Abstract
Amino acid sequence variants, especially variants containing non-canonical amino acids such as norleucine and norvaline, are a concern during therapeutic protein production in microbial systems. Substitution of methionine residues with norleucine in recombinant proteins produced in Escherichia coli is well known. Continuous feeding of amino acids such as methionine is commonly used in E. coli fermentation processes to control incorporation of norleucine in the recombinant protein. There are several disadvantages associated with continuous feeding during a fermentation process. For example, a continuous feed increases the operational complexity and cost of a manufacturing process and results in dilution of culture medium which could result in lower cell densities and product yields. To overcome the limitations of existing approaches to prevent norleucine incorporation during E. coli fermentations, a new approach using an engineered host was developed that overproduces methionine in the cell to prevent norleucine incorporation without negatively impacting fermentation process performance and product yields. In this commentary, the results on using methionine overproducing hosts for recombinant protein production in E. coli and some “watch outs” when using these hosts for recombinant protein production are discussed.
Keywords: fermentation, incorporation, methionine, norleucine, recombinant protein, sequence variant
Introduction
Protein based therapeutics have become a key component in the treatment of life-threatening diseases. To ensure the desired safety and efficacy of a therapeutic, it is important to reduce the formation of undesirable product variants during the manufacturing process. Amino acid sequence variants, which can occur as a result of DNA mutations, errors during transcription, translation, and/or post-translational processing, are one of the well-known product variants formed during manufacturing of recombinant protein therapeutics both in microbial1,2,3 and mammalian systems.2,4,5 Sequence variants have received considerable attention in recent years as there has been a tremendous advancement in the analytical techniques to detect these variants even at low levels.2,3,6 Sequence variants in the final drug product can have a potential impact on biological activity and could possibly result in immunogenicity when the product is in injected into patients. As a result of these undesirable substitutions, the product may require intensive analytical characterization, which could result in delays in product approvals by the regulatory health authorities. It is, therefore, important to develop and implement robust methods to prevent these undesirable variants during recombinant protein production.
Sequence variants arising as a result of norleucine incorporation in proteins produced in E. coli have been known for over 50 y7 Norleucine is an unnatural amino acid synthesized as a byproduct in the branched chain amino acid metabolism in E. coli.1 It is a structural analog of methionine and can substitute for methionine residues in proteins because methionyl-tRNA synthetase (MetRS) can use norleucine as a substrate, albeit at a lower efficiency when compared to methionine, to charge the methionyl-tRNA during the translation process.8,9 Several methods have been developed to prevent norleucine incorporation in recombinant proteins produced in E. coli namely: 1) altering codons in the DNA sequence coding for recombinant protein to remove methionine residues10; 2) expressing norleucine degrading enzymes11; 3) deleting the genes involved in biosynthesis of norleucine1; 4) supplementation of trace elements such as molybdenum, nickel and selenium, in the fermentation medium12; and 5) bolus addition or continuous feeding methionine or leucine during the fermentation.1 However most of these methods suffer from several limitations (discussed in the next paragraph) and are not ideal for the biotechnology industry.
Monoclonal antibodies, especially the IgG class, are rapidly growing class of human therapeutics in many disease areas including oncology, auto immune and infectious diseases.13 There are 2 highly conserved methionine residues in Fc region of human IgG1 that are important for binding to neonatal Fc receptor (FcRn) and as a result the serum half-life of IgG1.14-17 Altering methionine codons could potentially decrease serum half-life and hence, this approach may not be applicable for the production of Fc-containing antibodies in E. coli. Co-expressing a norleucine degrading enzyme (e.g. glutamate, leucine or valine dehydrogeneases) to prevent norleucine incorporation could reduce product yields due to co-expression. Supplementation of trace elements such as molybdenum, nickel and selenium, reduces norleucine incorporation under oxygen limited conditions, however a majority of E. coli fermentations in biotech industry are aerobic and are performed under oxygen excess conditions and hence this method may not be widely applicable. Deleting leucine biosynthetic genes and/or transaminases (genes involved in norleucine biosynthesis in the cell1) requires supplementation of high levels of leucine and other branched chain amino acids via continuous or bolus additions to the culture medium. Continuous or bolus additions of methionine during the fermentation process are commonly used methods to prevent norleucine incorporation in E. coli. Methionine supplementation reduces the likelihood of methionyl-tRNA mischarging with norleucine by MetRS during the translation process. However, continuous feeding of a nutrient during a fermentation process has several disadvantages: 1) an additional feed increases the operational complexity and cost (i.e. extra tankage, automation, preparation, sterilization, cleaning, etc.) of the manufacturing process; 2) feed rate and/or feed initiation timing (typically a few hours before product synthesis) are potential critical process parameters due to their impact on norleucine incorporation. Incorrect execution of the methionine feed (e.g., delay in feed initiation, flow control valve issues, transfer line leaks, etc.) during a manufacturing process requires additional discrepancy resolution overhead resulting in delays to product release; 3) continuous feeding or bolus additions during the fermentation process could impact cell densities and possibly product yields due to dilution of the culture medium; 4) an additional feed results in 2 additional parameters, feed rate and feed initiation timing, for evaluation during process characterization studies, which is a key step in the implementation of Quality by Design (QbD) for a biotech product.
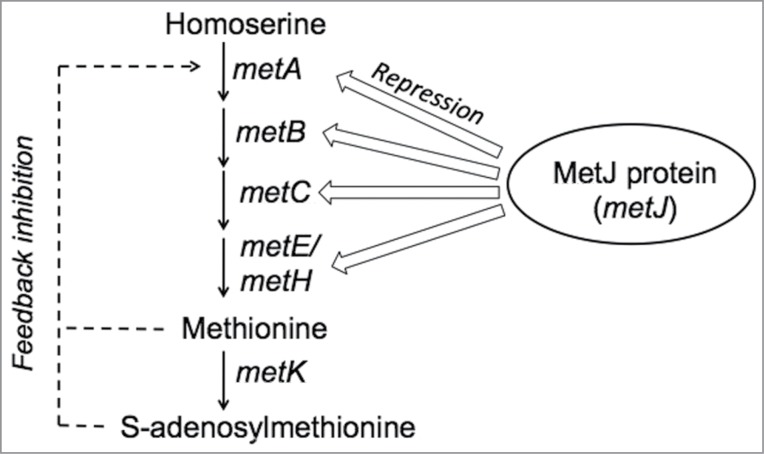
Recently, a new approach was developed which overcomes the limitations with the existing approaches to prevent norleucine incorporation during recombinant protein production in E.coli.18 An E. coli production host (60E4) was engineered to overproduce methionine by targeting the genes involved in methionine biosynthesis and regulation. Several hosts were constructed in the 60E4 host background containing mutations in the genes: metA (encodes for homoserine O-succinyltransferase which catalyzes conversion of homoserine to O-succinyl homoserine, the first unique step in the de novo methionine biosynthetic pathway19), and/or metK (encodes for methionyl adenosyltransferase, which catalyzes the formation of S-adenosyl methionine from methionine20) or metJ (encodes for transcriptional repressor of genes involved in biosynthesis and transport of methionine21) (Fig. 1). Comparable fermentation performance and product yields were obtained using methionine overproducing production hosts containing a metA(Y294C) allele (metA(Y294C) encodes for MetA protein with a Y294C mutation) that results in a MetA enzyme feedback resistant to methionine in 3 different recombinant protein production processes. As expected, the metA(Y294C) host accumulated high levels of intracellular (150–200 μM) and extracellular methionine (400 μM) during the recombinant protein production phase of the fermentation and hence prevented norleucine incorporation in the recombinant protein. The metA(Y294C) allele was introduced into 2 other production hosts, and the metA(Y294C) host fermentations showed comparable fermentation performance and product yields as their parent host fermentations in these 2 processes and the product obtained from metA(Y294C) host fermentations did not show norleucine incorporation.18 These results demonstrate the strength of a genetic engineering approach as it overcomes the limitations of existing approaches (e.g. lower costs, simpler processes, no dilution-related effects on yield, and no additional parameters to study during a process characterization exercise, etc.) in preventing norleucine incorporation during E. coli fermentations.
Figure 1.
Methionine biosynthesis and regulation in E. coli.
Fermentation process conditions and medium composition play an important role in the biosynthesis of methionine in the cell.22 For example, an elevated temperature could result in aggregation and proteolysis of MetA, or a pH that is sub-optimal for catalytic activity of an enzyme in the methionine biosynthetic pathway, could affect methionine biosynthesis.23,24 Similarly, dissolved oxygen and glucose concentrations in the medium affect methionine biosynthesis.25,26 Fermentation process conditions that affect methionine biosynthesis in the cell could potentially lead to norleucine incorporation in the recombinant protein when using a methionine overproducing host for recombinant protein production. Alternatively, process conditions that lead to increased biosynthesis of methionine could potentially have an impact on product yields due to a competition for glucose, which is a carbon and energy source in the cell, between pathways that lead to methionine overproduction and product synthesis. For demonstrating overall robustness of methionine overproducing hosts in preventing norleucine incorporation, a bolus of norleucine was added (final concentration of 0.15 mM in the culture medium) during the fermentation process using a metA(Y294C) host a few hours before synthesis of the recombinant protein.18 Levels of norleucine in the recombinant protein were <0.5%, suggesting that the host makes sufficient methionine to control the extent of methionyl tRNA mischarging with norleucine to very low levels even under these conditions. It may be worthwhile to evaluate the metA(Y294C) allele containing production hosts under a range of process conditions typically performed during process development and characterization studies and analyze for product yields and norleucine in the recombinant protein before utilizing these hosts for biologic manufacturing processes. Studies along these lines are on-going in our laboratory.
Incorporating a heterologous methionyl-tRNA synthetase or an engineered native MetRS, which cannot charge the methionyl tRNA with norleucine, into the E. coli chromosome could be a powerful approach to eliminate norleucine incorporation. While there is not much evidence on the existence of a heterologous methionyl-tRNA synthetase enzyme with no activity toward norleucine, protein engineers over the last couple of decades have developed and established methods to engineer tRNA synthetases for incorporation of a wide variety of non-canonical amino acids into proteins for studying protein structure and function and adding unique functionality to proteins.27-29 The E. coli MetRS enzyme has been engineered to efficiently incorporate a norleucine analog, azido norleucine, into proteins thus providing structural insights into the residues that are important for binding of azido norleucine in the active site of MetRS.30 Given that the crystal structures of E. coli MetRS with31 and without32 bound methionine and several methionine analogs33 are solved and residues that are critical for binding of azido norleucine is known,30 it should be possible to exploit this wealth of structural data and engineer the enzyme's active site to decrease or even eliminate its activity toward norleucine.
In addition to norleucine substitutions, multiple other sequence variants have been observed at relatively low levels (<0.2%) in the recombinant proteins purified from the metA(Y294C) host fermentations. However, similar levels of sequence variants were also observed in the wild-type metA+ host fermentations which used continuous methionine feeding indicating that these sequence variants are unrelated to methionine overproduction in the cell. These low level sequence variants are consistent with recent studies which demonstrated that many low level sequence variants exist in both recombinant and endogenous proteins produced in microbial systems.2,3
In summary, we conclude that utilizing methionine overproducing hosts for manufacturing recombinant proteins in E. coli is a promising approach to prevent norleucine incorporation and will lead to simpler and more cost effective manufacturing processes. Demonstrating process robustness with methionine overproducing hosts under a range of process conditions will help usher the microbial fermentation community toward a new era of using methionine overproducing hosts for E. coli recombinant protein production processes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Bogosian G, Violand BN, Dorward-King EJ, Workman WE, Jung PE, Kane JF. Biosynthesis and incorporation into protein of norleucine by Escherichia coli. J Biol Chem 1989; 264:531-9; PMID:2642478 [PubMed] [Google Scholar]
- 2. Zhang Z, Shah B, Bondarenko PV. G/U and certain wobble position mismatches as possible main causes of amino acid misincorporations. Biochemistry 2013; 52:8165-76; PMID:24128183; http://dx.doi.org/ 10.1021/bi401002c [DOI] [PubMed] [Google Scholar]
- 3. Harris RP, Mattocks J, Green PS, Moffatt F, Kilby PM. Determination and control of low-level amino acid misincorporation in human thioredoxin protein produced in a recombinant Escherichia coli production system. Biotechnol Bioeng 2012; 109:1987-95; PMID:22334292 [DOI] [PubMed] [Google Scholar]
- 4. Guo D, Gao A, Michels DA, Feeney L, Eng M, Chan B, Laird MW, Zhang B, Yu XC, Joly J, et al. Mechanisms of unintended amino acid sequence changes in recombinant monoclonal antibodies expressed in Chinese Hamster Ovary (CHO) cells. Biotechnol Bioeng 2010; 107:163-71; PMID:20506532; http://dx.doi.org/ 10.1002/bit.22780 [DOI] [PubMed] [Google Scholar]
- 5. Feeney L, Carvalhal V, Yu XC, Chan B, Michels DA, Wang YJ, Shen A, Ressl J, Dusel B, Laird MW. Eliminating tyrosine sequence variants in CHO cell lines producing recombinant monoclonal antibodies. Biotechnol Bioeng 2013; 110:1087-97; PMID:23108857; http://dx.doi.org/ 10.1002/bit.24759 [DOI] [PubMed] [Google Scholar]
- 6. Yu XC, Borisov OV., Alvarez M, Michels DA, Wang YJ, Ling V. Identification of codon-specific serine to asparagine mistranslation in recombinant monoclonal antibodies by high-resolution mass spectrometry. Anal Chem 2009; 81:9282-90; PMID:19852494 [DOI] [PubMed] [Google Scholar]
- 7. Cohen GN, Munier R. Incorporation of structural analogues of amino acids in bacterial proteins. Biochim Biophys Acta 1956; 21:592-3; PMID:13363976 [DOI] [PubMed] [Google Scholar]
- 8. Budisa N, Steipe B, Demange P, Eckerskorn C, Kellermann J, Huber R. High-level biosynthetic substitution of methionine in proteins by its analogs 2-aminohexanoic acid, selenomethionine, telluromethionine and ethionine in Escherichia coli. Eur J Biochem 1995; 230:788-96; PMID:7607253 [DOI] [PubMed] [Google Scholar]
- 9. Van Hest JCM, Kiick KL, Tirrell DA. Efficient incorporation of unsaturated methionine analogues into proteins in vivo. J Am Chem Soc 2000; 122:1282-8. [Google Scholar]
- 10. Brunner DP, Harbour GC, Kirschner RJ, Pinner JF, Garlick KL, inventors; Pharmacia and Upjohn company, assignee Fermentation media and methods for controlling norleucine in polypeptides.United States patent US 5,698,418. 1997. Dec 17. [Google Scholar]
- 11. Bogosian G, O’ Neil JP, Smith HQ, inventors; Monsanto Technology LLC, assignee Prevention of incorporation of non-standard amino acids into protein. Unites states patent US 8,603,781 B2. 2013. Dec 10. [Google Scholar]
- 12. Biermann M, Linnemann J, Knüpfer U, Vollstädt S, Bardl B, Seidel G, Horn U. Trace element associated reduction of norleucine and norvaline accumulation during oxygen limitation in a recombinant Escherichia coli fermentation. Microb Cell Fact 2013; 12:116.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol 2010; 10:301-16; PMID:20414204; http://dx.doi.org/ 10.1038/nri2761 [DOI] [PubMed] [Google Scholar]
- 14. Gao X, Ji J, Veeravalli K, Wang YJ, Zhang T, Mcgreevy W, Zheng K, Kelley RF, Laird MW, Liu J, et al. Effect of Individual Fc Methionine Oxidation on FcRn Binding: Met252 Oxidation Impairs FcRn Binding More Profoundly than Met428 Oxidation. J Pharm Sci 2014; 1-10; PMID:25175600; http://dx.doi.org/ 10.1002/jps.24136 [DOI] [PubMed] [Google Scholar]
- 15. Bertolotti-Ciarlet A, Wang W, Lownes R, Pristatsky P, Fang Y, McKelvey T, Li Y, Li Y, Drummond J, Prueksaritanont T, et al. Impact of methionine oxidation on the binding of human IgG1 to Fc Rn and Fc gamma receptors. Mol Immunol 2009; 46:1878-82; PMID:19269032 [DOI] [PubMed] [Google Scholar]
- 16. Pan H, Chen K, Chu L, Kinderman F, Apostol I, Huang G. Methionine oxidation in human IgG2 Fc decreases binding affinities to protein A and FcRn. Protein Sci 2009; 18:424-33; PMID:19165723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang W, Vlasak J, Li Y, Pristatsky P, Fang Y, Pittman T, Roman J, Wang Y, Prueksaritanont T, Ionescu R. Impact of methionine oxidation in human IgG1 Fc on serum half-life of monoclonal antibodies. Mol Immunol 2011; 48:860-6; PMID:21256596; http://dx.doi.org/ 10.1016/j.molimm.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 18. Veeravalli K, Laird MW, Fedesco M, Zhang Y, Yu XC. Strain engineering to prevent norleucine incorporation during recombinant protein production in Escherichia coli. Biotechnol Prog 2014; 31:204-11; PMID:25315437; http://dx.doi.org/ 10.1002/btpr.1999 [DOI] [PubMed] [Google Scholar]
- 19. Born TL, Blanchard JS. Enzyme-catalyzed acylation of homoserine: Mechanistic characterization of the Escherichia coli metA-encoded homoserine transsuccinylase. Biochemistry 1999; 38:14416-23; PMID:10572016; http://dx.doi.org/ 10.1021/bi991710o] [DOI] [PubMed] [Google Scholar]
- 20. Markham GD, Hafner EW, Tabor CW, Tabor H. S-Adenosylmethionine synthetase from Escherichia coli. J Biol Chem 1980; 255:9082-92; PMID:6251075 [PubMed] [Google Scholar]
- 21. Marincs F, Manfield IW, Stead JA, McDowall KJ, Stockley PG. Transcript analysis reveals an extended regulon and the importance of protein-protein co-operativity for the Escherichia coli methionine repressor. Biochem J 2006; 396:227-34; PMID:16515535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gomes J, Kumar D. Production of L-methionine by submerged fermentation: A review. Enzyme Microb. Technol. 2005; 37:3-18. [Google Scholar]
- 23. Gur E, Biran D, Gazit E, Ron EZ. In vivo aggregation of a single enzyme limits growth of Escherichia coli at elevated temperatures. Mol Microbiol 2002; 46:1391-7; PMID:12453224 [DOI] [PubMed] [Google Scholar]
- 24. Biran D, Gur E, Gollan L, Ron EZ. Control of methionine biosynthesis in Escherichia coli by proteolysis. Mol Microbiol 2000; 37:1436-43; PMID:10998174 [DOI] [PubMed] [Google Scholar]
- 25. Sharma S, Gomes J. Effect of Dissolved Oxygen on Continuous Production of Methionine. Eng Life Sci 2001; 1:69-73. [Google Scholar]
- 26. Ranjan AP, Gomes J. Simultaneous dissolved oxygen and glucose regulation in fed-batch methionine production using decoupled input-output linearizing control. J Process Control 2009; 19:664-77. [Google Scholar]
- 27. Wang L, Xie J, Schultz PG. Expanding the genetic code. Annu Rev Biophys Biomol Struct 2006; 35:225-49; PMID:16689635 [DOI] [PubMed] [Google Scholar]
- 28. Dougherty D. Unnatural amino acids as probes of protein structure and function. Curr Opin Chem Biol 2000; 4:645-52. [DOI] [PubMed] [Google Scholar]
- 29. Cornish VW, Mendel D, Schultz PG. Probing Protein Structure and Function with an Expanded Genetic Code. Angew Chemie Int Ed English Internet. 1995; 34:621-33. [Google Scholar]
- 30. Link AJ, Vink MKS, Agard NJ, Prescher JA, Bertozzi CR, Tirrell DA. Discovery of aminoacyl-tRNA synthetase activity through cell-surface display of noncanonical amino acids. Proc Natl Acad Sci U S A 2006; 103:10180-5; PMID:16801548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serre L, Verdon G, Choinowski T, Hervouet N, Risler JL, Zelwer C. How methionyl-tRNA synthetase creates its amino acid recognition pocket upon L-methionine binding. J Mol Biol 2001; 306:863-76; PMID:11243794 [DOI] [PubMed] [Google Scholar]
- 32. Mechulam Y, Schmitt E, Maveyraud L, Zelwer C, Nureki O, Yokoyama S, Konno M, Blanquet S. Crystal structure of Escherichia coli methionyl-tRNA synthetase highlights species-specific features. J Mol Biol 1999; 294:1287-97; PMID:10600385 [DOI] [PubMed] [Google Scholar]
- 33. Crepin T, Schmitt E, Mechulam Y, Sampson PB, Vaughan MD, Honek JF, Blanquet S. Use of analogues of methionine and methionyl adenylate to sample conformational changes during catalysis in Escherichia coli methionyl-tRNA synthetase. J Mol Biol 2003; 332:59-72; PMID:12946347 [DOI] [PubMed] [Google Scholar]