Abstract
Epithelial repair in the Drosophila embryo is achieved through 2 dynamic cytoskeletal machineries: a contractile actomyosin cable and actin-based cellular protrusions. Rho family small GTPases (Rho, Rac, and Cdc42) are cytoskeletal regulators that control both of these wound repair mechanisms. Cdc42 is necessary for cellular protrusions and, when absent, wounds are slow to repair and never completely close. Rac proteins accumulate at specific regions in the wound leading edge cells and Rac-deficient embryos exhibit slower repair kinetics. Mutants for both Rho1 and its effector Rok impair the ability of wounds to close by disrupting the leading-edge actin cable. Our studies highlight the importance of these proteins in wound repair and identify a downstream effector of Rho1 signaling in this process.
Keywords: Cdc42, cytoskeleton, Drosophila, epithelial wound repair, Rac, Rho, Rho family GTPases, Rok
Abbreviations
- GAPs
GTPase activating proteins
- GEFs
guanine nucleotide exchange factors
- GDIs
guanine nucleotide dissociation inhibitors
- RBD
Rho Binding Domain
Upon injury, wound repair is an essential process for continued cellular, tissue and organismal survival. In particular, epithelial tissue repair must occur rapidly and robustly to ensure that this tissue restores its function as a barrier to microbial invasion. Studies in a variety of organisms have shown that dynamic cytoskeletal processes, including leading edge cellular protrusions, a leading-edge contractile actomyosin purse-string, and in some cases, contraction of underlying tissue, drive this embryonic repair response.1-5 We have found that epithelial wound repair in stage 15–16 Drosophila embryos is actively driven by both actomyosin cable contraction and crawling via actin-rich cellular protrusions.3 The actomyosin purse-string is formed by the recruitment of actin and myosin to the apical, leading edge of the wound where they form a supracellular, contractile actomyosin array that is linked cell-to-cell through adherens junctions.3,6 Simultaneously, leading edge cells form dynamic actin-rich cellular protrusions, which contribute to wound repair by making contact with contralateral and adjacent protrusions and/or cells and subsequently pulling these regions toward each other. Other processes such as cell shape changes and rearrangements, both at the leading edge of the wound and several rows of cells away from the wound, also play supporting roles in these active processes such that the epithelial sheet is stretched to close the wound in the absence of cell division.7
One family of proteins essential to these dynamic cytoskeletal changes observed during proper wound repair is the Rho family of small GTPases.1,2,8–10 The 3 founding members of this protein family, Rho, Rac and Cdc42, are also necessary for a variety of other cellular and developmental functions through their activities in modulating the actin and microtubule cytoskeleton.11,12 Rho GTPases are G-protein switch molecules that cycle between GDP- and GTP-bound states.13 When GTP-bound these proteins undergo a conformational change that allows them to interact with downstream proteins through their exposed effector domains and are termed ‘activated’.14,15 Rho family GTPases are activated by guanine nucleotide exchange factors (RhoGEFs) that promote the exchange of GDP to GTP, and inactivated by GTPase-activating proteins (RhoGAPs) that promote the conversion of GTP to GDP and Rho GDP-dissociation inhibitors (RhoGDIs) that remove these proteins from the membrane and prevent GDP-GTP exchange.11-13,16 In addition, these proteins show context-dependent ‘crosstalk’ among themselves, as well as transmitting and receiving signals from the cytoskeleton.10,17,18
The role of Rho family GTPases in multicellular wound repair was initially explored in the embryonic chick wing bud using C3 exoenzyme and a dominant inhibitory Rac protein (DN-Rac) to inhibit Rho and Rac, respectively.1 Wing buds treated with C3-containing media did not readily repair as a result of their inability to form an actomyosin purse-string, while DN-Rac did not affect wound repair. A subsequent study used the dorsal closure-staged Drosophila embryo that, unlike the chick embryo wing bud, is amenable to live imaging and has stratified tissues with the outermost layer being a simple epithelial sheet.2 These studies with Drosophila embryos indicated that Rho1 and Cdc42 were necessary for proper wound repair, whereas the 3 Rac proteins (Rac1, Rac2 and Mtl) were dispensable.2 In particular, Cdc42 was shown to be essential late in the repair process for the final resealing of the epithelial sheet. Interestingly, upon wounding, Rho1 mutant embryos were observed to pause for 2 hours during which no changes in leading edge cell shape were noted. Following this 2-hour lag phase, wounds were observed to undergo repair with normal kinetics. Recently, we examined repair in Cdc42 mutants and found an additional role for this protein in the earlier contraction phase of embryo epithelial wound repair.3 Here we re-examine the roles of the other Rho family GTPases in Drosophila embryo epithelial wound repair and show that, in contrast to previous studies, Rho1 mutants exhibit altered repair dynamics (without the 2 hour lag phase) and the presence of Rac proteins are required for normal embryonic epithelial repair (Fig. 1A-J; Movie 1). In addition, we examined the localization of each of these GTPases in response to wounding, and show that Rho1 is likely signaling through multiple effectors, one of which is the myosin light chain (MLC) phosphorylating kinase, Rok.
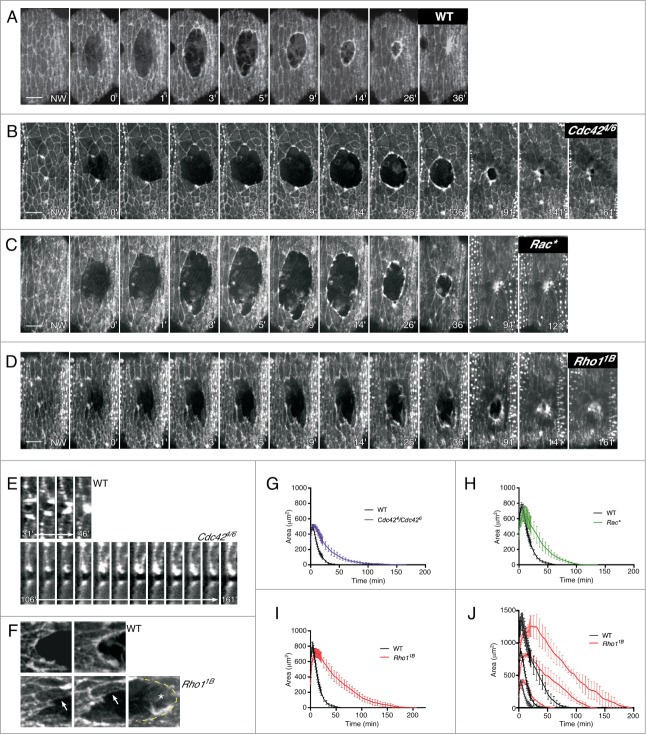
Figure 1.
Rho family GTPases are essential for efficient epithelial repair in the dorsal closure (stage 15) Drosophila embryo. (A-D) Time-lapse projections of embryos expressing an actin marker (sGMCA) in wildtype (A), Cdc424/Cdc426 (B), Rac1J10, Rac2Δ, MtlΔ (referred to as Rac*; C), and Rho11B (D) embryos during wound repair. Wildtype embryos display an actin cable and actin protrusions during wound repair (A). Cdc424/Cdc426 mutant embryos exhibit significantly less protrusions (B; adapted from3 and reproduced with permission from The Company of Biologists). Despite their significant delay in repair, Rac* mutant embryos do not show gross defects in actin cable formation or protrusions (C). Rho11B mutant embryos show incomplete actin cable formation and increased protrusions during wound repair (D). (E) Time series of size matched wildtype and Cdc424/Cdc426 wounds (orthogonal view) entering closure; Cdc424/Cdc426 mutants fail to reseal the epithelium. (F) Confocal projections of wounds in wildtype (top) and Rho11B (bottom) mutant embryos showing that Rho11 mutants fail to form a continuous actin cable along the lead edge (arrows) and do not become rounded (jagged leading edge) indicating a defective actomyosin purse string. Rho11B embryos have large protrusions (asterisk). (G–I) Quantification of wound area versus time in medium-size wounds showing that Rho11B, Cdc424/Cdc426, and Rac* mutant embryos exhibit delays throughout the repair process (wildtype, n = 10; Rho11B, n = 10; Cdc424/Cdc426, n = 6; Rac*, n = 6; results are given as means ± s.e.m.). (H) Quantification of wound area vs. time in small (<500 μm2), medium (500-1000 μm2), and large (1000-1500 μm2) wounds generated in wildtype or Rho11B mutant embryos showing that time of repair is scalar to wound size (all sizes: wildtype, n = 3; Rho11B, n = 3). Scale bar: 10 μm.
Using a conditional dominant-negative Cdc42 allele driven by the Engrailed Gal4 driver, it was originally shown that wounds in Cdc42 embryos were unable to undergo the last phase of wound repair needed to completely reseal the epithelial sheet.2 This defect was attributed to a lack of cellular protrusions such that the final hole could not be knit closed. Recently, we used the hetero-allelic combination of Cdc424/Cdc426 to explore the role of Cdc42 in wound repair.3 Same as originally reported, we found that these mutant embryos were unable to undergo the final process of resealing the epithelial sheet (Fig. 1B and E). The area of cellular protrusions present throughout repair was reduced and consistent with the lack of protrusions being responsible for the resealing defect.3 Significantly, we also found that these wounds show delayed repair kinetics throughout the entirety of the wound repair process compared to size-matched wildtype embryos (107.0 ± 12.3 minutes in Cdc424/Cdc426 mutants compared to 37.0 ± 5.1 minutes in wildtype; p = 0.0010) (Fig. 1B, G; Movie 1). This result shows that Cdc42 is necessary for the dynamic cellular protrusions required during wound contraction, in addition to those needed for the final resealing of the hole.
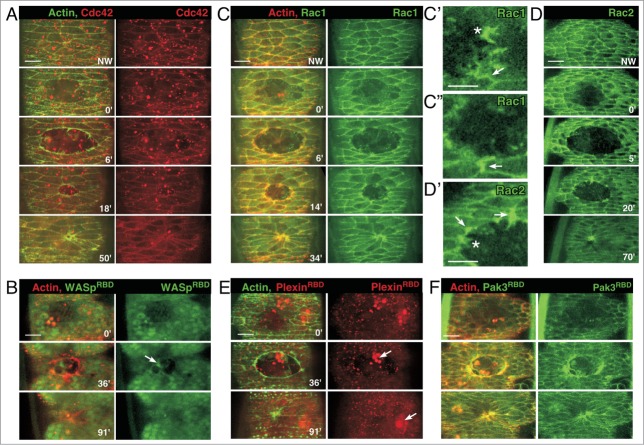
To further characterize the role of Cdc42, we looked for changes in localization and/or accumulations of Cdc42 in response to wounding. We wounded transgenic embryos expressing a previously described mChFP-Cdc42 fusion protein driven ubiquitously by the myosin spaghetti-squash (sqh) promoter.3,10 While we observed fluorescent Cdc42 fusion protein expression throughout the embryonic epithelia, there was no change in its localization or specific accumulation in response to wounding (Fig. 2A). This was somewhat surprising given the accumulation of Rho family GTPases at wounds during single cell repair and Cdc42s requirement for proper epithelial repair. One possible reason for this was that we could not detect the subset of Cdc42 that was being specifically ‘activated’ in response to the wound above the normal pool of Cdc42 protein present within each cell. To test this possibility, we wounded embryos expressing previously described GTPase biosensors comprised of simple fusions of GFP and the Rho family GTPase binding domain (RBD) from specific downstream effectors that allow visualization of activated GTPases. Each biosensor exhibited the expected developmental patterning and specifically bound their expected GTP-loaded Rho family GTPase unless otherwise noted.10 Using these biosensors, activated Rho family GTPase was detected as accumulation of signal above the background expression of these fusion-constructs. To determine the localization of activated Cdc42, a biosensor containing the RBD of Cdc42s effector, WASp, fused to GFP was used. While this biosensor functions developmentally in hemocytes (Fig. 2B, arrow),10 it does not accumulate at wounds (Fig. 2B). This lack of accumulation could be due to several possibilities: i) Cdc42 may not need to accumulate to appreciable levels to carry out its functions, ii) Cdc42 could be acting transiently, thus signal cannot sufficiently accumulate to be captured by this biosensor in any specific location, iii) activated Cdc42 may not be accumulating at the wound over the background level used for normal cell function, or iv) the biosensor could only act in a context-specific manner. We have previously shown a context-specific phenomenon in wound repair in the single-cell Drosophila embryo wherein an effector's RBD is able to function as a biosensor only if that effector is necessary for the repair process.10 It will be important to identify the effectors through which Cdc42 regulates the dynamic cellular protrusions of the leading edge necessary for normal wound repair. The design and use of bi-molecular biosensors in which fluorescence only occurs when the biosensor and Rho family GTPase members interact may provide additional insight for these abundant proteins.19
Figure 2.
Expression of Cdc42 and Rac1/2 fluorescent proteins and activity biosensors during epithelial repair. (A) Surface projections of wound repair in embryos expressing an actin marker (sGMCA) and fluorescent Cdc42; Cdc42 does not accumulate specifically in response to wounding. (B) Surface projections of wound repair in embryos expressing an actin marker (sChMCA) and the Cdc42 biosensor GFP-WASpRBD showing no accumulation indicative of activated Cdc42. This biosensor is functional as there is specific accumulation in the hemocytes responding to the wound (arrow). (C–C”) Surface projections of wound repair in embryos expressing an actin marker (sChMCA) and fluorescent Rac1; Rac1 accumulates in protrusions (asterisk; C’) and at some cell junctions (arrows; C’–C’’). (D–D’) Surface projections of wound repair in embryos expressing fluorescent Rac2. Rac2 accumulates in protrusions (asterisk; D’) and at some cell junctions (arrows; D’). (E–F) Surface projections of wound repair in embryos expressing an actin marker (sGMCA or sChMCA) and the Rac proteins biosensors ChFP-PlexinBRBD (E) or GFP-Pak3RBD (F) The ChFP-PlexinBRBD biosensor does not accumulate at the wound edge (E), whereas the GFP-Pak3RBD biosensor accumulates at the wound along portions of the leading edge (F). Scale bars: 10 μm (A–C, D, E, F) and 5 μm (C’, C’’, D’).
Rac proteins were previously reported to have no wound repair defect.2 This was unexpected given that Rac mutants exhibit a striking defect during dorsal closure, a morphogenetic process likened to epithelial wound repair wherein 2 lateral epithelial sheets zip together at the dorsal midline.20,21 In contrast to this report,2 we find that Rac family proteins are required for proper embryo epithelial wound repair. Using the same Rac1J1, Rac2Δ, MtlΔ (referred to as Rac*) triple mutant which combines mutations for all Drosophila Rac genes, we find that Rac* affects wound repair leading to a significant delay in the timing of wound closure relative to wildtype (106.2 ± 12.1 minutes in Rac* mutants compared to 51.0 ± 3.7 minutes in wildtype; p = 0.008) (Fig. 1C and H; Movie 1). Interestingly, we do not observe gross defects in leading edge morphology in these mutants (Fig. 1C). While both the actomyosin cable and cellular protrusions appear largely normal, we cannot rule out subtle defects to cellular protrusion efficacy or to actomyosin ring assembly, stability, or disassembly. Rac depletion may exert its effects through Rho1 and/or Cdc42 by regulating these proteins (cross-talk) leading to changes in their levels and/or activity that subtly disrupt how the proteins regulate the actomyosin purse-string and protrusions, respectively. An alternate possibility is that Rac affects cell rearrangements away from the wound. This would be consistent with studies in Drosophila embryos and pupae indicating that dynamic cell rearrangements several cells away from the wound are necessary for those epithelial cells to stretch across the closing wound and that reduced Rac activity inhibits dynamic cell rearrangement during Drosophila tracheal tubulogenesis.7,22 However, no significant defects in cell rearrangements were detected away from the wound in Rac* mutants (Fig. 1C; Movie 1).
To further examine the effects of the Rac proteins on epithelial wound repair, we wounded transgenic embryos expressing fluorescently tagged Rac1 or Rac2 under the control of their respective endogenous promoters. Before wounding, enrichment of Rac1 and Rac2 fluorescent fusion proteins was detected at apical and lateral cell membranes (Fig. 2C-D’). Upon wounding these Rac proteins accumulated at the wound edge in protrusions, especially where they emanated from the leading edge (Fig. 2C-D’). Interestingly, these Rac proteins also accumulated at the leading edge in discrete segments at cell-cell contacts and often extended along these contacts away from (perpendicular to) the wound (Fig. 2C-D’). To determine what subset of this Rac accumulation is the result of activated Rac protein, we examined Rac biosensors that had been generated using the RBD of 2 known Rac effectors, PlexinB and Pak3.15,23,24 Full-length fluorescent fusion proteins for PlexinB and Pak3 are unavailable for testing in this system. We have previously shown that PlexinB RBD binding is specific to GTP-bound Rac, whereas the Pak3 RBD binds Rac1 as well as Cdc42 (albeit with slightly lower affinity).10 The PlexinB-RBD biosensor does not accumulate or localize upon wounding (Fig. 2E), however, the biosensor is functional as can be observed from its accumulation in hemocytes (Fig. 2E, arrows). In contrast, the Pak3-RBD biosensor accumulates at the leading edge in a pattern consistent with that of Rac1 and Rac2 suggesting that the majority of the protein at the leading edge is activated (Fig. 2F). Interestingly, it was recently published that in Drosophila larval wounds Pak3 is the major kinase responsible for leading edge actomyosin integrity as RNAi knockdown of Pak3 specifically disrupts this structure in a Rac1 dependent manner compared to other candidate kinases, including Rok and MLCK.25 We are unable to assess the requirement of Pak3 in wound repair as an appropriate Pak3 mutant allele is not currently available. Nonetheless, consistent with our finding that Rac* is indeed required for proper epithelial wound repair, activated Rac* accumulates at the apical wound leading edge and in wound leading edge protrusions.
Rho1 hypomorphic mutants have been reported to affect epithelial wound repair: they assemble a disorganized actin cable such that wounds remain open for roughly 2 hours then close with normal kinetics.2 We were particularly intrigued by the 2 hour delay in repair because it suggested that Rho1 might be involved in recruiting or organizing molecules/machineries at the wound leading edge such that proper repair could begin. Alternatively, this delay might represent a lag needed by the wounded Rho1 mutant embryo to switch any machineries driving repair through the actomyosin cable contraction into repair driven primarily by cellular protrusions.2 To explore these possibilities, we wounded Rho1 null mutant embryos (Rho11B) and were surprised to find that we did not observe this 2 hour delay, rather wound repair kinetics were disrupted throughout the entire process in medium sized wounds (∼750 μm2) (Fig 1D and I; Movie 1). We hypothesized that this discrepancy in results might be due to disparities in the sizes of wounds being assayed, as wound size might decrease the frequency or increase distance upon which cellular protrusions would have to reach in order to make contact and create zippering events. To test this, we examined wounds in 3 different size ranges, small (<500 μm2), medium (500–1000 μm2), and large (1000–1500 μm2), with the expectation that an increase in wound size would lead to disproportionate defects in wound repair (Fig. 1J). However, we find that in both wildtype and Rho1B mutant embryos, wound repair is scalar to size and defects in Rho1 wound repair exist throughout the repair process – without a lag phase at any size tested (Fig. 1J). We find that medium size wounds in Rho1 mutant embryos take almost 3 times longer to heal than those in wildtype (129.0 ± 13.5 minutes in Rho1B mutants compared to 51.0 ± 3.7 minutes in wildtype; p = 0.0002) (Fig. 1D and I). One possible cause for the differences we have noted is our use of a more recently generated null-allele for Rho1 (Rho11B), rather than the Rho172R/Rho172O hetero-allelic hypomorphic mutant reported in the original study.2,26 Despite differences observed in the overall kinetics, we similarly observe that a considerable amount of wound contraction appears to be driven by protrusions along the leading edge. In Rho11B mutant embryos, large portions of the wound's leading edge lack a continuous actin cable and instead have large protrusions, as well as failing to form an ellipsoid shape for much of the repair process (Fig. 1D and F; Movie 1). We have previously observed similar phenotypes in myosin mutants,3 suggesting that the actomyosin purse-string is defective in Rho11B mutants. Interestingly, we also observed this aberrant repair phenotype with another actomyosin purse-string component, E-cadherin: E-cadherin knockdown leads to local accumulations of contractile actin along the leading edge.3 Consistent with this, Rho1 functions upstream of proteins that can potentially affect the actomyosin purse-string in myosin (Rok) and/or E-cadherin (α-catenin, p120-catenin) dependent manners.27,28
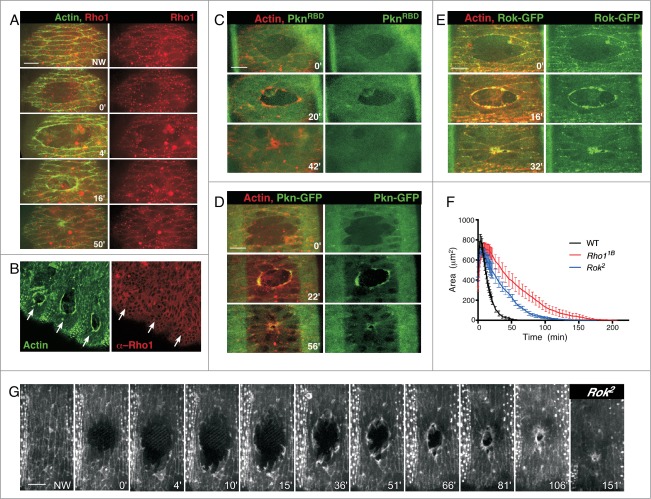
To further assess the manner in which Rho1 affected the actomyosin cable, we examined Rho1 localization during the repair process. We expected to observe accumulations of Rho1 along the leading edge, consistent with its promotion of the actomyosin cable, at cell-cell contacts along the leading edge modulating the junctions, or at both. Surprisingly, while Rho1 is expressed throughout the epithelia, it does not exhibit higher protein accumulation, changes in localization pattern, or associate with the actomyosin cable, junctions or protrusions during the repair process (Fig. 3A). This fluorescent Rho1 fusion protein is representative of endogenous Rho1 protein, as wounded embryos fixed then stained with a Rho1 antibody reveal no significant change in localization and/or accumulation of endogenous Rho1 protein in relation to the wound (Fig. 3B). Using a strategy similar to that described for Cdc42 and Rac*, we tested whether the inactive or background pools of Rho1 in each cell was masking the activity of Rho1 at the wound using a Pkn-RBD fluorescent fusion construct. This RBD protein fragment binds both Rho1 and Rac in vitro, however it binds Rho1 with much higher affinity.10 Similar to what we observed with the WASp-RBD biosensor for Cdc42, we were unable to detect any specific accumulation of this Pkn-RBD biosensor indicative of activated Rho1 at the wound (Fig. 3C). This was particularly surprising given that this biosensor not only accumulates during single-cell wound repair,10 but also full-length endogenous, fluorescent Pkn responds to wounds and localizes at the wound leading edge in regions where actin accumulation is highest (Fig. 3D).
Figure 3.
Expression of Rho1 and its downstream effectors and Rok mutant during wound repair. (A) Surface projections of wound repair in embryos expressing an actin marker (sGMCA) and mChFP-Rho1. Rho1 does not accumulate at the leading edge. (B) Confocal projection of 3 adjacent wounds in embryos expressing an actin marker (sGMCA) and stained with α-Rho1 antibody (P1D9; 1:50). Rho1 does not accumulate at the wound edge (arrows). (C–E) Surface projections of wound repair in embryos expressing an actin marker (sChMCA) and the Rho1 biosensor GFP-PknRBD (C) or the full-length Rho1 downstream effectors Pkn (Pkn-GFP; D) or Rok (Rok-GFP; E). The PknRBD biosensor does not accumulate at the wound edge (C), whereas the full-length Pkn and Rok effectors accumulate at the wound and co-localize with the actin cable (D–E). (F) Quantification of wound area versus time in medium-size wounds shows that Rok2 mutant embryos exhibit delays throughout the repair process, albeit less severe than that observed with Rho11B (wildtype, n = 10; Rho11B, n = 10; Rok2, n = 5; results are given as means ± s.e.m.). (G) Time-lapse projections of embryos expressing an actin marker (sGMCA) in wound Rok2 mutant embryos during wound repair. Rok2 mutant embryos show incomplete actin cable formation and increased cellular protrusions during wound repair similar to that observed with Rho11B.
While the effect of Pkn is currently unclear, as its substrates during wound repair have yet to be elucidated, another Rho1 effector, Rok, is known to phosphorylate the myosin light chain protein, spaghetti-squash, and was recently shown to function downstream of Rho1 during apical constriction.29,30 Indeed, we find that during epithelial wound repair full-length GFP-Rok co-localizes with actin along the majority of the leading edge (Fig. 3E). To test whether Rok might be responsible for the repair phenotype observed in Rho11B mutants, we examined wound repair in Rok2 mutant embryos (Fig. 3F and G; Movie 1). These embryos heal with delayed kinetics compared to wildtype (114.0 ± 9.8 minutes in Rok2 mutants compared to 51.0 ± 3.727 minutes in wildtype; p < 0.0001) and exhibit similar, albeit somewhat less severe, phenotypes to Rho1 (Fig. 3F and G). This suggests that Rok is acting downstream of Rho1, but is not likely the only Rho1 effector necessary during epithelial repair. Further testing using Rho1 point mutations that specifically inhibit the binding of certain effectors, as well as screening effector mutants, will elucidate what other Rho1 effector proteins might be necessary to recapitulate the severity of the Rho1 phenotype.
Here we showed that the 3 major Rho GTPases in Drosophila are all necessary for embryonic epithelial repair: Rho1, at least in part through its effector Rok, is required for proper actomyosin cable function; Cdc42 is required for the actin-based protrusions; and the absence of Rac proteins significantly delays normal repair without gross disruptions of the leading edge actin cytoskeleton. Surprisingly, Rac proteins are the only Rho family GTPase that accumulate appreciably at the wound. Interestingly, although Rho1 itself does not accumulate at the epithelial wounds, its downstream effector Rok does accumulate at wounds and, when absent, has a phenotype consistent with functioning downstream of Rho1. Recently, we showed that all 3 Rho family GTPases rapidly accumulate around the single cell wounds where they segregate into dynamic, partially overlapping, arrays.10 This raises the interesting question of why Rho family GTPases are so robustly recruited to single cell wounds, while Rac proteins are the only ones that accumulate during epithelial repair. In addition, the ability of the RBD of Pak3, but not PlexinB, to recapitulate Rac accumulation suggests that the RBD binding may be regulated such that only pertinent effectors are able to bind, similar to that observed in the Drosophila cell wound repair model.10 It will be interesting in the future to determine if and how crosstalk among these proteins influences their functions and modulates the cytoskeleton during the repair process.
Materials and Methods
Fly strains and genetics
Flies were cultured at 25°C on yeast-cornmeal-molasses-malt medium. The following stocks containing fluorescent fusion proteins were used: sGMCA,31 sChMCA,32 P{w[+mC] = mChFP-Rho1}21,10 P{w[+mC] = sqh-ChFP-Cdc42}23,3 P{w[+mC] = GFP-Rac1}20,10 P{w[+mC] = GFP-Rac2}21,10 P{w[+mC] = sqh-GFP-Rok}10,10 P{w[+mC] = sqh-Rok.RBD-GFP}30,10 GFP-Pkn (FlyTrap),33 P{w[+mC] = sqh-Pkn.RBD.G58A}212a/b,10 P{w[+mC] = sqh-GFP-Pak1}10,10 P{w[+mC] = sqh-Pak1.RBD-GFP}20,10 P{w[+mC] = sqh-mChFP-Pak3}8,10 P{w[+mC] = sqh-Pak3.RBD-GFP}30,10 P{w[+mC] = sqh-WASp.RBD-GFP}378a/b,10 and P{w[+mC] = sqh-PlexB.RBD-mChFP}356a/b.10
The following mutant alleles were used Rho11B;26 Cdc424 and Cdc426;34 Rac1J10, Rac2Δ, MtlΔ;35 and Rok2.36 Mutant alleles were crossed to the sGMCA; CyO-ChFP or sGMCA; TM3-ChFP balancer stocks to screen for homozygous mutants by selecting against the ChFP balancer. Mutant embryos for Cdc42 were generated using the hetero-allelic combination Cdc424/Cdc426.34 All mutant embryos express sGMCA allowing the actin cytoskeleton to be followed.
Embryo handling and preparation
Early embryos were collected for 0–1 hr at room temperature (23°C) then aged at 18°C for 21 hrs. The resulting stage 15 embryos were hand dechorionated, dried for 5 min and transferred individually with forceps onto strips of glue dried onto No. 1.5 coverslips, and covered with series 700 halocarbon oil (Halocarbon Products Corp).
Laser wounding
Wounds were generated using an N2 Micropoint laser (Photonic Instruments, St Charles, IL, USA) tuned to 405 nm and focused on the ventral surface of the embryo as previously described.23 A region of interest was selected and ablation was controlled by Volocity (v.5.3.0, Perkin Elmer, Waltham, MA, USA). On average, ablation time was less than 5 s, wounds were 750 μm2 (range: 500–1000 μm2), and time-lapse imaging was initiated immediately.
Embryo fixing and staining
Wounded embryos were collected and the halocarbon oil was removed with heptane. Embryos were then fixed and antibody staining was performed as described previously.37 Embryos were mounted in SlowFade Gold (Invitrogen) and imaged as previously described.32 Antibodies used were: mouse monoclonal Rho1 (P1D9; 1:50 dilution)27 and goat anti-mouse Alexa 568 (1:1000 dilution; Invitrogen).
Microscopy
All imaging was performed at room temperature (23°C) as previously described.3 The following microscopes were used:
Nikon TE2000-E stand (Nikon Instruments, Melville, NY, USA), with 40×/1.4 NA objective lens, controlled by Volocity software (v.5.3.0, Perkin Elmer, Waltham, MA, USA). Images were acquired with 491 nm and 561 nm lasers, with a Yokogawa CSU-10 confocal spinning disc head equipped with a 1.5 × magnifying lens, and a Hamamatsu C9100–13 EMCCD camera (Perkin Elmer, Waltham, MA, USA).
UltraVIEW VoX Confocal Imaging System (Perkin-Elmer, Waltham, MA, USA), in a Nikon Eclipse Ti stand (Nikon Instruments, Melville, NY, USA), with 60×/1.4 NA or 100×/1.4 NA objective lens and controlled by Volocity software (v.5.3.0, Perkin Elmer, Waltham, MA, USA). Images were acquired with 491 nm and 561 nm, with a Yokogawa CSU-X1 confocal spinning disc head equipped with a Hamamatsu C9100–13 EMCCD camera (Perkin-Elmer, Waltham, MA, USA).
Nikon LiveScan Swept Field Confocal (For Nikon by Prairie Technologies Inc.., Middleton, WI, USA) mounted on a Nikon Eclipse Ti (Nikon Instruments, Melville, NY, USA); with 60×/1.4 NA objectives lens, using the NIS-Elements AR 3.0 as acquisition software (Nikon Instruments, Melville, NY, USA). Images were acquired with a 491 nm laser, and a Photometrics QuantEM: 512SC EMCCD camera (Photometrics, Tucson, AZ, USA). All images acquired with a 40× or 60× objective lens are 25 μm stacks/0.5 μm steps, for the 100× images the stacks correspond to 1.5 μm /0.25 μm steps.
Image processing, analysis and quantification
Image series were either analyzed with Volocity software (v.5.3.0, Perkin Elmer, Waltham, MA, USA), or were exported as TIFF files then imported into ImageJ for processing. XY projections of 1–5 μm were generated. Wound areas were measured manually with ImageJ or NIS-Elements AR software (version 3.0, Nikon Instruments, Melville, NY, USA). A Student's t test with Welch's correction when applicable was used to analyze the data; p < 0.05 was considered to be statistically significant. All graphs present values ± s.e.m. All measurements were downloaded into Microsoft Excel and the data were graphed using Prism 5.0 c (GraphPad Software, Inc.).
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank M.T. Abreu-Blanco, C. Milligan, B. Sugumar, and members of the lab for their comments/advice. We are very grateful to L. Cooley, R. Fehon, FlyTrap, the Bloomington Stock Center, and the Murdock Foundation for flies and the microscope used for imaging.
Funding
This work was supported by NIH grants GM097083 and GM092731 to SMP.
References
- 1. Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol 1996; 135:1097-107; PMID:8922389; http://dx.doi.org/ 10.1083/jcb.135.4.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol 2002; 4:907-12; PMID:12402048; http://dx.doi.org/ 10.1038/ncb875 [DOI] [PubMed] [Google Scholar]
- 3. Abreu-Blanco MT, Verboon JM, Liu R, Watts JJ, Parkhurst SM. Drosophila embryos close epithelial wounds using a combination of cellular protrusions and an actomyosin purse string. J Cell Sci 2012; 125:5984-97; PMID:23038780; http://dx.doi.org/ 10.1242/jcs.109066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci U S A 2003; 100:10788-93; PMID:12960404; http://dx.doi.org/ 10.1073/pnas.1834401100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature 1992; 360:179-83; PMID:1436096; http://dx.doi.org/ 10.1038/360179a0 [DOI] [PubMed] [Google Scholar]
- 6. Danjo Y, Gipson IK. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J Cell Sci 1998; 111 (Pt 22):3323-32; PMID:9788874 [DOI] [PubMed] [Google Scholar]
- 7. Razzell W, Wood W, Martin P. Recapitulation of morphogenetic cell shape changes enables wound re-epithelialisation. Development 2014; 141:1814-20; PMID:24718989; http://dx.doi.org/ 10.1242/dev.107045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol 2005; 168:429-39; PMID:15684032; http://dx.doi.org/ 10.1083/jcb.200411109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clark AG, Miller AL, Vaughan E, Yu HY, Penkert R, Bement WM. Integration of single and multicellular wound responses. Curr Biol 2009; 19:1389-95; PMID:19631537; http://dx.doi.org/ 10.1016/j.cub.2009.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abreu-Blanco MT, Verboon JM, Parkhurst SM. Coordination of Rho family GTPase activities to orchestrate cytoskeleton responses during cell wound repair. Curr Biol 2014; 24:144-55; PMID:24388847; http://dx.doi.org/ 10.1016/j.cub.2013.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279:509-14; PMID:9438836; http://dx.doi.org/ 10.1126/science.279.5350.509 [DOI] [PubMed] [Google Scholar]
- 12. Machesky LM, Hall A. Rho: a connection between membrane receptor signalling and the cytoskeleton. Trends Cell Biol 1996; 6:304-10; PMID:15157438; http://dx.doi.org/ 10.1016/0962-8924(96)10026-X [DOI] [PubMed] [Google Scholar]
- 13. Narumiya S. The small GTPase Rho: cellular functions and signal transduction. J Biochem 1996; 120:215-28; PMID:8889802; http://dx.doi.org/ 10.1093/oxfordjournals.jbchem.a021401 [DOI] [PubMed] [Google Scholar]
- 14. Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 2000; 348(Pt 2):241-55; PMID:10816416; http://dx.doi.org/ 10.1042/0264-6021:3480241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 2007; 29:356-70; PMID:17373658; http://dx.doi.org/ 10.1002/bies.20558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J 2005; 390:1-9; PMID:16083425; http://dx.doi.org/ 10.1042/BJ20050104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vaughan EM, Miller AL, Yu HY, Bement WM. Control of local Rho GTPase crosstalk by Abr. Curr Biol 2011; 21:270-7; PMID:21295482; http://dx.doi.org/ 10.1016/j.cub.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol 2009; 11:71-7; PMID:19060892; http://dx.doi.org/ 10.1038/ncb1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pertz O. Spatio-temporal Rho GTPase signaling – where are we now? J Cell Sci 2010; 123:1841-50; PMID:20484664; http://dx.doi.org/ 10.1242/jcs.064345 [DOI] [PubMed] [Google Scholar]
- 20. Woolner S, Jacinto A, Martin P. The small GTPase Rac plays multiple roles in epithelial sheet fusion–dynamic studies of Drosophila dorsal closure. Dev Biol 2005; 282:163-73; PMID:15936337; http://dx.doi.org/ 10.1016/j.ydbio.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 21. Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development 2004; 131:3021-34; PMID:15197160; http://dx.doi.org/ 10.1242/dev.01253 [DOI] [PubMed] [Google Scholar]
- 22. Chihara T, Kato K, Taniguchi M, Ng J, Hayashi S. Rac promotes epithelial cell rearrangement during tracheal tubulogenesis in Drosophila. Development 2003; 130:1419-28; PMID:12588856; http://dx.doi.org/ 10.1242/dev.00361 [DOI] [PubMed] [Google Scholar]
- 23. Driessens MH, Hu H, Nobes CD, Self A, Jordens I, Goodman CS, Hall A. Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr Biol 2001; 11:339-44; PMID:11267870; http://dx.doi.org/ 10.1016/S0960-9822(01)00092-6 [DOI] [PubMed] [Google Scholar]
- 24. Lim L, Manser E, Leung T, Hall C. Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways. Eur J Biochem 1996; 242:171-85; PMID:8973630; http://dx.doi.org/ 10.1111/j.1432-1033.1996.0171r.x [DOI] [PubMed] [Google Scholar]
- 25. Baek SH, Cho HW, Kwon YC, Lee JH, Kim MJ, Lee H, Choe KM. Requirement for Pak3 in Rac1-induced organization of actin and myosin during Drosophila larval wound healing. FEBS Lett 2012; 586:772-7; PMID:22449966; http://dx.doi.org/ 10.1016/j.febslet.2012.01.061 [DOI] [PubMed] [Google Scholar]
- 26. Magie CR, Meyer MR, Gorsuch MS, Parkhurst SM. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development 1999; 126:5353-64; PMID:10556060 [DOI] [PubMed] [Google Scholar]
- 27. Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development 2002; 129:3771-82; PMID:12135916 [DOI] [PubMed] [Google Scholar]
- 28. Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene 2000; 19:6059-64; PMID:11146558; http://dx.doi.org/ 10.1038/sj.onc.1203987 [DOI] [PubMed] [Google Scholar]
- 29. Antunes M, Pereira T, Cordeiro JV, Almeida L, Jacinto A. Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding. J Cell Biol 2013; 202:365-79; PMID:23878279; http://dx.doi.org/ 10.1083/jcb.201211039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vasquez CG, Tworoger M, Martin AC. Dynamic myosin phosphorylation regulates contractile pulses and tissue integrity during epithelial morphogenesis. J Cell Biol 2014; 206:435-50; PMID:25092658; http://dx.doi.org/ 10.1083/jcb.201402004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol 2000; 149:471-90; PMID:10769037; http://dx.doi.org/ 10.1083/jcb.149.2.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abreu-Blanco MT, Verboon JM, Parkhurst SM. Cell wound repair in Drosophila occurs through three distinct phases of membrane and cytoskeletal remodeling. J Cell Biol 2011; 193:455-64; PMID:21518790; http://dx.doi.org/ 10.1083/jcb.201011018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buszczak M, Paterno S, Lighthouse D, Bachman J, Planck J, Owen S, Skora AD, Nystul TG, Ohlstein B, Allen A, et al. The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics 2007; 175:1505-31; PMID:17194782; http://dx.doi.org/ 10.1534/genetics.106.065961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Genova JL, Jong S, Camp JT, Fehon RG. Functional analysis of Cdc42 in actin filament assembly, epithelial morphogenesis, and cell signaling during Drosophila development. Dev Biol 2000; 221:181-94; PMID:10772800; http://dx.doi.org/ 10.1006/dbio.2000.9671 [DOI] [PubMed] [Google Scholar]
- 35. Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, Harms M, Nardine T, Luo L, Dickson BJ. Rac function and regulation during Drosophila development. Nature 2002; 416:438-42; PMID:11919634; http://dx.doi.org/ 10.1038/416438a [DOI] [PubMed] [Google Scholar]
- 36. Verdier V, Johndrow JE, Betson M, Chen GC, Hughes DA, Parkhurst SM, Settleman J. Drosophila Rho-kinase (DRok) is required for tissue morphogenesis in diverse compartments of the egg chamber during oogenesis. Dev Biol 2006; 297:417-32; PMID:16887114; http://dx.doi.org/ 10.1016/j.ydbio.2006.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rodriguez-Mesa E, Abreu-Blanco MT, Rosales-Nieves AE, Parkhurst SM. Developmental expression of Drosophila Wiskott-Aldrich Syndrome family proteins. Dev Dyn 2012; 241:608-26; PMID:22275148; http://dx.doi.org/ 10.1002/dvdy.23742 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.