Abstract
Legionella spp. are amoebae-resistant environmental bacteria that replicate in free-living protozoa in a distinct compartment, the Legionella-containing vacuole (LCV). Upon transmission of Legionella pneumophila to the lung, the pathogens employ an evolutionarily conserved mechanism to grow in LCVs within alveolar macrophages, thus triggering a severe pneumonia termed Legionnaires’ disease. LCV formation is a complex and robust process, which requires the bacterial Icm/Dot type IV secretion system and involves the amazing number of 300 different translocated effector proteins. LCVs interact with the host cell's endosomal and secretory vesicle trafficking pathway. Accordingly, in a proteomics approach as many as 12 small Rab GTPases implicated in endosomal and secretory vesicle trafficking were identified and validated as LCV components. Moreover, the small GTPase Ran and its effector protein RanBP1 have been found to decorate the pathogen vacuole. Ran regulates nucleo-cytoplasmic transport, spindle assembly, and cytokinesis, as well as the organization of non-centrosomal microtubules. In L. pneumophila-infected amoebae or macrophages, Ran and RanBP1 localize to LCVs, and the small GTPase is activated by the Icm/Dot substrate LegG1. Ran activation by LegG1 leads to microtubule stabilization and promotes intracellular pathogen vacuole motility and bacterial growth, as well as chemotaxis and migration of Legionella-infected cells.
Keywords: Acanthamoeba, bacterial effector protein, Dictyostelium, guanine nucleotide exchange factor, host-pathogen interactions, Legionella, macrophage, microtubules, pathogen vacuole, small GTPase, type IV secretion
Legionella pneumophila Forms a Distinct Pathogen Vacuole in Phagocytes
Gram-negative environmental bacteria of the genus Legionella resist degradation by free-living protozoa and grow within these phagocytic predators.1 Upon inhalation of aerosols contaminated with Legionella spp., the opportunistic pathogens reach the human lung and replicate in alveolar macrophages.2 Bacterial resistance of degradation by macrophages is a precondition to cause a severe pneumonia termed Legionnaires’ disease. Legionella-bearing aerosols can spread and sicken people several kilometres from their sources,3 which are frequently cooling towers of air conditioning systems or industrial plants. Legionella pneumophila causes up to 90% of the Legionnaires’ disease cases and therefore is the clinically most relevant species.2
To survive and replicate within amoebae or macrophages, L. pneumophila employs an apparently conserved mechanism that centers on the formation of a unique membrane-bound compartment, the Legionella-containing vacuole (LCV).4-6 LCVs interact with the endosomal pathway but do not fuse with bactericidal lysosomes. Instead, the pathogen vacuoles intercept early secretory vesicles emerging from endoplasmic reticulum (ER) exit sites and ultimately fuse with the ER. The fusion with the ER is not essential for the creation of a replication-permissive compartment, as L. pneumophila already grows within vacuoles that are attached to but not yet merged with the ER.7,8 In addition to subverting endosomal and secretory pathways, L. pneumophila also interferes with retrograde,9 lysosomal10 and autophagosomal trafficking.11
Environmental protozoa such as amoebae and ciliates are the natural hosts of Legionella species. Given the similarity of the infection process on a cellular level, amoebae including Acanthamoeba castellanii or Dictyostelium discoideum represent versatile models to analyze cell-autonomous Legionella-phagocyte interactions.12
Translocated Legionella Effector Proteins Determine Pathogen-Host Cell Interactions
LCV formation is an intricate and robust process, which is governed by the Legionella Icm/Dot type IV secretion system (T4SS) that translocates the astonishing number of ∼300 different “effector” proteins into host cells.5,13-15 Hence, L. pneumophila devotes at least 10% of its genome to define interactions with eukaryotic host cells. A number of effectors target phosphoinositide (PI) lipids, a family of low abundance glycerophospholipids, which play key roles in eukaryotic signal transduction and membrane dynamics.16-19 The L. pneumophila effectors anchor to the LCV membrane by selectively binding to phosphatidylinositol-4-phosphate (PtdIns(4)P) or PtdIns(3)P, which localize to LCVs and are hallmarks of the secretory or the endosomal pathway.8,20
The Icm/Dot-translocated ER interactor SidC and the Rab1 modulator SidM (see below) specifically bind to PtdIns(4)P and promote the tethering and fusion of ER-derived vesicles with the LCV, respectively.7,20-22 PtdIns(4)P might accumulate on LCV membranes either directly due to the activity of the Icm/Dot substrate SidF, a PI polyphosphate 3-phosphatase,23 or indirectly through the recruitment of OCRL1, a eukaryotic PI polyphosphate 5-phosphatase.24 Furthermore, the glucosyltransferase SetA25 and the retromer interactor RidL bind PtdIns(3)P9, which might be dephosphorylated to PtdIns by the Icm/Dot-translocated PI 3-phosphatase SidP.26
Several L. pneumophila effectors have been characterized that target small host GTPases implicated in the secretory or endosomal trafficking pathways.5,6,27,28 Some of these effectors exhibit novel activities, through which they modulate signal transduction and vesicle trafficking pathways. RalF or SidM (alias DrrA) function as guanine nucleotide exchange factors (GEFs) for the small GTPases Arf129 or Rab1,30,31 respectively. Intriguingly, Rab1 is targeted by at least six different Icm/Dot substrates. The small GTPase is activated by the GEF activity of SidM, and covalently modified through the adenosine monophosphate transferase (AMPylase) activity of SidM32,33 or by the phosphocholinase activity of AnkX,33,34 either of which prevents Rab1 inactivation. Furthermore, LidA functions as an activator/ stabilizer for the GTPase.31,35 The covalent modifications of Rab1 can be reverted by either the deAMPylase SidD36,37 or the dephosphocholinase Lem3,38,39 and the GTPase is inactivated by the GTPase-activating protein (GAP) LepB.40,41 Finally, the effector VipD tightly binds activated Rab5 and Rab22, thus impeding binding of Rab effectors and endosomal trafficking.42 VipD is a Rab5-activated phospholipase A, which catalyzes the removal of PtdIns(3)P from endosomal membranes and thereby protects LCVs from endosomal fusion.43
In addition to PI lipids or small GTPases, Icm/Dot substrates also specifically target other host trafficking components. The effector RidL binds to the Vps29 subunit of the heterotrimeric cargo recognition subunit of the retromer complex and interferes with retrograde endosome to Golgi trafficking.9 SidK interacts with the VatA subunit of the late endosomal/ lysosomal vacuolar H+-ATPase, thereby preventing acidification of the LCV.10 RavZ hydrolyzes the autophagy factor Atg8 at a C-terminal glycine, thus irreversibly removing a phosphatidylethanolamine residue and cleaving Atg8 from autophagosome membranes.11 Finally, LegS2 (a homolog of eukaryotic sphingosine-1-phosphate lyase with unknown function) localizes to mitochondria.44 Thus, host factors essential for different vesicle trafficking pathways that restrict the replication of intracellular pathogens are specifically targeted and modified by L. pneumophila effectors.
The Pathogen Vacuole is Decorated with Endosomal and Secretory Small GTPases
Groundbreaking early studies revealed that LCVs are decorated with the small GTPases Arf1 and Sar1,29,45 Rab146,47 as well as Rab7.48 In more recent studies, intact LCVs were isolated, and the host proteome of the purified pathogen vacuole preparations was determined by tandem mass spectrometry (MS).49-51 To this end, LCVs from infected D. discoideum amoebae were enriched, using an antibody against the PtdIns(4)P-binding Icm/Dot substrate SidC, which exclusively localizes to the pathogen vacuole membrane. The immuno-affinity separation step was then followed by Histodenz density gradient centrifugation.
In the proteomics approach, more than 560 D. discoideum proteins were initially identified as LCV components, including Arf1, Rab1, Rab7, Rab8 and Rab14.49 The proteomics data were validated by fluorescence microscopy using amoebae stably producing GFP fusion proteins. The purification protocol was subsequently adapted to enrich intact LCVs from RAW 264.7 macrophages, and the proteome was compared with LCVs isolated from D. discoideum.51 This analysis revealed more than 1150 (macrophages) or 670 (D. discoideum) host proteins, including Arf1, Sar1 (SarA), and 14 small GTPases of the Rab family. LCV localization of all Rab proteins except two (Rab6, Rab18) was confirmed by fluorescence microscopy. Therefore, Arf1, Sar1 and at least 12 different Rab GTPases (Rab1, Rab2, Rab4, Rab5, Rab7, Rab8, Rab9, Rab10, Rab11, Rab14, Rab21, and Rab32) localize to LCVs (Fig. 1). Most of these small GTPases (Arf1, Rab1, Rab2, Rab4, Rab8, Rab10, Rab11, Rab14, Rab21, Rab32) selectively accumulate on pathogen vacuoles containing wild-type L. pneumophila but not mutant bacteria lacking a functional Icm/Dot T4SS.29,45,49,51 In contrast, the late endosomal Rab proteins Rab7 and Rab9 (and to some extent also the early endosomal GTPase Rab5) are present on pathogen vacuoles harboring wild-type as well as ΔicmT mutant L. pneumophila.
Figure 1.
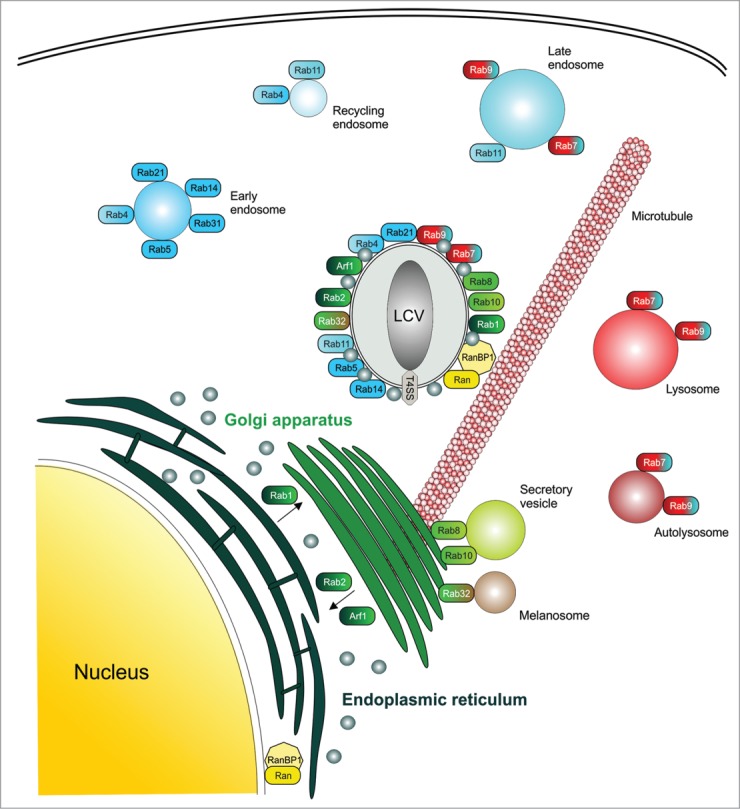
LCV localization and cellular functions of Rab and Ran GTPases. The LCV is decorated with small GTPases localizing to the secretory pathway (Arf1, Rab1, Rab2, Rab8, Rab10, Rab32), early endosomes (Rab4, Rab5, Rab14, Rab21), recycling endosomes (Rab4, Rab11), or late endosomes and lysosomes/ autolysosomes (Rab7, Rab9). The small GTPase Ran and its effector RanBP1 localize to LCVs and promote microtubule polymerization as well as nucleo-cytoplasmic transport.
Individual depletion of 20 small GTPases by RNA interference (RNAi) in epithelial cells revealed that endocytic GTPases (Rab5a, Rab14, and Rab21) restrict the intracellular growth of L. pneumophila, whereas secretory GTPases (Arf1, Rab8a, Rab10, and Rab32), implicated in Golgi to endosome trafficking, promote bacterial replication. Moreover, depletion of Arf1, Rab8a, Rab14, or Rab21 (but not Rab1 or Sar1) significantly decreased the number of SidC-positive LCVs, suggesting that these GTPases regulate the level of PtdIns(4)P on the pathogen vacuole.49,51 In summary, proteomics data and its validation revealed that at least 14 distinct small GTPases promoting different cellular pathways localize to LCVs. These findings corroborate the notion that LCVs communicate with many host cell compartments and vesicle trafficking pathways.
The Legionella Effector LegG1 Activates the Small GTPase Ran on the Pathogen Vacuole
The proteomics approach outlined above not only identified small GTPases of the Arf, Sar and Rab families on LCVs, but also indicated the presence of the small GTPase Ran and its effector Ran binding protein 1 (RanBP1), as well as α- and β-tubulin.49-51 Microtubules are implicated in the initial trafficking events of LCVs, taking place prior to the acquisition of the early secretory vesicle marker GFP-HDEL and the resident ER marker calnexin-GFP.52
The small GTPase Ran plays a fundamental role in many cellular processes, including nuclear pore translocation,53 or post-mitotic nuclear envelope formation and mitotic spindle assembly.54,55 Furthermore, Ran controls cytoplasmic events involving microtubules,56 e.g., organization of non-centrosomal microtubules,57 endocytic receptor trafficking,58 and retrograde signaling along microtubules in nerve axons.59 Ran is activated by a Ran GEF termed regulator of chromosome condensation 1 (RCC1),60 which localizes to the nucleus or the chromatin (in mitotic cells), respectively. Active Ran(GTP) is inactivated by cytoplasmic RanGAP1, together with RanBP1 that contains a Ran(GTP)-binding domain.59
The L. pneumophila Icm/Dot substrate LegG1 (Legionella eukaryotic gene G1; Lpg1976) shows amino acid sequence similarity with the eukaryotic Ran GEF RCC1.61-63 LegG1 (alias PieG) is encoded in the Pie (Plasticity island of effectors) gene cluster and localizes to punctate, vesicle-like structures upon ectopic production in CHO FcγRII cells.63 To target the bacterial protein to host membranes, LegG1/PieG is lipidated by the host prenylation machinery at a C-terminal CAAX tetrapeptide motif.64 Mutation of the conserved cysteine residue, as well as treatment with pharmacological inhibitors of isoprenoid biosynthesis (mevastatin) or geranylgeranyltransferase activity abolished the membrane localization of ectopically produced LegG1, suggesting that prenylation is a major membrane-targeting determinant. Thus, L. pneumophila employs at least four different strategies to specifically localize effector proteins to the LCV membrane: exploitation of PI metabolism (SidM, SidC, SetA, RidL), prenylation (LegG1), intrinsic hydrophobic domains (LepB, SidF) or a novel membrane sensor enriched in aromatic and positively charged amino acid residues (RalF).65
LCV localization of Ran and RanBP1, together with the prediction that LegG1 might function as a Ran activator, prompted us to validate the subcellular localization of these proteins, to investigate whether LegG1 indeed activates Ran and to analyze the consequences of Ran activation in L. pneumophila-infected cells.66 Ran and RanBP1 were found to localize to LCVs in an Icm/Dot-dependent manner in D. discoideum amoebae producing the corresponding GFP fusion proteins (Fig. 1), and M45-tagged LegG1 also accumulated on the pathogen vacuole membrane. Upon depletion of Ran or RanBP1 by RNAi, intracellular growth of L. pneumophila in A549 lung epithelial cells was significantly reduced. Furthermore, L. pneumophila lacking legG1 was compromised for intracellular growth in macrophages, and the mutant strain was efficiently out-competed by wild-type bacteria upon co-infection of A. castellanii.66 Yet, in absence of legG1, calnexin accumulation on LCVs was not affected, indicating that LegG1 does not affect the fusion of the pathogen vacuole with the ER.
LegG1 activated Ran GTPase on LCVs, since the Ran(GTP)-binding effector RanBP1 accumulated on D. discoideum LCVs harboring L. pneumophila wild-type, but not mutant bacteria lacking legG1 or a functional Icm/Dot T4SS, and purified LegG1 produced active Ran(GTP) in macrophage cell lysates.66 LegG1 represents the first Ran activator identified in a prokaryotic organism; yet the mechanism of Ran activation remains unclear. The effector might activate Ran directly through GEF activity, similar to the eukaryotic Ran GEFs RCC1 and RanBP10. However, in a nucleotide exchange assay using purified human Ran, His-tagged LegG1 showed no GEF activity, while purified RCC1 did. Perhaps, LegG1 activates Ran indirectly by stabilizing activated Ran(GTP), by inhibiting a Ran GAP, or by targeting a Ran-specific nucleotide release protein such as Mog1.67
To address downstream effects of Ran activation, we investigated microtubule polymerization. LegG1 stabilized microtubules in D. discoideum or macrophages throughout the host cell, as well as on the LCV membrane (Fig. 1), as indicated by confocal laser scanning microscopy and stimulated emission depletion (STED) microscopy, subcellular fractionation and western blot analysis. In an alternative approach, LegG1 was delivered into host cells by “microbial microinjection” using a Yersinia enterocolitica strain that produces the Ysc T3SS, yet lacks all endogenous T3SS effectors.68,69 Fusion proteins of the Y. enterocolitica effector YopE N-terminal translocation fragment with LegG1 (YopE1–53-LegG1) were injected into nocodazole-treated epithelial cells, where they elicited in a Ran-dependent manner a denser microtubule network, compared with uninfected cells or cells infected with bacteria producing YopE1–53.
Since LegG1 localizes to the LCV membrane, the Ran activator likely regulates the production of a Ran(GTP) gradient originating from this membrane compartment. Yet, the effector might not only act as a Ran activator in cis (on LCVs) but also in trans (in a distance from LCVs) to promote the formation of a replication-permissive pathogen vacuole and/or to affect other cellular processes regulated by the small GTPase. Given that the Icm/Dot substrate RomA (Regulator of methylation A), a L. pneumophila methyltransferase that modifies chromatin and gene expression, is targeted to the host cell nucleus,70 it is tempting to speculate that LegG1 might also control Ran-dependent nucleo-cytoplasmic transport.
The Ran Activator LegG1 Promotes Pathogen Vacuole Motility and Cell Migration
Shortly after phagosome closure LCVs move along microtubules within D. discoideum cells.52 Using real-time confocal laser scanning microscopy, we analyzed the dynamics of LCV transport along microtubules in D. discoideum producing either calnexin-GFP66 or GFP-α-tubulin.71 Under these conditions, LCVs harboring wild-type L. pneumophila were very motile and rapidly moved along microtubules. In contrast, LCVs harboring ΔlegG1 mutant bacteria were stalled, and the phenotype was complemented by plasmid-encoded LegG1. Microtubule-dependent LCV motility might position the pathogen vacuole in the vicinity of interacting compartments such as the ER. Alternatively, LegG1-dependent microtubule polymerization might promote vesicle trafficking processes in a distance from the pathogen vacuole, in order to promote fusion and fission events of vesicles communicating with the vacuole.
LegG1 not only promoted intracellular pathogen vacuole motility, but also chemotaxis and migration of eukaryotic cells.71 In under agarose assays, L. pneumophila inhibited in a dose- and T4SS-dependent manner the migration of D. discoideum amoebae toward folate, of murine RAW 264.7 macrophages toward the cytokines CCL5 and TNFα, or of primary human polymorphonuclear neutrophils (PMN) toward the peptide fMLP. L. pneumophila lacking legG1 hyper-inhibited the migration of the phagocytes, and the phenotype was reverted to an extent observed for mutant bacteria lacking a functional Icm/Dot T4SS by providing legG1 in trans. Similarly, LegG1 promoted random migration in scratch assays of L. pneumophila-infected macrophages and A549 epithelial cells in a Ran-dependent manner, or upon “microbial microinjection” into HeLa cells by Y. enterocolitica lacking endogenous effectors. Real-time single-cell tracking of L. pneumophila-infected phagocytes revealed that the velocity and directionality of the cells were decreased, and cell motility as well as microtubule dynamics were impaired.71
The above results are in agreement with the notion that LegG1 stimulates cell migration by antagonizing (unknown) Icm/Dot-translocated effectors inhibiting cell migration. L. pneumophila might benefit from the LegG1-dependent promotion of cell migration by counteracting Icm/Dot substrates, which destabilize microtubules and thus damage a number of essential cellular pathways such as phagocytosis, vesicle trafficking, cytokinesis and migration. Thus, by activating the Ran GTPase and consequent microtubule stabilization, LegG1 might dampen a deleterious impact of other effectors on the host cytoskeleton.
In summary, our results demonstrate that Ran and its eukaryotic effector RanBP1, as well as the L. pneumophila Icm/Dot substrate LegG1 localize to LCVs. LegG1 was characterized as the first bacterial Ran activator, which dependent on Ran GTPase and RanBP1 promotes microtubule stabilization, LCV motility and intracellular replication of L. pneumophila, as well as migration of infected cells. These findings pave the way for a detailed further analysis of the signal transduction pathways activated by Ran(GTP) in pathogen-infected phagocytes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by the German Research Foundation (DFG; HI 1511/1-1, SPP1580, SFB914), the “Bundesministerium für Bildung und Forschung” (BMBF; “Medical Infection Genomics” initiative, 0315834C) and the Swiss National Science Foundation (31003A-125369, CRSI33_130016).
References
- 1. Hilbi H, Hoffmann C, Harrison CF. Legionella spp. outdoors: colonization, communication and persistence. Environ Microbiol Rep 2011; 3:286-96; PMID:23761274; http://dx.doi.org/ 10.1111/j.1758-2229.2011.00247.x [DOI] [PubMed] [Google Scholar]
- 2. Newton HJ, Ang DK, van Driel IR, Hartland EL. Molecular pathogenesis of infections caused by Legionella pneumophila. Clin Microbiol Rev 2010; 23:274-98; PMID:20375353; http://dx.doi.org/ 10.1128/CMR.00052-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nguyen TM, Ilef D, Jarraud S, Rouil L, Campese C, Che D, Haeghebaert S, Ganiayre F, Marcel F, Etienne J, et al. A community-wide outbreak of legionnaires disease linked to industrial cooling towers–how far can contaminated aerosols spread? J Infect Dis 2006; 193:102-11; PMID:16323138; http://dx.doi.org/ 10.1086/498575 [DOI] [PubMed] [Google Scholar]
- 4. Isberg RR, O’Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol 2009; 7:13-24; PMID:19011659; http://dx.doi.org/ 10.1038/nrmicro1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hubber A, Roy CR. Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol 2010; 26:261-83; PMID:20929312; http://dx.doi.org/ 10.1146/annurev-cellbio-100109-104034 [DOI] [PubMed] [Google Scholar]
- 6. Hilbi H, Haas A. Secretive bacterial pathogens and the secretory pathway. Traffic 2012; 13:1187-97; PMID:22340894; http://dx.doi.org/ 10.1111/j.1600-0854.2012.01344.x [DOI] [PubMed] [Google Scholar]
- 7. Ragaz C, Pietsch H, Urwyler S, Tiaden A, Weber SS, Hilbi H. The Legionella pneumophila phosphatidylinositol-4 phosphate-binding type IV substrate SidC recruits endoplasmic reticulum vesicles to a replication-permissive vacuole. Cell Microbiol 2008; 10:2416-33; PMID:18673369; http://dx.doi.org/ 10.1111/j.1462-5822.2008.01219.x [DOI] [PubMed] [Google Scholar]
- 8. Weber S, Wagner M, Hilbi H. Live-cell imaging of phosphoinositide dynamics and membrane architecture during Legionella infection. MBio 2013; 5:e00839-13; PMID:24473127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finsel I, Ragaz C, Hoffmann C, Harrison CF, Weber S, van Rahden VA, Johannes L, Hilbi H. The Legionella effector RidL inhibits retrograde trafficking to promote intracellular replication. Cell Host Microbe 2013; 14:38–50; PMID:23870312; http://dx.doi.org/ 10.1016/j.chom.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 10. Xu L, Shen X, Bryan A, Banga S, Swanson MS, Luo ZQ. Inhibition of host vacuolar H+-ATPase activity by a Legionella pneumophila effector. PLoS Pathog 2010; 6:e1000822; PMID:20333253; http://dx.doi.org/ 10.1371/journal.ppat.1000822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choy A, Dancourt J, Mugo B, O’Connor TJ, Isberg RR, Melia TJ, Roy CR. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science 2012; 338:1072-6; PMID:23112293; http://dx.doi.org/ 10.1126/science.1227026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann C, Harrison CF, Hilbi H. The natural alternative: protozoa as cellular models for Legionella infection. Cell Microbiol 2014; 16:15-26; PMID:24168696; http://dx.doi.org/ 10.1111/cmi.12235 [DOI] [PubMed] [Google Scholar]
- 13. Zhu W, Banga S, Tan Y, Zheng C, Stephenson R, Gately J, Luo ZQ. Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS One 2011; 6:e17638; PMID:21408005; http://dx.doi.org/ 10.1371/journal.pone.0017638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gomez-Valero L, Rusniok C, Cazalet C, Buchrieser C. Comparative and functional genomics of Legionella identified eukaryotic like proteins as key players in host-pathogen interactions. Front Microbiol 2011; 2:208; PMID:22059087; http://dx.doi.org/ 10.3389/fmicb.2011.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lifshitz Z, Burstein D, Peeri M, Zusman T, Schwartz K, Shuman HA, Pupko T, Segal G. Computational modeling and experimental validation of the Legionella and Coxiella virulence-related type-IVB secretion signal. Proc Natl Acad Sci U S A 2013; 110:E707-15; PMID:23382224; http://dx.doi.org/ 10.1073/pnas.1215278110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haneburger I, Hilbi H. Phosphoinositide lipids and the Legionella pathogen vacuole. Curr Top Microbiol Immunol 2013; 376:155-73; PMID:23918172; http://dx.doi.org/ 10.1007/82_2013_341 [DOI] [PubMed] [Google Scholar]
- 17. Hilbi H, Weber S, Finsel I. Anchors for effectors: subversion of phosphoinositide lipids by Legionella. Front Microbiol 2011; 2:91; PMID:21833330; http://dx.doi.org/ 10.3389/fmicb.2011.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weber SS, Ragaz C, Hilbi H. Pathogen trafficking pathways and host phosphoinositide metabolism. Mol Microbiol 2009; 71:1341-52; PMID:19208094; http://dx.doi.org/ 10.1111/j.1365-2958.2009.06608.x [DOI] [PubMed] [Google Scholar]
- 19. Weber S, Dolinsky S, Hilbi H. Interactions of Legionella effector proteins with host phosphoinositide lipids. Methods Mol Biol 2013; 954:367-80; PMID:23150409; http://dx.doi.org/ 10.1007/978-1-62703-161-5_23 [DOI] [PubMed] [Google Scholar]
- 20. Weber SS, Ragaz C, Reus K, Nyfeler Y, Hilbi H. Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog 2006; 2:e46; PMID:16710455; http://dx.doi.org/ 10.1371/journal.ppat.0020046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brombacher E, Urwyler S, Ragaz C, Weber SS, Kami K, Overduin M, Hilbi H. Rab1 guanine nucleotide exchange factor SidM is a major phosphatidylinositol 4-phosphate-binding effector protein of Legionella pneumophila. J Biol Chem 2009; 284:4846-56; PMID:19095644; http://dx.doi.org/ 10.1074/jbc.M807505200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schoebel S, Blankenfeldt W, Goody RS, Itzen A. High-affinity binding of phosphatidylinositol 4-phosphate by Legionella pneumophila DrrA. EMBO Rep 2010; 11:598-604; PMID:20616805; http://dx.doi.org/ 10.1038/embor.2010.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsu F, Zhu W, Brennan L, Tao L, Luo ZQ, Mao Y. Structural basis for substrate recognition by a unique Legionella phosphoinositide phosphatase. Proc Natl Acad Sci U S A 2012; 109:13567-72; PMID:22872863; http://dx.doi.org/ 10.1073/pnas.1207903109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weber SS, Ragaz C, Hilbi H. The inositol polyphosphate 5-phosphatase OCRL1 restricts intracellular growth of Legionella, localizes to the replicative vacuole and binds to the bacterial effector LpnE. Cell Microbiol 2009; 11:442-60; PMID:19021631; http://dx.doi.org/ 10.1111/j.1462-5822.2008.01266.x [DOI] [PubMed] [Google Scholar]
- 25. Jank T, Böhmer KE, Tzivelekidis T, Schwan C, Belyi Y, Aktories K. Domain organization of Legionella effector SetA. Cell Microbiol 2012; 14:852-68; PMID:22288428; http://dx.doi.org/ 10.1111/j.1462-5822.2012.01761.x [DOI] [PubMed] [Google Scholar]
- 26. Toulabi L, Wu X, Cheng Y, Mao Y. Identification and structural characterization of a Legionella phosphoinositide phosphatase. J Biol Chem 2013; 288:24518-27; PMID:23843460; http://dx.doi.org/ 10.1074/jbc.M113.474239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Itzen A, Goody RS. Covalent coercion by Legionella pneumophila. Cell Host Microbe 2011; 10:89-91; PMID:21843863; http://dx.doi.org/ 10.1016/j.chom.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 28. Sherwood RK, Roy CR. A Rab-centric perspective of bacterial pathogen-occupied vacuoles. Cell Host Microbe 2013; 14:256-68; PMID:24034612; http://dx.doi.org/ 10.1016/j.chom.2013.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 2002; 295:679-82; PMID:11809974; http://dx.doi.org/ 10.1126/science.1067025 [DOI] [PubMed] [Google Scholar]
- 30. Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol 2006; 8:971-7; PMID:16906144; http://dx.doi.org/ 10.1038/ncb1463 [DOI] [PubMed] [Google Scholar]
- 31. Machner MP, Isberg RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell 2006; 11:47–56; PMID:16824952; http://dx.doi.org/ 10.1016/j.devcel.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 32. Müller MP, Peters H, Blümer J, Blankenfeldt W, Goody RS, Itzen A. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science 2010; 329:946-9; PMID:20651120; http://dx.doi.org/ 10.1126/science.1192276 [DOI] [PubMed] [Google Scholar]
- 33. Mukherjee S, Liu X, Arasaki K, McDonough J, Galán JE, Roy CR. Modulation of Rab GTPase function by a protein phosphocholine transferase. Nature 2011; 477:103-6; PMID:21822290; http://dx.doi.org/ 10.1038/nature10335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pan X, Lührmann A, Satoh A, Laskowski-Arce MA, Roy CR. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science 2008; 320:1651-4; PMID:18566289; http://dx.doi.org/ 10.1126/science.1158160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schoebel S, Cichy AL, Goody RS, Itzen A. Protein LidA from Legionella is a Rab GTPase supereffector. Proc Natl Acad Sci U S A 2011; 108:17945–50; PMID:22011575; http://dx.doi.org/ 10.1073/pnas.1113133108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan Y, Luo ZQ. Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature 2011; 475:506-9; PMID:21734656; http://dx.doi.org/ 10.1038/nature10307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neunuebel MR, Chen Y, Gaspar AH, Backlund PS, Jr., Yergey A, Machner MP. De-AMPylation of the small GTPase Rab1 by the pathogen Legionella pneumophila. Science 2011; 333:453-6; PMID:21680813; http://dx.doi.org/ 10.1126/science.1207193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tan Y, Arnold RJ, Luo ZQ. Legionella pneumophila regulates the small GTPase Rab1 activity by reversible phosphorylcholination. Proc Natl Acad Sci U S A 2011; 108:21212-7; PMID:22158903; http://dx.doi.org/ 10.1073/pnas.1114023109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goody PR, Heller K, Oesterlin LK, Müller MP, Itzen A, Goody RS. Reversible phosphocholination of Rab proteins by Legionella pneumophila effector proteins. EMBO J 2012; 31:1774-84; PMID:22307087; http://dx.doi.org/ 10.1038/emboj.2012.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ingmundson A, Delprato A, Lambright DG, Roy CR. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature 2007; 450:365-9; PMID:17952054; http://dx.doi.org/ 10.1038/nature06336 [DOI] [PubMed] [Google Scholar]
- 41. Gazdag ME, Streller A, Haneburger I, Hilbi H, Vetter IR, Goody RS, Itzen A. Mechanism of Rab1b deactivation by the Legionella pneumophila GAP LepB. EMBO Rep 2013; 14:199-205; PMID:23288104; http://dx.doi.org/ 10.1038/embor.2012.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ku B, Lee KH, Park WS, Yang CS, Ge J, Lee SG, Cha SS, Shao F, Heo WD, Jung JU, et al. VipD of Legionella pneumophila targets activated Rab5 and Rab22 to interfere with endosomal trafficking in macrophages. PLoS Pathog 2012; 8:e1003082; PMID:23271971; http://dx.doi.org/ 10.1371/journal.ppat.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gaspar AH, Machner MP. VipD is a Rab5-activated phospholipase A1 that protects Legionella pneumophila from endosomal fusion. Proc Natl Acad Sci U S A 2014; 111:4560-4565; PMID:24616501; http://dx.doi.org/ 10.1073/pnas.1316376111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Degtyar E, Zusman T, Ehrlich M, Segal G. A. Legionella effector acquired from protozoa is involved in sphingolipids metabolism and is targeted to the host cell mitochondria. Cell Microbiol 2009; 11:1219-35; PMID:19438520; http://dx.doi.org/ 10.1111/j.1462-5822.2009.01328.x [DOI] [PubMed] [Google Scholar]
- 45. Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol 2002; 4:945-54; PMID:12447391; http://dx.doi.org/ 10.1038/ncb883 [DOI] [PubMed] [Google Scholar]
- 46. Kagan JC, Stein MP, Pypaert M, Roy CR. Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J Exp Med 2004; 199:1201-11; PMID:15117975; http://dx.doi.org/ 10.1084/jem.20031706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Derré I, Isberg RR. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect Immun 2004; 72:3048-53; PMID:15102819; http://dx.doi.org/ 10.1128/IAI.72.5.3048-3053.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clemens DL, Lee BY, Horwitz MA. Mycobacterium tuberculosis and Legionella pneumophila phagosomes exhibit arrested maturation despite acquisition of Rab7. Infect Immun 2000; 68:5154-66; PMID:10948139; http://dx.doi.org/ 10.1128/IAI.68.9.5154-5166.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Urwyler S, Nyfeler Y, Ragaz C, Lee H, Mueller LN, Aebersold R, Hilbi H. Proteome analysis of Legionella vacuoles purified by magnetic immunoseparation reveals secretory and endosomal GTPases. Traffic 2009; 10:76-87; PMID:18980612; http://dx.doi.org/ 10.1111/j.1600-0854.2008.00851.x [DOI] [PubMed] [Google Scholar]
- 50. Shevchuk O, Batzilla C, Hägele S, Kusch H, Engelmann S, Hecker M, Haas A, Heuner K, Glöckner G, Steinert M. Proteomic analysis of Legionella-containing phagosomes isolated from Dictyostelium. Int J Med Microbiol 2009; 299:489-508; PMID:19482547; http://dx.doi.org/ 10.1016/j.ijmm.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 51. Hoffmann C, Finsel I, Otto A, Pfaffinger G, Rothmeier E, Hecker M, Becher D, Hilbi H. Functional analysis of novel Rab GTPases identified in the proteome of purified Legionella-containing vacuoles from macrophages. Cell Microbiol 2014; 16:1034-52; PMID:24373249 [DOI] [PubMed] [Google Scholar]
- 52. Lu H, Clarke M. Dynamic properties of Legionella-containing phagosomes in Dictyostelium amoebae. Cell Microbiol 2005; 7:995-1007; PMID:15953031; http://dx.doi.org/ 10.1111/j.1462-5822.2005.00528.x [DOI] [PubMed] [Google Scholar]
- 53. Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol 2007; 8:195-208; PMID:17287812; http://dx.doi.org/ 10.1038/nrm2114 [DOI] [PubMed] [Google Scholar]
- 54. Goodman B, Zheng Y. Mitotic spindle morphogenesis: Ran on the microtubule cytoskeleton and beyond. Biochem Soc Trans 2006; 34:716-21; PMID:17052181; http://dx.doi.org/ 10.1042/BST0340716 [DOI] [PubMed] [Google Scholar]
- 55. Clarke PR, Zhang C. Spatial and temporal coordination of mitosis by Ran GTPase. Nat Rev Mol Cell Biol 2008; 9:464-77; PMID:18478030; http://dx.doi.org/ 10.1038/nrm2410 [DOI] [PubMed] [Google Scholar]
- 56. Joseph J. Ran at a glance. J Cell Sci 2006; 119:3481-4; PMID:16931595; http://dx.doi.org/ 10.1242/jcs.03071 [DOI] [PubMed] [Google Scholar]
- 57. Schulze H, Dose M, Korpal M, Meyer I, Italiano JE, Jr., Shivdasani RA. RanBP10 is a cytoplasmic guanine nucleotide exchange factor that modulates noncentrosomal microtubules. J Biol Chem 2008; 283:14109-19; PMID:18347012; http://dx.doi.org/ 10.1074/jbc.M709397200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cheng H, Govindan JA, Greenstein D. Regulated trafficking of the MSP/Eph receptor during oocyte meiotic maturation in C. elegans. Curr Biol 2008; 18:705-14; PMID:18472420; http://dx.doi.org/ 10.1016/j.cub.2008.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yudin D, Fainzilber M. Ran on tracks–cytoplasmic roles for a nuclear regulator. J Cell Sci 2009; 122:587-93; PMID:19225125; http://dx.doi.org/ 10.1242/jcs.015289 [DOI] [PubMed] [Google Scholar]
- 60. Bischoff FR, Ponstingl H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature 1991; 354:80-2; PMID:1944575; http://dx.doi.org/ 10.1038/354080a0 [DOI] [PubMed] [Google Scholar]
- 61. de Felipe KS, Pampou S, Jovanovic OS, Pericone CD, Ye SF, Kalachikov S, Shuman HA. Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol 2005; 187:7716-26; PMID:16267296; http://dx.doi.org/ 10.1128/JB.187.22.7716-7726.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Felipe KS, Glover RT, Charpentier X, Anderson OR, Reyes M, Pericone CD, Shuman HA. Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog 2008; 4:e1000117; PMID:18670632; http://dx.doi.org/ 10.1371/journal.ppat.1000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ninio S, Celli J, Roy CR. A Legionella pneumophila effector protein encoded in a region of genomic plasticity binds to Dot/Icm-modified vacuoles. PLoS Pathog 2009; 5:e1000278; PMID:19165328; http://dx.doi.org/ 10.1371/journal.ppat.1000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ivanov SS, Charron G, Hang HC, Roy CR. Lipidation by the host prenyltransferase machinery facilitates membrane localization of Legionella pneumophila effector proteins. J Biol Chem 2010; 285:34686-98; PMID:20813839; http://dx.doi.org/ 10.1074/jbc.M110.170746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Folly-Klan M, Alix E, Stalder D, Ray P, Duarte LV, Delprato A, Zeghouf M, Antonny B, Campanacci V, Roy CR, et al. A novel membrane sensor controls the localization and ArfGEF activity of bacterial RalF. PLoS Pathog 2013; 9:e1003747;PMID:24244168; http://dx.doi.org/ 10.1371/journal.ppat.1003747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rothmeier E, Pfaffinger G, Hoffmann C, Harrison CF, Grabmayr H, Repnik U, Hannemann M, Wölke S, Bausch A, Griffiths G, et al. Activation of Ran GTPase by a Legionella effector promotes microtubule polymerization, pathogen vacuole motility and infection. PLoS Pathog 2013; 9:e1003598; PMID:24068924; http://dx.doi.org/ 10.1371/journal.ppat.1003598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Steggerda SM, Paschal BM. The mammalian Mog1 protein is a guanine nucleotide release factor for Ran. J Biol Chem 2000; 275:23175-80; PMID:10811801; http://dx.doi.org/ 10.1074/jbc.C000252200 [DOI] [PubMed] [Google Scholar]
- 68. Trülzsch K, Roggenkamp A, Aepfelbacher M, Wilharm G, Ruckdeschel K, Heesemann J. Analysis of chaperone-dependent Yop secretion/translocation and effector function using a mini-virulence plasmid of Yersinia enterocolitica. Int J Med Microbiol 2003; 293:167-77; PMID:12868653; http://dx.doi.org/ 10.1078/1438-4221-00251 [DOI] [PubMed] [Google Scholar]
- 69. Wölke S, Heesemann J. Probing the cellular effects of bacterial effector proteins with the Yersinia toolbox. Future Microbiol 2012; 7:449–56; PMID:22439722; http://dx.doi.org/ 10.2217/fmb.12.16 [DOI] [PubMed] [Google Scholar]
- 70. Rolando M, Sanulli S, Rusniok C, Gomez-Valero L, Bertholet C, Sahr T, Margueron R, Buchrieser C. Legionella pneumophila effector RomA uniquely modifies host chromatin to repress gene expression and promote intracellular bacterial replication. Cell Host Microbe 2013; 13:395-405; PMID:23601102; http://dx.doi.org/ 10.1016/j.chom.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 71. Simon S, Wagner MA, Rothmeier E, Müller-Taubenberger A, Hilbi H. Icm/Dot-dependent inhibition of phagocyte migration by Legionella is antagonized by a translocated Ran GTPase activator. Cell Microbiol 2014; 16: 977-92; PMID:24397557 [DOI] [PubMed] [Google Scholar]


