Abstract
Background
The pathogenesis of esophagus carcinoma involves a cascade process consisting of multiple factors and accumulation of gene mutations. It is known that vascular endothelial growth factor (VEGF) mainly regulates de novo vascular formation while B-cell lymphoma-2 (BCL-2) gene exerts a tumor-suppressing effect. The prominent expression of VEGFA and BCL-2 genes, along with the most famous tumor-suppressor gene, TP53, raise the possibly of gene interaction. This study therefore investigated the effect and correlation of TP53, BCL-2, and VEGFA genes on cell proliferation and apoptosis of esophagus carcinoma.
Material/Methods
A total of 30 male rats were prepared by subcutaneous injection of methyl-benzyl-nitrosamine (MBNA) to induce esophagus cancer, along with 30 controlled rats which received saline instead. After 4, 10, 20, or 30 weeks, rats were sacrificed to observe the morphological changes of esophageal mucosa. Cell apoptosis was quantified by terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labeling (TUNEL) assay. Immunohistochemical (IHC) staining was employed to examine the expression of TP53, BCL-2 and VEGFA genes.
Results
With the progression of cancer, pathological damages of esophageal tissue aggravated while the cancer cell apoptosis gradually decreased compared to controlled animals. Protein levels of p53, Bcl-2, and VEGF in the model group were significantly elevated at each time point. Positive correlations existed between p53 and Bcl-2 or VEGF.
Conclusions
Abnormally elevated expression of TP53, BCL-2, and VEGFA genes may participate in the proliferation of esophagus cancer cells in a synergistic manner.
MeSH Keywords: Barrett Esophagus, Proto-Oncogene Proteins c-bcl-6, Tumor Suppressor Protein p53
Background
As a common malignant tumor in the digestive tract, esophagus carcinoma derives from a cascade process which is dependent on multiple factors. Cells in the precancerous lesion are able to re-differentiate into normal cells or transform into primary carcinoma. Therefore, elucidation of the molecular mechanism of esophagus carcinoma pathogenesis and the establishment of drug targets are critically important for the prevention and treatment of disease [1,2]. Both the occurrence and progression of malignant tumors are correlated with cell proliferation/apoptosis [3,4], which is mainly regulated by tumor suppressor gene B-cell lymphoma-2 (BCL-2). The over-expression of Bcl-2 proteins in tumor cells may promote the growth of lesions, suggesting the correlation of BCL-2 and biological behavior of esophagus cancer cells [4]. Another tumor suppressor gene, TP53, may induce oncogenesis when it has been mutated. Vascular epithelial growth factor (VEGF) is also known to be related to the invasion and metastasis of malignant tumors. Studies have revealed the expression of TP53, BCL-2, and VEGFA genes in both esophagus tumor and adjacent tissues [5,6], suggesting the biological significance of those 3 factors in esophagus cancer. This study therefore investigated the effect of TP53, BCL-2, and VEGFA genes on the proliferation and apoptosis of esophagus carcinoma cells, via a methyl-benzyl-nitrosamine (MBNA)-induced rat esophagus cancer model, in an attempt to investigate the potential molecular mechanism underlying cancer pathogenesis.
Material and Methods
Animal model
A cohort of 60 male Wistar rats (7~8 weeks old, body weight between 180 and 200 grams) were provided by the Animal Center of the Institute of Oncology, Shandong University. Animals were kept in a specific pathogen-free (SPF) grade facility with food and water provided ad libitum. The experimental protocol was pre-approved by the ethics committee of our hospital.
After acclimation, all animals were randomly divided into control and model groups (N=30 each). Rats in the model group received bi-weekly subcutaneous injection of 0.15% MBNA (at 3.5 mg/kg, Sigma, USA) in 0.9% saline. Controlled animals received an equal volume of 0.9% saline. General condition of animals was daily observed during the whole experiment, which lasted 30 consecutive weeks.
Morphological observation
Animals from both groups were sacrificed at week 4, 10, 20, and 30 (N=7 each). Esophagus tissues were longitudinally dissected for examination of mucosa morphology. Tissue samples were then fixed in 10% neutral buffered formalin (NBF) solution and stained by HE method. Microscopic morphology was then examined under a light-field microscope.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labeling (TUNEL) assay
We also quantified the condition of tumor cell apoptosis using a TUNEL kit (Boster Biotech, China) following the manual instruction. A total of 100 nuclei were observed from each of 5 randomly selected fields from 1 slide. The apoptosis index (AI) was calculated as: number of apoptotic cells/total cell number ×100%.
Immunohistochemical (IHC) staining
Esophagus tissues were fixed in NBF, embedded in paraffin, and sectioned. After dewaxing and rehydration, mouse anti-p53 monoclonal antibody (1:100, Boster Biotech, China), mouse anti-Bcl-2 monoclonal antibody (1:60, Boster Biotech, China), or mouse anti-VEGF monoclonal antibody (1:100, Boster Biotech, China) were added for overnight incubation. Goat anti-mouse IgG secondary antibody conjugated with horseradish peroxidase (Boster Biotech, China) was applied, followed by color development using DAB substrate.
Positive signals were deduced as yellow/brown granules in the cytoplasm (for VEGF and Bcl-2) or the nucleus (for p53). The relative staining intensity was calculated by the averaged optical density (OD) value. The staining scoring is described in Table 1.
Table 1.
Standards and scores of IHC staining.
| Intensity score | Percentage score | Staining score=(1)×(2) | |||
|---|---|---|---|---|---|
| Score | Criteria | Score | Criteria | Score | Description |
| 0 | No staining | 0 | No positive cell | 0 | Negative (−) |
| 1 | Light yellow | 1 | 0~25% positive cells | 1~3 | Weak positive (+) |
| 2 | Brown yellow | 2 | 26~50% positive cells | 4~5 | Moderate (++) |
| 3 | Dark brown | 3 | 51~75% positive cells | 6~7 | Strong (+++) |
| 4 | >75% positive cells | ||||
Statistical analysis
The SPSS 19.0 software package was used to analyze all collected data, of which measurement data with normal distribution are presented as mean ± standard deviation (SD). Between-group comparison was done by t-test or one-way analysis of variance (ANOVA). Significance of correlation between parameters was performed by Spearman correlation analysis. A statistical significance was defined when p<0.05.
Results
Morphological features of esophagus mucosa
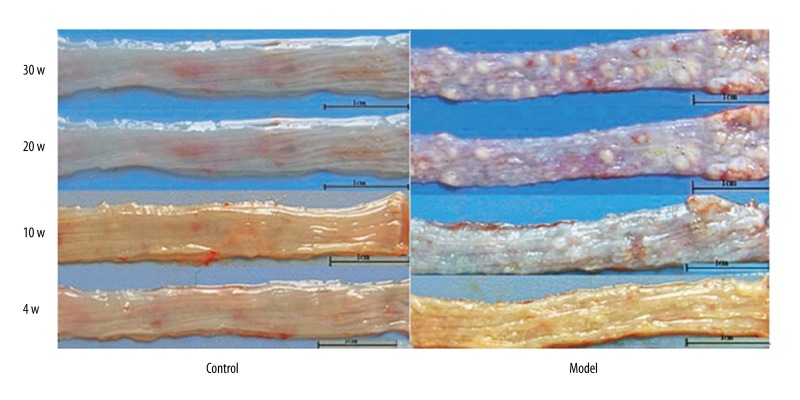
At each time point, control rats had smooth mucosa with no significant abnormality in the esophagus (Figure 1A). After 4 weeks, small granules occurred in the rough mucosa of rat esophagus in the model group (Figure 1B). Papillomas were observed from esophagus mucosa from 10 weeks, and were aggravated at 20 and 30 weeks.
Figure 1.
Morphological changes of rat esophagus. Representative images of rat esophagus from control (A) and model (B) groups. Magnification, ×10.
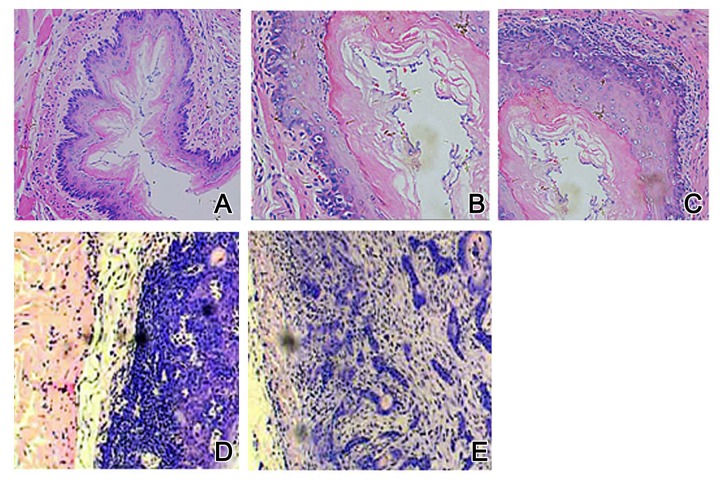
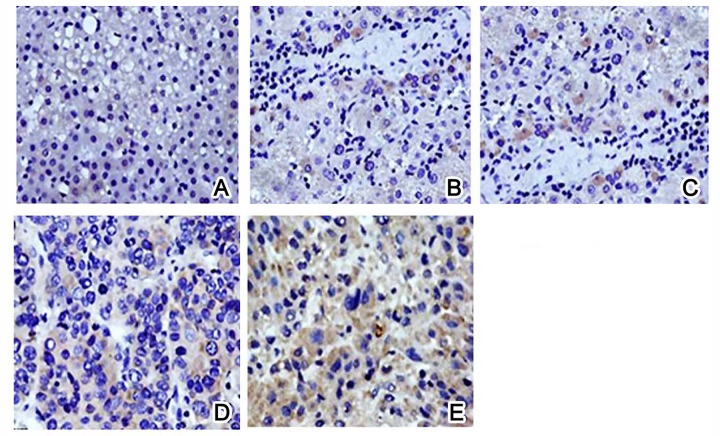
We further checked the morphological features of esophagus mucosa under the microscope. Control rats had a basal cell layer in a regular arrangement, with evenly distributed epithelial cells (Figure 2A). Model rats, however, had inflammation from 4 weeks, as shown by irregular arranged epithelial cells, hyperplasia nucleus containing granular chromatin, and unclear cell border (Figure 2B). Esophagus tissue at 10 weeks had atypical proliferation, loosely arranged cells, and enlarged nuclei containing granular chromatin (Figure 2C). At 20 weeks, atypical proliferation was aggravated, as shown by cells with discrete sizes, enlarged and darkened nuclei, and abnormal nucleus/cytoplasm ratio (Figure 2D). After 30 weeks, features of squamous carcinoma could be identified by irregular cell arrangement, abnormal cell shape, elevated chromatin, and nuclei with dark color and abnormal shape (Figure 2E).
Figure 2.
Pathological alternations of rat esophagus by HE staining. (A) Control group: (B) Model group at 4 weeks; (C) Model group at 10 weeks; (D) Model group at 20 weeks; (E) Model group at 30 weeks. Magnification, ×200.
Expression of p53, Bcl-2 and VEGF proteins
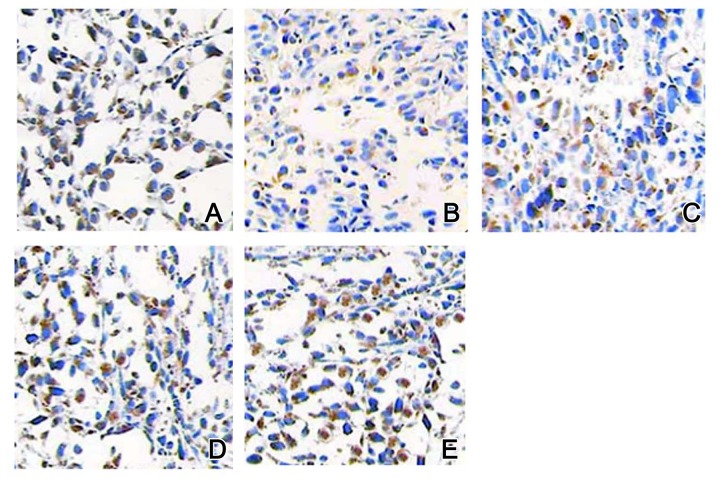
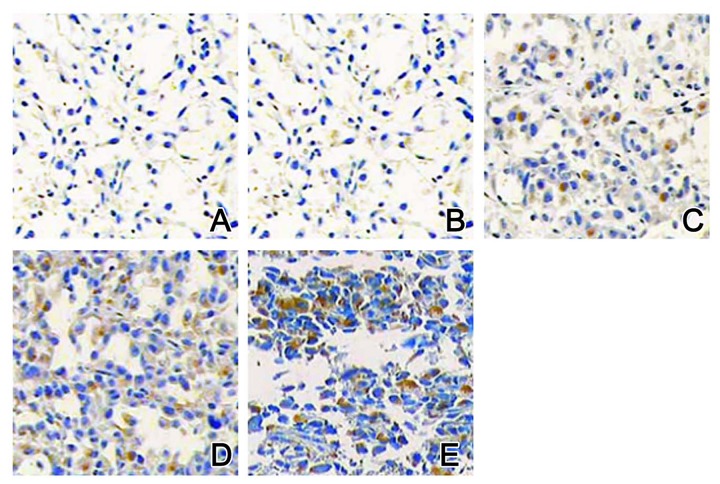
IHC staining results showed that Bcl-2 proteins were mainly identified in the cell membrane and cytoplasm, VEGF proteins existed in the cytoplasm, and p53 proteins occurred in the nucleus. In the control group, all those 3 proteins had lower expression levels (Figures 3A, 4A, and 5A). In the model group, after 4 weeks of MBNA-induction, expression levels of p53, Bcl-2, and VEGF proteins were all elevated (Figures 3B, 4B, and 5B). With the progression of the disease, expression levels of those 3 proteins were further elevated (Figures 3C–3E, 4C–4E, and 5C–5E) in a time-dependent manner.
Figure 3.
Expression of Bcl-2 proteins by IHC staining. (A) Control group: (B) Model group at 4 weeks; (C) Model group at 10 weeks; (D) Model group at 20 weeks; (E) Model group at 30 weeks. Magnification, ×200.
Figure 4.
Expression of VEGF proteins by IHC staining. (A) Control group: (B) Model group at 4 weeks; (C) Model group at 10 weeks; (D) Model group at 20 weeks; (E) Model group at 30 weeks. Magnification, ×200.
Figure 5.
Expression of p53 proteins by IHC staining. (A) Control group: (B) Model group at 4 weeks; (C) Model group at 10 weeks; (D) Model group at 20 weeks; (E) Model group at 30 weeks. Magnification, ×200.
Cell apoptosis
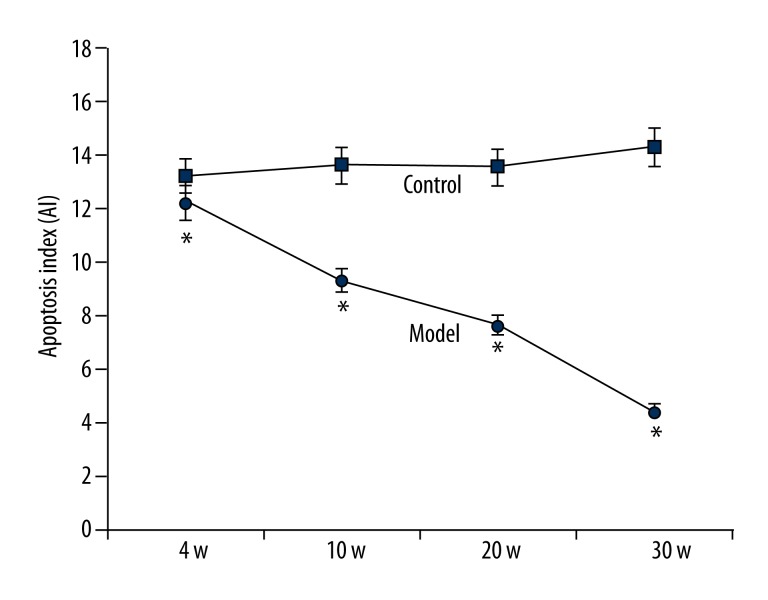
With the elongation of induction, model rats had decreased levels of esophagus carcinoma cells, as supported by the significantly suppressed AI compared to the control group (p<0.05, Figure 6).
Figure 6.
Apoptosis of esophagus carcinoma cells. The condition of cell apoptosis in both control and model rat esophagus tissues is presented as apoptosis index (AI). * p<0.05 compared to controlled animals at the same time point.
Correlation analysis
We analyzed the correlation among p53, Bcl-2, and VEGF proteins in esophagus carcinoma tissues. Results showed a significant positive correlation between p53 and Bcl-2 or VEGF proteins (r=0.753 and 0.762, p<0.05 in both cases). The correlation between Bcl-2 and VEGF proteins was not significant (r=0.109, p>0.05).
Discussion
The pathogenesis of esophagus carcinoma is closely related with the imbalance of cell apoptosis. Under normal physiological conditions, a dynamic balance exists between cell proliferation and apoptosis. The dysregulation of apoptosis, therefore, may lead to various diseases, including malignant tumors. Actually, it has been well established that the inactivation of tumor-suppressing genes and activation of oncogenes are molecular mechanisms underlying the immortality of cancer cells. Tumor-suppressor genes inhibit the formation of tumors via its participation in cell cycle-related signal transduction, cell apoptosis, and differentiation regulation. It has been shown that inhibition of cell apoptosis play an important role in the pathogenesis of esophagus carcinoma [7,8]. The most widely studied tumor-related genes in esophagus cancer are TP53 and BCL-2 genes. As an anti-apoptotic gene, the BCL-2 gene locates at chromosome 14q18 and encodes a membrane protein to prolong the cell survival period. Bcl-2 protein is an oncogene that inhibits cell death due to various causes. The over-expression of BCL-2 gene in tumor cells can cause resistance to chemotherapy. The elevated expression of BCL-2 gene in esophagus carcinoma cells may sustain the cell cycle via its inhibition on intracellular Ca2+, blocking nucleus transportation and anti-oxidation [9,10].
The wild-type TP53 gene encodes p53 protein, which can suppress tumor formation by its surveillance on cell growth and genome, along with the induction of apoptosis of cells with mutated DNA. Bcl-2 protein expression can be down-regulated by wild-type TP53 gene to facilitate cell apoptosis, accompanied with elevated expression of bax proteins [11,12]. VEGF, on the other hand, directly induces the tumor angiogenesis by the elevated permeability of micro-vessels, thereby providing a favorable environment for endothelial cell proliferation. The highly specific induction of vascular endothelial cell mitosis by VEGF directly facilitates de novo formation of tumor vessels. It has been reported that both tumor proliferation and invasion are correlated with elevated VEGF expression, which can be regulated by the TP53 gene [13,14]. Various oncology studies all suggested the correlation between TP53 and BCL-2/VEGFA expression and the existence of a possibly shared transcriptional activation pathway. This study thus investigated the role and correlation of p53, Bcl-2, and VEGF in the apoptosis of esophagus carcinoma.
Our results showed significantly lowered apoptosis level in the esophagus model group compared to control animals. With the disease progression, esophagus cell apoptosis decreased gradually, as shown by lowered AI, suggesting the potential involvement of apoptosis in esophagus cancer. Compared to those in the control group, model rats had elevated expression of Bcl-2, p53, and VEGF proteins in their esophagus tissues, in addition to the existence of inflammatory lesions and atypical hyperplasia. Expression of these proteins also increased with the progression of disease, in a time-dependent manner. All those results suggest the involvement of these proteins in the dysregulation of cell apoptosis and further tumor progression.
In esophagus carcinoma tissues, TP53 gene expression level was positively correlated with Bcl-2 or VEGF proteins, suggesting the role of p53 in the directing apoptotic pathway in the occurrence of esophagus cancer. Because elevated Bcl-2 may keep cells in the active cycle and decrease apoptotic level, it can increase tumor cell proliferation. VEGF also facilitates tumor progression by regulating angiogenesis. Our results thus suggest the synergistic effect of TP53, BCL-2, and VEGFA genes in esophagus cancer occurrence.
The possible cellular pathway among those proteins has been reported previously: Bcl-2 altered the redox status of mitochondrial and membrane potential, thus releasing apoptotic protein precursor Apaf-1, cytochrome C and activating caspase-9; p53 induced the death receptor via cascade activation of Fas, DR5 and DR4 to facilitate cell apoptosis [15,16]. p53 also regulated the endogenous apoptotic pathway as an upstream factor of the Bax/Bak pathway to mediate Bcl-2 protein expression [17,18]. As the downstream effector of p53 gene, both Bax and Bcl-2 can be selectively regulated by p53. The imbalance between Bcl-2 and Bax gene expression may favor the occurrence of esophagus cancer. Further studies supported the role of p53 in tumor angiogenesis via its regulation of VEGF expression [19,20]. Our study provided further evidence, as the expression of p53 was closely related with Bcl-2 in esophagus carcinoma tissues, suggesting potential synergistic effects.
Previous study of genomic screening in esophagus cancer patients revealed the loss of chromosome 17p, where TP53 gene localizes in cancer patients, suggesting the genetic grounds of oncogenesis [21]. Moreover, the gene polymorphism of the TP53 gene may also reside in the tumor pathogenesis as the so-called unique tumor principle, in which each individual tumor may have various tumor suppressor gene polymorphisms, in addition to certain epigenetic regulations, such as methylation patterns [22]. Therefore, the expressional regulation of TP53 gene is of crucial importance in the development of esophagus tumor. Our study provides further evidence regarding the role of TP53 in BCL-2 gene expression regulation.
Such selective modulation of TP53 on BAX and BCL-2 gene expression may be affected by environmental factors, leaving the detailed cellular pathway unclear. It is commonly believed that TP53 gene mutation and protein deposition occur early in the oncogenesis of esophagus cancer. The wild-type TP53 gene may inhibit tumor angiogenesis via its down-regulation of VEGF proteins; however, mutant p53 can facilitate the proliferation and metastasis of tumor cells. These phenomena suggest the existence of a common pathway that is regulated by both p53 and VEGF. The illustration of a synergistic effect between VEGF and p53 proteins requires further knowledge about the role of p53 in mediating tumor angiogenesis [20]. It has been postulated that p53 may regulate tumor vascular formation via the increase of protein kinase C activity and modulating VEGF expression. Wild-type TP53 gene may inhibit VEGF expression via negative feedback in its transcription, thereby inhibiting tumor vessel formation. Mutant TP53 gene, however, loses its ability to inhibit VEGF transcription, as does the inhibition of vascular formation and tumor invasiveness. Our results revealed that a time-dependent relationship between p53 and Bcl-2 or VEGF protein levels, suggesting their close relationship with tumor cell proliferation and apoptosis.
Conclusions
The abnormally elevated expression of TP53, BCL-2, and VEGFA genes in esophagus carcinoma suggest potential synergistic effects between these proteins, which may all participate in the proliferation and apoptosis of tumor cells.
Footnotes
Source of support: Departmental sources
References
- 1.Zhang J, Zhu Z, Liu Y, et al. Diagnostic value of multiple tumor markers for patients with esophageal carcinoma. PLoS One. 2015;10(2):e0116951. doi: 10.1371/journal.pone.0116951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba Y, Ishimoto T, Harada K, et al. Molecular characteristics of basaloid squamous cell carcinoma of the esophagus: Analysis of KRAS, BRAF, and PIK3CA mutations and LINE-1 methylation. Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-4445-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Ning Z, Zhu H, Li F, et al. Tumor suppression by miR-31 in esophageal carcinoma is p21-dependent. Genes Cancer. 2014;5(11–12):436–44. doi: 10.18632/genesandcancer.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lv H, Liu R, Fu J, et al. Epithelial cell-derived periostin functions as a tumor suppressor in gastric cancer through stabilizing p53 and E-cadherin proteins via the Rb/E2F1/p14ARF/Mdm2 signaling pathway. Cell Cycle. 2014;13(18):2962–74. doi: 10.4161/15384101.2014.947203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nason KS. Predicting response to neoadjuvant therapy in esophageal cancer with p53 genotyping: a fortune-teller’s crystal ball or a viable prognostic tool? J Thorac Cardiovasc Surg. 2014;148(5):2286–87. doi: 10.1016/j.jtcvs.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Chang Z, Zhang W, Chang Z, et al. Expression characteristics of FHIT, p53, BRCA2 and MLH1 in families with a history of oesophageal cancer in a region with a high incidence of oesophageal cancer. Oncol Lett. 2015;9(1):430–36. doi: 10.3892/ol.2014.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao W, Qin X, Qi B, et al. Association of p53 expression with prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7(10):7158–63. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Xia J, Wang K, Zhang J. Serum autoantibodies in the early detection of esophageal cancer: a systematic review. Tumour Biol. 2015;36(1):95–109. doi: 10.1007/s13277-014-2878-9. [DOI] [PubMed] [Google Scholar]
- 9.Shang L, Liu HJ, Hao JJ, et al. A panel of overexpressed proteins for prognosis in esophageal squamous cell carcinoma. PLoS One. 2014;9(10):e111045. doi: 10.1371/journal.pone.0111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buas MF, Levine DM, Makar KW, et al. Integrative post-genome-wide association analysis of CDKN2A and TP53 SNPs and risk of esophageal adenocarcinoma. Carcinogenesis. 2014;35(12):2740–47. doi: 10.1093/carcin/bgu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu XL, Zheng WH, Tao KY, et al. p53 is an independent prognostic factor in operable esophageal squamous cell carcinoma: a large-scale study with a long follow-up. Med Oncol. 2014;31(11):257. doi: 10.1007/s12032-014-0257-4. [DOI] [PubMed] [Google Scholar]
- 12.Appelman HD, Matejcic M, Parker MI, et al. Progression of esophageal dysplasia to cancer. Ann NY Acad Sci. 2014;1325:96–107. doi: 10.1111/nyas.12523. [DOI] [PubMed] [Google Scholar]
- 13.Huang K, Chen L, Zhang J, et al. Elevated p53 expression levels correlate with tumor progression and poor prognosis in patients exhibiting esophageal squamous cell carcinoma. Oncol Lett. 2014;8(4):1441–46. doi: 10.3892/ol.2014.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandioler D, Schoppmann SF, Zwrtek R, et al. The biomarker TP53 divides patients with neoadjuvantly treated esophageal cancer into 2 subgroups with markedly different outcomes. A p53 Research Group study. J Thorac Cardiovasc Surg. 2014;148(5):2280–86. doi: 10.1016/j.jtcvs.2014.06.079. [DOI] [PubMed] [Google Scholar]
- 15.Yu JP, Lu WB, Wang JL, et al. Pathologic response during chemo-radiotherapy and variation of serum VEGF levels could predict effects of chemo-radiotherapy in patients with esophageal cancer. Asian Pac J Cancer Prev. 2015;16(3):1111–16. doi: 10.7314/apjcp.2015.16.3.1111. [DOI] [PubMed] [Google Scholar]
- 16.Xu YW, Peng YH, Chen B, et al. Autoantibodies as potential biomarkers for the early detection of esophageal squamous cell carcinoma. Am J Gastroenterol. 2014;109(1):36–45. doi: 10.1038/ajg.2013.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z, Wang YG, Su K. VEGF-C and VEGF-D expression and its correlation with lymph node metastasis in esophageal squamous cell cancer tissue. Asian Pac J Cancer Prev. 2015;16(1):271–74. doi: 10.7314/apjcp.2015.16.1.271. [DOI] [PubMed] [Google Scholar]
- 18.Omoto I, Matsumoto M, Okumura H, et al. Expression of vascular endothelial growth factor-C and vascular endothelial growth factor receptor-3 in esophageal squamous cell carcinoma. Oncol Lett. 2014;7(4):1027–32. doi: 10.3892/ol.2014.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang PW, Hsieh MS, Huang YC, et al. Genetic variants of EGF and VEGF predict prognosis of patients with advanced esophageal squamous cell carcinoma. PLoS One. 2014;9(6):e100326. doi: 10.1371/journal.pone.0100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song HY, Deng XH, Yuan GY, et al. Expression of bcl-2 and p53 in induction of esophageal cancer cell apoptosis by ECRG2 in combination with cisplatin. Asian Pac J Cancer Prev. 2014;15(3):1397–401. doi: 10.7314/apjcp.2014.15.3.1397. [DOI] [PubMed] [Google Scholar]
- 21.Dulak AM, Schumacher SE, van Lieshout J, et al. Gastrointestinal adenocarcinomas of the esophagus, stomach and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res. 2012;72(17):4383–93. doi: 10.1158/0008-5472.CAN-11-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogino S, Fuchs CS, Giovannucci E. How many molecular subtypes? Implications of the unique tumor principle in personalized medicine. Expert Rev Mol Diagn. 2012;12(6):621–28. doi: 10.1586/erm.12.46. [DOI] [PMC free article] [PubMed] [Google Scholar]