603 [DOI] [PubMed] [Google Scholar]
- 73. Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol 2007; 178:5635-42; PMID:17442946; http://dx.doi.org/ 10.4049/jimmunol.178.9.5635 [DOI] [PubMed] [Google Scholar]
- 74. Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, Garcia de Frutos P, Lemke G. TAM receptor function in the retinal pigment epithelium. Mol Cell Neurosci 2006; 33:96-108; PMID:16901715; http://dx.doi.org/ 10.1016/j.mcn.2006.06.011 [DOI] [PubMed] [Google Scholar]
- 75. Hanayama R, Miyasaka K, Nakaya M, Nagata S. MFG-E8-dependent clearance of apoptotic cells, and autoimmunity caused by its failure. Curr Dir Autoimmun 2006; 9:162-72; PMID:16394660 [DOI] [PubMed] [Google Scholar]
- 76. Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC. Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci U S A 2007; 104:12005-10; PMID:17620600; http://dx.doi.org/ 10.1073/pnas.0704756104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Angelillo-Scherrer A, Burnier L, Flores N, Savi P, DeMol M, Schaeffer P, Herbert JM, Lemke G, Goff SP, Matsushima GK, et al. . Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J Clin Invest 2005; 115:237-46; PMID:15650770; http://dx.doi.org/ 10.1172/JCI22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Burstyn-Cohen T, Lew ED, Traves PG, Burrola PG, Hash JC, Lemke G. Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron 2012; 76:1123-32; PMID:23259948; http://dx.doi.org/ 10.1016/j.neuron.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dupuy AG, Caron E. Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci 2008; 121:1773-83; PMID:18492791; http://dx.doi.org/ 10.1242/jcs.018036 [DOI] [PubMed] [Google Scholar]
- 80. Albert ML, Kim JI, Birge RB. αvβ5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol 2000; 2:899-905; PMID:11146654; http://dx.doi.org/ 10.1038/35046549 [DOI] [PubMed] [Google Scholar]
- 81. Akakura S, Singh S, Spataro M, Akakura R, Kim JI, Albert ML, Birge RB. The opsonin MFG-E8 is a ligand for the αvβ5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp Cell Res 2004; 292:403-16; PMID:14697347; http://dx.doi.org/ 10.1016/j.yexcr.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 82. Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J Biol Chem 2006; 281:8836-42; PMID:16439364; http://dx.doi.org/ 10.1074/jbc.M510972200 [DOI] [PubMed] [Google Scholar]
- 83. Mao Y, Finnemann SC. Essential diurnal Rac1 activation during retinal phagocytosis requires αvβ5 integrin but not tyrosine kinases focal adhesion kinase or Mer tyrosine kinase. Mol Biol Cell 2012; 23:1104-14; PMID:22262456; http://dx.doi.org/ 10.1091/mbc.E11-10-0840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nakaya M, Kitano M, Matsuda M, Nagata S. Spatiotemporal activation of Rac1 for engulfment of apoptotic cells. Proc Natl Acad Sci U S A 2008; 105:9198-203; PMID:18591655; http://dx.doi.org/ 10.1073/pnas.0803677105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tosello-Trampont AC, Nakada-Tsukui K, Ravichandran KS. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J Biol Chem 2003; 278:49911-9; PMID:14514696; http://dx.doi.org/ 10.1074/jbc.M306079200 [DOI] [PubMed] [Google Scholar]
- 86. Erwig LP, McPhilips KA, Wynes MW, Ivetic A, Ridley AJ, Henson PM. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc Natl Acad Sci U S A 2006; 103:12825-30; PMID:16908865; http://dx.doi.org/ 10.1073/pnas.0605331103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Leverrier Y, Ridley AJ. Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr Biol 2001; 11:195-9; PMID:11231156; http://dx.doi.org/ 10.1016/S0960-9822(01)00047-1 [DOI] [PubMed] [Google Scholar]
- 88. Leverrier Y, Lorenzi R, Blundell MP, Brickell P, Kinnon C, Ridley AJ, Thrasher AJ. Cutting edge: the Wiskott-Aldrich syndrome protein is required for efficient phagocytosis of apoptotic cells. J Immunol 2001; 166:4831-4; PMID:11290758; http://dx.doi.org/ 10.4049/jimmunol.166.8.4831 [DOI] [PubMed] [Google Scholar]
- 89. Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, et al. . CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 2001; 107:27-41; PMID:11595183; http://dx.doi.org/ 10.1016/S0092-8674(01)00520-7 [DOI] [PubMed] [Google Scholar]
- 90. Lu M, Kinchen JM, Rossman KL, Grimsley C, Hall M, Sondek J, Hengartner MO, Yajnik V, Ravichandran KS. A Steric-inhibition model for regulation of nucleotide exchange via the Dock180 family of GEFs. Curr Biol 2005; 15:371-7; PMID:15723800; http://dx.doi.org/ 10.1016/j.cub.2005.01.050 [DOI] [PubMed] [Google Scholar]
- 91. Lu M, Kinchen JM, Rossman KL, Grimsley C, deBakker C, Brugnera E, Tosello-Trampont AC, Haney LB, Klingele D, Sondek J, et al. . PH domain of ELMO functions in trans to regulate Rac activation via Dock180. Nat Struct Mol Biol 2004; 11:756-62; PMID:15247908; http://dx.doi.org/ 10.1038/nsmb800 [DOI] [PubMed] [Google Scholar]
- 92. Tosello-Trampont AC, Kinchen JM, Brugnera E, Haney LB, Hengartner MO, Ravichandran KS. Identification of two signaling submodules within the CrkII/ELMO/Dock180 pathway regulating engulfment of apoptotic cells. Cell Death Differ 2007; 14:963-72; PMID:17304244 [DOI] [PubMed] [Google Scholar]
- 93. Wu Y, Singh S, Georgescu MM, Birge RB. A role for Mer tyrosine kinase in αvβ5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci 2005; 118:539-53; PMID:15673687; http://dx.doi.org/ 10.1242/jcs.01632 [DOI] [PubMed] [Google Scholar]
- 94. Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J 2003; 22:4143-54; PMID:12912913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol 2000; 150:1299-310; PMID:10995436; http://dx.doi.org/ 10.1083/jcb.150.6.1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. May RC, Caron E, Hall A, Machesky LM. Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat Cell Biol 2000; 2:246-8; PMID:10783245; http://dx.doi.org/ 10.1038/35008673 [DOI] [PubMed] [Google Scholar]
- 97. Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J 1998; 17:6932-41; PMID:9843499; http://dx.doi.org/ 10.1093/emboj/17.23.6932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Abou-Kheir W, Isaac B, Yamaguchi H, Cox D. Membrane targeting of WAVE2 is not sufficient for WAVE2-dependent actin polymerization: a role for IRSp53 in mediating the interaction between Rac and WAVE2. J Cell Sci 2008; 121:379-90; PMID:18198193; http://dx.doi.org/ 10.1242/jcs.010272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Coppolino MG, Krause M, Hagendorff P, Monner DA, Trimble W, Grinstein S, Wehland J, Sechi AS. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcγ receptor signalling during phagocytosis. J Cell Sci 2001; 114:4307-18; PMID:11739662 [DOI] [PubMed] [Google Scholar]
- 100. Dart AE, Donnelly SK, Holden DW, Way M, Caron E. Nck and Cdc42 co-operate to recruit N-WASP to promote FcγR-mediated phagocytosis. J Cell Sci 2012; 125:2825-30; PMID:22454526; http://dx.doi.org/ 10.1242/jcs.106583 [DOI] [PubMed] [Google Scholar]
- 101. Park H, Cox D. Cdc42 regulates Fcγ receptor-mediated phagocytosis through the activation and phosphorylation of Wiskott-Aldrich syndrome protein (WASP) and neural-WASP. Mol Biol Cell 2009; 20:4500-8; PMID:19741094; http://dx.doi.org/ 10.1091/mbc.E09-03-0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Colucci-Guyon E, Niedergang F, Wallar BJ, Peng J, Alberts AS, Chavrier P. A role for mammalian diaphanous-related formins in complement receptor (CR3)-mediated phagocytosis in macrophages. Curr Biol 2005; 15:2007-12; PMID:16303559; http://dx.doi.org/ 10.1016/j.cub.2005.09.051 [DOI] [PubMed] [Google Scholar]
- 103. Masters TA, Pontes B, Viasnoff V, Li Y, Gauthier NC. Plasma membrane tension orchestrates membrane trafficking, cytoskeletal remodeling, and biochemical signaling during phagocytosis. Proc Natl Acad Sci U S A 2013; 110:11875-80; PMID:23821745; http://dx.doi.org/ 10.1073/pnas.1301766110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wu H, Turner C, Gardner J, Temple B, Brennwald P. The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol Biol Cell 2010; 21:430-42; PMID:19955214; http://dx.doi.org/ 10.1091/mbc.E09-06-0501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Flannagan RS, Harrison RE, Yip CM, Jaqaman K, Grinstein S. Dynamic macrophage “probing” is required for the efficient capture of phagocytic targets. J Cell Biol 2010; 191:1205-18; PMID:21135140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mao YS, Yamaga M, Zhu X, Wei Y, Sun HQ, Wang J, Yun M, Wang Y, Di Paolo G, Bennett M, et al. . Essential and unique roles of PIP5K-γ and -α in Fcγ receptor-mediated phagocytosis. J Cell Biol 2009; 184:281-96; PMID:19153220; http://dx.doi.org/ 10.1083/jcb.200806121 [DOI] [PMC free article] [PubMed] [Google Scholar]