Abstract
The budding yeast Saccharomyces cerevisiae is a valuable model system for studying prion-prion interactions as it contains multiple prion proteins. A recent study from our laboratory showed that the existence of Swi1 prion ([SWI+]) and overproduction of Swi1 can have strong impacts on the formation of 2 other extensively studied yeast prions, [PSI+] and [PIN+] ([RNQ+]) (Genetics, Vol. 197, 685–700). We showed that a single yeast cell is capable of harboring at least 3 heterologous prion elements and these prions can influence each other's appearance positively and/or negatively. We also showed that during the de novo [PSI+] formation process upon Sup35 overproduction, the aggregation patterns of a preexisting inducer ([RNQ+] or [SWI+]) can undergo significant remodeling from stably transmitted dot-shaped aggregates to aggregates that co-localize with the newly formed Sup35 aggregates that are ring/ribbon/rod- shaped. Such co-localization disappears once the newly formed [PSI+] prion stabilizes. Our finding provides strong evidence supporting the “cross-seeding” model for prion-prion interactions and confirms earlier reports that the interactions among different prions and their prion proteins mostly occur at the initiation stages of prionogenesis. Our results also highlight a complex prion interaction network in yeast. We believe that elucidating the mechanism underlying the yeast prion-prion interaction network will not only provide insight into the process of prion de novo generation and propagation in yeast but also shed light on the mechanisms that govern protein misfolding, aggregation, and amyloidogenesis in higher eukaryotes.
Keywords: [PIN+], [PSI+], [SWI+], amyloid, prion, protein aggregation, prion interactions, Saccharomyces cerevisiae, yeast
The Effect of Overexpression in Prionogenesis
It was thought that rapid synthesis of a prion protein would lead to its misfolding, aggregation, and thus a higher frequency of prion de novo formation. However, the efficiency of such an overproduction event in promoting prion conversion is not clear. Our recent finding that Swi1 aggregates formed from transient Swi1 overproduction were not inheritable suggests that Swi1 overproduction is not an effective means to induce [SWI+] de novo formation.1 We also observed that Swi1 overproduction in [pin−] cells caused Rnq1 aggregation. We failed, however, to obtain prion-like aggregates of Rnq1. In addition, we found that Rnq1 overproduction alone dramatically increases its own aggregation, however, only 3.3% of these aggregates are inheritable.1 Our findings are in agreement with a previous report that overproduction of Sup35 alone in a non-prion strain is ineffective in inducing Sup35 aggregation.2,3 Together, these results suggest that prion protein overproduction in non-prion cells is not an effective way to promote prion-prone aggregation. Even in the presence of [PIN+], most sup35 amyloids formed upon overproduction are shown to be non-inheritable.3 Thus, without a positive selection system, it would be difficult to obtain prions by simply tracing the aggregates generated upon overproduction. An earlier study suggests that overproduction caused non-inheritable Sup35 aggregates are actually SDS-resistant amyloids that cannot be shorn by chaperones thus cannot propagate.2 It is unknown if the non-inheritable Swi1 and Rnq1 aggregates are SDS-resistant amyloids or just amorphous aggregates. In either case, these non-inheritable aggregates may represent distinct conformations with poor seeding capacity compared to that of prion-prone aggregates (Fig. 1).
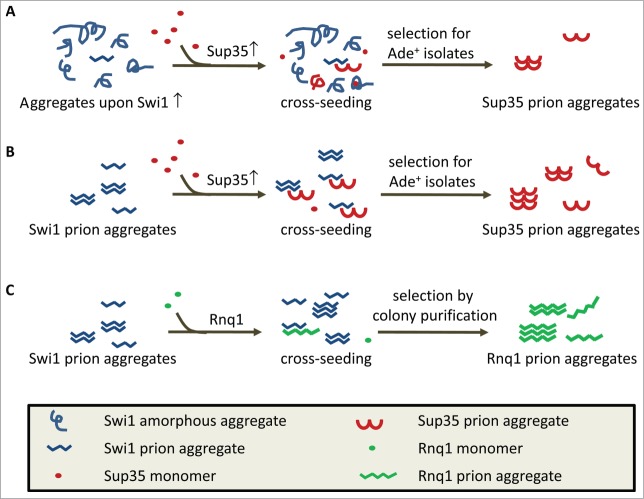
Figure 1.
Cross-seeding models to interpret [PSI+] and [PIN+] de novo formation promoted by [SWI+] and Swi1 overproduction. (A) [PSI+] inducibility by Swi1 overproduction. Swi1 aggregates formed upon overproduction are mostly non-inheritable. The small amounts of Swi1 aggregates that can be used to cross-seed de novo [PSI+] formation may explain the low efficiency of Swi1 overproduction in promoting [PSI+] de novo appearance. (B) [PSI+] inducibility by [SWI+]. Since the amyloidogenic [SWI+] aggregates can be used as an imperfect template to directly cross-seed Sup35 for [PSI+] de novo formation, [SWI+] is a better Pin+ factor than Swi1 overproduction as more templates are available for cross-seeding Sup35. (C) [PIN+] induction by [SWI+] without Rnq1 overproduction. Rnq1 has a complex prion domain with an amino acid composition more similar to that of Swi1 compared to that of Sup35. Thus, [SWI+] amyloids might have a higher cross-seeding ability to Rnq1 than to Sup35 resulting in [PIN+] formation even in the absence of Rnq1 overproduction.
While overproduction of Sup35 alone is not effective in promoting [PSI+] conversion, a series of elegant studies by the Liebman group demonstrated that [PSI+] de novo formation upon Sup35 overproduction can be dramatically promoted by a pre-existing prion [RNQ+] or [URE3], or co-overproduction of one of the several non-Sup35 Q/N-rich proteins they examined, including Ure2, Swi1, Cyc8 or New1.3,4 This [PSI+]-promoting phenotype is called Pin+, for [PSI+] inducibility. Intriguingly, although [RNQ+] is an effective Pin+ factor, Rnq1 was not on the list of the identified Q/N-proteins from a systematic genetic screen for Pin+ factors upon overexpression. We also observed that the Pin+ activity associated with Swi1 overproduction is at least 10 times lower than that of [SWI+],1 demonstrating that a pre-existing prion is a significantly stronger Pin+ factor than overproduction of its corresponding protein determinant. It is worth noting that Pin+ activities were also observed for overproduction events of Mod5, a non-Q/N rich prion protein,5 and the polyglutamine (Q) containing domain of huntingtin,6 suggesting that that neither prion-formation nor a Q/N-rich feature is essential for the Pin+ function.
Although the molecular mechanism underlying these observed Pin+ phenomena remains elusive, 2 models, “cross-seeding” and “titration,” have been proposed to explain the [PSI+] inducibility by [RNQ+] or an overproduction event of a Q/N-rich protein.4,7 In the cross-seeding model, a direct protein-protein contact is considered the basis of the observed Pin+ function (Fig. 1). A pre-existing amyloid prion or amyloid-like aggregates resulting from overproduction of a protein, such as polyQ, might serve as templates to allow de novo formation of a new prion.6 In the case of [PSI+] induction upon co-overproduction of Swi1 and Sup35, it is likely that only a very small portion of Swi1 aggregates formed under the overproduction condition are prion-prone amyloids and they are most often buried among the massive amorphous non-inheritable aggregates, therefore the [PSI+] inducibility is insufficient (Fig. 1A). In [SWI+] cells, however, the availability of templates that can be cross-seeded with Sup35 is significantly increased and thus [PSI+] is more efficiently induced (Fig. 1B). A similar explanation can be used to interpret the different Pin+ activities observed between Rnq1 overproduction alone and in the presence of [PIN+] prion. Alternatively, the titration model predicts that pre-existing prion aggregates, or newly formed protein aggregates by overproduction, may compete for binding, or perhaps sequester anti-prion cellular factors, such as chaperones and proteases, and thereby increase the likelihood of a new prion conversion.7 Indeed, it was recently shown that aggregation of several Q/N-rich Pin+ factors upon overproduction results in chaperones being sequestered from Sup35 aggregates and in some cases alters the chaperone levels in a [PSI+] strain.8 Similar chaperone sequestration events may also occur when Pin+ factors, such as Q/N-rich proteins, are overproduced in a [psi−] strain thereby enhancing the susceptibility of yeast to prion formation. It is also possible that the low Pin+ activity and poor inheritability of Swi1 or Rnq1 aggregates formed upon overproduction may be partly attributable to the toxic effects that are associated with overproduction, which has been broadly noted to be stressful to yeast. Taken together, our results suggest that the cross-seeding and titration models are not mutually exclusive. Overproduction of a prion protein may increase the amount of misfolded prion protein(s) but may not effectively promote prion formation. The availability of amyloid-like templates capable of cross-seeding is also essential for efficient prion de novo formation.
Interactions of [SWI+], [PIN+], and [PSI+]
Early studies showed that [PSI+] and [PIN+] can promote each other's de novo appearance.3,4 Besides [PIN+], other prions such as [URE3] and [NU+] can also promote the de novo appearance of [PSI+].4,7 Subsequent studies showed that prion-prion interactions can be also mutually antagonistic, suggesting that there is a complicated interaction network among heterologous prions.9-11 The discovery of [SWI+] has provided us with an additional system to investigate interactions among heterologous prions. There are several unique properties of [SWI+] that are different from those of [PSI+] or [PIN+]. First, Swi1 is a nuclear protein involved in chromatin remodeling. Second, a Swi1 region less than 40 amino acid residues that is free of glutamine but rich in asparagine is enough to maintain and propagate [SWI+].12,13 Third, the maintenance of [SWI+] requires a more delicate molecular chaperone network than that of [PSI+] and [PIN+].14,15 Thus, it is of interest to investigate the effect of [SWI+] on the de novo formation of [PSI+] and [PIN+]. In our recent report in Genetics,1 we showed that [SWI+] could facilitate [PSI+] and [PIN+] conversion, but weaken the Pin+ function of [PIN+] when [SWI+] and [PIN+] co-exist. We were also able to confirm an earlier observation that [PSI+] and [PIN+] mutually promote each other's de novo appearance.
There are at least 8 amyloid prions that have been discovered in budding yeast.31 The discovery of [PIN+] showed for the first time that one yeast cell could harbor 2 different prions ([PSI+] and [PIN+]).3,16 We showed that a single yeast cell can harbor at least 3 different prions: [SWI+], [PSI+], and [PIN+] simultaneously.1 Although [SWI+] could be stably maintained in more than 95% of cells that contain [PSI+] or [PIN+] in our tested conditions, we showed that [SWI+] became significantly unstable in cells containing all 3 prions, while the transmission of [PSI+] or [PIN+] was not affected. While more systematic studies are needed to address the interesting question of how many heterologous prion species one yeast cell can harbor concurrently, our results seem to suggest that it would be difficult for one yeast cell to harbor more than 3 heterologous prions. In our study, the unstable propagation of [SWI+] is probably not due to direct interactions among the 3 prions because no co-localization of these prion aggregates was observed in cells carrying the 3 prions. Instead, toxic effects and stability of individual prion species might have determined the prion-harboring capacity of a cell and the compatibility of the co-existing prion species. Co-existence of 3 prion species or more may cause an unbearable stress to the cell, leading to cellular toxicity. This cellular stress will likely modulate the steady-state level of molecular chaperones resulting in the collapse of proteostasis.
We showed that [SWI+] has a significantly weaker Pin+ function than that of [PIN+]. Interestingly, although overproduction of Sup35 or its prion domain is required for a detectable [PSI+] de novo formation in [PIN+] or [SWI+] cells, [SWI+] can promote a significant amount of [PIN+] appearance without Rnq1 overproduction. These results suggest that [SWI+] is a stronger inducer of [PIN+] than that of [PSI+]. As shown is Figure 1C, this difference might be explained by the fact that the asparagine-rich PrD of Swi1 has a higher homology to that of the Rnq1 PrD than that of the Sup35 PrD.12,13,17 The amino acid compositional differences of these PrDs may result in differences in their amyloid core structures, which in turn determine their cross-seeding abilities on different prion conformations. Furthermore, the Rnq1 PrD has a complex sequencing feature including 4 distinct and semi-independent aggregation determinants that may provide more opportunities for cross-seeding,18 which may explain why [PIN+] is a stronger Pin+ factor than [SWI+]. The observed antagonistic effect of [SWI+] on the Pin+ function of [PIN+] might be a result of competition between the 2 [PSI+] facilitators, [SWI+] and [PIN+], for free Sup35. The binding of Sup35 by [SWI+] aggregates may have reduced the amount of free Sup35 molecules to be cross-seeded by [PIN+], a stronger Pin+ factor, thereby reducing the [PSI+] initiation efficiency of [PIN+].
Prion Aggregate Remodeling During the Prionogenesis of Another Prion—a Timed Interaction to Support the Cross-seeding Model
Earlier studies showed that overproduction of Sup35PrD-GFP in [PIN+] cells can result in formation of both dot-shaped and ring/ribbon/rod-like aggregates, and only the ring/ribbon/rod-like aggregates are prone to establish stable [PSI+].4,19-22 Interestingly, the Sup35 aggregates in mature [PSI+] cells are also dot-shaped.1,4,19 While it is unclear how the ring/ribbon/rod-shaped Sup35 aggregates are processed to the final dot-shaped prion aggregates, it is reasonable to speculate that the newly formed non-inheritable Sup35 dots formed upon overproduction are structurally distinct from the dot-shaped aggregates in the mature [PSI+] cells. Similarly, we found that Swi1 or Rnq1 aggregates formed upon overproduction are mostly dot-like and non-inheritable, distinct from the prion-prone ring/ribbon/rod-like aggregates.1 The fact that both the ring/ribbon/rod-shaped and mature dot-like Sup35 foci in [PSI+] cells have similar bundled-fibrillar structure22,23 may explain why the ring/ribbon/rod-like aggregates of Sup35NMGFP can be further processed to mature stable [PSI+]. Interestingly, forming ring/ribbon/rod-like aggregates seems to be a shared feature by several Q/N-rich candidate proteins besides Sup35 and Rnq1.24
Our results indicate that the transition from ring/ribbon/rod-like aggregation to dot-shaped stable prion foci requires many generations.1 In the [PSI+] de novo formation process, co-localization of a pre-existing Pin+ factor and the newly generated ring/ribbon/rod-like Sup35 aggregates upon Sup35 overproduction has been only observed at the early initiation stage of [PSI+] prionogenesis.1,6,25 Our finding that the prion aggregates of both Rnq1 and Swi1 in [PIN+] and [SWI+] cells formed a beads-on-string organization with the newly formed Sup35 ring/ribbon/rod-shaped aggregates demonstrates that these preexisting prion aggregates (beads) are physically associated with the newly formed prionogenic Sup35 aggregates (string) (see Fig 2, middle panel), providing direct evidence supporting the cross-seeding model for prionogenesis. In addition, we observed that the colocalization frequency of the Pin+ factor and newly formed Sup35 aggregation is positively correlated with the prion promoting activity of the Pin+ factor,1 implicating that a direct contact of the Pin+ factor and SUp35 is essential in [PSI+] induction. It is worth emphasizing that direct associations between 2 heterologous prions are rarely seen when they co-exist as mature prions. When a newly formed prion is stabilized, the interaction between the newly formed prion and its facilitator is rarely detectable.1 These observations are consistent with an earlier report that [PIN+] is only required for [PSI+] de novo formation but not for its propagation.4,26 Once [PSI+] is established, it can stably propagate in the absence of [PIN+].
Figure 2.
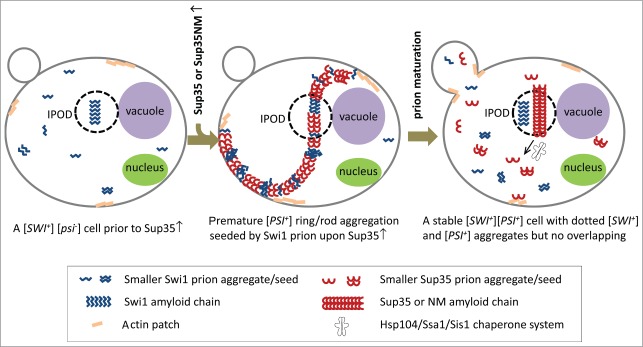
Morphological remodeling of [SWI+] prion aggregation during the initiation and maturation processes of [PSI+]. Prior to Sup35 overproduction, [SWI+] exists as multiple dot-like aggregates in [SWI+][psi−] cells (left). When Sup35 (or its prion domain) is overproduced, Sup35 ring/ribbon/rod-shaped aggregates are formed, presumably through cross-seeding with the preexisting Swi1 prion amyloids at IPOD or multiple cellular sites. At the same time, [SWI+] prion aggregates undergo significant morphological remodeling from multiple distinct dots to be ring/ribbon/rod-shaped. The remodeled [SWI+] prion aggregation is drastically co-localized with the newly formed Sup35 ring/ribbon/rod-shaped aggregates to form a beads-on-string organization (middle), supporting the cross-seeding model. During the maturation process, Sup35 ring/ribbon/rod-shaped structures are processed into dotted mature prion aggregates likely through the action of chaperones such as Hsp104, Ssa1, and Sis1. These dotted aggregates can serve as seeds for prion transmission during cell division. In mature [PSI+] cells, the aggregates of [SWI+] and [PSI+] are mainly dot-shaped and do not interact with the possible exception in the IPOD (right).
It has been proposed that when Sup35 is overproduced in [PIN+] cells, Sup35 ring/ribbon/rods are produced in and elongated from an ancient protein quality control compartment, IPOD (insoluble protein deposit), which is adjacent to the vacuole.22 IPOD may serve as a reservoir that retains multiple heterologous amyloid species, including prion aggregates,22 therefore the occasionally observed overlapping between mature heterologous prion aggregates may occur in IPOD. It seems that IPOD could serve as an ideal site for de novo heterologous prion cross-seeding, filamentous growth, and elongation of prion chain.22 Though the newly formed Sup35 ring/ribbon/rod-shaped aggregation is found interacting with IPOD, whether prion de novo formation initiates in IPOD needs to be further investigated.22 Our observation that in [SWI+] or [PIN+] cells, multiple Swi1 or Rnq1 aggregates were overlapping with the newly formed ring/ribbon/rod structures of Sup35 to form the beads-on-string-like organization beyond IPOD argues that cross-seeding might happen at multiple cellular sites. IPOD may be just one of the possible sites for cross-seeding and prionogenesis. The newly formed Sup35 rings are located in the cell periphery, and actin cytoskeleton is believed to be critical in this earlier ring-processing event, perhaps by serving as a platform for prion initiation.20,27,28 Figure 2 illustrates a likely scenario where prion-prion interactions might occur in the prion initiation and maturation processes.
In the prion maturation process, smaller dot-like aggregates can be derived from the ring/ribbon/rod-shaped prion aggregates. This remodeling occurs through processing of large amyloid aggregates into smaller aggregates by the action of a group of chaperones, including Hsp104, Ssa1, and Sis1. Some of these smaller aggregates serve as seeds for prion transmission as they are believed to be distributed to daughter cells when cells divide. Intriguingly, the ring-like aggregates are frequently observed for [PSI+] but rarely seen for [PIN+] at the initiation stage of prion de novo formation. The [PIN+] prion aggregates appear mostly rod-like, not ring-like, suggesting a structural difference between the 2 premature prion aggregates. It may also suggest that the elongated Sup35 and Rnq1 pre-prion aggregates have distinct binding affinities to actin patches and/or other cytoskeleton components.
Once again, our data, together with other accumulating evidence,1,6,25 support the cross-seeding model in terms of mutually promoting prion-prion interactions in yeast. For example, Rnq1 can be immunocaptured with Sup35 during the de novo induction of [PSI+] in [PIN+] cells, indicating a direct physical association of Sup35 and Rnq1.29 The fact that preformed Rnq1 amyloid fibrils could be used as templates to cross-seed soluble Sup35NM in vitro also supports the cross-seeding model.6 However, our results do not exclude other possible mechanisms, such as those proposed in the titration model.
Closing Remarks
The prion concept has been recently extended beyond the territory of proteinaceous pathogen of PrPSc and protein conformation based fungal epigenetic elements. There are ample data suggesting that many amyloidogenic proteins can be transmitted in a way similar to that of PrPSc and are now considered as “prions.” They can be either functional protein aggregates or disease-associated pathogenesis,30,31 including β-amyloid, α-synuclein, tau, and mutant SOD1.32 Aggregation of one amyloidogenic protein might trigger a complex interaction among multiple aggregation-prone proteins. As a consequence, formation of one prion can lead to modulation of one or more biological pathways to result in either beneficial phenotypes or pathogenic disorders. Studying prion-prion interactions in yeast might provide valuable information to aid our understanding of not only the prion phenomena in yeast but also the mechanisms underlying protein folding, aggregation, and prionogenesis in mammals.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The related work was supported by grants from the US. National Institutes of Health (R01GM110045) and US National Science Foundation (MCB 1122135) to LL.
References
- 1. Du Z, Li L. Investigating the interactions of yeast prions: [SWI+], [PSI+], and [PIN+]. Genetics 2014; 197:685-700; PMID:24727082; http://dx.doi.org/ 10.1534/genetics.114.163402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salnikova AB, Kryndushkin DS, Smirnov VN, Kushnirov VV, Ter-Avanesyan MD. Nonsense suppression in yeast cells overproducing Sup35 (eRF3) is caused by its non-heritable amyloids. J Biol Chem 2005; 280:8808-12; PMID:15618222; http://dx.doi.org/ 10.1074/jbc.M410150200 [DOI] [PubMed] [Google Scholar]
- 3. Derkatch IL, Bradley ME, Zhou P, Chernoff YO, Liebman SW. Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics 1997; 147:507-19; PMID:9335589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Derkatch IL, Bradley ME, Hong JY, Liebman SW. Prions affect the appearance of other prions: the story of [PIN+]. Cell 2001; 106:171-82; PMID:11511345; http://dx.doi.org/ 10.1016/S0092-8674(01)00427-5 [DOI] [PubMed] [Google Scholar]
- 5. Suzuki G, Shimazu N, Tanaka M. A yeast prion, Mod5, promotes acquired drug resistance and cell survival under environmental stress. Science 2012; 336:355-9; PMID:22517861; http://dx.doi.org/ 10.1126/science.1219491 [DOI] [PubMed] [Google Scholar]
- 6. Derkatch IL, Uptain SM, Outeiro TF, Krishnan R, Lindquist SL, Liebman SW. Effects of Q/N-rich, polyQ, and non-polyQ amyloids on the de novo formation of the [PSI+] prion in yeast and aggregation of Sup35 in vitro. Proc Natl Acad Sci U S A 2004; 101:12934-9; PMID:15326312; http://dx.doi.org/ 10.1073/pnas.0404968101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osherovich LZ, Weissman JS. Multiple Gln/Asn-rich prion domains confer susceptibility to induction of the yeast [PSI+] prion. Cell 2001; 106:183-94; PMID:11511346; http://dx.doi.org/ 10.1016/S0092-8674(01)00440-8 [DOI] [PubMed] [Google Scholar]
- 8. Derkatch IL, Liebman SW. The story of stolen chaperones: how overexpression of Q/N proteins cures yeast prions. Prion 2013; 7:294-300; PMID:23924684; http://dx.doi.org/ 10.4161/pri.26021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradley ME, Edskes HK, Hong JY, Wickner RB, Liebman SW Interactions among prions and prion "strains" in yeast. Proc Natl Acad Sci U S A 2002; 99 Suppl 4:16392-9; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwimmer C, Masison DC. Antagonistic interactions between yeast [PSI+] and [URE3] prions and curing of [URE3] by Hsp70 protein chaperone Ssa1p but not by Ssa2p. Mol Cell Biol 2002; 22:3590-8; PMID:11997496; http://dx.doi.org/ 10.1128/MCB.22.11.3590-3598.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bradley ME, Liebman SW. Destabilizing interactions among [PSI+] and [PIN+] yeast prion variants. Genetics 2003; 165:1675-85; PMID:14704158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du Z, Crow ET, Kang HS, Li L. Distinct subregions of Swi1 manifest striking differences in prion transmission and SWI/SNF function. Mol Cell Biol 2010; 30:4644-55; PMID:20679490; http://dx.doi.org/ 10.1128/MCB.00225-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crow ET, Du Z, Li L. A small, glutamine-free domain propagates the [SWI+] prion in budding yeast. Mol Cell Biol 2011; 31:3436-44; PMID:21670156; http://dx.doi.org/ 10.1128/MCB.05338-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Du Z, Park KW, Yu H, Fan Q, Li L. Newly identified prion linked to the chromatin-remodeling factor Swi1 in Saccharomyces cerevisiae. Nat Genet 2008; 40:460-5; PMID:18362884; http://dx.doi.org/ 10.1038/ng.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hines JK, Li X, Du Z, Higurashi T, Li L, Craig EA. [SWI+], the prion formed by the chromatin remodeling factor Swi1, is highly sensitive to alterations in Hsp70 chaperone system activity. PLoS genetics 2011; 7:e1001309; PMID:21379326; http://dx.doi.org/ 10.1371/journal.pgen.1001309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG, Liebman SW. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics 1996; 144:1375-86; PMID:8978027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Du Z. The complexity and implications of yeast prion domains. Prion 2011; 5:311-6; PMID:22156731; http://dx.doi.org/ 10.4161/pri.18304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kadnar ML, Articov G, Derkatch IL. Distinct type of transmission barrier revealed by study of multiple prion determinants of Rnq1. PLoS genetics 2010; 6:e1000824; PMID:20107602; http://dx.doi.org/ 10.1371/journal.pgen.1000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou P, Derkatch IL, Liebman SW. The relationship between visible intracellular aggregates that appear after overexpression of Sup35 and the yeast prion-like elements [PSI+] and [PIN+]. Mol Microbiol 2001; 39:37-46; PMID:11123686; http://dx.doi.org/ 10.1046/j.1365-2958.2001.02224.x [DOI] [PubMed] [Google Scholar]
- 20. Ganusova EE, Ozolins LN, Bhagat S, Newnam GP, Wegrzyn RD, Sherman MY, Chernoff YO. Modulation of prion formation, aggregation, and toxicity by the actin cytoskeleton in yeast. Mol Cell Biol 2006; 26:617-29; PMID:16382152; http://dx.doi.org/ 10.1128/MCB.26.2.617-629.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathur V, Taneja V, Sun Y, Liebman SW. Analyzing the birth and propagation of two distinct prions, [PSI+] and [Het-s](y), in yeast. Mol Biol Cell 2010; 21:1449-61; PMID:20219972; http://dx.doi.org/ 10.1091/mbc.E09-11-0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tyedmers J, Treusch S, Dong J, McCaffery JM, Bevis B, Lindquist S. Prion induction involves an ancient system for the sequestration of aggregated proteins and heritable changes in prion fragmentation. Proc Natl Acad Sci U S A 2010; 107:8633-8; PMID:20421488; http://dx.doi.org/ 10.1073/pnas.1003895107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Basso E, Antas P, Marijanovic Z, Goncalves S, Tenreiro S, Outeiro TF. PLK2 Modulates α-Synuclein Aggregation in Yeast and Mammalian Cells. Mol Neurobiol 2013; PMID:23677647 [DOI] [PubMed] [Google Scholar]
- 24. Alberti S, Halfmann R, King O, Kapila A, Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 2009; 137:146-58; PMID:19345193; http://dx.doi.org/ 10.1016/j.cell.2009.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bagriantsev S, Liebman SW. Specificity of prion assembly in vivo. [PSI+] and [PIN+] form separate structures in yeast. J Biol Chem 2004; 279:51042-8; PMID:15465809 [DOI] [PubMed] [Google Scholar]
- 26. Derkatch IL, Bradley ME, Masse SV, Zadorsky SP, Polozkov GV, Inge-Vechtomov SG, Liebman SW. Dependence and independence of [PSI+] and [PIN+]: a two-prion system in yeast? EMBO J 2000; 19:1942-52; PMID:10790361; http://dx.doi.org/ 10.1093/emboj/19.9.1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manogaran AL, Hong JY, Hufana J, Tyedmers J, Lindquist S, Liebman SW. Prion formation and polyglutamine aggregation are controlled by two classes of genes. PLoS genetics 2011; 7:e1001386; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chernova TA, Romanyuk AV, Karpova TS, Shanks JR, Ali M, Moffatt N, Howie RL, O'Dell A, McNally JG, Liebman SW, et al. . Prion induction by the short-lived, stress-induced protein Lsb2 is regulated by ubiquitination and association with the actin cytoskeleton. Mol Cell 2011; 43:242-52; PMID:21777813; http://dx.doi.org/ 10.1016/j.molcel.2011.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharma J, Liebman SW. Exploring the basis of [PIN+] variant differences in [PSI+] induction. J Mol Biol 2013; 425:3046-59; PMID:23770111; http://dx.doi.org/ 10.1016/j.jmb.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crow ET, Li L. Newly identified prions in budding yeast, and their possible functions. Semin Cell Dev Biol 2011; 22:452-9; PMID:21397710; http://dx.doi.org/ 10.1016/j.semcdb.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cascarina SM, Ross ED. Yeast prions and human prion-like proteins: sequence features and prediction methods. Cellular and molecular life sciences : CMLS 2014; 71:2047-63; PMID:24390581; http://dx.doi.org/ 10.1007/s00018-013-1543-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prusiner SB. Biology and genetics of prions causing neurodegeneration. Annu Rev Genet 2013; 47:601-23; PMID:24274755; http://dx.doi.org/ 10.1146/annurev-genet-110711-155524 [DOI] [PMC free article] [PubMed] [Google Scholar]