Abstract
Signaling via the Rho GTPases provides crucial regulation of numerous cell polarization events, including apicobasal (AB) polarity, polarized cell migration, polarized cell division and neuronal polarity. Here we review the relationships between the Rho family GTPases and epithelial AB polarization events, focusing on the 3 best-characterized members: Rho, Rac and Cdc42. We discuss a multitude of processes that are important for AB polarization, including lumen formation, apical membrane specification, cell-cell junction assembly and maintenance, as well as tissue polarity. Our discussions aim to highlight the immensely complex regulatory mechanisms that encompass Rho GTPase signaling during AB polarization. More specifically, in this review we discuss several emerging common themes, that include: 1) the need for Rho GTPase activities to be carefully balanced in both a spatial and temporal manner through a multitude of mechanisms; 2) the existence of signaling feedback loops and crosstalk to create robust cellular responses; and 3) the frequent multifunctionality that exists among AB polarity regulators. Regarding this latter theme, we provide further discussion of the potential plasticity of the cell polarity machinery and as a result the possible implications for human disease.
Keywords: apicobasal, Cdc42, epithelia, GTPases, junction, par, polarity, Rac, Rho
Abbreviations
- AB
Apicobasal
- α-cat
Alpha-catenin
- AJ
Adherens junction
- Amot
Angiomotin
- aPKC
Atypical Protein Kinase C
- Arp2/3
Actin-related protein-2/3
- Baz
Bazooka
- β-cat
Beta-Catenin
- β2-syn
Beta-2-syntrophin
- Caco2
Human colon carcinoma
- C. elegans
Caenorhabditis elegans
- CA
Constitutively-active
- CD2AP
CD2-associated protein
- Dia1
Diaphanous-related formin 1
- DN
Dominant-negative
- Drosophila
Drosophila melanogaster
- Dys-β
Dystrobrevin-β
- Cora
Coracle
- Crb
Crumbs
- Dlg
Discs large
- ECM
Extracellular matrix
- Ect2
Epithelial cell transforming sequence 2 oncogene
- Eya1
Eyes absent 1
- F-actin
Filamentous actin
- FRET
Fluorescence resonance energy transfer
- GAP
GTPase-activating protein
- GDI
Guanine nucleotide dissociation inhibitor
- GEF
Guanine nucleotide exchange factor
- JACOP
Junction-associated coiled-coiled protein
- JAM
Junctional adhesion molecule
- Lgl
Lethal giant larvae
- LKB1
Liver kinase B1
- MDCK
Madin-Darby canine kidney
- MTOC
Microtubule-organizing center
- NrxIV
Neurexin IV
- Pals1
Protein associated with Lin-7 1
- Par
Partitioning-defective
- Patj
Pals1-associated TJ protein
- Rich1
RhoGAP interacting with CIP4 homologues
- ROCK
Rho-associated kinase
- S. cerevisiae
Saccharomyces cerevisiae
- Scrib
Scribble
- SH3BP1
SH3-domain binding protein 1
- S. pombe
Schizosaccharomyces pombe
- Std
Stardust
- Tiam1
T-cell lymphoma invasion and metastasis-inducing protein 1
- TEM4
Tumor endothelial marker 4
- TJ
Tight junction
- WASp
Wiskott-aldrich syndrome protein
- Yrt
Yurt
- ZA
zonula adherens
- ZO
Zonula occludens
Introduction
Cell polarity refers to the presence of asymmetry within a cell that can be generated by spatial differences in a variety of subcellular components. Cells must acquire particular types of polarity in order to perform specialized cell functions. The Rho family of GTPases are evolutionarily conserved regulators of numerous types of cell polarity. They act as molecular switches through their ability to cycle between active (GTP-bound) and inactive (GDP-bound) states.1 Their activation status is mediated by association with one of their many guanine nucleotide exchange factors (GEFs) or GTPase activating proteins (GAPs) that promote activation or inactivation, respectively. Additionally, association with guanine dissociation inhibitors (RhoGDIs) can maintain their inactive state,2 and various post-translational modifications can also control their activities.3,4 Furthermore, the importance of spatiotemporal regulation of their activities is becoming increasingly apparent, which often appears to be achieved through a complex regulation of both GEFs and GAPs. This review discusses the role of the Rho family GTPases in epithelial apicobasal polarity, focusing upon the 3 best-characterized members, Rho, Rac and Cdc42.
Apicobasal (AB) polarity is the generation of asymmetry along the apical-basal cell axis and is a key feature of epithelial cells. Epithelial cells arrange into multi- or mono-layered sheets in vivo and the formation and continued integrity of these sheets relies on cell-cell adhesion and the establishment and maintenance of AB polarity. This type of polarity occurs upon the generation of 2 distinct membrane domains: the apical membrane faces either the external environment or an organ lumen, while the basolateral membrane contacts neighboring cells or the underlying extracellular matrix (ECM). The acquisition of AB polarity is essential for normal epithelial cell shape, proliferation, organization and function, as well as for the maintenance of overall tissue architecture. Consequently, AB polarity defects have been associated with various types of human disease, particularly cancer. Loss of AB polarity is frequently observed in human tumors and is found to be associated with disease progression.5
The process of establishing AB polarity is highly interconnected with that of numerous cellular processes, including the establishment of intercellular adhesion protein complexes, the regulation of the actomyosin network, vesicle trafficking, and even spindle orientation during cell division. Consequently, to date, a wide array of signaling pathways have been implicated in the regulation of AB polarity and Rho, Rac and Cdc42, are all commonplace. Hereafter, we discuss the intimate relationship between the Rho GTPases and AB polarity, highlighting the interdependent nature of their regulation and drawing upon evidence from a variety of systems and experimental approaches.
Establishing Apicobasal Polarity
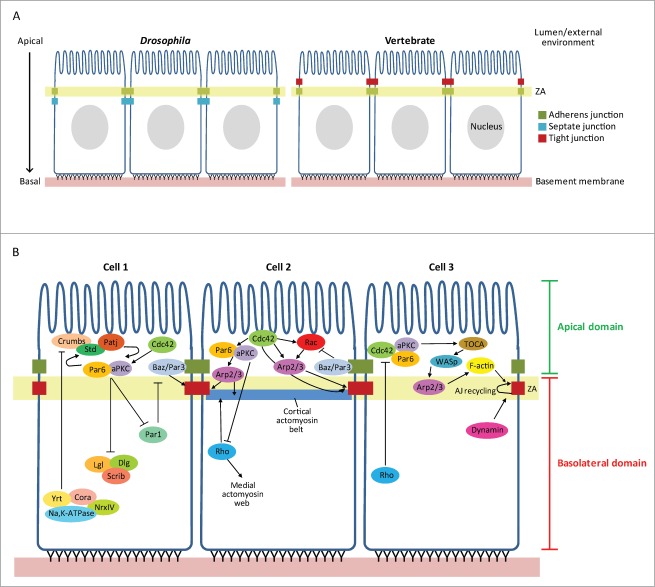
The establishment of epithelia with correct AB polarity requires the generation of distinct apical and basolateral membrane domains (Fig. 1B). These domains are designated by the correct localization of particular polarity proteins and by the formation of cell-cell interactions, which mature into specialized cell-cell junctions (Fig 1A). Cell-cell interactions are particularly important for polarity establishment. It has been shown that cell-cell contact can trigger the correct segregation of apical and basal proteins to their corresponding domains in MDCK (Madin-Darby canine kidney) cells.6
Figure 1.
Schematic representations of epithelial apicobasal polarity. (A) Epithelial junction organization in Drosophila and vertebrate cells. (B) Epithelial apicobasal polarity is governed by numerous signaling pathways: Cell 1: conserved protein complexes are required to establish and maintain apicobasal polarity within the cell. Apical and basolateral polarity proteins act antagonistically to one another around the adherens junction (AJ), thereby forming distinct apical and basolateral domains within the cell; Cell 2: the cytoskeleton is also polarized and is regulated by several polarity proteins and Rho GTPases. This spatial regulation of the cytoskeleton is required to maintain cell shape and cell-cell junctions, and is therefore essential for epithelial integrity; Cell 3: Cdc42-Par6-aPKC is required to maintain AJ integrity by promoting the dynamin-mediated endocytosis of junction material, via TOCA proteins and Arp2/3. This allows AJ recycling, thereby promoting junction plasticity.
It has long been established in a wide variety of systems that AB polarity establishment relies on the mutual exclusion of proteins that define the apical and basolateral domains of a cell (Fig. 1B, Cell 1).7 The apical Par proteins: Bazooka (Baz)/Par3, atypical Protein Kinase C (aPKC)/PKCζ, Par6 (Drosophila/vertebrate; note that / distinguishes Drosophila from vertebrate orthologues hereafter); and the Crumbs complex: Crumbs, Stardust/Pals1, and Discs Lost/Patj, play a role in defining the apical domain. On the other hand, the Scribble complex (lgl, dlg, and scrib),8 and the Yurt (Yrt)/Coracle (Cora) group: Yrt/EBP41L5, Cora/EPB41, Na(+),K(+)-ATPase, Neurexin IV (NrxIV),9,10 together with Par1,11 establish the basolateral domain (Fig. 1B, Cell 1). Interactions between these functional modules generate zones of mutual exclusion around epithelial junctions: tight junctions (TJs) in vertebrates, adherens junctions (AJs) in invertebrates, to generate an AB asymmetry (Fig. 1A and B, Cell 1). This complex process requires many concurrent events that are controlled in a spatiotemporal manner. Rho, Rac and Cdc42 have all been implicated in various stages of AB polarity generation, with substantial evidence coming from both Drosophila and mammalian cell culture studies, as discussed below.
Lumen Formation
When cultured in a 3-dimensional matrix, epithelial cells form spherical cyst-like structures, comprising of a single-layer epithelium surrounding a single central lumen, with their apical domains facing the lumen and their basal domains on the outer surface. This in vitro assay effectively recapitulates the organization of epithelial tissues found within the human body. Disruption of AB polarity perturbs this organization, resulting in lumen defects, often manifested as multiple-lumen or no-lumen cysts. Consequently, this assay has been used to identify many regulators of AB polarity, including the Rho GTPases. Here we discuss the various mechanisms by which Rho, Rac and Cdc42 regulate the establishment of AB polarization, drawing upon evidence from lumen formation assays.
Signaling through Rac is important for directing where the apical domain develops, since expression of dominant-negative (DN)-Rac causes a striking inversion of apical polarity in MDCK cell cysts.12 Rac is thought to achieve proper apical polarity by signaling downstream of β1-integrin to promote surface laminin assembly,12-14 and also by antagonising Rho-dependent actomyosin contractility.15
Interestingly, during AB polarization, Rac activity becomes differentially regulated along the apical-basal axis, a step that is required for proper polarization.16,17 Using a Rac-FRET biosensor to directly visualize Rac activity in live polarizing MDCK cells, Mack et al. demonstrated higher Rac activity at adherens junctions (AJs) and lower activity more apically at tight junctions (TJs).16 Low Rac activity at TJs was expected since Chen and Macara had previously reported Par3-mediated inhibition of Tiam1-Rac activity and shown this to be important for TJ assembly.18 However, Mack et al. also identified β2-syntrophin as an important activator of the Rac-GEF Tiam1 at AJs and showed that this Tiam1 activator (like Par3)19 was required for correct AB polarization, since β2-syntrophin knockdown or the mistargeting of constitutively-active (CA)-Rac to TJs, resulted in cysts with multiple lumens. Consistent with this, Yagi et al. observed lower Rac activity at the apical membrane compared with the lateral, and found that increased apical Rac activity produced cysts with cells within the luminal space.17 Additionally, they reported that Chimaerin, a GAP for Rac, may be reducing Rac activity apically.20 This differential regulation of Rac activity has also been observed in other systems. In flies, Baz/Par3 was found to inhibit Rac activity apically, via the inhibition of SIF/Tiam1,21 consistent with the results from Chen and Macara using mammalian cells (Fig. 1B, Cell 2).18 Gon et al. also reported a similar differential regulation of Rac activity in intestinal epithelial cells, and intriguingly also found that Rho activity is differentially regulated, but in the opposite direction to Rac.22 Additionally they suggested that Wnt5a signaling promotes polarization of Rac and Rho through Tiam1 and p190RhoGAP, respectively. Together these studies demonstrate the importance of spatiotemporal regulation of Rho GTPase signaling for correct AB polarization.
Cdc42 function is also critical for the polarization of MDCK cells grown in 3D. The following molecular mechanism has been elucidated, whereby PTEN-mediated generation of apically localized PIP2 recruits annexin2, which subsequently binds Cdc42 that then recruits aPKC and Par6.23 All these components were required to establish the apical membrane and for normal lumen formation. More recently, Cdc42-mediated generation of the apical membrane and a single lumen was shown to require cooperation with membrane trafficking machineries. A Rab11a-directed network was delineated, involving Cdc42, its GEF Tuba, annexin2 and the Par complex, which ultimately directs Cdc42-dependent exocytosis and leads to the delivery of apical markers to the plasma membrane, generating the apical domain.24
Cdc42 also regulates lumen formation through its control of mitotic spindle orientation, which must be tightly controlled so that cells remain in the plane of the epithelium following cell division, thereby maintaining tissue structure and polarity. Caco2 (Human colon carcinoma) cells depleted of Cdc42 produce multiple lumen cysts, as a result of misoriented mitotic spindles.25 Moreover, 2 Cdc42-specific GEFs, Intersectin 2 and Tuba, have both been shown to regulate mitotic spindle orientation and are both essential for normal lumen formation in MDCK cysts.26,27 Furthermore, an ECM-Rho-RhoK pathway was recently described that promotes recruitment of the spindle-anchoring complex to align the spindle parallel to the apical surface in MDCK cells.28 Par1B overexpression was shown to inhibit this pathway, leading to tilted spindles and lateral lumen polarity, phenotypes reminiscent of hepatocytic epithelia. Consistent with this, Par1B depletion in hepatocytes promoted MDCK-like spindle alignment and apical polarity through changes in Rho activity.
All this work follows on from the classic experiments in Caenorhabditis elegans (C. elegans), which first identified the PAR (partitioning-defective) proteins: a set of evolutionarily conserved proteins that are necessary for establishing an anterior-posterior cortical polarity axis prior to the first zygotic division.29 Subsequent studies showed how both Cdc42 and the PAR proteins regulate mitotic spindle orientation and are involved in the asymmetric positioning of the mitotic spindle during zygotic cell division.30-33 These findings suggest an evolutionarily conserved mechanism for determining mitotic spindle orientation, involving both the Par polarity proteins and the Rho GTPases, which play a key role in determining mitotic spindle orientation in multiple cell types.
It should also be noted that the budding yeast Saccharomyces cerevisiae (S. cerevisiae) and the fission yeast Schizosaccharomyces pombe (S. pombe) also rely on Cdc42 activity for their polarized cell growth and division.34 In S. cerevisiae, the polarized localization of active Cdc42 defines the new bud site, from which Cdc42 subsequently drives polarized cell growth through its regulation of the actin cytoskeleton, the septins and polarized secretion.
In summary, the Rho family GTPases regulate lumen polarity through a variety of mechanisms, including: spatiotemporal regulation of their activities, as well as the regulation of apical identity, intracellular trafficking, and mitotic spindle orientation. These distinct, yet interconnected cellular events, work together in order to correctly generate and assemble the distinct domains that define the AB polarized state, which in the context of lumen formation, means the formation of a normal single lumen cyst. Intriguingly, close cooperation between the Rho GTPases and the Par proteins is a common theme throughout.
Cdc42-driven Apical Differentiation
The function of Cdc42 in the establishment of AB polarity is largely dependent upon its interaction with the polarity protein Par6, and we have already discussed above its role in regulating apical determination required for normal lumen formation in cultured mammalian cells.23,24 Importantly, the Cdc42-Par6 interaction is highly conserved as it has been found in vertebrates as well as C. elegans and Drosophila epithelia.33,35-39
In agreement with the lumen formation studies,23,24 analysis of Drosophila embryogenesis has also revealed an essential role for Cdc42-Par6 in apical determination. Establishment of the epithelial apical domain occurs following cellularisation of the Drosophila syncytium, at which point all 3 members of the Par complex (Par6, Baz and aPKC) can be found at the apical cell cortex. Drosophila embryos expressing mutant Par6 that cannot bind Cdc42 fail to accumulate Par6 or Baz apically and consequently AJs are not formed.39 Similarly, expression of DA- or DN-Cdc42 prevents the apical localization of Par6 and leads to loss of epithelial polarity. In addition, it has been shown that loss of Cdc42 in mature epithelia leads to the loss of both Par6 and aPKC at the apical cortex.40 Cdc42 and Par6 are also likely to regulate AB polarity in Drosophila neuroblasts. Despite Hutterer et al. reporting no effect on Par6 localization and polarity by expression of DA- or DN-Cdc42 in neuroblasts,39 Atwood and colleagues later reported that neuroblasts mutant for Cdc42 or expressing mutant Par6 that cannot bind to Cdc42, both failed to localize Par6 at the apical cortex and had lost polarity.41
In addition to Cdc42s role in correctly localizing apical determinants, some evidence indicates that Cdc42 may also promote apical differentiation through enhancing aPKC activity. In vertebrates, aPKC activity was found to be increased when in a complex with Cdc42 and Par6.42 Additional in vitro and mammalian cell culture studies showed how Cdc42 binding can cause a conformational change in Par6 that may alter its affinity for aPKC substrates.43-45 Consequently, there is speculation that Cdc42-Par6 may promote the phosphorylation of aPKC substrates such as Baz, Crumbs and lgl, which are all important for polarity establishment. Supporting this view, Drosophila Cdc42 activates aPKC in vitro via Par6, and also the aPKC substrate, Mira, displays an unpolarised localization following loss of the Cdc42-Par6 interaction in Drosophila mitotic neuroblasts.41 In contrast however, lgl phosphorylation was still observed in Drosophila embryos expressing Par6 that is mutant for Cdc42 binding, indicating that aPKC activity is not impaired.39 Also, aPKC-mediated Baz phosphorylation is reportedly retained following loss of the Cdc42-Par6 interaction during Drosophila photoreceptor remodelling.46 Ultimately, the importance of Cdc42-Par6 in stimulating aPKC-mediated phosphorylation events required for AB polarization currently remains unclear.
Another mechanism by which Cdc42-Par6 may promote apical differentiation is through regulation of apical Baz/Par3 exclusion (Fig. 2). In Drosophila epithelial cells, both aPKC and Crumbs mediate the exclusion of Baz from the apical domain, causing it to localize basal to Par6 and aPKC, and thereby defining the apical-lateral border.47 This requires aPKC-mediated phosphorylation of Baz at the conserved serine 980 to disrupt the Baz-aPKC interaction. In addition, it requires Crumbs-mediated prevention of the Baz-Par6 interaction. In the absence of these events, Baz is mislocalised, causing AJ components to localize apically, resulting in loss of the apical domain and expansion of the lateral. A similar mechanism has also been described to drive Drosophila photoreceptor remodelling, except Cdc42 was also implicated.46 Cdc42 binding to Par6 was required for the correct apical localization of Crumbs, a step necessary for Baz exclusion and the subsequent separation of the subapical membrane from the AJ.
Figure 2.

Apical exclusion of Baz/Par3 defines the apical-lateral border. Cdc42-mediated signaling through Par6-aPKC and Crumbs excludes Baz/Par3 from the apical domain and thereby defines the apical-lateral border.46,47,49. This process involves 2 mechanisms: 1. aPKC-mediated phosphorylation of Baz at Serine 980 to disrupt the Baz-aPKC interaction, and 2. Crumbs-mediated inhibition of the Baz-Par6 interaction. P = phosphorylation.
Furthermore, several lines of evidence suggest similar mechanisms also operate in vertebrates. In MDCK cells, Rab11a-positive vesicles have been found to deliver the Crumbs-Pals1-Patj complex to early lumens,48 and Cdc42 and its GEF, Tuba, are required to mediate transport of apical determinants from these vesicles.24 Moreover, another Cdc42 GEF, Dbl3, was recently implicated in driving apical Cdc42 activity during apical differentiation of cultured mammalian epithelial cells.49 Enhanced Dbl3/Cdc42 signaling increased apical aPKC and therefore also Par3 phosphorylation, leading to repositioning of the apical-lateral border.
It should also be noted that Drosophila Baz and vertebrate Par3 localize to different junctional structures, with Baz localizing to AJs (Fig. 2, left-hand cell),50 whereas Par3 localizes to TJs through binding to JAM (Fig. 2, right-hand cell).16,51 However, in both cases Baz/Par3 is localized to the most apical region of the cell-cell junctions, where it functions to define the apical-lateral border (Fig. 2). In summary, the evidence suggests that apical differentiation is controlled by an evolutionarily conserved mechanism involving signaling through a Cdc42-Par6-aPKC-Crumbs-Baz/Par3 pathway, resulting in destabilisation of the Par complex and subsequent segregation of Par6-aPKC into the apical domain, and Baz/Par3 to the apical-lateral border (Fig. 2).
Cell-cell Junctions
At distinct locations along the epithelial AB axis different types of cell-cell junctions can be found, including AJs, TJs (or septate junctions in Drosophila), gap junctions and desmosomes. AJs and TJs are important for establishing and maintaining AB polarity. AJs are particularly important for tissue integrity as they provide strong intercellular connections. However, TJs act as a “gate” by allowing selective passage of molecules through the intercellular space, and additionally act as a “fence” by preventing diffusion of molecules between the apical and basolateral domains.
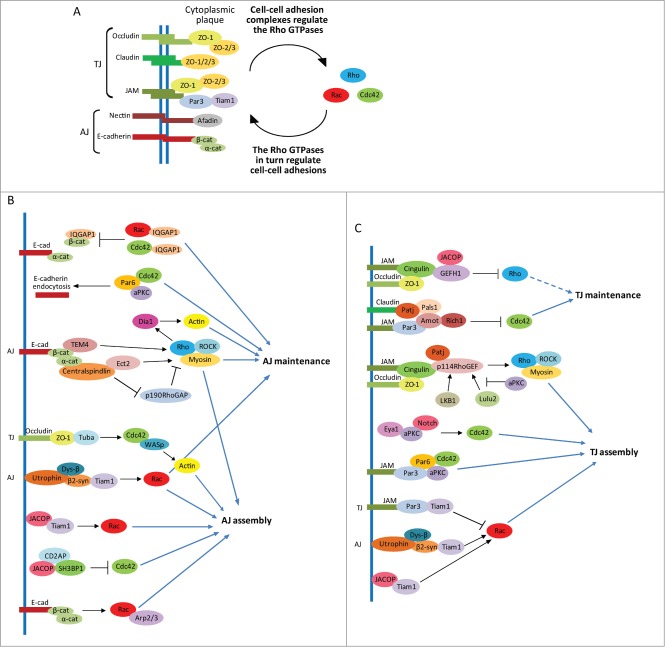
Both AJs and TJs are composed of adhesion molecules with extracellular domains that form homophilic interactions with adhesion molecules on neighboring cells and with intracellular domains that associate with various cytoplasmic scaffolding and signaling complexes in a so-called cytoplasmic plaque, which subsequently associate with and regulate the Rho GTPases (Fig. 3A). In turn, signaling through the Rho GTPases regulates the junctions (Fig. 3A). These junctions also connect to the actomyosin network, forming a belt-like ring around the cell perimeter that provides junctional tension (Fig. 1B, Cell 2). AJs are comprised of cadherin- and nectin-based adhesion molecules, whereas TJs are made up of Occludin, JAM and Claudin (Fig. 3A). E-cadherin is important for the establishment of cell-cell junctions and AB polarity in MDCK cells, however intriguingly it is somewhat dispensable for the integrity of mature junctions.52
Figure 3.
Epithelial cell-cell junctions are regulated by multiple signaling pathways that include the Rho family GTPases. (A) Shown are the main constituents of epithelial adherens junctions (AJs) and tight junctions (TJs) that connect to numerous cytoplasmic signaling and scaffolding molecules in a cytoplasmic plaque, which subsequently link the junctions with the Rho family GTPases. (B and C) Shown are the numerous molecular mechanisms involving the Rho GTPases that control epithelial AJs (B), and TJs (C). Dotted line represents a speculated link.
The assembly of AJs and TJs are highly interconnected, with the general consensus being that immature AJs are a prerequisite for TJ formation. Epithelial cells initiate their E-cadherin-mediated cell-cell contacts via membrane protrusions. These initial contacts require a local activation of Rac, which drives the formation of actin-based protrusions that carry E-cadherin.53-58 The immature cadherin-based contacts then locally induce de novo formation of both lamellipodia and filopodia53,54,59,60 through remodelling of the local actin cytoskeleton, thereby promoting further E-cadherin interactions. This demonstrates that the promotion of nascent cell-cell contacts requires an intimate relationship between Rho GTPase activity, and the correct localization of both E-cadherin and regulators of the actin cytoskeleton. Active Rac and Cdc42 have been shown to recruit a host of proteins to the site of cell-cell contact (including cortactin, Mena, PAK4, formin-1, and the Arp2/3 complex) all of which promote actin nucleation and remodelling, thereby promoting junction maturation.53-55,61,62 The importance of Rho family GTPase activities is maintained as the AJ matures, with the subsequent establishment of both TJs and AB polarity relying on Rho, Rac and Cdc42 activity, as discussed below.
Adherens Junctions
In the mid 1990s, the regulation of AJs by the Rho family of GTPases first began to be appreciated. Rho, Rac and Cdc42 were all shown to localize to and regulate E-cadherin based cell-cell adhesions.63-68 Subsequent studies confirmed their roles in AJ formation. In particular, Rac was found to be important during the early stages. Several reports have shown that Rac is recruited to and activated by nascent AJs58,69,70 and interestingly, its activation is reduced as AJs mature.71-73 The initial burst of Rac activity stimulates lamellipodia, which as described above, help initiate cell-cell contacts. However, this burst of Rac activity, specifically localized to de novo cell-cell contacts, is short-lived. Rac activity rapidly diminishes as E-cadherin accumulates at the initial site of contact. However, since active Rac promotes lamellipodia formation, new sites of cell-cell contact form at the periphery, leading to a wave of Rac activation flowing outwards from the initial contact site and thereby driving junction expansion.54 Further, Rho and actomyosin contractility are also activated at the contact edges, with Rho activity more peripheral than Rac.74 Rac activity localization correlated with that of lamellipodia and Arp2/3 activity, whereas Rho with phosphorylated myosin and its associated actomyosin contractility. Therefore, Rac and Rho activity, through their respective regulation of actin and actomyosin, are required to drive expansion of cell-cell adhesion. Earlier studies also suggest that Rac-driven actin protrusions precede or occur in concert with, E-cadherin engagement,59,60,75-77 and that Rho-driven actomyosin contractility is important for cell-cell adhesion regulation.78-81 It is therefore believed that Rac-driven lamellipodia and Rho-driven actomyosin contractility cooperate during the initial assembly and expansion of AJs.
Filopodia may also drive AJ assembly by embedding into neighboring cells with cadherin complexes clustered at their tips.53,82,83 This method of cell-cell contact has been shown to be especially important when attempting to bring the free edges of epithelial sheets together, both during embryonic development in C. elegans and Drosophila,82-84 and during wound healing in the chick.85 These filopodia may be Cdc42-driven, since depletion of one of its GAPs, SH3BP1, increased filopodia formation at immature cell-cell contacts, and interestingly impeded subsequent junction maturation.86 SH3BP1 forms a complex with JACOP/paracingulin and CD2AP, which are both required for normal Cdc42 signaling and junction assembly (Fig. 3B). These findings suggest that Cdc42-driven filopodia may be important for driving initial cell-cell contact formation, but then Cdc42 activity must be down-regulated in a spatiotemporal manner for subsequent junction maturation. Consistent with a role in the early stages of AJ assembly, Cdc42 activation has been observed in response to E-cadherin-mediated adhesion in MCF-7 cells.87 However, in contrast others have reported Cdc42 is not activated,72 or that reduced Cdc42 activity may be necessary for AJ assembly.88,89 Possibly the need for both activation and down-regulation of Cdc42 activity at different stages of AJ assembly may explain these discrepancies. Furthermore, in contrast to the SH3BP1 study, Cdc42 is reportedly crucial for AJ maturation in keratinocytes through regulation of aPKCζ.90 Although the role of Cdc42 activity in AJ assembly remains somewhat unclear, a common theme does seem to emerge from the plethora of experiments undertaken in multiple systems: the tight spatiotemporal regulation of Cdc42, Rac and Rho activity seems to be critical to drive the initiation and expansion of cell-cell contacts, as well as the subsequent maturation of the AJ.
In addition to regulating AJ assembly and maturation, the Rho GTPases have also been implicated in the control of AJ integrity and maintenance. Confluent monolayers of MDCK cells have higher Rac and Cdc42 activity than subconfluent cells, suggesting their activities promote junction maintenance.72 Moreover, Tiam1-Rac activity seems to be especially important, since it restores AJs and an epithelioid morphology in several cell types,68,91 and Tiam1 degradation at AJs is required for Src-induced AJ disassembly.92 IQGAP, an effector for Rac and Cdc42, negatively regulates AJ stability by dissociating α-catenin from the cadherin-catenin complex.93 Cdc42 and Rac were found to impede the IQGAP-β-catenin interaction, thereby stabilizing AJs (Fig. 3B).94 Furthermore, a Rac-PAK1-Ajuba feedback loop has been described, that reinforces junction-associated Rac activity and thereby promotes E-cadherin stabilization (Fig. 3B).95 Cadherin-induced Rac-PAK1 signaling leads to phosphorylation of the Rac-interacting scaffold protein, Ajuba. Phosphorylated Ajuba preferentially binds to activated Rac, thereby helping to maintain active Rac at the junctions.
Rho signaling can also help stabilize AJs. The Rho GEF, Ect2, is specifically recruited to AJs and activated by the centralspindlin complex.96 Subsequently, myosin IIA is recruited and activated, resulting in the stabilization of both E-cadherin and junctional tension (Fig. 3B). Moreover, centralspindlin enriches Rho activity at AJs via an interaction with α-catenin and also prevents Rho inhibition through blocking p190RhoGAP recruitment (Fig. 3B), a process involving reduced Rac signaling. Intriguingly, this molecular ensemble also operates at the contractile furrow during cell division.97 More recently, another Rho GEF, TEM4, was also found to regulate AJ integrity through associating with the cadherin-catenin complex (Fig. 3B).98 Additionally, several other studies have indicated that Rho activity promotes AJs: RhoA has been found to help maintain AJs via Dia199 and non-muscle myosin II (Fig. 3B).81 Smutny et al. highlighted the importance of myosin II-mediated contraction in promoting junction stability, with different isoforms promoting E-cadherin homophilic adhesion (myosin IIA), and supporting the integrity of the apical cortical actin ring (myosin IIB).100 Numerous additional studies, carried out both in vivo and in vitro, demonstrate the importance of Rho activity and actomyosin contractility in maintaining cell-cell junction integrity in both stable and remodelling epithelia.74,99,101-109
In order for epithelial integrity to be maintained, it is now known that even within a mature, stable epithelium, epithelial AJs must be plastic: they require an ability to continually form and disassemble. This is vital, as within a living epithelial sheet there are constant changes in tissue organization due to cell division, cell death and delamination. The inherent plasticity of AJs allows the epithelial sheet to accommodate these changes, as well as when required, to carry out more dramatic morphogenetic movements during tissue remodelling events. It is now known, through studies in several systems, that E-cadherin is constantly being turned over at the junction.110-115
Cdc42 activity and the apical Par proteins have been shown to maintain AJ integrity through their control of E-cadherin endocytosis (Figs. 1B, Cell 3, and Fig. 3B). Two studies in the pupal dorsal thorax of Drosophila showed that loss of Cdc42, Par6 or aPKC function led to a break-up of the AJ. This was due to disrupted endocytosis in these mutants. Cdc42, Par6 and aPKC therefore appear to promote AJ turnover.40,116 In contrast, in the ventral ectoderm of the Drosophila embryo, Cdc42 and Par proteins appear to regulate the trafficking of AJ components and apical polarity proteins by limiting endocytosis from the apical membrane.117 This raises the possibility of tissue specific roles for the Cdc42-Par6-aPKC module in the regulation of junction turnover.
Cdc42 is known to regulate the actin cytoskeleton through the activation of WASp, which in turn promotes actin nucleation via the Arp2/3 complex. Evidence suggests that the Cdc42-Par6-aPKC module can promote AJ endocytosis by remodelling the actin cytoskeleton through the local activation of WASp, which drives the dynamin-mediated endocytosis of junctional material (Fig. 1B, Cell 3).40,116 Additionally, studies in the Drosophila eye have identified extensive crosstalk between Cdc42 and Rho signaling for the regulation of AJs and cell shape in remodelling epithelial cells.118,119 Firstly, Cdc42 limits Rho-mediated apical tension by localizing Par6-aPKC to the AJs, where aPKC inhibits Rho activity (Fig. 1B, Cell 2).118 On the other hand, Rho signaling limits Cdc42-Par6-aPKC-driven cadherin endocytosis, which thereby helps to maintain AJs (Fig. 1B, Cell 3).119 The above work demonstrates a surprising level of molecular complexity to simply maintain AJ integrity, with extensive cross-talk between the Rho GTPases, the Par proteins, regulators of the actin cytoskeleton and endocytic pathways.
The Rho GTPases may also stabilize AJs by helping them tether to the actin cytoskeleton. For many years this was believed to be achieved through actin binding directly to the cadherin-catenin complex via α-catenin. However, this model was challenged when it was revealed that actin doesn't directly bind α-catenin at AJs.120 So exactly how AJs physically connect to the actin cytoskeleton remains unknown. The many other actin binding proteins that are known to regulate and/or localize to AJs may be responsible; such as Nectins,121 Ena/VASP proteins,122 formin-1,123 Arp2/3, Utrophin,16 and TEM4,98 and intriguingly all of these have been linked to Rho GTPase signaling.
There is also evidence that limiting the extent of Rho family GTPase activities is also important for AJ stability. Many studies have shown that increased Rac signaling can disrupt AJs.124-131 In fact, under some physiological situations high Rac activity appears to drive AJ disassembly, for example during cell scattering132,133 and potentially tumourigenesis.134 Limitation of Rho activity at AJs by various Rho-specific GAPs has also been suggested to be important for their stabilization.86,135 Intriguingly, Rho-ROCK mediated actomyosin contractility may also disrupt AJs.99 Together these findings suggest that Rho and Rac must be carefully controlled, likely in a spatiotemporal manner, for proper AJ regulation.
Tight Junctions
The Rho family of GTPases have also been implicated in the control of TJs. Treatment of cultured epithelial cells with CNF-1, which activates Rho, Rac and Cdc42, was found to perturb TJ function and cause displacement of TJ proteins.136 Moreover, expression of either DN or CA versions of Rho, Rac or Cdc42 can all perturb TJ function.137-139
Rho in particular is a key regulator of TJs. Back in 1995, inhibition of Rho signaling with the C3 toxin was found to reduce TJ function in human intestinal cells.140 Since then numerous regulators of TJ-associated Rho signaling have been identified, many of which promote TJ assembly through control of the Rho-ROCK-myosin signaling pathway. For example, p114RhoGEF specifically activates Rho at TJs through its interaction with the TJ-associated adaptor cingulin.141 Depletion of p114RhoGEF mislocalises Rho-mediated myosin phosphorylation, and consequently disrupts TJ assembly (Fig. 3C). Moreover, other Rho GEFs also promote TJs by stimulating Rho-myosin signaling.142,143 Further mechanistic details of p114RhoGEF regulation at TJs have also been uncovered.144,145 Its localization is regulated by binding the apical polarity protein, Patj,144 whereas its activity is promoted through binding the FERM-domain containing protein Lulu2,144 or the polarity regulator and tumor suppressor LKB1 (Fig. 3C).145 Interestingly, the Lulu2 interaction can be negatively regulated by aPKC.144 Further, Rho may also be stimulated at TJs by heterotrimeric G proteins.146,147
Rac signaling is also important for proper TJ assembly. Inhibition of Tiam1-Rac signaling by Par3 was found to be required for efficient TJ assembly in MDCKII cells.18 As discussed above, Mack et al. found this occurs in concert with β2-syntrophin-mediated activation of Tiam1-Rac at AJs, which is necessary to drive proper TJ assembly and AB polarization (Fig. 3C).16 Using epidermal keratinocytes, Mertens et al. provide additional evidence that the regulation of Tiam1-Rac by the Par polarity complex is important for TJ biogenesis.148,149 Moreover, the recruitment of Tiam1 to junctions through its association with JACOP/paracingulin also appears to be important for proper regulation of Tiam1-Rac signaling during AJ/TJ assembly.149
Cdc42 also regulates TJs via numerous signaling complexes. In MDCK cells, the classic Cdc42-Par6-Par3 polarity complex was found to promote TJ assembly.35 Additionally, Cdc42 activity induced by an Eya1-aPKC-Notch1 signaling pathway (Fig. 3C),150 as well as signaling through its effectors PAK4 and Par6B,62 have also been implicated in TJ assembly. Moreover, the Cdc42 GEF, Tuba, localizes to TJs through its interaction with ZO-1, where it regulates junctional configuration as well as the early stages of AJ formation, through stimulation of Cdc42-WASp-driven actin polymerisation (Fig. 3B).151
Additionally, there is evidence to suggest that Rho family GTPase signaling can negatively regulate epithelial TJs. For example, Cdc42 activity can promote HGF-induced TJ disassembly,152 and increased Tiam1-Rac signaling can perturb TJ assembly.16,18 Moreover, in contrast to p114RhoGEF, another Rho GEF, GEFH1, is inhibited by its association with cingulin,153 in addition to its association with JACOP/paracingulin,149 and its activity can reportedly promote TJ disassembly,154 as well as increase paracellular permeability for small tracers.155 Thus, we speculate that cingulin- or JACOP/paracingulin-mediated inhibition of GEFH1 may have a role in TJ maintenance (Fig. 3C). This is in addition to its proposed role in mediating confluency-induced Rho inactivation.153,156 Unlike Rac and Cdc42 activities, Rho signaling is downregulated in confluent cells,72 which ultimately impedes cell proliferation.157 Also important for TJ integrity is a complex containing the Cdc42 RhoGAP, Rich1, and the scaffolding protein Amot that associates with some key polarity proteins including Pals1, Patj and Par3 (Fig. 3C).158 These studies suggest that Rho GTPase activities must also be limited for TJ maintenance.
In summary, Rho, Rac and Cdc42 are critical regulators of epithelial cell-cell junctions, but their junction-associated activities must be carefully balanced for proper control of junction assembly, maturation and maintenance. Cells achieve this balancing act through a multitude of junction-associated Rho GTPase regulators, including many GEFs and GAPs, which cooperate over space and time (Fig. 3B and C). Additionally, many of these studies again highlight the tight relationship between Rho GTPase activities and cell polarity determinants.
Tissue Polarity
As an epithelium develops, individual cells must acquire AB polarity in a coordinated fashion in order to produce an organized, fully differentiated and functional tissue. For example, the development of organized cell-cell junctions gives the epithelium strength and integrity. Additionally, during organism development many morphogenetic changes take place, which rely on cell-cell adhesion and changes in cell shape to determine the final size and shape of a tissue. Once again an intricate interplay between Rho GTPases, polarity proteins and regulators of the actin cytoskeleton is key in driving these morphogenetic processes to change the shape and/or position of individual cells within a tissue. The establishment and maintenance of correct AB polarity is required for correct cell shape and to form and position cell-cell junctions. Consequently, the regulation of AB polarization within single epithelial cells has major implications for the whole tissue, which therefore implicates the Rho GTPases as important regulators of tissue polarity.
In particular, Cdc42 can control a cell's height as well as its apex size. In the Drosophila wing, Cdc42 promotes AB elongation,66 whereas in the Drosophila notum Cdc42 is required to restrict cell height as well as apical constriction.21,40 Many other studies also support a role for Cdc42 in restricting apical constriction,40,116,159 which it achieves through antagonising Rho activity at AJs to limit apical tension (Fig. 1B, Cell 2).118
Several other lines of evidence also show the importance of junction-associated Rho-mediated actomyosin contractility in controlling tissue polarity, particularly from the study of several developmental processes that involve extensive tissue remodelling. For example, during Drosophila germband extension, the tissue doubles in length but its width is halved,160 which is achieved by a pattern of shrinking and growing cell-cell junctions, resulting in cell rearrangements to reorganise the tissue.104,106,161 Interestingly, in this intercalating ectoderm, Rho-mediated actomyosin contractility is concentrated cortically at shrinking cell-cell junctions (Fig. 1B, Cell 2), promoting their contraction, and driving tissue remodelling. Moreover, Rho-mediated actomyosin contractility is also required to promote coordinated apical constriction during Drosophila gastrulation.105,162-164 Gastrulation requires a coordinated apical constriction in the mesoderm, which is thought to be mediated by pulses of actomyosin contractility, this time driven by a medial actomyosin web-like network (Fig. 1B, Cell 2).108 Therefore, mesoderm cells undergoing apical constriction utilize a different actomyosin network to the intercalating cells of the ectoderm, thereby explaining the disparity in the cell shape changes observed between the 2 cell populations.
The Drosophila gastrulation process involves a cell-lengthening phase, whereby individual cells elongate concomitantly with apical constriction, and a subsequent cell-shortening phase, whereby cells shorten as the tissue invaginates.165 Interestingly, He et al. report that the lateral membranes of individual mesoderm cells are dispensable for the cell-lengthening process.165 They present a model whereby the cytoplasm of multiple cells provides a collective force that actively promotes lengthening.
The actomyosin web-like network is also required for apical constriction in amnioserosa cells, which is necessary for Drosophila dorsal closure.166,167 Repeated cycles of assembly and disassembly of the apical actomyosin network produces pulses of contractility that ultimately drive apical constriction.168 However, the involvement of Rho activity is unclear since DN-Rho does not prevent dorsal closure.169 Intriguingly though, Rac-mediated actin regulation has been implicated in this process.169 Moreover, the Par complex appears to be important for the dynamics of the actomyosin pulses. Baz, Par6 and aPKC accumulate at apical puncta that transiently associate with the actomyosin network.170 Baz promotes the duration of the pulses, whereas Par6-aPKC regulates the time between pulses.
In summary, the morphogenetic movement generated relies on the specific location of Rho family GTPase activities and the type of actomyosin contractility, which determines cell shape change and ultimately shapes the developing tissue.171 All cell shape changes fundamentally rely on asymmetry: an asymmetry of tension (mediated by Rho activity, myosin and actin filaments; Fig. 1B, Cell 2), and an asymmetry of cell-cell adhesion (mediated by cadherin and its recycling at the junction; Fig. 1B, Cell 3). Central to all this are the polarity proteins, which provide the positional information that is required to generate these asymmetries.
Discussion
Several themes are evident surrounding the function of Rho GTPases in epithelial AB polarization. The most obvious being the need for a complex spatiotemporal regulation of their activities; the large number of GEFs and GAPs within the cell is conducive to such complex regulatory mechanisms. Through their scaffolding and signaling functions they can permit activation and/or inactivation of the Rho GTPases at specific locations and times, thereby fine-tuning their activities and providing an effective control of the polarization process.
Signaling feedback loops,23,95 as well as crosstalk and interdependence between the Rho family GTPases are also common. In particular, Rac and Rho frequently function antagonistically,15,96 or their activities are distinctly separated,74 with Rac activity often enriched basolaterally16 and Rho activity apically.22 Antagonism between Rho and Cdc42 is important for controlling apical tension,118 whereas on the other hand, Rac and Cdc42 frequently cooperate.21,94 These mechanisms provide immense robustness to AB polarization.
There is also tight cooperation between distinct cellular processes, including: intercellular adhesion, membrane protrusion dynamics, actomyosin contractility, membrane trafficking, and mitotic spindle orientation, in order to achieve and maintain a correctly AB polarized state at both the single cell and whole tissue level. Bearing all of the above in mind, it is not surprising that overstimulation as well as inhibition of Rho GTPase signaling, often through the expression of DN and CA forms of the GTPases, have been found to perturb AB polarization.86,137-139 These findings likely reflect the multifunctional nature of the Rho GTPases, as well as their interdependence, and also the need for their activities to be correctly balanced.
Polarity Plasticity
In addition to their roles in epithelial AB polarization, the Rho family GTPases have also been widely implicated in various other types of cell polarity, including endothelial AB polarity,98,172,173 front-rear polarity,174-179 polarized cell division,34,180 polarized secretion,181 planar cell polarity,182 and neuronal polarity.103,143,183 Intriguingly, this is also the case for many other polarity regulators, notably the Par proteins.184-186 In fact, many of the same signaling complexes/pathways that are critical in regulating AB polarity have also been implicated in multiple cell polarization events. We would particularly like to draw attention to the analogies between AB polarization and front-rear polarity, necessary for directed cell migration (Fig. 4).
Figure 4.
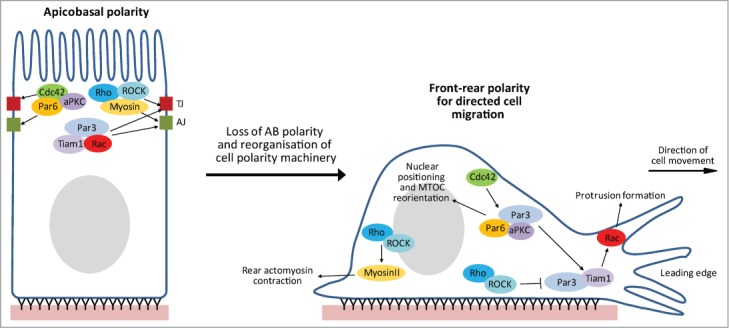
Schematic representation of epithelial cell polarity plasticity. Many aspects of an epithelial cells’ polarity machinery are multi-functional, potentially permitting a switch from promoting apicobasal polarity to front-rear polarity, which promotes directed cell migration. Shown are some of the Rho GTPase signaling complexes that have been implicated in the regulation of both types of polarization, and are therefore possible candidates for mediating such a polarity switch.
We discussed earlier the evidence implicating Par3-Tiam1-Rac signaling in TJ assembly and AB polarization.16,18,148 Similarly, Par3-Tiam1-Rac signaling also promotes front-rear polarity and directed cell migration in multiple cell types (Fig. 4).175,176 Pegtel et al. showed that the Par-Tiam1 complex promotes persistent migration of keratinocytes by stabilizing front-rear polarity through increasing microtubule stability.176 More recently, Wang et al. reported that the Par-Tiam1 complex is recruited to a subset of adhesions in a talin-dependent manner, where it mediates integrin-induced Rac activation and regulates adhesion turnover necessary for polarized migration.175
Cdc42-mediated signaling via the Par complex has also been implicated in polarized cell migration (Fig. 4). In fibroblasts, Cdc42 has been shown to regulate reorientation of the microtubule organizing center (MTOC), an important event in establishing front-rear polarity, through 2 mechanisms: 1. by promoting rearward nuclear movement via an MRCK-myosin-actin pathway, and 2. by maintaining a centroid MTOC localization through a Par6-PKCζ-Dynein pathway.174 This latter mechanism likely also operates in migrating astrocytes downstream of integrin activation.187 Cdc42 association with the Par complex also reinforces Par-Tiam1-Rac signaling in polarized migrating cells.177
Rho-ROCK signaling also functions in both AB polarization and polarized cell migration (Fig. 4). The Rho-ROCK-myosin pathway promotes actomyosin contractility at the rear of polarized migrating cells.188,189 In addition, ROCK-mediated phosphorylation of Par3 inhibits Par complex formation and consequently leads to Rac inactivation at the leading edge, as well as in central and rear regions of migrating cells.177
Also noteworthy, is the specific spatiotemporal regulation of Rho, Rac and Cdc42,190 which includes the distinct separation of Rac and Rho activities,191 as well as their interdependence;192 this tight regulation is required to control membrane protrusion dynamics at the leading edge, necessary for directed cell migration. Furthermore, like AB polarity, polarized cell migration also requires the cooperation of adhesions,175 membrane protrusion dynamics,191 actomyosin contractility193 and membrane trafficking.194 Consequently, the regulatory mechanisms controlling the establishment and maintenance of both AB polarity and polarized cell migration are remarkably similar.
We propose the evidence suggests that there is a general “hub” of cell polarity machinery, comprised of numerous polarity regulators that are capable of promoting multiple cell polarization events. We envisage this would provide cells with “polarity plasticity," meaning that they can efficiently reorganise their polarity in response to various stimuli or environmental changes. In fact, major changes in cell polarity are required for numerous physiological events, including cell division, various developmental events and tumor metastasis. For example, during dorsal closure of the Drosophila embryo, cells at the leading edge must lose AB polarity and take on front-rear polarity in order to migrate. Subsequently however, as opposing cells meet and adhere to one another to seal the gap, AB polarity must be re-established. Interestingly, the Rac and Cdc42 effector, Pak, is important for both polarized cell migration and restoration of AB polarity during this process.195
We suspect that the type of polarization stimulated is context-dependent. For example, the presence of strong intercellular contacts should promote AB polarity (Fig. 4, left-hand cell), whereas in their absence, or following some perturbation of AB polarity (which frequently occurs during tumor progression),196 the same polarity machinery may become free to take up alternative localisations and instead promote front-rear polarity and consequently directed cell migration (Fig. 4, right-hand cell). Therefore, this proposed phenomenon could have important implications for our understanding of tumor progression and what we consider to be oncogenes or tumor suppressors. During tumor progression, tumor cells frequently lose AB polarity, become disorganized, divide rapidly, and may eventually invade the surrounding tissue and metastasise through polarized cell migration.196 We envisage that through these events the cell's polarity machinery is rearranged, thereby encouraging drastic changes in cellular function and disease progression. We therefore speculate that some so-called tumor suppressors may also have oncogenic functions, for example, if they are capable of promoting both AB polarity and polarized cell migration.
In support of our hypothesis, several epithelial AB polarity regulators have been observed to be mislocalised in human cancers, with possible consequences for disease progression. For example, reduced membrane-localized Par3 has been found associated with potentially more aggressive invasive breast cancers.197 Reduced membrane-localized β2-syntrophin appears to be associated with more aggressive prostate cancers.16 Scribble has been found to be mislocalised away from cell-cell junctions in breast198 and prostate199 cancers, the latter also being associated with poor survival. A mutant version of Scribble that cannot localize to cell-cell junctions was also recently shown to promote mammary tumourigenesis in mice.200 Although these findings support their correct cortical localization as being tumor suppressive, they also support the possibility that once mislocalised they may actively participate in alternative cellular functions that actually encourage tumor progression.
There is also substantial evidence from a variety of human cancers that expression of the Rac activator, Tiam1, can correlate with metastasis or poor survival.201-205 In cancers where Tiam1 is over-expressed, these findings could reflect the reportedly negative effect of increased Tiam1-Rac signaling on cell-cell adhesions and AB polarity.16,18 However, they also support the possibility of a more direct function for Tiam1-Rac signaling in promoting metastasis, through its reportedly positive role in promoting polarized cell migration.175,176
We propose that a detailed understanding of the extent of multifunctionality among cell polarity regulators is required. Future research investigating the potential direct oncogenic activities of mislocalised polarity regulators in human cancers could have huge implications for our understanding of tumor progression and metastasis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We apologize for any omissions when citing relevant literature due to space restrictions.
Funding
Research in the lab of MG is supported by a Career Establishment Award from Cancer Research UK.
References
- 1. Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer 2002; 2:133-42; PMID:12635176; http://dx.doi.org/ 10.1038/nrc725 [DOI] [PubMed] [Google Scholar]
- 2. Boulter E, Garcia-Mata R. Analysis of the role of RhoGDI1 and isoprenylation in the degradation of RhoGTPases. Methods Mol Biol 2012; 827:97-105; PMID:22144270; http://dx.doi.org/ 10.1007/978-1-61779-442-1_7 [DOI] [PubMed] [Google Scholar]
- 3. Castillo-Lluva S, Tatham MH, Jones RC, Jaffray EG, Edmondson RD, Hay RT, Malliri A. SUMOylation of the GTPase Rac1 is required for optimal cell migration. Nat Cell Biol 2010; 12:1078-85; PMID:20935639; http://dx.doi.org/ 10.1038/ncb2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castillo-Lluva S, Tan CT, Daugaard M, Sorensen PH, Malliri A. The tumour suppressor HACE1 controls cell migration by regulating Rac1 degradation. Oncogene 2013; 32:1735-42; PMID:22614015; http://dx.doi.org/ 10.1038/onc.2012.189 [DOI] [PubMed] [Google Scholar]
- 5. Lo P, Hawrot H, Georgiou M. Apicobasal polarity and its role in cancer progression. Biomol Concept 2012; 3:505-21; http://dx.doi.org/ 10.1515/bmc-2012-0020 [DOI] [PubMed] [Google Scholar]
- 6. Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci 1990; 95 (Pt 1):137-51; PMID:2351699 [DOI] [PubMed] [Google Scholar]
- 7. Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta 2008; 1778:614-30; PMID:18005931; http://dx.doi.org/ 10.1016/j.bbamem.2007.08.029 [DOI] [PubMed] [Google Scholar]
- 8. Yamanaka T, Ohno S. Role of LglDlgScribble in the regulation of epithelial junction, polarity and growth. Front Biosci: J Virtual Libr 2008; 13:6693-707; PMID:18508688; http://dx.doi.org/ 10.2741/3182 [DOI] [PubMed] [Google Scholar]
- 9. Laprise P, Lau KM, Harris KP, Silva-Gagliardi NF, Paul SM, Beronja S, Beitel GJ, McGlade CJ, Tepass U. Yurt, Coracle, Neurexin IV and the Na(+),K(+)-ATPase form a novel group of epithelial polarity proteins. Nature 2009; 459:1141-5; PMID:19553998; http://dx.doi.org/ 10.1038/nature08067 [DOI] [PubMed] [Google Scholar]
- 10. Tepass U. FERM proteins in animal morphogenesis. Curr Opin Genet Dev 2009; 19:357-67; PMID:19596566; http://dx.doi.org/ 10.1016/j.gde.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 11. Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit BazookaPAR-3 to establish complementary cortical domains in polarized cells. Cell 2003; 115:691-704; PMID:14675534; http://dx.doi.org/ 10.1016/S0092-8674(03)00938-3 [DOI] [PubMed] [Google Scholar]
- 12. O’Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol 2001; 3:831-8; PMID:11533663; http://dx.doi.org/ 10.1038/ncb0901-831 [DOI] [PubMed] [Google Scholar]
- 13. Yu W, Datta A, Leroy P, O’Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell 2005; 16:433-45; PMID:15574881; http://dx.doi.org/ 10.1091/mbc.E04-05-0435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu KD, Datta A, Yu W, Brakeman PR, Jou TS, Matthay MA, Mostov KE. Rac1 is required for reorientation of polarity and lumen formation through a PI 3-kinase-dependent pathway. Am J Physiol Renal Physiol 2007; 293:F1633-40; PMID:17804488; http://dx.doi.org/ 10.1152/ajprenal.00053.2007 [DOI] [PubMed] [Google Scholar]
- 15. Yu W, Shewan AM, Brakeman P, Eastburn DJ, Datta A, Bryant DM, Fan QW, Weiss WA, Zegers MM, Mostov KE. Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity. EMBO Rep 2008; 9:923-9; PMID:18660750; http://dx.doi.org/ 10.1038/embor.2008.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mack NA, Porter AP, Whalley HJ, Schwarz JP, Jones RC, Khaja AS, Bjartell A, Anderson KI, Malliri A. beta2-syntrophin and Par-3 promote an apicobasal Rac activity gradient at cell-cell junctions by differentially regulating Tiam1 activity. Nat Cell Biol 2012; 14:1169-80; PMID:23103911; http://dx.doi.org/ 10.1038/ncb2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yagi S, Matsuda M, Kiyokawa E. Suppression of Rac1 activity at the apical membrane of MDCK cells is essential for cyst structure maintenance. EMBO Rep 2012; 13:237-43; PMID:22261715; http://dx.doi.org/ 10.1038/embor.2011.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol 2005; 7:262-9; PMID:15723052; http://dx.doi.org/ 10.1038/ncb1226 [DOI] [PubMed] [Google Scholar]
- 19. Horikoshi Y, Suzuki A, Yamanaka T, Sasaki K, Mizuno K, Sawada H, Yonemura S, Ohno S. Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J Cell Sci 2009; 122:1595-606; PMID:19401335; http://dx.doi.org/ 10.1242/jcs.043174 [DOI] [PubMed] [Google Scholar]
- 20. Yagi S, Matsuda M, Kiyokawa E. Chimaerin suppresses Rac1 activation at the apical membrane to maintain the cyst structure. PloS One 2012; 7:e52258; PMID:23284959; http://dx.doi.org/ 10.1371/journal.pone.0052258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Georgiou M, Baum B. Polarity proteins and Rho GTPases cooperate to spatially organise epithelial actin-based protrusions. J Cell Sci 2010; 123:1089-98; PMID:20197404; http://dx.doi.org/ 10.1242/jcs.060772 [DOI] [PubMed] [Google Scholar]
- 22. Gon H, Fumoto K, Ku Y, Matsumoto S, Kikuchi A. Wnt5a signaling promotes apical and basolateral polarization of single epithelial cells. Mol Biol Cell 2013; 24:3764-74; PMID:24088568; http://dx.doi.org/ 10.1091/mbc.E13-07-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 2007; 128:383-97; PMID:17254974; http://dx.doi.org/ 10.1016/j.cell.2006.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 2010; 12:1035-45; PMID:20890297; http://dx.doi.org/ 10.1038/ncb2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol 2008; 183:625-33; PMID:19001128; http://dx.doi.org/ 10.1083/jcb.200807121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodriguez-Fraticelli AE, Vergarajauregui S, Eastburn DJ, Datta A, Alonso MA, Mostov K, Martin-Belmonte F. The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J Cell Biol 2010; 189:725-38; PMID:20479469; http://dx.doi.org/ 10.1083/jcb.201002047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qin Y, Meisen WH, Hao Y, Macara IG. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J Cell Biol 2010; 189:661-9; PMID:20479467; http://dx.doi.org/ 10.1083/jcb.201002097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lazaro-Dieguez F, Cohen D, Fernandez D, Hodgson L, van Ijzendoorn SC, Musch A. Par1b links lumen polarity with LGN-NuMA positioning for distinct epithelial cell division phenotypes. J Cell Biol 2013; 203:251-64; PMID:24165937; http://dx.doi.org/ 10.1083/jcb.201303013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 1988; 52:311-20; PMID:3345562; http://dx.doi.org/ 10.1016/S0092-8674(88)80024-2 [DOI] [PubMed] [Google Scholar]
- 30. Etemad-Moghadam B, Guo S, Kemphues KJ. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 1995; 83:743-52; PMID:8521491; http://dx.doi.org/ 10.1016/0092-8674(95)90187-6 [DOI] [PubMed] [Google Scholar]
- 31. Grill SW, Gonczy P, Stelzer EH, Hyman AA. Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature 2001; 409:630-3; PMID:11214323; http://dx.doi.org/ 10.1038/35054572 [DOI] [PubMed] [Google Scholar]
- 32. Hung TJ, Kemphues KJ. PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development 1999; 126:127-35; PMID:9834192 [DOI] [PubMed] [Google Scholar]
- 33. Gotta M, Abraham MC, Ahringer J. CDC-42 controls early cell polarity and spindle orientation in C. elegans. Curr Biol: CB 2001; 11:482-8; PMID:11412997; http://dx.doi.org/ 10.1016/S0960-9822(01)00142-7 [DOI] [PubMed] [Google Scholar]
- 34. Perez P, Rincon SA. Rho GTPases: regulation of cell polarity and growth in yeasts. The Biochemical journal 2010; 426:243-53; PMID:20175747; http://dx.doi.org/ 10.1042/BJ20091823 [DOI] [PubMed] [Google Scholar]
- 35. Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2000; 2:531-9; PMID:10934474; http://dx.doi.org/ 10.1038/35019573 [DOI] [PubMed] [Google Scholar]
- 36. Johansson A, Driessens M, Aspenstrom P. The mammalian homologue of the Caenorhabditis elegans polarity protein PAR-6 is a binding partner for the Rho GTPases Cdc42 and Rac1. J Cell Sci 2000; 113 (Pt 18):3267-75; PMID:10954424 [DOI] [PubMed] [Google Scholar]
- 37. Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42Rac1 and aPKC signalling and cell polarity. Nat Cell Biol 2000; 2:540-7; PMID:10934475; http://dx.doi.org/ 10.1038/35019592 [DOI] [PubMed] [Google Scholar]
- 38. Qiu RG, Abo A, Steven Martin G. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr Biol: CB 2000; 10:697-707; PMID:10873802; http://dx.doi.org/ 10.1016/S0960-9822(00)00535-2 [DOI] [PubMed] [Google Scholar]
- 39. Hutterer A, Betschinger J, Petronczki M, Knoblich JA. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell 2004; 6:845-54; PMID:15177032; http://dx.doi.org/ 10.1016/j.devcel.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 40. Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp23-mediated endocytosis to control local adherens junction stability. Curr Biol: CB 2008; 18:1631-8; PMID:18976918; http://dx.doi.org/ 10.1016/j.cub.2008.09.029 [DOI] [PubMed] [Google Scholar]
- 41. Atwood SX, Chabu C, Penkert RR, Doe CQ, Prehoda KE. Cdc42 acts downstream of Bazooka to regulate neuroblast polarity through Par-6 aPKC. J Cell Sci 2007; 120:3200-6; PMID:17726059; http://dx.doi.org/ 10.1242/jcs.014902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T, Ishikawa H, et al. . PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells: Dev Mol Cell Mech 2001; 6:721-31; PMID:11532031; http://dx.doi.org/ 10.1046/j.1365-2443.2001.00453.x [DOI] [PubMed] [Google Scholar]
- 43. Garrard SM, Capaldo CT, Gao L, Rosen MK, Macara IG, Tomchick DR. Structure of Cdc42 in a complex with the GTPase-binding domain of the cell polarity protein, Par6. EMBO J 2003; 22:1125-33; PMID:12606577; http://dx.doi.org/ 10.1093/emboj/cdg110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hurd TW, Gao L, Roh MH, Macara IG, Margolis B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol 2003; 5:137-42; PMID:12545177; http://dx.doi.org/ 10.1038/ncb923 [DOI] [PubMed] [Google Scholar]
- 45. Penkert RR, DiVittorio HM, Prehoda KE. Internal recognition through PDZ domain plasticity in the Par-6-Pals1 complex. Nat Struct Mol Biol 2004; 11:1122-7; PMID:15475968; http://dx.doi.org/ 10.1038/nsmb839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Walther RF, Pichaud F. CrumbsDaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr Biol: CB 2010; 20:1065-74; PMID:20493700; http://dx.doi.org/ 10.1016/j.cub.2010.04.049 [DOI] [PubMed] [Google Scholar]
- 47. Morais-de-Sa E, Mirouse V, St Johnston D. aPKC phosphorylation of Bazooka defines the apicallateral border in Drosophila epithelial cells. Cell 2010; 141:509-23; PMID:20434988; http://dx.doi.org/ 10.1016/j.cell.2010.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schluter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, Fan S, Liu CJ, Margolis B. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell 2009; 20:4652-63; PMID:19776356; http://dx.doi.org/ 10.1091/mbc.E09-02-0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zihni C, Munro PM, Elbediwy A, Keep NH, Terry SJ, Harris J, Balda MS, Matter K. Dbl3 drives Cdc42 signaling at the apical margin to regulate junction position and apical differentiation. J Cell Biol 2014; 204:111-27; PMID:24379416; http://dx.doi.org/ 10.1083/jcb.201304064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McKinley RF, Yu CG, Harris TJ. Assembly of Bazooka polarity landmarks through a multifaceted membrane-association mechanism. J Cell Sci 2012; 125:1177-90; PMID:22303000; http://dx.doi.org/ 10.1242/jcs.091884 [DOI] [PubMed] [Google Scholar]
- 51. Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol 2001; 154:491-7; PMID:11489913; http://dx.doi.org/ 10.1083/jcb.200103047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell 2007; 18:189-200; PMID:17093058; http://dx.doi.org/ 10.1091/mbc.E06-05-0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 2000; 100:209-19; PMID:10660044; http://dx.doi.org/ 10.1016/S0092-8674(00)81559-7 [DOI] [PubMed] [Google Scholar]
- 54. Ehrlich JS, Hansen MD, Nelson WJ. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev Cell 2002; 3:259-70; PMID:12194856; http://dx.doi.org/ 10.1016/S1534-5807(02)00216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp23 complex to direct actin assembly in nascent adhesive contacts. Curr Biol 2002; 12:379-82; PMID:11882288; http://dx.doi.org/ 10.1016/S0960-9822(02)00661-9 [DOI] [PubMed] [Google Scholar]
- 56. Lambert M, Choquet D, Mege RM. Dynamics of ligand-induced, Rac1-dependent anchoring of cadherins to the actin cytoskeleton. J Cell Biol 2002; 157:469-79; PMID:11970959; http://dx.doi.org/ 10.1083/jcb.200107104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gavard J, Lambert M, Grosheva I, Marthiens V, Irinopoulou T, Riou JF, Bershadsky A, Mege RM. Lamellipodium extension and cadherin adhesion: two cell responses to cadherin activation relying on distinct signalling pathways. J Cell Sci 2004; 117:257-70; PMID:14657280; http://dx.doi.org/ 10.1242/jcs.00857 [DOI] [PubMed] [Google Scholar]
- 58. Hoshino T, Shimizu K, Honda T, Kawakatsu T, Fukuyama T, Nakamura T, Matsuda M, Takai Y. A novel role of nectins in inhibition of the E-cadherin-induced activation of Rac and formation of cell-cell adherens junctions. Mol Biol Cell 2004; 15:1077-88; PMID:14699074; http://dx.doi.org/ 10.1091/mbc.E03-05-0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol 1998; 142:1105-19; PMID:9722621; http://dx.doi.org/ 10.1083/jcb.142.4.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Krendel MF, Bonder EM. Analysis of actin filament bundle dynamics during contact formation in live epithelial cells. Cell Motil Cytoskel 1999; 43:296-309; PMID:10423271; http://dx.doi.org/ 10.1002/(SICI)1097-0169(1999)43:4%3c296::AID-CM3%3e3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- 61. Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol 2004; 6:21-30; PMID:14647292; http://dx.doi.org/; http://dx.doi.org/ 10.1038/ncb1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wallace SW, Durgan J, Jin D, Hall A. Cdc42 regulates apical junction formation in human bronchial epithelial cells through PAK4 and Par6B. Mol Biol Cell 2010; 21:2996-3006; PMID:20631255; http://dx.doi.org/ 10.1091/mbc.E10-05-0429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kuroda S, Fukata M, Fujii K, Nakamura T, Izawa I, Kaibuchi K. Regulation of cell-cell adhesion of MDCK cells by Cdc42 and Rac1 small GTPases. Biochem Biophys Res Commun 1997; 240:430-5; PMID:9388496; http://dx.doi.org/ 10.1006/bbrc.1997.7675 [DOI] [PubMed] [Google Scholar]
- 64. Takaishi K, Sasaki T, Kameyama T, Tsukita S, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene 1995; 11:39-48; PMID:7624130 [PubMed] [Google Scholar]
- 65. Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol 1997; 139:1047-59; PMID:9362522; http://dx.doi.org/ 10.1083/jcb.139.4.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Eaton S, Auvinen P, Luo L, Jan YN, Simons K. CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing disc epithelium. J Cell Biol 1995; 131:151-64; PMID:7559772; http://dx.doi.org/ 10.1083/jcb.131.1.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol 1997; 137:1421-31; PMID:9182672; http://dx.doi.org/ 10.1083/jcb.137.6.1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hordijk PL, ten Klooster JP, van der Kammen RA, Michiels F, Oomen LC, Collard JG. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science 1997; 278:1464-6; PMID:9367959; http://dx.doi.org/ 10.1126/science.278.5342.1464 [DOI] [PubMed] [Google Scholar]
- 69. Kovacs EM, Ali RG, McCormack AJ, Yap AS. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J Biol Chem 2002; 277:6708-18; PMID:11744701; http://dx.doi.org/ 10.1074/jbc.M109640200 [DOI] [PubMed] [Google Scholar]
- 70. Kawakatsu T, Shimizu K, Honda T, Fukuhara T, Hoshino T, Takai Y. Trans-interactions of nectins induce formation of filopodia and Lamellipodia through the respective activation of Cdc42 and Rac small G proteins. J Biol Chem 2002; 277:50749-55; PMID:12379640; http://dx.doi.org/ 10.1074/jbc.M209846200 [DOI] [PubMed] [Google Scholar]
- 71. Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci 2001; 114:1829-38; PMID:11329369 [DOI] [PubMed] [Google Scholar]
- 72. Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem 2001; 276:33305-8; PMID:11457821; http://dx.doi.org/ 10.1074/jbc.C100306200 [DOI] [PubMed] [Google Scholar]
- 73. Kitt KN, Nelson WJ. Rapid suppression of activated Rac1 by cadherins and nectins during de novo cell-cell adhesion. PloS One 2011; 6:e17841; PMID:21412440; http://dx.doi.org/ 10.1371/journal.pone.0017841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol 2007; 178:517-27; PMID:17646397; http://dx.doi.org/ 10.1083/jcb.200701058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McNeill H, Ryan TA, Smith SJ, Nelson WJ. Spatial and temporal dissection of immediate and early events following cadherin-mediated epithelial cell adhesion. J Cell Biol 1993; 120:1217-26; PMID:8436592; http://dx.doi.org/ 10.1083/jcb.120.5.1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Adams CL, Nelson WJ, Smith SJ. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell-cell adhesion. J Cell Biol 1996; 135:1899-911; PMID:8991100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Adams CL, Nelson WJ. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr Opin Cell Biol 1998; 10:572-7; PMID:9818166 [DOI] [PubMed] [Google Scholar]
- 78. Krendel M, Gloushankova NA, Bonder EM, Feder HH, Vasiliev JM, Gelfand IM. Myosin-dependent contractile activity of the actin cytoskeleton modulates the spatial organization of cell-cell contacts in cultured epitheliocytes. Proc Nat Acad Sci U S A 1999; 96:9666-70; PMID:10449751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ivanov AI, McCall IC, Parkos CA, Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell 2004; 15:2639-51; PMID:15047870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell 2005; 16:2636-50; PMID:15800060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell 2005; 16:4531-42; PMID:16030252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Raich WB, Agbunag C, Hardin J. Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr Biol: CB 1999; 9:1139-46; PMID:10531027 [DOI] [PubMed] [Google Scholar]
- 83. Jacinto A, Wood W, Balayo T, Turmaine M, Martinez-Arias A, Martin P. Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr Biol: CB 2000; 10:1420-6; PMID:11102803 [DOI] [PubMed] [Google Scholar]
- 84. Jacinto A, Woolner S, Martin P. Dynamic analysis of dorsal closure in Drosophila: from genetics to cell biology. Dev Cell 2002; 3:9-19; PMID:12110163 [DOI] [PubMed] [Google Scholar]
- 85. Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol 1996; 135:1097-107; PMID:8922389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Elbediwy A, Zihni C, Terry SJ, Clark P, Matter K, Balda MS. Epithelial junction formation requires confinement of Cdc42 activity by a novel SH3BP1 complex. J Cell Biol 2012; 198:677-93; PMID:22891260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kim SH, Li Z, Sacks DB. E-cadherin-mediated cell-cell attachment activates Cdc42. J Biol Chem 2000; 275:36999-7005; PMID:10950951 [DOI] [PubMed] [Google Scholar]
- 88. Erasmus J, Aresta S, Nola S, Caron E, Braga VM. Newly formed E-cadherin contacts do not activate Cdc42 or induce filopodia protrusion in human keratinocytes. Biol Cell Under Auspices Eur Cell Biol Organ 2010; 102:13-24 [DOI] [PubMed] [Google Scholar]
- 89. Barcellos KS, Bigarella CL, Wagner MV, Vieira KP, Lazarini M, Langford PR, Machado-Neto JA, Call SG, Staley DM, Chung JY, et al. . ARHGAP21 protein, a new partner of alpha-tubulin involved in cell-cell adhesion formation and essential for epithelial-mesenchymal transition. J Biol Chem 2013; 288:2179-89; PMID:23235160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Du D, Pedersen E, Wang Z, Karlsson R, Chen Z, Wu X, Brakebusch C. Cdc42 is crucial for the maturation of primordial cell junctions in keratinocytes independent of Rac1. Exp Cell Res 2009; 315:1480-9; PMID:19100259 [DOI] [PubMed] [Google Scholar]
- 91. Malliri A, van Es S, Huveneers S, Collard JG. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol Chem 2004; 279:30092-8; PMID:15138270; http://dx.doi.org/ 10.1074/jbc.M401192200 [DOI] [PubMed] [Google Scholar]
- 92. Woodcock SA, Rooney C, Liontos M, Connolly Y, Zoumpourlis V, Whetton AD, Gorgoulis VG, Malliri A. SRC-induced disassembly of adherens junctions requires localized phosphorylation and degradation of the rac activator tiam1. Mol Cell 2009; 33:639-53; PMID:19285946; http://dx.doi.org/ 10.1016/j.molcel.2009.02.012 [DOI] [PubMed] [Google Scholar]
- 93. Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, et al. . Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science 1998; 281:832-5; PMID:9694656; http://dx.doi.org/ 10.1126/science.281.5378.832 [DOI] [PubMed] [Google Scholar]
- 94. Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, Matsuura Y, Yonehara S, Fujisawa H, Kikuchi A, et al. . Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. J Biol Chem 1999; 274:26044-50; PMID:10473551; http://dx.doi.org/ 10.1074/jbc.274.37.26044 [DOI] [PubMed] [Google Scholar]
- 95. Nola S, Daigaku R, Smolarczyk K, Carstens M, Martin-Martin B, Longmore G, Bailly M, Braga VM. Ajuba is required for Rac activation and maintenance of E-cadherin adhesion. J Cell Biol 2011; 195:855-71; PMID:22105346; http://dx.doi.org/ 10.1083/jcb.201107162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and alpha-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol 2012; 14:818-28; PMID:22750944; http://dx.doi.org/ 10.1038/ncb2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Su KC, Takaki T, Petronczki M. Targeting of the RhoGEF Ect2 to the equatorial membrane controls cleavage furrow formation during cytokinesis. Dev Cell 2011; 21:1104-15; PMID:22172673; http://dx.doi.org/ 10.1016/j.devcel.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 98. Ngok SP, Geyer R, Kourtidis A, Mitin N, Feathers R, Der C, Anastasiadis PZ. TEM4 is a junctional Rho GEF required for cell-cell adhesion, monolayer integrity and barrier function. J Cell Sci 2013; 126:3271-7; PMID:23729734; http://dx.doi.org/ 10.1242/jcs.123869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol 2002; 4:408-15; PMID:11992112; http://dx.doi.org/ 10.1038/ncb796 [DOI] [PubMed] [Google Scholar]
- 100. Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol 2010; 12:696-702; PMID:20543839; http://dx.doi.org/ 10.1038/ncb2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Katayama K, Melendez J, Baumann JM, Leslie JR, Chauhan BK, Nemkul N, Lang RA, Kuan CY, Zheng Y, Yoshida Y. Loss of RhoA in neural progenitor cells causes the disruption of adherens junctions and hyperproliferation. Proc Nat Acad Sci U S A 2011; 108:7607-12; PMID:21502507; http://dx.doi.org/ 10.1073/pnas.1101347108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bonacci TM, Hirsch DS, Shen Y, Dokmanovic M, Wu WJ. Small GTPase Rho regulates R-cadherin through Dia1profilin-1. Cell Signalling 2012; 24:2102-10; PMID:22820501; http://dx.doi.org/ 10.1016/j.cellsig.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 103. Herzog D, Loetscher P, van Hengel J, Knusel S, Brakebusch C, Taylor V, Suter U, Relvas JB. The small GTPase RhoA is required to maintain spinal cord neuroepithelium organization and the neural stem cell pool. J Neurosci: Off J Soci Neurosci 2011; 31:5120-30; PMID:21451048; http://dx.doi.org/ 10.1523/JNEUROSCI.4807-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 2004; 429:667-71; PMID:15190355; http://dx.doi.org/ 10.1038/nature02590 [DOI] [PubMed] [Google Scholar]
- 105. Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development 2005; 132:4165-78; PMID:16123312; http://dx.doi.org/ 10.1242/dev.01938 [DOI] [PubMed] [Google Scholar]
- 106. Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell 2006; 11:459-70; PMID:17011486; http://dx.doi.org/ 10.1016/j.devcel.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 107. Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, Mavria G. VE-Cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol 2009; 19:668-74; PMID:19345098; http://dx.doi.org/ 10.1016/j.cub.2009.02.057 [DOI] [PubMed] [Google Scholar]
- 108. Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 2009; 457:495-9; PMID:19029882; http://dx.doi.org/ 10.1038/nature07522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A 2010; 107:9944-9; PMID:20463286; http://dx.doi.org/ 10.1073/pnas.0914547107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 2002; 4:222-31; PMID:11836526; http://dx.doi.org/ 10.1038/ncb758 [DOI] [PubMed] [Google Scholar]
- 111. Pilot F, Philippe JM, Lemmers C, Lecuit T. Spatial control of actin organization at adherens junctions by a synaptotagmin-like protein Btsz. Nature 2006; 442:580-4; PMID:16862128; http://dx.doi.org/ 10.1038/nature04935 [DOI] [PubMed] [Google Scholar]
- 112. Cavey M, Rauzi M, Lenne PF, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 2008; 453:751-6; PMID:18480755; http://dx.doi.org/ 10.1038/nature06953 [DOI] [PubMed] [Google Scholar]
- 113. de Beco S, Gueudry C, Amblard F, Coscoy S. Endocytosis is required for E-cadherin redistribution at mature adherens junctions. Proc Natl Acad Sci U S A 2009; 106:7010-5; PMID:19372377; http://dx.doi.org/ 10.1073/pnas.0811253106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol 1999; 146:219-32; PMID:10402472; http://dx.doi.org/ 10.1083/jcb.146.1.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaiche Y. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell 2005; 9:355-76; PMID:16224820; http://dx.doi.org/ 10.1016/j.devcel.2005.07.013 [DOI] [PubMed] [Google Scholar]
- 116. Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol: CB 2008; 18:1639-48; PMID:18976911; http://dx.doi.org/ 10.1016/j.cub.2008.09.063 [DOI] [PubMed] [Google Scholar]
- 117. Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol 2008; 183:1129-43; PMID:19064670; http://dx.doi.org/ 10.1083/jcb.200807020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Warner SJ, Longmore GD. Cdc42 antagonizes Rho1 activity at adherens junctions to limit epithelial cell apical tension. J Cell Biol 2009; 187:119-33; PMID:19805632; http://dx.doi.org/ 10.1083/jcb.200906047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Warner SJ, Longmore GD. Distinct functions for Rho1 in maintaining adherens junctions and apical tension in remodeling epithelia. J Cell Biol 2009; 185:1111-25; PMID:19506041; http://dx.doi.org/ 10.1083/jcb.200901029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell 2005; 123:889-901; PMID:16325582; http://dx.doi.org/ 10.1016/j.cell.2005.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Miyoshi J, Takai Y. Nectin and nectin-like molecules: biology and pathology. Am J Nephrol 2007; 27:590-604; PMID:17823505; http://dx.doi.org/ 10.1159/000108103 [DOI] [PubMed] [Google Scholar]
- 122. Furman C, Sieminski AL, Kwiatkowski AV, Rubinson DA, Vasile E, Bronson RT, Fassler R, Gertler FB. EnaVASP is required for endothelial barrier function in vivo. J Cell Biol 2007; 179:761-75; PMID:17998398; http://dx.doi.org/ 10.1083/jcb.200705002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Ann Rev Biochem 2007; 76:593-627; PMID:17373907; http://dx.doi.org/ 10.1146/annurev.biochem.75.103004.142647 [DOI] [PubMed] [Google Scholar]
- 124. Yano T, Yamazaki Y, Adachi M, Okawa K, Fort P, Uji M, Tsukita S. Tara up-regulates E-cadherin transcription by binding to the Trio RhoGEF and inhibiting Rac signaling. J Cell Biol 2011; 193:319-32; PMID:21482718; http://dx.doi.org/ 10.1083/jcb.201009100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hage B, Meinel K, Baum I, Giehl K, Menke A. Rac1 activation inhibits E-cadherin-mediated adherens junctions via binding to IQGAP1 in pancreatic carcinoma cells. Cell Commun Signaling: CCS 2009; 7:23; PMID:19737400; http://dx.doi.org/ 10.1186/1478-811X-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lozano E, Frasa MA, Smolarczyk K, Knaus UG, Braga VM. PAK is required for the disruption of E-cadherin adhesion by the small GTPase Rac. J Cell Sci 2008; 121:933-8; PMID:18319303; http://dx.doi.org/ 10.1242/jcs.016121 [DOI] [PubMed] [Google Scholar]
- 127. Braga VM, Betson M, Li X, Lamarche-Vane N. Activation of the small GTPase Rac is sufficient to disrupt cadherin-dependent cell-cell adhesion in normal human keratinocytes. Mol Biol Cell 2000; 11:3703-21; PMID:11071901; http://dx.doi.org/ 10.1091/mbc.11.11.3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Akhtar N, Hotchin NA. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell 2001; 12:847-62; PMID:11294891; http://dx.doi.org/ 10.1091/mbc.12.4.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Fischer RS, Quinlan MP. Identification of a novel mechanism of regulation of the adherens junction by E1A, Rac1, and cortical actin filaments that contributes to tumor progression. Cell Growth Differ: Mol Biol J Am Assoc Cancer Res 1998; 9:905-18 [PubMed] [Google Scholar]
- 130. Quinlan MP. Rac regulates the stability of the adherens junction and its components, thus affecting epithelial cell differentiation and transformation. Oncogene 1999; 18:6434-42; PMID:10597245; http://dx.doi.org/ 10.1038/sj.onc.1203026 [DOI] [PubMed] [Google Scholar]
- 131. Kawasaki Y, Sato R, Akiyama T. Mutated APC and Asef are involved in the migration of colorectal tumour cells. Nat Cell Biol 2003; 5:211-5; PMID:12598901; http://dx.doi.org/ 10.1038/ncb937 [DOI] [PubMed] [Google Scholar]
- 132. Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factorscatter factor-induced adherens junction disassembly. Mol Biol Cell 1998; 9:2185-200; PMID:9693375; http://dx.doi.org/ 10.1091/mbc.9.8.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shintani Y, Wheelock MJ, Johnson KR. Phosphoinositide-3 kinase-Rac1-c-Jun NH2-terminal kinase signaling mediates collagen I-induced cell scattering and up-regulation of N-cadherin expression in mouse mammary epithelial cells. Mol Biol Cell 2006; 17:2963-75; PMID:16624865; http://dx.doi.org/ 10.1091/mbc.E05-12-1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yagi R, Waguri S, Sumikawa Y, Nada S, Oneyama C, Itami S, Schmedt C, Uchiyama Y, Okada M. C-terminal Src kinase controls development and maintenance of mouse squamous epithelia. EMBO J 2007; 26:1234-44; PMID:17304209; http://dx.doi.org/ 10.1038/sj.emboj.7601595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Holeiter G, Bischoff A, Braun AC, Huck B, Erlmann P, Schmid S, Herr R, Brummer T, Olayioye MA. The RhoGAP protein Deleted in Liver Cancer 3 (DLC3) is essential for adherens junctions integrity. Oncogenesis 2012; 1:e13; PMID:23552697; http://dx.doi.org/ 10.1038/oncsis.2012.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci 2003; 116:725-42; PMID:12538773; http://dx.doi.org/ 10.1242/jcs.00300 [DOI] [PubMed] [Google Scholar]
- 137. Bruewer M, Hopkins AM, Hobert ME, Nusrat A, Madara JL. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am J Physiol Cell Physiol 2004; 287:C327-35; PMID:15044152; http://dx.doi.org/ 10.1152/ajpcell.00087.2004 [DOI] [PubMed] [Google Scholar]
- 138. Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol 1998; 142:101-15; PMID:9660866; http://dx.doi.org/ 10.1083/jcb.142.1.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rojas R, Ruiz WG, Leung SM, Jou TS, Apodaca G. Cdc42-dependent modulation of tight junctions and membrane protein traffic in polarized Madin-Darby canine kidney cells. Mol Biol Cell 2001; 12:2257-74; PMID:11514615; http://dx.doi.org/ 10.1091/mbc.12.8.2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, Lemichez E, Boquet P, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Nat Acad Sci U S A 1995; 92:10629-33; PMID:7479854; http://dx.doi.org/ 10.1073/pnas.92.23.10629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol 2011; 13:159-66; PMID:21258369; http://dx.doi.org/ 10.1038/ncb2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Itoh M, Tsukita S, Yamazaki Y, Sugimoto H. Rho GTP exchange factor ARHGEF11 regulates the integrity of epithelial junctions by connecting ZO-1 and RhoA-myosin II signaling. Proc Nat Acad Sci U S A 2012; 109:9905-10; PMID:22665792; http://dx.doi.org/ 10.1073/pnas.1115063109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Herder C, Swiercz JM, Muller C, Peravali R, Quiring R, Offermanns S, Wittbrodt J, Loosli F. ArhGEF18 regulates RhoA-Rock2 signaling to maintain neuro-epithelial apico-basal polarity and proliferation. Development 2013; 140:2787-97; PMID:23698346; http://dx.doi.org/ 10.1242/dev.096487 [DOI] [PubMed] [Google Scholar]
- 144. Nakajima H, Tanoue T. Lulu2 regulates the circumferential actomyosin tensile system in epithelial cells through p114RhoGEF. J Cell Biol 2011; 195:245-61; PMID:22006950; http://dx.doi.org/ 10.1083/jcb.201104118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Xu X, Jin D, Durgan J, Hall A. LKB1 controls human bronchial epithelial morphogenesis through p114RhoGEF-dependent RhoA activation. Mol Cell Biol 2013; 33:2671-82; PMID:23648482; http://dx.doi.org/ 10.1128/MCB.00154-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Terry S, Nie M, Matter K, Balda MS. Rho signaling and tight junction functions. Physiol (Bethesda) 2010; 25:16-26; PMID:20134025; http://dx.doi.org/ 10.1152/physiol.00034.2009 [DOI] [PubMed] [Google Scholar]
- 147. Hasegawa H, Fujita H, Katoh H, Aoki J, Nakamura K, Ichikawa A, Negishi M. Opposite regulation of transepithelial electrical resistance and paracellular permeability by Rho in Madin-Darby canine kidney cells. J Biol Chem 1999; 274:20982-8; PMID:10409646; http://dx.doi.org/ 10.1074/jbc.274.30.20982 [DOI] [PubMed] [Google Scholar]
- 148. Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol 2005; 170:1029-37; PMID:16186252; http://dx.doi.org/ 10.1083/jcb.200502129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Guillemot L, Paschoud S, Jond L, Foglia A, Citi S. Paracingulin regulates the activity of Rac1 and RhoA GTPases by recruiting Tiam1 and GEF-H1 to epithelial junctions. Mol Biol Cell 2008; 19:4442-53; PMID:18653465; http://dx.doi.org/ 10.1091/mbc.E08-06-0558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. El-Hashash AH, Turcatel G, Varma S, Berika M, Al Alam D, Warburton D. Eya1 protein phosphatase regulates tight junction formation in lung distal epithelium. J Cell Sci 2012; 125:4036-48; PMID:22685326; http://dx.doi.org/ 10.1242/jcs.102848 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 151. Otani T, Ichii T, Aono S, Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J Cell Biol 2006; 175:135-46; PMID:17015620; http://dx.doi.org/ 10.1083/jcb.200605012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Togawa A, Sfakianos J, Ishibe S, Suzuki S, Fujigaki Y, Kitagawa M, Mellman I, Cantley LG. Hepatocyte Growth Factor stimulated cell scattering requires ERK and Cdc42-dependent tight junction disassembly. Biochem Biophys Res Commun 2010; 400:271-7; PMID:20728428; http://dx.doi.org/ 10.1016/j.bbrc.2010.08.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Aijaz S, D’Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1S phase transition. Dev Cell 2005; 8:777-86; PMID:15866167; http://dx.doi.org/ 10.1016/j.devcel.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 154. Samarin SN, Ivanov AI, Flatau G, Parkos CA, Nusrat A. RhoRho-associated kinase-II signaling mediates disassembly of epithelial apical junctions. Mol Biol Cell 2007; 18:3429-39; PMID:17596509; http://dx.doi.org/ 10.1091/mbc.E07-04-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Benais-Pont G, Punn A, Flores-Maldonado C, Eckert J, Raposo G, Fleming TP, Cereijido M, Balda MS, Matter K. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J Cell Biol 2003; 160:729-40; PMID:12604587; http://dx.doi.org/ 10.1083/jcb.200211047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Nie M, Aijaz S, Leefa Chong San IV, Balda MS, Matter K. The Y-box factor ZONABDbpA associates with GEF-H1Lfc and mediates Rho-stimulated transcription. EMBO Rep 2009; 10:1125-31; PMID:19730435; http://dx.doi.org/ 10.1038/embor.2009.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Coleman ML, Marshall CJ, Olson MF. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat Rev Mol Cell Biol 2004; 5:355-66; PMID:15122349; http://dx.doi.org/ 10.1038/nrm1365 [DOI] [PubMed] [Google Scholar]
- 158. Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, et al. . A Rich1Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell 2006; 125:535-48; PMID:16678097; http://dx.doi.org/ 10.1016/j.cell.2006.02.045 [DOI] [PubMed] [Google Scholar]
- 159. Yang L, Wang L, Zheng Y. Gene targeting of Cdc42 and Cdc42GAP affirms the critical involvement of Cdc42 in filopodia induction, directed migration, and proliferation in primary mouse embryonic fibroblasts. Mol Biol Cell 2006; 17:4675-85; PMID:16914516; http://dx.doi.org/ 10.1091/mbc.E06-05-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Irvine KD, Wieschaus E. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development 1994; 120:827-41; PMID:7600960 [DOI] [PubMed] [Google Scholar]
- 161. Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell 2004; 6:343-55; PMID:15030758; http://dx.doi.org/ 10.1016/S1534-5807(04)00060-7 [DOI] [PubMed] [Google Scholar]
- 162. Tan JL, Ravid S, Spudich JA. Control of nonmuscle myosins by phosphorylation. Ann Rev Biochem 1992; 61:721-59; PMID:1497323; http://dx.doi.org/ 10.1146/annurev.bi.61.070192.003445 [DOI] [PubMed] [Google Scholar]
- 163. Nikolaidou KK, Barrett K. A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Curr Biol: CB 2004; 14:1822-6; PMID:15498489; http://dx.doi.org/ 10.1016/j.cub.2004.09.080 [DOI] [PubMed] [Google Scholar]
- 164. Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 1997; 91:905-15; PMID:9428514; http://dx.doi.org/ 10.1016/S0092-8674(00)80482-1 [DOI] [PubMed] [Google Scholar]
- 165. He B, Doubrovinski K, Polyakov O, Wieschaus E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature 2014; 508:392-6; PMID:24590071; http://dx.doi.org/ 10.1038/nature13070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Gorfinkiel N, Blanchard GB, Adams RJ, Martinez Arias A. Mechanical control of global cell behaviour during dorsal closure in Drosophila. Development 2009; 136:1889-98; PMID:19403661; http://dx.doi.org/ 10.1242/dev.030866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 2009; 137:1331-42; PMID:19563762; http://dx.doi.org/ 10.1016/j.cell.2009.03.050 [DOI] [PubMed] [Google Scholar]
- 168. David DJ, Tishkina A, Harris TJ. The PAR complex regulates pulsed actomyosin contractions during amnioserosa apical constriction in Drosophila. Development 2010; 137:1645-55; PMID:20392741; http://dx.doi.org/ 10.1242/dev.044107 [DOI] [PubMed] [Google Scholar]
- 169. Harden N, Ricos M, Yee K, Sanny J, Langmann C, Yu H, Chia W, Lim L. Drac1 and Crumbs participate in amnioserosa morphogenesis during dorsal closure in Drosophila. J Cell Sci 2002; 115:2119-29; PMID:11973353 [DOI] [PubMed] [Google Scholar]
- 170. David DJ, Tishkina A, Harris TJ. The PAR complex regulates pulsed actomyosin contractions during amnioserosa apical constriction in Drosophila. Development 2010; 137:1645-55; PMID:20392741; http://dx.doi.org/ 10.1242/dev.044107 [DOI] [PubMed] [Google Scholar]
- 171. Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol 2011; 192:907-17; PMID:21422226; http://dx.doi.org/ 10.1083/jcb.201009141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ngok SP, Geyer R, Liu M, Kourtidis A, Agrawal S, Wu C, Seerapu HR, Lewis-Tuffin LJ, Moodie KL, Huveldt D, et al. . VEGF and Angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. J Cell Biol 2012; 199:1103-15; PMID:23253477; http://dx.doi.org/ 10.1083/jcb.201207009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci 2008; 121:989-1001; PMID:18319301; http://dx.doi.org/ 10.1242/jcs.020693 [DOI] [PubMed] [Google Scholar]
- 174. Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 2005; 121:451-63; PMID:15882626; http://dx.doi.org/ 10.1016/j.cell.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 175. Wang S, Watanabe T, Matsuzawa K, Katsumi A, Kakeno M, Matsui T, Ye F, Sato K, Murase K, Sugiyama I, et al. . Tiam1 interaction with the PAR complex promotes talin-mediated Rac1 activation during polarized cell migration. J Cell Biol 2012; 199:331-45; PMID:23071154; http://dx.doi.org/ 10.1083/jcb.201202041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Pegtel DM, Ellenbroek SI, Mertens AE, van der Kammen RA, de Rooij J, Collard JG. The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr Biol: CB 2007; 17:1623-34; PMID:17825562; http://dx.doi.org/ 10.1016/j.cub.2007.08.035 [DOI] [PubMed] [Google Scholar]
- 177. Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, Amano M, Kaibuchi K. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell 2008; 14:205-15; PMID:18267089; http://dx.doi.org/ 10.1016/j.devcel.2007.11.021 [DOI] [PubMed] [Google Scholar]
- 178. Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol 2004; 265:23-32; PMID:14697350; http://dx.doi.org/ 10.1016/j.ydbio.2003.06.003 [DOI] [PubMed] [Google Scholar]
- 179. Friedl P, Wolf K, Zegers MM. Rho-directed forces in collective migration. Nat Cell Biol 2014; 16:208-10; PMID:24576897; http://dx.doi.org/ 10.1038/ncb2923 [DOI] [PubMed] [Google Scholar]
- 180. Johnson DI. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev: MMBR 1999; 63:54-105; PMID:10066831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ory S, Gasman S. Rho GTPases and exocytosis: what are the molecular links? Semin Cell Dev Biol 2011; 22:27-32; PMID:21145407; http://dx.doi.org/ 10.1016/j.semcdb.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 182. Mayor R, Theveneau E. The role of the non-canonical Wnt-planar cell polarity pathway in neural crest migration. Biochem J 2014; 457:19-26; PMID:24325550; http://dx.doi.org/ 10.1042/BJ20131182 [DOI] [PubMed] [Google Scholar]
- 183. Tahirovic S, Bradke F. Neuronal polarity. Cold Spring Harbor Perspect Biol 2009; 1:a001644; PMID:20066106; http://dx.doi.org/ 10.1101/cshperspect.a001644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Macara IG. Par proteins: partners in polarization. Curr Biol: CB 2004; 14:R160-2; PMID:15027470; http://dx.doi.org/ 10.1016/j.cub.2004.01.048 [DOI] [PubMed] [Google Scholar]
- 185. Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell 2007; 13:609-22; PMID:17981131; http://dx.doi.org/ 10.1016/j.devcel.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Nance J, Zallen JA. Elaborating polarity: PAR proteins and the cytoskeleton. Development 2011; 138:799-809; PMID:21303844; http://dx.doi.org/ 10.1242/dev.053538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 2001; 106:489-98; PMID:11525734; http://dx.doi.org/ 10.1016/S0092-8674(01)00471-8 [DOI] [PubMed] [Google Scholar]
- 188. Kolega J. Asymmetric distribution of myosin IIB in migrating endothelial cells is regulated by a rho-dependent kinase and contributes to tail retraction. Mol Biol Cell 2003; 14:4745-57; PMID:12960430; http://dx.doi.org/ 10.1091/mbc.E03-04-0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol 2001; 154:147-60; PMID:11448997; http://dx.doi.org/ 10.1083/jcb.200103048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 2006; 440:1069-72; PMID:16547516; http://dx.doi.org/ 10.1038/nature04665 [DOI] [PubMed] [Google Scholar]
- 191. Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature 2009; 461:99-103; PMID:19693013; http://dx.doi.org/ 10.1038/nature08242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. El-Sibai M, Pertz O, Pang H, Yip SC, Lorenz M, Symons M, Condeelis JS, Hahn KM, Backer JM. RhoAROCK-mediated switching between Cdc42- and Rac1-dependent protrusion in MTLn3 carcinoma cells. Exp Cell Res 2008; 314:1540-52; PMID:18316075; http://dx.doi.org/ 10.1016/j.yexcr.2008.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Petrie RJ, Yamada KM. At the leading edge of three-dimensional cell migration. J Cell Sci 2012; 125:5917-26; PMID:23378019; http://dx.doi.org/ 10.1242/jcs.093732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Osmani N, Peglion F, Chavrier P, Etienne-Manneville S. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol 2010; 191:1261-9; PMID:21173111; http://dx.doi.org/ 10.1083/jcb.201003091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Bahri S, Wang S, Conder R, Choy J, Vlachos S, Dong K, Merino C, Sigrist S, Molnar C, Yang X, et al. . The leading edge during dorsal closure as a model for epithelial plasticity: pak is required for recruitment of the Scribble complex and septate junction formation. Development 2010; 137:2023-32; PMID:20501591; http://dx.doi.org/ 10.1242/dev.045088 [DOI] [PubMed] [Google Scholar]
- 196. Royer C, Lu X. Epithelial cell polarity: a major gatekeeper against cancer? Cell Death Differ 2011; 18:1470-7; PMID:21617693; http://dx.doi.org/ 10.1038/cdd.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Xue B, Krishnamurthy K, Allred DC, Muthuswamy SK. Loss of Par3 promotes breast cancer metastasis by compromising cell-cell cohesion. Nat Cell Biol 2013; 15:189-200; PMID:23263278; http://dx.doi.org/ 10.1038/ncb2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, Allred C, Muthuswamy SK. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell 2008; 135:865-78; PMID:19041750; http://dx.doi.org/ 10.1016/j.cell.2008.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Pearson HB, Perez-Mancera PA, Dow LE, Ryan A, Tennstedt P, Bogani D, Elsum I, Greenfield A, Tuveson DA, Simon R, et al. . SCRIB expression is deregulated in human prostate cancer, and its deficiency in mice promotes prostate neoplasia. J Clin Invest 2011; 121:4257-67; PMID:21965329; http://dx.doi.org/ 10.1172/JCI58509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Feigin ME, Akshinthala SD, Araki K, Rosenberg AZ, Muthuswamy LB, Martin B, Lehmann BD, Berman HK, Pietenpol JA, Cardiff RD, et al. . Mislocalization of the cell polarity protein scribble promotes mammary tumorigenesis and is associated with Basal breast cancer. Cancer Res 2014; 74:3180-94; PMID:24662921; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Liu S, Li Y, Qi W, Zhao Y, Huang A, Sheng W, Lei B, Lin P, Zhu H, Li W, et al. . Expression of Tiam1 predicts lymph node metastasis and poor survival of lung adenocarcinoma patients. Diagn Pathol 2014; 9:69; PMID:24661909; http://dx.doi.org/ 10.1186/1746-1596-9-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Wang S, Li S, Yang X, Yang S, Liu S, Liu B, Liu J. Elevated expression of T-lymphoma invasion and metastasis inducing factor 1 in squamous-cell carcinoma of the head and neck and its clinical significance. Eur J Cancer 2014; 50:379-87; PMID:24189000; http://dx.doi.org/ 10.1016/j.ejca.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 203. Qi Y, Huang B, Yu L, Wang Q, Lan G, Zhang Q. Prognostic value of Tiam1 and Rac1 overexpression in nasopharyngeal carcinoma. ORL; J Oto-Rhino-Laryngol Its Relat Spec 2009; 71:163-71; PMID:19506399; http://dx.doi.org/ 10.1159/000223440 [DOI] [PubMed] [Google Scholar]
- 204. Liu N, Tang LL, Sun Y, Cui RX, Wang HY, Huang BJ, He QM, Jiang W, Ma J. MiR-29c suppresses invasion and metastasis by targeting TIAM1 in nasopharyngeal carcinoma. Cancer Lett 2013; 329:181-8; PMID:23142282; http://dx.doi.org/ 10.1016/j.canlet.2012.10.032 [DOI] [PubMed] [Google Scholar]
- 205. Lane J, Martin TA, Mansel RE, Jiang WG. The expression and prognostic value of the guanine nucleotide exchange factors (GEFs) Trio, Vav1 and TIAM-1 in human breast cancer. Int Semin Surg Oncol: ISSO 2008; 5:23; PMID:18925966; http://dx.doi.org/ 10.1186/1477-7800-5-23 [DOI] [PMC free article] [PubMed] [Google Scholar]