Abstract
Repair of wounds to single cells involves dynamic membrane and cytoskeletal rearrangements necessary to seal the wound and repair the underlying cytoskeleton cortex. One group of proteins essential to the cortical remodeling is the Rho family of small GTPases. Recently we showed that the founding members of this GTPases family, Rho, Rac, and Cdc42, are all essential for normal single cell wound repair and accumulate at the wound periphery in distinct temporal/spatial patterns in the Drosophila cell wound model. In addition, these proteins communicate with one another and with the cytoskeleton to regulate their distribution in response to wounds. Unexpectedly, we found evidence for context specific Rho GTPase binding to downstream targets or “effectors” which cannot be explained solely by means of local GTPase activation. Here we discuss these observations in relation to similar studies in single cell wound repair in the Xenopus oocyte, and highlight how these cell wound models serve as powerful tools to understand both cell wound repair and Rho GTPase biology.
Keywords: cell wound repair, cytoskeleton, Cdc42, Drosophila, Rho family GTPases, Rho, Rac, Xenopus
Abbreviations
- GAPs
GTPase activating proteins
- GEFs
guanine nucleotide exchange factors
- GDIs
guanine nucleotide dissociation inhibitors
- RBD
Rho Binding Domain
The Rho, Cdc42, and Rac proteins, collectively known as Rho family GTPases, are switch molecules (cycling between GDP- and GTP- bound states) that serve as key regulators of the cytoskeleton.1-3 Rho family GTPases are activated by Rho guanine nucleotide exchange factors (GEFs) that exchange GDP for GTP, and turned-off by Rho GTPase activating proteins (GAPs) that increase the rate of GTP conversion to GDP.1,3,4 Rho guanine nucleotide dissociation inhibitors (GDIs) can also bind to prenylated Rho family GTPases and regulate GDP/GTP exchange, thereby providing another level of regulation.5 Activated (GTP-bound) Rho family GTPases undergo a conformational change that allows them to interact with downstream effector proteins (cellular target proteins) to drive a wide spectrum of biological responses including reorganization of the actin and/or microtubule cytoskeleton affecting cell shape changes, cell polarity, movement, adhesion and cytokinesis during normal development, as well as when co-opted during infection, oncogenic transformation, and wound repair.1,6-12
Work in the last 2 decades has highlighted the necessity of Rho family GTPases for proper wound repair, in the context of both single cells and tissues.13-17 Single cell wound repair is a particularly powerful platform to study the roles and regulation of this protein family, as cell wounds can be induced, are highly reproducible, involve coordinated cytoskeletal responses, and heal on the scale of minutes.18 The ability to visualize the repair process by high-speed time-lapse confocal microscopy, combined with the ever-growing number of diverse fluorescent reporters, mutant alleles, and inhibitors, now allows a broad range of analyses on the repair process to be performed. Two model systems that are highly amenable to live imaging have come to the forefront for studying single cell wound repair and, in particular, the role of Rho family GTPases in this process: the Xenopus oocyte and Drosophila syncytial embryo.19,20 Here we discuss our recent findings using the Drosophila syncytial embryo single cell repair model, compare them to what is known from studies using the Xenopus oocyte cell repair model,13,19,21-24 and highlight key features in each model.
Rho Family GTPases Regulate Cortical Cytoskeletal Remodeling During Cell Wound Repair
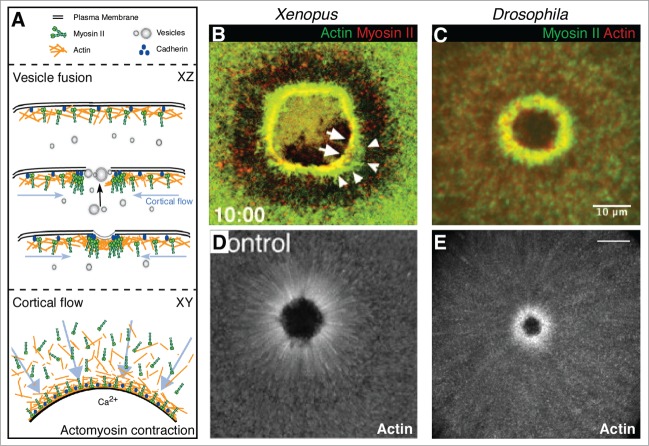
Upon a breach of the plasma membrane, wounded single cells must be rapidly repaired to avoid a toxic influx of extracellular molecules and ions, loss of cytoplasm, and ultimately to maintain viability. This repair is composed of 2 main processes: fusion of vesicles to form a temporary membrane plug and the subsequent remodeling of the membrane and underlying cortical cytoskeleton (Fig. 1A).13,18 Both processes are downstream of calcium ion influx, which has been proposed as the initial signal for wound repair.18,25,26 The source of the membrane vesicles that respond to the calcium signal to form the plug is likely context dependent, as endosomes, lysosomes, enlargesomes and yolk granules have all been implicated in varying contexts.18,25,27-29 Subsequent to the membrane plug being formed, work using the Xenopus oocyte and our work using the early Drosophila syncytial embryo has shown that cortical cytoskeletal remodeling is largely driven by the assembly and contraction of an actomyosin ring (Fig. 1B-C), as well as cortical flow of actin and myosin toward the wounded area (Fig. 1D-E).20,22 We have shown in the Drosophila model that the actomyosin cable is, at least in part, linked to the overlying plasma membrane by the adherens junction protein E-cadherin (Fig. 1A).20 The mode of tethering the actomyosin cable to the plasma membrane in the Xenopus oocyte model is not yet known.
Figure 1.
Mechanisms of single cell repair. (A) Schematic depicting single cell repair mechanisms including vesicle fusion and cortical cytoskeleton remodeling by cortical flow and actomyosin contraction. While XY views depict the overall repair process, a 'behind the scenes' account of processes such as vesicles trafficking toward the wound from an internal membrane source are visible in XZ views (black arrow). Arrows (blue) in XY and XZ views show cortical flow as polarized trafficking of actin and myosin toward the wound. (B-D) Fluorescent micrographs of actomyosin contraction (B and C) or cortical flow (D and E) in single cell repair. An actomyosin array is formed at the wound edge in the Xenopus oocyte ((B) © 2001 Rockefeller University Press. Originally published in Journal of Cell Biology 154: 785–797) and the Drosophila syncytial embryo (C). Cortical flow of actin contributes to cortical remodeling in Xenopus (D; © 2005 Rockefeller University Press. Originally published in Journal of Cell Biology 168: 429–439) and Drosophila (E) single cell repair.
The necessity for Rho family GTPases in cell wound repair was initially shown by examining wound repair under conditions where the levels of these proteins were altered. In Xenopus oocytes this has been accomplished using inhibitors and/or recombinant dominant negative and constitutively active GTPases, whereas the Drosophila model has relied on the use of loss-of-function mutant alleles and/or inhibitors (Table 1).13 Specifically, in the Xenopus model, RhoA is necessary for cortical flow, whereas Cdc42 regulates actin cable assembly, and both are involved in actomyosin contractility. A role for Rac proteins in this process has not been reported. Our recent work with the Drosophila model has implicated the same Rho family GTPases (Rho1 and Cdc42), as well as the 3 fly Rac proteins (Rac1, Rac2, and Mtl) in cell wound repair, albeit with somewhat divergent roles.17 Addition of the Rho inhibitor C3 exoenzyme disrupts cortical flow in the Drosophila model similar to that observed in Xenopus; however, this phenotype is not recapitulated in Drosophila Rho1 loss-of-function mutants.13,17 Instead, we found that Rac proteins are necessary for cortical flow: both loss-of-function mutations (Rac1 Rac2 Mtl triple mutants in which all 3 Rac genes are removed) and the Rac inhibitor NSC23766 abrogate cortical flow. In addition, we found that Cdc42 plays a complementary role in cortical flow by orienting the Rac-dependent flow toward the wound.17 Cdc42 is specifically important to stabilize the actin ring at the wound, whereas Rho1 is involved in actin-myosin II stabilization and ring assembly. Intriguingly, when we inhibited all 3 GTPases simultaneously (Rho1, 3 Rac GTPases, and Cdc42), wound repair morphology and dynamics were completely altered, yet some organization of the actin cytoskeleton could be observed suggesting that other classes of cytoskeletal modulators are likely working in parallel with the Rho family GTPases to achieve proper cellular repair.17
Table 1.
Summary of Rho family GTPase accumulation in wildtype and various backgrounds in response to cell wounds
| Rho Accumulation |
Rac Accumulation |
Cdc42 Accumulation |
|||||
|---|---|---|---|---|---|---|---|
| Genotype | Xenopusa | Drosophilab | Xenopusa | Drosophilab | Xenopusa | Drosophilab | |
| WT | Array of active RhoA in ring; interior to and no overlap with active Cdc42 array | Array of Rho1 in ring; partially interior to Cdc42 and Rac arrays; Array of active Rho1 at innermost part of Rho1 ring | ND | Array of Rac in ring & halo; overlapping with Cdc42 array at ring; partial overlap with Rho1 at inner ring edge | Array of active Cdc42 in ring; exterior to and no overlap with RhoA array | Array of Cdc42 in ring; overlapping with Rac at outer ring edge; partial overlap with Rho1 at inner ring edge | |
| Inhibitors/Mutants c | |||||||
| Rho | C3 toxin; LOF mutant | Rho array fails to form | Rho array fails to form | ND | Lower level in array; Elevated background level; array broadens | Increased level in array; array broadens | Array narrows |
| CA | Elevated background level; array formation impaired | ND | ND | ND | Array broadens | ND | |
| Cdc42 | DN; LOF mutant | Array fails to form | ND | ND | ND | Array fails to form | ND |
| CA | Array broadens | ND | ND | ND | ND | ||
| Rac | NSC27366; LOF mutant | ND | Lower level in array | ND | Decreased c | ND | Lower level in array |
| Actin | Jasplakinolide | Outward broadening of array; array does not translocate | Lower level in array; array does not translocate | ND | Lower level in array; array does not translocate; accumulation delayed | Outward broadening of array; rapid turnover; array does not translocate | Lower level in array; array does not translocate; accumulation delayed; array is narrower |
| LatrunculinB; Concanavalin A | Elevated background level; array broadens; array does not translocate | ND | ND | ND | Decreased background level; array broadens; array does not translocate | ND | |
| Myosin | Y27632 ; Blebbistatin | Array translocation rate slowed | Lower level in array; array does not translocate; array broadens | ND | Lower level in array; array does not translocate; accumulation delayed | Array translocation rate slowed | Lower level in array; array does not translocate; accumulation delayed |
References cited.13,21,22,24
References cited.17,20
ND: Not Done; WT: wildtype; LOF: loss-of-function; CA: constitutively active; DN: dominant negative.`
NSC27366 cannot be concentrated to levels sufficient to remove Rac1-GFP in addition to endogenous Rac1, Rac2, and MTL.
The differences in phenotype observed when inhibiting Rho1 function using C3 exoenzyme or with loss of function mutants is interesting and could be the result of 2 phenomena we have observed: crosstalk between the Rho and Rac GTPases and/or the chronic versus catastrophic means by which Rho1 activity is reduced. As we discuss later, Rac proteins at the wound leading edge are reduced indirectly when Rho1 activity is inhibited, and may be responsible for the cortical flow defect.17 Alternatively, immediate ‘catastrophic’ reduction of Rho1 proteins (with inhibitors) may occur within a time-frame that does not allow the embryo enough time to correct disruptions to the normally balanced system. Consistent with established work, Rho family GTPases are in a delicate balance with each other and with other proteins such as Rho GDIs.30 ‘Chronic’ reduction of Rho1 (i.e., when using loss of function mutants) allows the embryo time to shift toward an alternative equilibrium, perhaps permitting Rac activity at the wound. Future experiments comparing Rac localization and activity in a Rho1 mutant background vs. Rho1 removal by C3 inhibition during wound repair will be necessary to test this hypothesis.
Rho Family GTPases Localize at the Wound in Distinct Spatial and Temporal Patterns
Consistent with their cell wound repair phenotypes, Rho GTPases exhibit dynamic localization at the wound site. In the Xenopus oocyte, probes for the activated RhoA and Cdc42 are immediately localized at the wound, and by one minute post-wounding have formed discrete concentric rings or arrays with the ring of activated Rho interior to that of activated Cdc42 (Fig. 2A and H).13 We have recently observed a similar response wherein full-length fluorescent fusion proteins of the Drosophila Rho family GTPases (Rho1, Rac1, Rac2, and Cdc42) are recruited to cellular wounds in the syncytial Drosophila embryo (Fig. 2B and C).17 In the Drosophila model, Rho family GTPases are not only coordinated spatially, but also temporally. Rho1 accumulates first and almost immediately post wounding (by 30 seconds), Cdc42 accumulates next (60–90 seconds), and Rac 1 and Rac 2 accumulate last (90–135 seconds).17 Similar to that observed in Xenopus, Rho1 accumulates as the innermost array with only its outermost edge overlapping with the actomyosin ring. Cdc42 and Rac co-localize with the actomyosin ring, with Rac also forming a broader region (halo) of slightly elevated accumulation surrounding the actomyosin ring. There is significant overlap of Rho accumulation with Rac and Cdc42 accumulation (Fig. 2B-C and H), however, this accumulation represents the inactive as well as activated pools of each GTPase. We examined the localization of activated Rho GTPases using a series of biosensors consisting of the Rho Binding Domain (RBD) of known downstream effectors for each of the Rho family GTPases fused to a fluorescent protein (GFP or mChFP).17 Surprisingly, while all of the biosensors recapitulated developmental Rho family GTPase expression, only a subset accumulated at the wound. Rho1 biosensors using the RBD of Rok, Dia, and PKN (but not Wash or Capu) responded upon wounding and formed a narrow ring largely interior to actin accumulation. As none of the biosensors generated for Cdc42 or Rac (WASp, PlexB, Pak1, Pak3) accumulated upon wounding, it was not possible to determine if the activated forms of Rho, Cdc42, and Rac form discrete concentric rings similar to that observed in Xenopus oocytes. However, activated Rho1 is enriched in a narrower region toward the interior of overall Rho1 accumulation, suggesting it is possible that Rho1 and Cdc42/Rac form exclusive rings in Drosophila as well. Cdc42 and Rac1 exhibit essentially complete co-localization making it unlikely that these 2 proteins segregate in a similar manner. To further address this point, it will be necessary to identify biosensors specific for Cdc42 and Rac that respond to the cell wound repair process.
Figure 2.
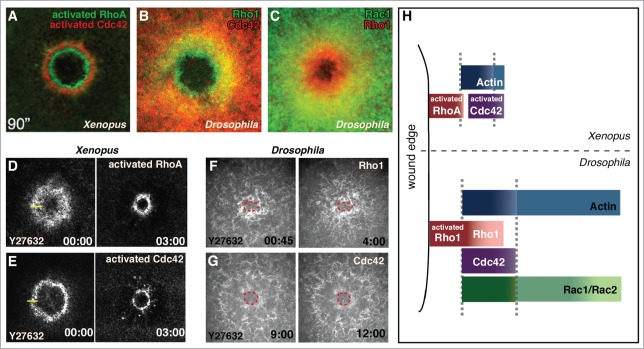
GTPase arrays form in response to cell wound repair the Xenopus oocyte and Drosophila syncytial embryo cell repair models. (A, D, and E) Fluorescent reporters for Xenopus studies use biosensor reporters depicting activated Rho family GTPases. (B, C, F, and G) Drosophila Rho GTPase fluorescent probes report all protein (not just the activated forms). (A) Activated RhoA and Cdc42 form non-overlapping concentric rings in the Xenopus oocyte (© 2005 Rockefeller University Press. Originally published in Journal of Cell Biology 168: 429–439). (B) Rho1 and Cdc42 form overlapping arrays in the Drosophila syncytial embryo with the Rho1 array interior to Cdc42. (C) Rac1 and Rho1 form overlapping arrays in the Drosophila syncytial embryo with the Rho1 array interior to Rac1. (D and E) RhoA and Cdc42 arrays in the Xenopus model translocate upon inhibition of contraction with the Rok inhibitor Y27632 (adapted from21 and reproduced with permission from Elsevier). (F and G) Rho1 and Cdc42 arrays in the Drosophila model are unable to translocate when contraction is inhibited with Y27632 (wound edge outlined in red). (H) Schematic depicting the relative localization of Rho family GTPases in the Xenopus and Drosophila single cell wound repair models (adapted from17 and reproduced with permission from Elsevier).
The ability of only certain RBD constructs to bind to Rho1 in specific contexts is intriguing. Conventional models for the on/off nature of Rho GTPases suggest that when the proteins are activated they are accessible for binding by any available binding partner/effector.9,12,31 However, as only a subset of the biosensors respond to wound repair, and considering that our panel of RBD-fusion proteins all expressed under control of the same ubiquitous promoter, are able to specifically bind to GTP-loaded Rho GTPases in pull-down assays, and display Rho1 GTPase developmental patterns, our work suggests a previously unexplored avenue of Rho family GTPase-effector specificity. Future experiments using alternative biosensor designs, such as uni- and/or bi-molecular FRET probes that do not require an accumulation of signal but allow for immediate and precise visualization of activated GTPases, will be necessary to explore this specificity.31 It may become necessary in the future to design biosensors that can function in a context-dependent manner.
Crosstalk Among Rho GTPases Family Members and with the Cytoskeleton
Segregation of the Rho GTPases into separate zones in both cell wound repair model systems suggests that there is crosstalk among GTPases through regulation of each other, through close spatial/temporal regulation by GEF/GAPs (a process known as ‘GTPase flux'), and/or indirectly through feedback with the cytoskeleton.24,32 One set of experiments used in both the Xenopus and Drosophila models to explore these possibilities compared the localization of Rho GTPase arrays in controls to their localization upon altered Rho GTPase levels using drugs, dominant negative, and constitutively active proteins.13,17 In general, the results have suggested that the disruption of one GTPase alters the array localization of the other Rho GTPases (Table 1). While these studies can neither rule in favor of nor against direct regulation of Rho GTPases by each other, there is evidence of GTPase flux. In Xenopus, candidate screening of Rho GTPase GEFs and GAPs identified Abr, a protein with both GEF and GAP activity that localizes at the wound and upon knockdown leads to aberrant repair.24 Further analysis by over-expression and specific mutagenesis of either the GEF or GAP domain showed that Abr acts to amplify local RhoA activity through its GEF domain while maintaining a barrier to the spreading of activated Cdc42 through its GAP domain.24 Subsequent studies using photo-activatable probes have demonstrated that within these zones there is dynamic RhoA and Cdc42 activation and inactivation with peak half-lives of 8–12 seconds.21 While a similar mechanism has not yet been identified in Drosophila, proteins with both GEF and GAP activity exist (i.e., RhoGAP1A) that will be interesting to examine in the context of cell wound repair.
The influence of the cytoskeleton on GTPase array formation was examined using an inhibitor-based approach: cytoskeleton components including actin, microtubules, and myosin, were perturbed then the ensuing effects on Rho GTPase array formation were examined.13,17 One difference between these studies is that in Xenopus the Rho GTPase arrays were assayed using probes for activated GTPases, whereas in Drosophila the entire pool of each GTPase was assessed. Nonetheless, these results have highlighted several important similarities and differences between repair in the Xenopus and Drosophila models (Table 1). First, dynamic F-actin is necessary in both Xenopus and Drosophila for the translocation of the GTPase arrays, however, stabilization of F-actin by Jasplakinolide has non-conserved effects on recruitment amount and timing, as well as localization of the GTPase arrays between the 2 systems (Table 1). Microtubules are also necessary for the proper localization of the GTPase arrays in Xenopus,13 while their role in Drosophila remains to be tested. The most striking difference between the wound-induced Xenopus and Drosophila Rho GTPase arrays is apparent when contractility is impaired by inhibition of active myosin II. In Xenopus, impairment of myosin II by either the Rok inhibitor Y27632 or the ATPase inhibitor blebbistatin fails to prevent the forward translocation of the GTPase arrays, rather it only slows the rate of repair (Fig. 2D-E).21 This surprising finding has been proposed to be a result of signal ‘treadmilling', wherein preferential leading edge activation of Cdc42 and trailing edge inactivation of RhoA within their activity zones drives the contractile machineries forward during wound closure and has been confirmed in the aforementioned studies using photoactivatable Rho GTPases.21 In contrast, impairment of myosin II by Y27632 in the Drosophila model led to an inability of the Rho GTPase arrays to translocate (Fig. 2F-G),17 suggesting that while the molecules and machineries (Rho family GTPases, a contractile ring, and dynamic F-actin) are highly conserved, the mode of their employment may differ among organisms (Table 1). While this result precludes ‘treadmilling' in the Drosophila model, it remains possible that in addition to disrupting actomyosin contraction Y27632 indirectly affects Rho1 in this model. Assays similar to those performed in Xenopus accessing the translocation of photoactivated Rho GTPases should clarify this important point.
Conclusions and Future Directions
Single cell wounds in Xenopus and Drosophila are proving to be invaluable models for the study of the repair process, as well as Rho GTPase biology. Findings such as direct evidence for Rho GTPase flux and signal treadmilling in the Xenopus model, and our new evidence for context-dependent Rho GTPase-effector specificity in the Drosophila model have broad implications for Rho family GTPase functions.17,21,24 Although the spatial and temporal dynamics of the GTPase arrays are different in the 2 cell wound models, they both achieve the same end result: organization of a workable and highly animated repair machinery. These differences highlight many important remaining questions. How is the initial wounding signal differentially propagated such that the GTPases bind only a subset of their downstream effector proteins? What is the significance of the differences in temporal localization of Rho1 accumulation at the wound significantly before that of the Rac family proteins and Cdc42? Are the effectors used by each GTPase also spatially segregated within each Rho GTPases activity zone or are these effectors competing for binding? Finally, how are wounds in which all 3 Rho GTPases are inhibited able to close and what alternative cytoskeletal remodelers might be involved? Future studies promise to provide critical insights into this important process of cellular repair and will be informative to the numerous cellular and developmental processes for which the Rho GTPases are critical.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank M.T. Abreu-Blanco, C. Milligan, and members of the lab for their comments/advice.
Funding
This work was supported by NIH grants GM097083 and GM092731 to S.M.P.
References
- 1. Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279:509-14; PMID:9438836; http://dx.doi.org/ 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 2. Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol 2001; 11:471-7; PMID:11719051; http://dx.doi.org/ 10.1016/S0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- 3. Narumiya S. The small GTPase Rho: cellular functions and signal transduction. J Biochem 1996; 120:215-28; PMID:8889802; http://dx.doi.org/ 10.1093/oxfordjournals.jbchem.a021401. [DOI] [PubMed] [Google Scholar]
- 4. Machesky LM, Hall A. Rho: a connection between membrane receptor signalling and the cytoskeleton. Trends in cell Biol 1996; 6:304-10; PMID:15157438; http://dx.doi.org/ 10.1016/0962-8924(96)10026-X. [DOI] [PubMed] [Google Scholar]
- 5. Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J 2005; 390:1-9; PMID:16083425; http://dx.doi.org/ 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy AM, Montell DJ. Cell type-specific roles for Cdc42, Rac, and RhoL in Drosophila oogenesis. J Cell Biol 1996; 133:617-30; PMID:8636236; http://dx.doi.org/ 10.1083/jcb.133.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zohn IM, Campbell SL, Khosravi-Far R, Rossman KL, Der CJ. Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene 1998; 17:1415-38; PMID:9779988; http://dx.doi.org/ 10.1038/sj.onc.1202181. [DOI] [PubMed] [Google Scholar]
- 8. Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 2000; 406:532-5; PMID:10952316; http://dx.doi.org/ 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 9. Bishop AL, Hall A. Rho GTPases and their effector proteins. The Biochemical journal 2000; 348 Pt 2:241-55; http://dx.doi.org/ 10.1042/0264-6021:3480241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Settleman J. Rho GTPases in development. Progress in molecular and subcellular biology 1999; 22:201-29; PMID:10081071; http://dx.doi.org/ 10.1007/978-3-642-58591-3_10. [DOI] [PubMed] [Google Scholar]
- 11. Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annual review of cell and developmental biology 2005; 21:247-69; PMID:16212495; http://dx.doi.org/ 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 12. Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. BioEssays : news and reviews in molecular, cellular and developmental biology 2007; 29:356-70; PMID:17373658; http://dx.doi.org/ 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol 2005; 168:429-39; PMID:15684032; http://dx.doi.org/ 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol 1996; 135:1097-107; PMID:8922389; http://dx.doi.org/ 10.1083/jcb.135.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nature cell Biol 2002; 4:907-12; PMID:12402048; http://dx.doi.org/ 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 16. Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial "leader" cells during wound healing. Proc Natl Acad Sci U S A 2003; 100:10788-93; PMID:12960404; http://dx.doi.org/ 10.1073/pnas.1834401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abreu-Blanco MT, Verboon JM, Parkhurst SM. Coordination of Rho family GTPase activities to orchestrate cytoskeleton responses during cell wound repair. Curr Biol 2014; 24:144-55; PMID:24388847; http://dx.doi.org/ 10.1016/j.cub.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McNeil PL, Terasaki M. Coping with the inevitable: how cells repair a torn surface membrane. Nat Cell Biol 2001; 3:E124-9; PMID:11331898; http://dx.doi.org/ 10.1038/35074652. [DOI] [PubMed] [Google Scholar]
- 19. Bement WM, Yu HY, Burkel BM, Vaughan EM, Clark AG. Rehabilitation and the single cell. Curr opin Cell Biol 2007; 19:95-100; PMID:17174083; http://dx.doi.org/ 10.1016/j.ceb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abreu-Blanco MT, Verboon JM, Parkhurst SM. Cell wound repair in Drosophila occurs through three distinct phases of membrane and cytoskeletal remodeling. J Cell Biol 2011; 193:455-64; PMID:21518790; http://dx.doi.org/ 10.1083/jcb.201011018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burkel BM, Benink HA, Vaughan EM, von Dassow G, Bement WM. A Rho GTPase signal treadmill backs a contractile array. Dev Cell 2012; 23:384-96; PMID:22819338; http://dx.doi.org/ 10.1016/j.devcel.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mandato CA, Bement WM. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J Cell Biol 2001; 154:785-97; PMID:11502762; http://dx.doi.org/ 10.1083/jcb.200103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mandato CA, Bement WM. Actomyosin transports microtubules and microtubules control actomyosin recruitment during Xenopus oocyte wound healing. Curr Biol 2003; 13:1096-105; PMID:12842008; http://dx.doi.org/ 10.1016/S0960-9822(03)00420-2. [DOI] [PubMed] [Google Scholar]
- 24. Vaughan EM, Miller AL, Yu HY, Bement WM. Control of local Rho GTPase crosstalk by Abr. Curr Biol 2011; 21:270-7; PMID:21295482; http://dx.doi.org/ 10.1016/j.cub.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terasaki M, Miyake K, McNeil PL. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. J Cell Biol 1997; 139:63-74; PMID:9314529; http://dx.doi.org/ 10.1083/jcb.139.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trump BF, Berezesky IK. Calcium-mediated cell injury and cell death. FASEB J 1995; 9:219-28; PMID:7781924. [DOI] [PubMed] [Google Scholar]
- 27. Chestkov VV, Radko SP, Cho MS, Chrambach A, Vogel SS. Reconstitution of calcium-triggered membrane fusion using “reserve” granules. J Biol Chem 1998; 273:2445-51; PMID:9442095; http://dx.doi.org/ 10.1074/jbc.273.4.2445. [DOI] [PubMed] [Google Scholar]
- 28. Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell 2001; 106:157-69; PMID:11511344; http://dx.doi.org/ 10.1016/S0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 29. Togo T, Alderton JM, Bi GQ, Steinhardt RA. The mechanism of facilitated cell membrane resealing. J Cell Sci 1999; 112 ( Pt 5):719-31; PMID:9973606. [DOI] [PubMed] [Google Scholar]
- 30. Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, Burridge K. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol 2010; 12:477-83; PMID:20400958; http://dx.doi.org/ 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pertz O. Spatio-temporal Rho GTPase signaling - where are we now? J Cell Sci 2010; 123:1841-50; PMID:20484664; http://dx.doi.org/ 10.1242/jcs.064345. [DOI] [PubMed] [Google Scholar]
- 32. Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol 2009; 11:71-7; PMID:19060892; http://dx.doi.org/ 10.1038/ncb1814. [DOI] [PMC free article] [PubMed] [Google Scholar]