Abstract
Mortality associated with mucormycosis remains high despite current antifungals. Iron-starvation strategies have been shown to have promising activity against Mucorales. We hypothesized that iron starvation enhances apoptosis in Rhizopus oryzae. Apoptosis was characterized in R. oryzae transformed with RNAi plasmid targeting FTR1 expression (iron permease mutant) or empty plasmid grown in iron rich (0.125% FeCl3) and iron depleted media (YNB+1mM ferrozine and 1 mM ascorbic acid). Increased apoptosis was observed with dihydrorhodamine-123 and rhodamine-123 staining in the iron starved mutant FTR1 when compared to empty plasmid, followed by increased extracellular ATP levels. In addition, DNA fragmentation and metacaspase activity were prominent in FTR1. In contrast, Rhizopus strains grown in iron-rich medium displayed minimal apoptosis. Our results demonstrate a metacaspase dependent apoptotic process in iron deprived condition and further support the role of iron starvation strategies as an adjunct treatment for mucormycosis, a mechanism by which iron starvation affects R. oryzae.
Keywords: apoptosis, iron starvation, iron permease, rhizopus oryzae, reactive oxygen species
Introduction
Mucormycosis is an emerging life-threatening mold infection in immunocompromised patients, as well as patients with diabetes increased levels of available serum iron, or trauma with Rhizopus and Mucor species account for more than 70% of such infections.1,2 Unfortunately, despite surgical debridement and systemic antifungal therapy, the overall mortality rate for mucormycosis remains high and may approach 100% in patients with disseminated disease, or persistent neutropenia.2,3,4 Thus, new strategies to prevent and treat mucormycosis are urgently needed.
The higher rate of infection by R. oryzae in patients with elevated levels of free iron in serum highlights the central role of iron metabolism in the fungal pathogenesis.5 Ibrahim et al.1 found that the iron chelator deferasirox, unlike the deferoxamine, does not act as an iron siderophore for Rhizopus. On the contrary, deferasirox demonstrates cidal activity against Mucorales in vitro and treatment of Rhizopus-infected mice with deferasirox markedly improves survival, reduces fungal burden in target organs, and enhances the host inflammatory response to mucormycosis, further reinforcing the role of iron in pathogenesis.1 These results were also corroborated by our findings that deferasirox protects Drosophila from mucormycosis infection.6
Fu et al.7 cloned the high-affinity iron permease (FTR1) of R. oryzae, which is a primary effector for organism ability to acquire iron in iron-limited environments such as those found in susceptible hosts. Iron starvation causes the rapid expression of FTR1 in R. oryzae, while excess iron in the form of ferric chloride reduces this expression.1,3
Apoptosis has been studied in many higher eukaryotes but also have been observed in lower eukaryotes, including yeasts and filamentous fungi.8-11 Rhizopus exhibit apoptotic markers that are similar to those of mammalian cells, including phosphatidylserine externalization, reactive oxygen species (ROS) accumulation, mitochondrial membrane potential dissipation, and DNA condensation and fragmentation.10,11 Apoptosis can be induced in R. oryzae by oxidative stress and antifungal agents such as posaconazole and itraconazole.10,11 However, it is unclear whether apoptotic effects in Rhizopus are related to iron starvation or iron availability. We hypothesized that iron starvation leads to enhanced apoptosis in R. oryzae with reduced ability to acquire iron due to inhibition of FTR1 expression.
Results
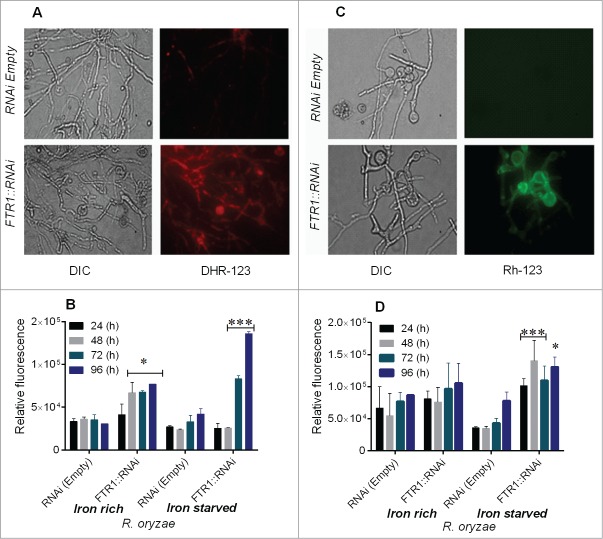
We first studied the effect of iron starvation on mitochondrial membrane potential and ROS accumulation. We found that staining of R. oryzae FTR1:RNAi germlings with DHR-123 and Rh-123 was most prominent in the iron-starved condition as shown by increased red and green fluorescence, respectively compared with the control (Fig. 1 A–D). A relatively small percentage of germlings were positively stained when grown in iron-rich conditions (SupplementaryFig. 1 A, B). Similarly, accumulation of ROS was increased markedly as illustrated by increased fluorescence (approximately fold4-) in R. oryzae FTR1::RNAi grown in the iron-deprived condition for 72 to 96 h (Fig. 1B). However, in R. oryzae FTR1::RNAi grown in iron-rich media, only a 2.fold5- increase (relative 151.0% compared to control)in fluorescence was observed, whereas no changes in fluorescence were observed in the control strain (Fig. 1B, Supplementary Fig. 1A).
Figure 1.
Iron starvation leads to ROS accumulation and mitochondrial membrane damage in R. oryzae FTR1::RNAi mutant (inhibition of FTR1 expression) compared with control (RNAi empty). DHR-123 and Rh123 were used to measure ROS and mitochondrial membrane damage, respectively, using fluorescence microscopy and fluorescence spectrophotometry. Fluorescent images of R. oryzae FTR1::RNAi and control strain grown for 96 h in iron-deprived condition, stained with DHR-123 (A) and Rh-123 (C). Relative fluorescence of R. oryzae FTR1::RNAi and control strains stained with DHR-123 (B) and Rh-123 (D) grown in iron-deprived and iron-rich conditions. DIC, differential interference contrast. *P <0.05; ***P<0.0001 (compared with control strain).
To further assess whether iron deprivation was associated with changes in depolarization of mitochondrial membrane potential in R. oryzae FTR1:RNAi, we used the Rh-123 staining method. Rh-123 staining showed a fold4- increase in fluorescence in R. oryzae FTR1::RNAi germlings grown for 96 h compared with the fluorescence observed in the control germlings grown in the iron-deprivation condition (Fig. 1D). Both R. oryzae strains grown in iron-rich media had no change in membrane potential (relative 21.0% compared to control). (Supplementary Fig. 1B). These results are consistent with the notion that iron starvation leads to physiological stress and apoptosis in mitochondrial-dependent pathways in FTR1::RNAi strain with reduced ability to acquire iron.
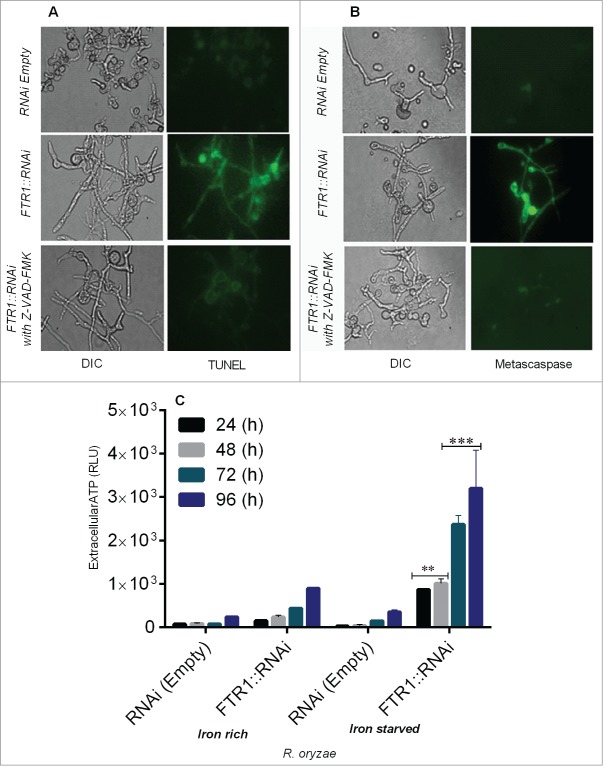
Next we studied DNA fragmentation by TUNEL assay using fluorescence microscopy in R. oryzae FTR1::RNAi strain and found that DNA degradation was prominent in iron-starved cells grown for 96 h compared with the control (Fig. 2A, Supplementary Fig. 1C).
Figure 2.
R. oryzae germlings deprived of iron display typical morphological changes associated with apoptosis, including nuclear degradation (by TUNEL assay), caspase-like activity (by CaspACE FITC-VAD-FMK), and extracellular ATP release (by CellTiter-Glo luminescent assay) in R. oryzae FTR1::RNAi and control strains grown for 24–96 h. Fluorescent images of R. oryzae FTR1::RNAi and control strains grown for 96 h in iron-deprived condition, stained with TUNEL (A), CaspACE FITC-VAD-FMK (B). Extracellular ATP release is higher in R. oryzae FTR1::RNAi mutant than control strain grown in iron-starved and iron-rich conditions, respectively (C). DIC, differential interference contrast). **p <0.001; ***p<0.0001 (compared with control).
Caspases are activated in the early stages of apoptosis and play a central role in the apoptotic cascade. Caspase-like activity (metacaspase) can be assessed by using the in situ detection marker CaspACE FITC-VAD-FMK.10,11 The lack of iron led to cleavage of the metacaspase, suggesting that the apoptosis observed after iron deprivation was metacaspase-dependent (Fig. 2B, Supplementary Fig. 1D). To further support the concept that caspase-like activity are involved in apoptosis under iron starvation, we assessed the metacaspase activity in Z-VAD-FMK–treated and untreated samples of the R. oryzae germlings and found no DNA fragmentation and metacaspase activity (Fig. 2 A and B), which indicate improved survival and metacaspase dependent cell death under iron starvation.
The major source of energy for metabolic processes in most cells is derived from oxidative phosphorylation, and these processes depend on iron availability.12 Maintaining the ratio of ATP to ADP is essential to maintaining mitochondrial membrane potential; thus, depletion of ATP leads to apoptosis.12 To determine whether depletion of iron was associated with alterations in ATP levels and apoptosis, we measured extracellular ATP levels and the association of these levels with apoptosis. The extracellular levels of ATP were significantly increased (P = <0.0001) in iron starved FTR1::RNAi germlings when grown in iron-starved condition as compared with levels observed when FTR1::RNAi germlings were grown in iron-containing medium (Fig. 2C), which caused mitochondrial damage and apoptosis that were effectively reversed by media supplemented with iron (Fig. 2A, B). These results are consistent with a rapid increase in the mitochondrial membrane permeability of germlings as detected by ROS accumulation, reduced mitochondrial membrane potential and metacaspase-dependent cell death.
Discussion
In this study, we investigated the activation and execution of apoptosis in the R. oryzae grown under iron-starved condition. We utilized R. oryzae iron permease mutant (with RNAi-mediated inhibition of FTR1 expression) to elucidate the morphological and biochemical characteristics of apoptosis in response to iron starvation.
In mitochondria, iron is required for electron transfer reactions, including oxidative phosphorylation, which are involved in the generation of hydroxyl radicals.12,13 Iron starvation triggered acute physiological stress and led to apoptotic phenomena induced by a mitochondrial-dependent pathway. Changes in mitochondrial biogenesis and functions are hallmarks of all forms of apoptosis.12 An initial event of apoptosis is a decrease in mitochondrial membrane potential, which is required to maintain an asymmetric distribution of charges between the inner and outer mitochondrial membranes.12,13 Reductions in mitochondrial membrane potential result in the loss of outer mitochondrial membrane permeability. We found increased ROS accumulation and decreased mitochondrial potential in R. oryzae FTR1::RNAi mutant in the iron-depleted condition, which indicated that apoptosis can be induced by iron depletion and is associated with damage of the mitochondria.
Our results agree with prior results which showed that iron deprivation induced apoptosis in murine lymphoma cells.14 We showed that loss of iron and induction of apoptosis might be associated with impaired function of the respiratory chain required to generate ATP and the subsequent damage to mitochondria. Mitochondrial dysfunction and the loss of membrane potential appear to result from the failure to maintain proper exchange of ATP and ADP.12 An increase in extracellular ATP levels seen after iron depletion should thus lead to marked alterations in the ATP/ADP exchange, mitochondrial inner membrane hyperpolarization, and matrix swelling. These, changes would ultimately compromise the function of the organelle.
Previous studies have reported accumulating evidence that different stimuli induce different apoptotic pathways in fungi that are regulated by activation of metacaspase, which cleave specific substrates and trigger apoptotic death.15 Here, we have shown that iron deprivation induces metacaspase-dependent pathways that led to apoptosis, and moreover we have identified iron as a critical component to prevent apoptosis.
Our results introduce a mechanism by which iron starvation by chelation therapy (e.g. by the use of deferasirox) is cidal against R. oryzae as well as other agents of mucormycosis in vitro and is protective against experimental mucormycosis.1 However, a small phase II, double-blind, randomized, placebo-controlled clinical trial of 20 patients with mucormycosis treated with deferasirox combined with standard therapy of liposomal amphotericin B (LAmB) versus the polyene alone (Placebo) failed to demonstrate any benefit of using the iron chelator.16 In fact significantly higher mortality rates were found in patients randomized to receive deferasirox at 30 (45% vs. Eleven%) and 90 d (82% vs. Twenty-two%, P = 0 .01) compared to those treated with LAmB alone.16 It is imperative to point that this study suffered from shortcomings including patients in the deferasirox arm were more likely than placebo patients to have active malignancy, neutropenia, corticosteroid therapy, and less likely to have received additional antifungals. All of these factors contribute to higher mortaility in patients with mucormycosis and make the results of this small pilot trial hard to interpret.17 Only a large, Phase III trial, potentially enrolling only diabetic or corticosteroid-treated patients (since animal model and case studies suggested a benefit of using deferasirox in this patient populations),1,18 and excluding cancer/neutropenia patients, could further elucidate the safety and efficacy of initial, adjunctive deferasirox for the treatment of mucormycosis.
In conclusion, we have shown, to our knowledge for the first time that lethality of iron depletion in R. oryzae is, at least partially, driven by metacaspase-dependent apoptosis. The results presented here suggest that iron depletion is a viable potential therapeutic strategy for mucormycosis for which treatment options are extremely limited.
Materials and Methods
R. oryzae transformed with RNAi plasmid targeting FTR1 expression (FTR1::RNAi, iron permease mutant with >90% inhibited FTR1 expression) or empty plasmid (control) were described before.3 Spores of R. oryzae strains RNAi empty or FTR1::RNAi were collected from 4–5 d old plates that have been incubated at 37oC on yeast nitrogen base (YNB) agar plates supplemented with amino acids without uracil and containing either 1 mM ascorbic acid and ferrozine (iron-starved conditions) or 0.125% FeCl3 (iron-rich conditions), as previously described.1,19 Spores were washed in phosphate buffer saline (PBS) and enumerated in a hemocytometer.
For apoptosis experiments, spores of R. oryzae strains (1 × 106 /mL) were suspended in RPMI 1640 media containing 1 mM ascorbic acid and 1 mM ferrozine (for iron-depleted conditions) or 0.125% FeCl3 (for iron-rich conditions at which FTR1 is weakly expressed7 and grown for 24–96 h at 37ºC with shaking. At selected time intervals, samples of R. oryzae cells were collected and assessed for apoptosis markers.
Intracellular ROS levels in germlings of R. oryzae strains (RNAi empty (control) or FTR1::RNAi) were measured as previously described.10,11 R. oryzae spores were inoculated in RPMI 1640 medium with 0.125% FeCl3 or without iron (1 mM ascorbic acid and ferrozine) and were incubated for 24–96 h at 37°C, and then spiked with DHR-123 (5 μg/ml). After incubation for 2h at RT, cells were harvested after centrifugation at 10,000 × g for 5 min and observed with a Nikon Microphot SA fluorescence microscope (excitation, 488 nm; emission, 520 nm). For quantitative assays, fluorescence intensity values were recorded using a POLARstar Galaxy microplate reader (excitation, 490 nm; emission, 590 nm; BMG LABTECH, Offenburg, Germany).
Mitochondrial membrane depolarization was assessed by staining with rhodamine (Rh)-123, a fluorescent dye that is distributed in the mitochondrial matrix as previously described.10,11 Briefly, germlings were incubated for 24–96 hours at 37°C and were harvested via centrifugation, washed twice, and resuspended in PBS. Rh-123 was added to the final concentration of 10 μM, and then the mixture was incubated for 30 min in the dark at RT. Fluorescence intensity was measured as described above.
We measured intracellular ATP efflux from germlings grown at 37°C for 24–96 h as an indication of cell membrane damage, cytoplasmic and mitochondrial membrane leakage.11,20 The cells were removed by centrifugation, and the supernatants were assayed for ATP using the CellTiter-Glo luminescent kit (Promega, Madison, WI). Data were collected with a microplate luminometer (Spectramax M5; Molecular Devices, Sunnyvale, CA).
DNA fragmentation and nuclear condensation, a characteristic change in apoptosis was detected using a terminal deoxynucelotidyltransferase-mediated dUTP nick end-labeling (TUNEL) assay and DAPI staining in R. oryzae RNAi empty (control) or FTR1::RNAi. Germlings grown for 24–96 h with and without iron at 37°C were fixed with 3.7% formaldehyde for 30 min on ice and were analyzed as described by Shirazi et al.10,11 The cells were observed for fluorescence with excitation and emission wavelengths of 488 nm and 520 nm.
Detection of active metacaspases in R. oryzae germlings was performed using the CaspACE FITC-VAD-FMK (Promega) according to the manufacturer's instructions.10,11 Briefly, germlings incubated for 24–96 h at 37°C were collected, washed in PBS, resuspended in 10 μM FITC-VAD-FMK, and incubated for 2 h at 30°C. Inhibition of apoptosis was performed by incubating R. oryzae germlings in the presence of the caspase-1 inhibitor Z-VAD-FMK (Sigma) to final concentrations of 40 μM. After incubation, germlings were washed twice in PBS and were observed microscopically for fluorescence with excitation and emission settings of 488 nm and 520 nm.
For all assays, 3 independent experiments conducted on different days in triplicates. Multiple groups were compared with use of the Kruskall-Wallis test and post-hoc paired comparisons were made with Dunnett's tests. Calculations were made with InStat (GraphPad Software). All results were expressed as means ± standard deviations. Two-tailed P values of less than 0.05 were considered statistically significant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
D.P.K. acknowledges the Frances King Black Endowed Professorship for Cancer Research.
funding
This work was partially supported by Public Health Service grant AI063503 to A.S.I.
Références
- 1. Ibrahim AS, Gebermariam T, Fu Y, Lin L, Husseiny MI, French SW, Schwartz J, Skory CD, Edwards JE, Jr, Spellberg BJ. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest 2007; 117:2649-657; PMID:17786247; http://dx.doi.org/ 10.1172/JCI32338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev 2005; 18:556-69; PMID:16020690; http://dx.doi.org/ 10.1128/CMR.18.3.556-569.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ibrahim A, Gebremariam T, Lin L. The high affinity iron permease is a key virulence factor required for rhizopus oryzae pathogenesis. Mol Microbiol 2010; 77:587-604; PMID:20545847; http://dx.doi.org/ 10.1111/j.1365-2958.2010.07234.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kontoyiannis DP, Wessel VC, Bodey GP, Rolston KV. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis 2000; 30:851-56; PMID:10852735; http://dx.doi.org/ 10.1086/313803 [DOI] [PubMed] [Google Scholar]
- 5. Ibrahim AS, Edwards JE, Jr, Filler SG. Zygomycosis. in clinical mycology. Dismukes W.E., Pappas P.G., and Sobel J.D. (eds). New York, NY:Oxford University Press, 2003; 241-51. [Google Scholar]
- 6. Chamilos G, Lewis RE, Hu Jianhua, Xiao Lianchun, Zal T, Gillet M, Halder G, Kontoyiannis DP. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc Natl Acad Sci USA 2008; 105:9367-372; PMID:18583479; http://dx.doi.org/ 10.1073/pnas.0709578105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu Y, Lee H, Collins M, Tsai HF, Spellberg B, Edwards JE, Jr, Kwon-Chung KJ, Ibrahim AS. Cloning and functional characterization of the rhizopus oryzae high affinity iron permease (rFTR1) gene. FEMS Microbiol Lett 2004; 235:169-76; PMID:15158278; http://dx.doi.org/ 10.1111/j.1574-6968.2004.tb09583.x [DOI] [PubMed] [Google Scholar]
- 8. Almeida B, Silva A, Mesquita A, Sampaio-Marques B, Rodrigues F, Ludovico P. Drug-induced apoptosis in yeast. Biochim Biophysica Acta Mol Cell Res 2008; 1783:1436-448; PMID:18252203; http://dx.doi.org/ 10.1016/j.bbamcr.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 9. Ramsdale M. Programmed cell death in pathogenic fungi. Biochim Biophys Acta 2008; 1783:1369-380; PMID:18294459; http://dx.doi: 10.1016/j.bbamcr.2008.01.021 [DOI] [PubMed] [Google Scholar]
- 10. Shirazi F, Kontoyiannis DP. Mitochondrial respiratory pathways inhibition in rhizopus oryzae potentiates activity of posaconazole and itraconazole via apoptosis. PLoS One 2013; 8:e63393; PMID:23696824; http://dx.doi.org/ 10.1371/journal.pone.0063393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shirazi F, Kontoyiannis DP. The calcineurin pathway inhibitor tacrolimus enhances the in vitro activity of azoles against mucorales via apoptosis. Eukaryot Cell 2013; 12: 1225-234; PMID:23851337; http://dx.doi.org/ 10.1128/EC.00138-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maclean K, Yang H, Cleveland JL. Serum suppresses myeloid progenitor apoptosis by regulating iron homeostasis. J Cell Biochem 2001; 82:171-86; PMID:11400174; http://dx.doi.org/ 10.1002/jcb.1111 [DOI] [PubMed] [Google Scholar]
- 13. Simbula G, Glascott PA, Akita S, Hoek JB, Farber JL. Two mechanisms by which ATP depletion potentiates induction of the mitochondrial permeability transition. Am J Physiol 1997; 273:C479-88; PMID:9277345 [DOI] [PubMed] [Google Scholar]
- 14. Kovar J, Stunz LL, Stewart BC, Kriegerbeckova K, Ashman RF, Kemp JD. Direct evidence that iron deprivation induces apoptosis in murine lymphoma 38C13. Pathobiol 1997; 65:61-8; PMID:9253029; http://dx.doi.org/ 10.1159/000164105 [DOI] [PubMed] [Google Scholar]
- 15. Hamann A, Brust D, Osiewacz HD. Apoptosis pathways in fungal growth, development and ageing. Trend Microbiol 2008; 16:276-83; PMID:18440231; http://dx.doi.org/ 10.1016/j.tim.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 16. Spellberg B, Ibrahim AS, Chin-Hong PV, Kontoyiannis DP, Morris MI, Perfect JR, Fredricks D, Brass EP. The deferasirox-amBisome therapy for mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother 2012; 67:715-22; PMID:21937481; http://dx.doi.org/ 10.1093/jac/dkr375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donnelly JP, Lahav M. Deferasirox as adjunctive therapy for mucormycosis. J Antimicrob Chemother. 2012; 67:519-20; PMID:22186877; http://dox.doi:org/ 10.1093/jac/dkr540 [DOI] [PubMed] [Google Scholar]
- 18. Spellberg B, Andes D, Perez M, Anglim A, Bonilla H, Mathisen GE, Walsh TJ, Ibrahim AS. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother 2009; 53:3122-5; PMID:19433555; http://dox. doi:org/ 10.1128/AAC.00361-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewis RE, Pongas GN, Albert N, Ben-Ami R, Walsh T, Kontoyiannis DP. Activity of deferasirox in mucorales: influences of species and exogenous iron. Antimirob Agents Chemother 2011; 55:411-13; PMID:20956598; http://dox. doi: 10.1128/AAC.00792-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ben-Ami R, Lewis RE, Tarrand J, Leventakos K, Kontoyiannis DP. Antifungal activity of colistin against mucorales species in vitro and in a murine model of rhizopus oryzae pulmonary infection. Antimicrob Agent Chemother 2010; 54:484-90; PMID:19858263; http://dx.doi.org/ 10.1128/AAC.00956-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.