Abstract
Rac and PI3Ks are intracellular signal transducers able to regulate multiple signaling pathways fundamental for cell behavior. PI3Ks are lipid kinases that produce phosphorylated lipids which, in turn, transduce extracellular cues within the cell, while Rac is a small G protein that impacts on actin organization. Compelling evidence indicates that in multiple circumstances the 2 signaling pathways appear intermingled. For instance, phosphorylated lipids produced by PI3Ks recruit and activate GEF and GAP proteins, key modulators of Rac function. Conversely, PI3Ks interact with activated Rac, leading to Rac signaling amplification. This review summarizes the molecular mechanisms underlying the cross-talk between Rac and PI3K signaling in 2 different processes, cell migration and ROS production.
Keywords: actin polymerization, inflammation, migration, PI3K, rho GTPase, ROS production
The Rho Family GTPases
Rho GTPases constitute one of the 5 distinct families of the Ras superfamily.1 Members of the Rho GTPase family are found in all eukaryotic organisms and have been implicated in the regulation of several aspects of intracellular actin dynamics. Rho, Rac and Cdc42 members show common intracellular functions as they promote cell growth, inhibit apoptosis and regulate gene expression.2 They also promote actin cytoskeleton reorganization, although each isoform affects distinct features of cell shape and movement.3
Different plasma membrane receptors, including tyrosine kinase (RTKs), G protein-coupled (GPCRs) and cytokine receptors can initiate the signaling cascade engaging Rho GTPases. Rho GTPase activation is mediated by a multistep reaction involving guanine exchange factor proteins (GEFs) that favor the exchange between GDP and GTP on the RhoGTPase.4 GEF proteins do not stimulate the direct binding of the GTPase to GDP/GTP, but stabilize the nucleotide-free state of the enzyme. Since the free nucleotide-binding site of the GTPase has similar affinity for GTP and GDP, the binding of the GTPase to GTP is thus only determined by the high intracellular concentration of this nucleotide which is 10-fold more abundant than GDP.5 Subsequently, the binding of GTP displaces the GEF and frees the GTPase to interact with effectors.6-8 Similar to many other signaling enzymes, the activation status of Rho GTPases is finely regulated. Rho GTPases possess an inefficient intrinsic GTPase activity that is enhanced by GTPase Activating Proteins (GAPs) which promote the formation of the inactive GDP-bound form.6,9,10 In this context, GEFs and GAPs represent key determinants and regulators of Rho GTPase activity. Another level of regulation of Rho GTPases involves a class of inhibitory proteins defined GDP dissociation inhibitors (GDIs). GDIs negatively regulate Rho GTPases through 2 different mechanisms. Firstly, they bind to the GTPase thus impeding the dissociation of GDP. Secondly, since the membrane localization of Rho GTPases is essential for their biological function, GDIs can inhibit Rho GTPases by interacting with prenylated Rho GTPases and subsequently sequestering them in the cytosol.11,12
Based on the primary amino acid sequence, the structural motifs and the biological function, the Rho family can be divided into 6 subfamilies, and among them, the RhoA-related, the Rac1-related and the Cdc42-related subfamilies are the best characterized.3 Rac GTPases are intracellular signal transducers implicated in controlling actin cytoskeleton organization, cell migration, proliferation and survival in mammalian cells.3,13 The Rac subfamily includes different members, encoded by distinct genes: the ubiquitously expressed Rac1, the widely expressed RhoG, the haematopoietic -specific Rac2, and Rac3 which is predominantly expressed in the brain. Although they appear to have redundant functions, in some circumstances they play isoform-specific roles.2 Rac1 predominantly regulates actin organization of cytoskeleton and cell adhesion and its genetic ablation results in embryonic lethality during gastrulation, due to a defective formation of the 3 germ layers.14 Conversely, the absence of Rac2 expression is compatible with life, but results in deficiencies of haematopoietic cells, including severe neutrophilic, phagocytic and lymphocytic defects15-18 Rac3 isoform is not strictly required for normal development in utero, but may be relevant to later events in the development of a functional nervous system.19 RhoG deficiency produces a mild phenotype in lymphocytes where it is abundantly expressed, indicating a functional redundancy of RhoG with other Rac proteins in these cells.20
In its active state, Rac signals by recruiting and activating numerous proteins, including, protein kinases, lipid kinases, hydrolases, phosphatases, actin-binding proteins and the actin cytoskeleton.21 Some of these Rac effectors are bound directly by Rac-GTP and, among them, PAK and MLK22 regulate actin polymerization, PAK23 and p70S6K24 control cell proliferation and transcription, and p67phox25 controls ROS production.21,26,27 In other contexts, Rac-GTP promotes the activation of indirect targets such as MEKK128 and WAVE.29 Finally, inactive GDP-bound Rac is also able to indirectly trigger downstream effectors, such as IQGAP1/2.30
Class I PI3K
The PI3K family includes 3 different classes of enzymes able to phosphorylate the D3 position of the inositol ring of phosphatidylinositol (PtdIns) as well as its phosphorylated derivatives. Among them, class I PI3K represents the most characterized group of enzymes that produce PtdIns(3,4,5)P3 from PtdIns(4,5)P2. They operate downstream of different cell surface receptors and, upon activation, they exert their role through the recruitment of signaling effectors carrying phosphoinositide-recognition modules, such as the FYVE zinc finger (Fab-1, YGL023, Vps27, and EEA1), the PH (pleckstrin homology), the GRAM (Glucosyltransferases, Rab-like GTPase activators and Myotubularins), the GLUE (GRAM-like, ubiquitin-binding on Eap45) and the PX (phosphoinositide-binding) domains.31
Class I PI3Ks are dimeric enzymes composed by a 110 kDa catalytic subunit and a regulatory adaptor. On the basis of the different regulatory subunit, class I is further divided into class IA, represented by PI3Kα, PI3Kβ and PI3Kδ, and class IB whose unique member is PI3Kγ. Class IA catalytic subunits, p110α, p110β and p110δ associate with 5 different regulatory subunits, p85β, p55γ, p85α and its splice variants p55α and p50α. All catalytic subunits can form dimers with any of the type IA regulatory subunit.32 Class IA enzymes exist in a heterodimeric form uniquely, since monomeric p85 is unstable and loss of p110α and p110β results in a concomitant reduction of p85 expression levels.33-35 PI3K regulatory subunits can affect the activity of the p110 catalytic subunit by promoting its stabilization, by inhibiting its enzymatic activity and by recruiting it to phosphorylated tyrosine residues on RTKs or on adaptor proteins, such as the insulin receptor substrate (IRS). The full length p85 subunit is structurally characterized by distinct domains, including an N-terminal SH3 (Src homolory-3) region, a BCR (breakpoint cluster region) and 2 SH2 domains, respectively at the N-terminus (n-SH2) and C-terminus of the protein (c-SH2), separated by an intervening coiled-coil domain (iSH2). On the contrary, the p50α, p55α and p55γ are shorter variants of the regulatory subunits and they maintain only the 2 SH2 domains and the iSH2.36,37 Class I catalytic subunits share common domains represented by an N-terminal adaptor binding domain (ABD), a Ras binding domain (RBD), a C2 domain (C2), a helical domain and a C-terminal kinase domain. During the formation of the heterodimer, the regulatory subunit contacts, with high affinity, the ABD domain of the p110 catalytic subunit through the iSH2.38,39
Among class IA PI3Ks, the PI3Kβ isoform is the unique member to be recruited downstream of GPCR and to be activated by the direct binding of Gβγ.40,41 The Gβγ subunit directly binds p110β within a region located between the C2 and the helical domains.42 Intriguingly, Gβγ is able to stimulate p110β activity even in the absence of p85 and phosphotyrosine.42
Differently from class IA enzymes, the single class IB isotype, p110γ, interacts with either a p101 or a p84/87 regulatory subunit.43-45 Both p101 and p84/87 facilitate the activation of the p110γ enzymatic activity downstream of GPCRs and determine the substrate specificity in vitro.46 The N- and C-terminal regions of p101 are critically involved in the binding to p110γ and to Gβγ subunits of GPCRs, respectively, and the residues required for this interaction have been recently characterized.47
Another important mechanism of PI3K activation involves the small GTPase Ras. Activation of PI3K by Ras was demonstrated for p110α and p110γ and was shown to be critical for regulation of cell growth, survival, cytoskeleton reorganization, and metabolism.48,49 Notably, Ras binds to the p110 catalytic subunit directly and independently of the p85 adaptor.50,51
Class II PI3Ks are 3 monomeric enzymes, PI3KC2α, PI3KC2β and PI3KC2γ, composed by a Ras binding domain, 2 C2 domains, a PX domain and a catalytic domain. Recently, this class has emerged as a key regulator of vesicle trafficking and signal transduction downstream of RTKs. In particular, PI3KC2α52,53 and PI3KC2β54,55 activate Rho family GTPases at the plasma membrane and in endosomes, through a lipid-dependent mechanism. Class III PI3Ks comprise only one protein, Vps34, that is composed by a C2 and a catalytic domain, associated with a regulatory subunit named p150, and is responsible for the production of PtdIns(3)P in endosomes to stimulate vesicle transport and autophagy.56
PtdIns(3,4,5)P3 as a Linker for Rac Activation
The understanding of the role of class I PI3K, and of its lipid product PtdIns(3,4,5)P3, in Rac signaling is mainly due to studies using selective PI3K inhibitors. In particular, the treatment with the fungal metabolite Wortmannin and the expression of dominant negative enzymes have allowed the identification of a functional link between PI3K- and Rac-mediated signaling pathways in response to extracellular cues.57-59 Once activated, class I PI3Ks produce PtdIns(3,4,5)P3 which functions as a second messenger that recruits proteins containing phosphoinositide-binding modules, like the PH domain. The PH domain was also identified in several members of the GEF family, including Dbl proteins.60,61 All members of the Dbl family share structural similarities and, in particular, they possess common modules such as the DH (Dbl homology) domain, a 240 residue region involved in the GTP exchange reaction, and a C-terminal PH domain.62-64 The identification of a PH domain, able to bind membrane phosphoinositides in different GEFs, has initially provided a possible mechanism whereby cytosolic proteins like GEFs are translocated to the plasma membrane upon receptor activation.65 For instance, the T-cell lymphoma invasion and metastasis 1 protein (TIAM1) presents 2 different PH domains, one at the N terminus and the other one at the C terminus, associated with the DH domain. The N terminal PH region interacts with PtdIns(3,4,5)P3 and enhances Rac1 activation, both in vitro and in vivo. However, TIAM1 localization on the plasma membrane appears to be independent of PtdIns(3,4,5)P3 as the presence of PtdIns(3,4,5)P3 is not sufficient to recruit to the plasma membrane an isolated TIAM1 PH domain.66 On the contrary, the class III PI3K lipid product, the PtdIns(3)P, seems to be sufficient for membrane recruitment of TIAM to the early endosomes.67 Accordingly, further analysis demonstrates that the PH domain of GEFs possesses only a micromolar affinity for phosphorylated lipids (summarized in Table 1) compared to the PH domains found in other proteins,68 thus suggesting a lipid-independent mechanism for membrane recruitment and a different role for the PH domain of GEFs.69 The membrane localization of the GEF upon GPCR stimulation can be instead mediated by the interaction between the Gβγ subunits of the activated G protein and the nucleotide exchanger, such as in the case of the PtdIns(3,4,5)P3-dependent Rac exchanger 1 protein (P-Rex).70 The analysis of the crystal structure of the GEF-Rho GTPase complex, demonstrates a role for the PH domain not only as a docking module, but also as an intra-molecular inhibitor of the DH domain activity. This model was demonstrated for TIAM and for the dual Rho-Ras GEF Son of Sevenless (SOS), also acting as a GEF for Rac, and it is supported by the finding that deletion of the PH domain within these proteins leads to constitutive in vitro and in vivo activation of Rac.47,53,71 Relief of this autoinhibition is a major mechanism of GEF regulation and is accomplished by phosphorylation of specific residues and/or protein-protein interactions that in certain conditions can involve phosphoinositide binding. The ability of PtdIns(3,4,5)P3 to release the inhibitory effect of the PH domain has been demonstrated for the protein P-REX. The binding of PtdIns(3,4,5)P3 to this GEF results in the inhibition of the DH-PH interaction and increases the GEF activity by 10 folds.72 Notably, a synergistic cooperation between PtdIns(3,4,5)P3 and the Gβγ subunits of the GPCR is able to induce a 50-fold change in GEF activity.72 Another example is represented by the Vav protein whose activity is regulated by phosphorylation of the Tyr174 residue,73,54 and in some circumstances can be potentiated by the binding of PtdIns(3,4,5)P3 and inhibited by the interaction with PtdIns(4,5)P2.39 In particular, the binding of PtdIns(3,4,5)P3 to the PH domain of Vav releases the inhibitory action and, at the same time, induces the exposure of the Tyr174 residue that is promptly phosphorylated by Src-family kinases, with consequent activation of the GEF activity.74 Alternatively, the activity of some GEFs can be modulated by specific protein-protein interactions: for instance, the nucleotide exchange of SOS is enhanced when PI3K is recruited to the Eps8-Abi1-Sos complex.75,76 Non-universal mechanisms of GEF activation are also reported. For instance, the non-canonical GEF protein DOCK180 is recruited to the plasma membrane by a PtdIns(3,4,5)P3-dependent mechanism, but its full activation is lipid-independent and strictly relies on a protein-protein interaction with the PH domain of ELMO.77,78 Another example is provided by the atypical GEF protein Swap-70 whose PH domain is required for the membrane translocation of the protein after EGF stimulation, through a Ras-independent mechanism. In primary fibroblasts, the binding of Swap-70 to membrane PtdIns(3,4,5)P3 stimulates nucleotide exchange and eventually results in increased membrane ruffling.79 Altogether, these data are consistent with a model where PtdIns(3,4,5)P3 can concomitantly favor the membrane translocation of GEFs and positively regulate the exchange reaction. In addition, PtdIns(3,4,5)P3 can promote GEF activity also by interacting with the GEF itself and driving a conformational change of the protein which ultimately allows the binding with Rac and the ensuing stabilization in the nucleotide-free conformation.
Table 1.
Molecular interactions between membrane lipids and RacGEFs
|
GEF |
Lipid Binding Domain(s) |
PI3K lipid product |
Effect(s) |
| Tiam 1 | DH-PH, PH N-ter and PH C-ter | PtdIns3P144–146 PtdIns(3,4)P2144 PtdIns(3,4,5)P3144 |
No effect on GEF activity146 Enhanced Guanine Nucleotide Exchange activity in vitro 66 Stimulated GDP/GTP exchange on Rac1 in vivo 147 |
| Intersectin | DH-PH | PtdIns3P145 PtdIns(3,4)P2145 |
No effect145,148 |
| Dbs | DH-PH | PtdIns3P145,149 PtdIns(3,4)P2145 PtdIns(3,4,5)P3145 |
PH domain is required for efficient exchange in vivo69 PH-DH domains may promote guanine nucleotide exchange of Rho GTPases150 The PH domain contributes independently to membrane targeting151 |
| Vav1 | FL | PtdIns(3,4,5)P3132 | PtdIns(3,4,5)P3 enhances phosphorylation and activation of Vav by Lck132 Recruitment to the plasma membrane152 |
| P-Rex1 | FL and DH-PH | PtdIns(3,4)P2 PtdIns(3,4,5)P3 |
Stimulate the GEF activity153,72 |
| Dbl | PH | PtdIns(3,4,5)P3 | The binding to PtdIns(3,4,5)P3 inhibits the GEF activity 154 The PH domain contributes to the membrane recruitment of Dbl154 |
The analysis of knockout mouse models of different GEFs demonstrated a functional redundancy between these proteins. For instance, the absence of Dbl, Vav1/2/3, Tiam1 and P-Rex1 expression does not result in embryonic lethality or developmental defects.80-85 Likewise, genetic inactivation of all 3 Vav genes induces alteration in the haematopoietic lineage83 and only the simultaneous loss of Vav1 and Vav2 leads to B cell defects.81,86
While compelling evidence supports the role of PtdIns(3,4,5)P3 as positive regulator of Rac activity, several studies demonstrate an additional function as inhibitor of Rac signaling. On these grounds, PtdIns(3,4,5)P3 has been involved in the activation of Rac-GAPs, since some isoforms present a lipid-binding domain able to activate and localize the enzyme at the plasma membrane. A prototype of GAP proteins regulated by lipid interaction is represented by the cdGAPs family. cdGAPs contain a polybasic region (PBR) able to interact with PtdIns(3,4,5)P3 and this binding is required for full inactivation of Rac1 signaling.87,88 PtdIns(3,4,5)P3-binding domains have been also identified in other GAPs, such as p190 Rho-GAPs that possess a PBR (polybasic region) domain able to recognize phosphorylated lipids,89,90 and the ArhGAP family, characterized by a PH domain.91 In particular, ArhGAP15 has been suggested to be activated downstream of PI3Kγ and to negatively regulate Rac activity in C5a-stimulated bone marrow-derived macrophages.92
All these data thus support a fundamental interplay between PI3K lipid kinase activity and Rac signaling, with the PI3K lipid product affecting crucial modulators of Rac activity.
GTPases as Linkers for PI3K Activation
Although a large body of evidence identifies the PI3K lipid product as a key activator of Rac signaling, several works define PI3K as a possible direct effector of activated Rho-GTPases. The first evidence that PI3K can be activated by small GTPases comes from the finding that PI3K interacts with the GTP-bound forms of CDC42Hs and Rac1, via the p85 regulatory subunit, and serves as an effector of these 2 GTPases.93 Another study demonstrates that Rac1 and Rac2 specifically bind to PI3K in an equimolar complex. Rac interacts with the BCR homology domain of the PI3K regulatory subunit p8593 and such interaction is markedly enhanced when Rac is in the GTP-bound state, thus suggesting that PI3K is a direct target of activated Rac.94 However, evidence indicates that PI3K cannot be directly triggered by Rho-GTPases as GTP[S]-Cdc42 fails to activate recombinant PI3K in vitro and thus suggests that other mechanisms underlie the interaction between Rho-GTPase and PI3K signaling.
Intriguingly, Rho-GTPase and PI3K are causally linked by a feedback loop that is critically involved in the establishment and the maintenance of leukocyte polarity. In particular, through the binding with p85, Rac contributes to the recruitment of PI3K and the ensuing production of PtdIns(3,4,5)P3 at the leading edge of migrating cells. This increase in PtdIns(3,4,5)P3 levels induces GEF-Rac activation that in turn sustains the formation of directional protrusions.95 Experiments in differentiated HL60 cells demonstrate that Rac activity is necessary and sufficient for the accumulation of PtdIns(3,4,5)P3 at the leading edge.96 Likewise, both genetic and pharmacological ablation of different PI3K isoforms results in defective leukocyte migration. For instance, PI3Kγ-deficient neutrophils show loss of directionality during N-formyl-Met-Leu-Phe-(fMLP)-induced chemotaxis.97 In addition, PI3Kδ inhibitors impair PtdIns(3,4,5)P3 accumulation at the leading edge of migrating neutrophils and eventually result in defective chemotaxis.98 Nevertheless, in particular conditions, PI3K activity is dispensable for directional cell migration. The emerging view is that, although PI3K is necessary to accelerate neutrophil migration, PI3K is instead not required for long-term fMLP-driven neutrophil chemotaxis, thus indicating that alternative pathways overcome PI3K and critically control late events during cell migration.99 The feedback loop linking PI3Ks and RhoGTPase signaling pathways appears to be cell-specific as in Hela and NIH3t3 cells, conditional activation of individual members of RhoGTPases, including Rac1, Cdc42 and RhoG, is not sufficient to increase PI3K activity.100 In addition, in Zebrafish, the expression of a genetically-encoded photoactivable version of Rac, namely PA-Rac, rescues the protrusion defects but not the migration defects induced by PI3K inhibition, thereby demonstrating that PI3K activity is essential for neutrophil polarity and motility in vivo.101 Altogether, these works suggest that in vivo other components are required to allow the feedback loop between Rac and PI3K. For instance, simultaneous activation of Rac1, Cdc42 and RhoG, triggers PI3K through a Grb2 and Ras-independent mechanism96,102,103 Therefore, combined engagement of multiple small GTPases is necessary for the establishment of the feedback loop culminating in PI3K activation.100
Activation of the Rac/PI3K feedback loop also involves the process of actin polymerization as cells expressing constitutively active Rac display elevated levels of PtdIns(3,4,5)P3 which can be reduced by actin depolimerization. In addition, experiments using a pharmacological cocktail that inhibits actin dynamics while preserving cell function and cytoskeleton integrity, demonstrate that actin remodeling is required for the spatial persistence of Rac activity.104
Although compelling evidence indicates that the interaction between PI3K and Rac is primarily mediated by the PI3K regulatory subunit p85, the emerging view is that Rac can also interact with PI3K catalytic subunits. A recent report demonstrates that Rac1 and Cdc42, but not Ras, directly associate with the Ras binding domain (RBD) of the PI3K catalytic subunit, p110β. Such association occurs even in the absence of the BH domain of p85, while it is abrogated by point mutations in the RBD of p110β. This work elegantly demonstrates that GPCRs couple to PI3K via Dock180/Elmo1-mediated Rac activation and binding to p110β and that the interaction between Rac and p110β is a crucial determinant of fibroblast migration. Accordingly constitutively active Rac stimulates migration in absence of chemoattractants, but in presence of stimuli triggering p110β, while mutations in the RBD domain of p110β abolish the effect of constitutively active Rac.105
Altogether, these findings clearly support a key role of GTP-bound Rac in the activation of the PI3K signaling via feedback mechanisms, but this activation is context-dependent and requires the action of actin dynamics. In the following sections, we will discuss how Rac and PI3K signaling pathways cooperate in the regulation of essential functions of immune cells, such as migration and ROS production.
PtdIns(3,4,5)P3 Delimits Actin Polymerization at the Leading Edge
Cell migration strictly depends on the ability of cells to polarize. During polarization cells respond to chemoattractants by forming a leading edge where actin is rapidly polymerized and a trailing edge where actin is more stable. This process promotes the formation of protrusions and focal adhesions at the leading edge which, in turn, allow cells to attach to the substrate matrix and to contract the trailing edge.106-108
PI3Ks cooperate with Rac to regulate cell polarization in response to different stimuli and, in particular, to identify and organize the leading edge of migrating cells (Fig. 1).108 The functional interaction between PI3Ks and Rac has a major role in neutrophils, a subset of immune cells that need to rapidly respond to extracellular cues. In general, chemoattractants such as CXCL12, CXCL2 and fMLP activate neutrophil GPCRs leading to the dissociation of the G protein into Gαi and Gßγ subunits. While historically Gαi appeared to be not necessary for cell migration,109 recent evidence points to a key role of this protein in the regulation of directionality and termination of neutrophil chemotaxis.110,111 On the other hand, Gßγ activates major intracellular pathways governing migration, via downstream modulation of PI3Ks and Rac. More recently, a Gβγ effector, named ElmoE, was shown to associate with Dock-like proteins in Dictyostelium to activate Rac and promote actin polymerization. In these cells, ElmoE associates with both Arp2/3 and F-actin, thus serving as a molecular link between GPCR, G proteins and actin cytoskeleton, at least in Dictyostelium, although it is plausible to envisage a similar mechanism in mammalian cells.112,113
Figure 1.
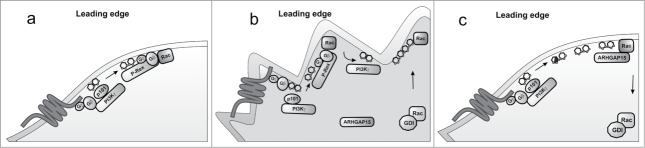
Schematic representation of signaling events activated by PI3K leading to cell polarization. GPCR activation stimulates the lipid kinase activity of PI3K to activate Rac through the GEF protein P-Rex (A) A positive feedback loop mediated by the interaction between PI3K and activated Rac sustains cell polarization (B) ArhGAP15 binds to PtdIns(3,4,5)P3 produced by PI3K to terminate Rac signaling at the leading edge (C).
In unstimulated neutrophils, the lipid product of PI3Ks, PtdIns(3,4,5)P3, is nearly absent, but increases rapidly and dramatically after stimulation with chemoattractants.114 Notably, the increase of PtdIns(3,4,5)P3 concurs to enhanced actin polymerization. This indicates that the burst of PtdIns(3,4,5)P3 is coupled with Rac activation and the ensuing actin polymerization. Consistent with this view, manipulation of PI3K activity, using pharmacological inhibitors or dominant-negative PI3K proteins, results in defective actin polymerization and cell polarization.59,115,116 Conversely, enhancement of intracellular amount of PtdIns(3,4,5)P3, using membrane-permeant lipids, promotes migration and cell polarization.117 Experiments with fluorescent probes for PI3K and Rac activity (AKT-PH domain and PAK-PBD, respectively) demonstrate that PtdIns(3,4,5)P3 production colocalizes with sites of Rac activation at the leading edge during cell polarization.96,118 The asymmetric distribution of phosphorylated lipids between the leading and the trailing edge of polarizing cells is due, at least in part, to the ability of Gßγ subunits to localize and activate class IB PI3Kγ at the leading edge.92,119,120 PI3Kγ is the major responsible for PtdIns(3,4,5)P3 synthesis in neutrophils after stimulation with tripeptide formyl-Met-Leu-Phe (fMLP), interleukin 8 and C5a. Accordingly, neutrophils lacking PI3Kγ have reduced migration, both in vitro and in vivo.121-123 Similarly, the use of a PI3Kγ selective inhibitor (AS252424) reduces directional migration of mouse neutrophils.124,125 Other PI3K isoforms can cooperate with PI3Kγ in regulating neutrophil migration. Both human and mouse cells treated with PI3Kβ inhibitors (TGX-115 and TGX 221) show impaired chemotaxis, while PI3Kδ inhibition with IC87114 affects migration only in human neutrophils.98,125
Two different proteins, which antagonize PI3K-dependent activation of Rac signaling, further contribute to the establishment of an asymmetric distribution of PtdIns(3,4,5)P3 in migrating cells: PTEN and SHIP1. PTEN and SHIP1 act as phosphatases to dephosphorylate the 3′ and the 5′ phosphate, respectively, of PtdIns(3,4,5)P3, leading to the formation of PtdIns(4,5)P2 or PtdIns(3,4)P2.126,127 Neutrophils lacking PTEN display normal migration in response to a single chemoattractant, but fail to respond to gradients of multiple chemoattractants, indicating that PTEN functions to 'prioritize' chemotactic cues and prevent 'distraction' in migrating neutrophils.128 On the contrary, ablation of SHIP1 dramatically impairs neutrophil chemotaxis toward a single chemoattractant, as a result of unpolarized PtdIns(3,4,5)P3 localization.129 The different phenotypes observed in SHIP1- and PTEN-knockouts are due to the fact that the 2 phosphatases affect PtdIns(3,4,5)P3 distribution through different mechanisms. While PTEN localizes to the rear of neutrophils and facilitates the accumulation of phosphorylated lipids at the leading edge, SHIP1 is active at the cell–matrix interface to abolish the gradient of PtdIns(3,4,5)P3 induced by integrin activation.130
PtdIns(3,4,5)P3 Burst ROS Production
In professional phagocytes, superoxide production from oxygen and NADPH by activated NADPH oxidase is an obligated step in destruction of invading microorganisms. The NADPH oxidase complex is composed by a catalytic core, including gp91 and p22phox, and by a regulatory part composed by p40phox, p47phox, p67phox and Rac. Upon cell stimulation, the degranulation of intracellular granules brings the catalytic subunits to the plasma membrane and concomitantly induces the translocation of the regulatory elements from the cytosol to the plasma membrane in order to form an active complex.131 Phosphoinositides regulate the assembly of the regulatory components of the NADPH oxidase in different ways, such as phosphorylation of p47phox by phosphoinositide-dependent kinases, activation of Rac via phosphoinositide-dependent GEF or direct interaction of cytosolic subunits (p40phox, p47phox and Rac) with phosphoinositides (Fig. 2).
Figure 2.
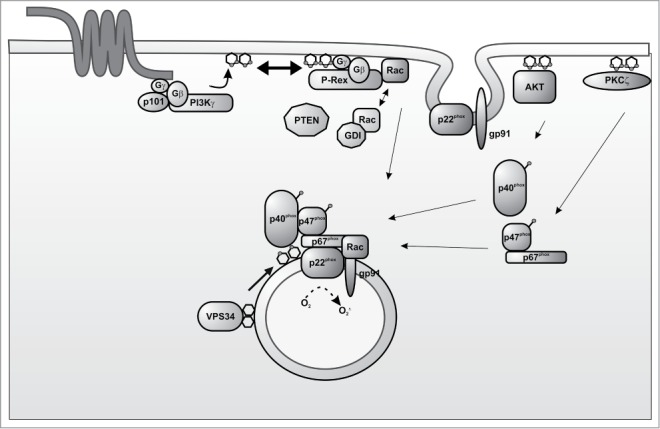
Schematic representation of signaling events activated by PI3K leading to ROS production. GPCR activation stimulates the production of PtdIns(3,4,5)P3 at the plasma membrane to activate Rac through the GEF protein P-Rex. The phosphorylated lipids produced by PI3K activate p40phox and p47phox through Akt and PKC phosphorylation. The assembly of the NADPH oxidase complex on the phagosomal membrane is favored by the PtdIns(3)P produced by Vps34.
p67phox is one of the cytosolic members of the NADPH oxidase complex and is strictly required for O2 radical production in a reconstituted cell-free system.132,133 The activation of p67phox is regulated in vitro by the interaction between p40phox and p47phox, which localize the heterotrimeric complex to the phagosomal membrane. In vitro p40phox associates with p67phox with high affinity134,135 and it is possible that, in vivo, this interaction is regulated by PI3K-dependent phosphorylation of p40phox.136 A similar scenario has been described for the interaction between p47phox and p67phox. p47phox presents 4 different domains, a PX in the N-terminal region, followed by 2 SH3 and a prolin rich region that binds to p67phox. The SH3 domains interact with both the PX and the proline-rich domains within p47phox, thereby driving the auto-inhibition of the protein that is retained in the cytosol and does not associate with p67phox. The interaction with both phosphorylated lipids at the plasma membrane and p67phox is promoted by phosphorylation events controlled by several kinases, such as PKC, MAPK as well as the PI3K effector, Akt.137,138 In line with a fundamental role of PI3K in NADPH oxidase activation, inhibition of PI3K decreases ROS production in neutrophils stimulated with immune complexes.122,139 In addition, class III PI3Ks critically contribute to the localization of the NADPH cytosolic complex to phagosomal membrane by producing PtdIns(3)P that functions as a docking site for p40phox. Accordingly, neutrophils expressing a mutant p40phox, carrying a point mutation in the lipid-binding domain, display defective ROS production.140
Besides PI3K-mediated mechanisms, the assembly and activation of the NADPH complex is strictly controlled by Rac, at least in response to serum-opsonized Staphylococcus Aureus.141 The mechanism whereby Rac controls NADPH assembly and ROS production is still debated. The current view is that Rac, activated via a PI3K-dependent mechanism, either stabilizes the interaction between the regulatory complex and the catalytic core of the oxidase or participates to the electron transfer.142
Concluding Remarks
PI3K and Rac are tightly connected due to the ability to regulate each other in a lipid-dependent (PI3K upstream) or -independent (Rac upstream) manner. However, the comprehension of the functional impact of these processes in vivo appears more complicated. The major drawback of genetic approaches is that inactivation of distinct Rac isoforms in mouse models may lead to compensatory upregulation of other isoenzymes, thus confusing the resulting phenotype.143 A further level of complexity is provided by the intricate network of feedback regulatory mechanisms whereby PI3K controls both positive and negative regulators of Rac which in turn fosters PI3K activity. In recent years, the availability of inducible systems to control PI3K-Rac axis have dramatically improved our understanding of the spatial and temporal regulation of the connection between PI3K and Rac signaling pathways and, of feedback mechanisms of regulation in isolated systems. If the lessons learnt in cells could be applied to the tight control of cell behavior in intact organisms will be investigated in the near future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Firb 2010 Futuro In Ricerca, Prin 2009, Leducq Foundation (09CVD02) and ISHR/Servier Research Fellowship.
References
- 1. Corbetta S, Gualdoni S, Ciceri G, Monari M, Zuccaro E, Tybulewicz VL, de Curtis I. Essential role of rac1 and rac3 GTPases in neuronal development. FASEB J 2009; 23:1347-57; PMID:19126596; http://dx.doi.org/ 10.1096/fj.08-121574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wennerberg K, Der CJ. Rho-family GTPases: it's not only rac and rho (and I like it). J Cell Sci 2004; 117:1301-12; PMID:15020670; http://dx.doi.org/ 10.1242/jcs.01118 [DOI] [PubMed] [Google Scholar]
- 3. Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629-35; PMID:12478284; http://dx.doi.org/ 10.1038/nature01148 [DOI] [PubMed] [Google Scholar]
- 4. Lenzen C, Cool RH, Prinz H, Kuhlmann J, Wittinghofer A. Kinetic analysis by fluorescence of the interaction between ras and the catalytic domain of the guanine nucleotide exchange factor cdc25Mm. Biochemistry 1998; 37:7420-30; PMID:9585556; http://dx.doi.org/ 10.1021/bi972621j [DOI] [PubMed] [Google Scholar]
- 5. Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 2007; 129:865-77; PMID:17540168; http://dx.doi.org/ 10.1016/j.cell.2007.05.018 [DOI] [PubMed] [Google Scholar]
- 6. Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev 2013; 93:269-309; PMID:23303910; http://dx.doi.org/ 10.1152/physrev.00003.2012 [DOI] [PubMed] [Google Scholar]
- 7. Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 2005; 6:167-80; PMID:15688002; http://dx.doi.org/ 10.1038/nrm1587 [DOI] [PubMed] [Google Scholar]
- 8. Erickson JW, Cerione RA. Structural elements, mechanism, and evolutionary convergence of rho protein-guanine nucleotide exchange factor complexes. Biochemistry 2004; 43:837-42; PMID:14744125; http://dx.doi.org/ 10.1021/bi036026v [DOI] [PubMed] [Google Scholar]
- 9. Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol 2003; 13:13-22; PMID:12480336; http://dx.doi.org/ 10.1016/S0962-8924(02)00004-1 [DOI] [PubMed] [Google Scholar]
- 10. DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in rho GTPase activation. Trends Cell Biol 2005; 15:356-63; PMID:15921909; http://dx.doi.org/ 10.1016/j.tcb.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 11. Molnar G, Dagher MC, Geiszt M, Settleman J, Ligeti E. Role of prenylation in the interaction of rho-family small GTPases with GTPase activating proteins. Biochemistry 2001; 40:10542-9; PMID:11523996; http://dx.doi.org/ 10.1021/bi011158e [DOI] [PubMed] [Google Scholar]
- 12. Garcia-Mata R, Boulter E, Burridge K. The 'invisible hand': regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol 2011; 12:493-504; PMID:21779026; http://dx.doi.org/ 10.1038/nrm3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall A. Rho family GTPases. Biochem Soc Trans 2012; 40:1378-82; PMID:23176484; http://dx.doi.org/ 10.1042/BST20120103 [DOI] [PubMed] [Google Scholar]
- 14. Sugihara K, Nakatsuji N, Nakamura K, Nakao K, Hashimoto R, Otani H, Sakagami H, Kondo H, Nozawa S, Aiba A, et al. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene 1998; 17:3427-33; PMID:10030666; http://dx.doi.org/ 10.1038/sj.onc.1202595 [DOI] [PubMed] [Google Scholar]
- 15. Ambruso DR, Knall C, Abell AN, Panepinto J, Kurkchubasche A, Thurman G, Gonzalez-Aller C, Hiester A, deBoer M, Harbeck RJ, et al. Human neutrophil immunodeficiency syndrome is associated with an inhibitory rac2 mutation. Proc Natl Acad Sci U S A 2000; 97:4654-9; PMID:10758162; http://dx.doi.org/ 10.1073/pnas.080074897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kasper B, Tidow N, Grothues D, Welte K. Differential expression and regulation of GTPases (rhoA and rac2) and GDIs (LyGDI and rhoGDI) in neutrophils from patients with severe congenital neutropenia. Blood 2000; 95:2947-53; PMID:10779444 [PubMed] [Google Scholar]
- 17. Li B, Yu H, Zheng W, Voll R, Na S, Roberts AW, Williams DA, Davis RJ, Ghosh S, Flavell RA. Role of the guanosine triphosphatase rac2 in T helper 1 cell differentiation. Science 2000; 288:2219-22; PMID:10864872; http://dx.doi.org/ 10.1126/science.288.5474.2219 [DOI] [PubMed] [Google Scholar]
- 18. Williams DA, Tao W, Yang F, Kim C, Gu Y, Mansfield P, Levine JE, Petryniak B, Derrow CW, Harris C, et al. Dominant negative mutation of the hematopoietic-specific rho GTPase, rac2, is associated with a human phagocyte immunodeficiency. Blood 2000; 96:1646-54; PMID:10961859 [PubMed] [Google Scholar]
- 19. Corbetta S, Gualdoni S, Albertinazzi C, Paris S, Croci L, Consalez GG, de Curtis I. Generation and characterization of rac3 knockout mice. Mol Cell Biol 2005; 25:5763-76; PMID:15964829; http://dx.doi.org/ 10.1128/MCB.25.13.5763-5776.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vigorito E, Bell S, Hebeis BJ, Reynolds H, McAdam S, Emson PC, McKenzie A, Turner M. Immunological function in mice lacking the rac-related GTPase rhoG. Mol Cell Biol 2004; 24:719-29; PMID:14701744; http://dx.doi.org/ 10.1128/MCB.24.2.719-729.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cotteret S, Chernoff J. The evolutionary history of effectors downstream of cdc42 and rac. Genome Biol 2002; 3:REVIEWS0002; PMID:11864373; http://dx.doi.org/ 10.1186/gb-2002-3-2-reviews0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burbelo PD, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both cdc42 and rac GTPases. J Biol Chem 1995; 270:29071-4; PMID:7493928; http://dx.doi.org/ 10.1074/jbc.270.49.29071 [DOI] [PubMed] [Google Scholar]
- 23. Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by cdc42 and rac1. Nature 1994; 367:40-6; PMID:8107774; http://dx.doi.org/ 10.1038/367040a0 [DOI] [PubMed] [Google Scholar]
- 24. Chou MM, Blenis J. The 70 kDa S6 kinase complexes with and is activated by the rho family G proteins cdc42 and rac1. Cell 1996; 85:573-83; PMID:8653792; http://dx.doi.org/ 10.1016/S0092-8674(00)81257-X [DOI] [PubMed] [Google Scholar]
- 25. Diekmann D, Abo A, Johnston C, Segal AW, Hall A. Interaction of rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science 1994; 265:531-3; PMID:8036496; http://dx.doi.org/ 10.1126/science.8036496 [DOI] [PubMed] [Google Scholar]
- 26. Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the rho/rac family: regulation, effectors and functions in vivo. Bioessays 2007; 29:356-70; PMID:17373658; http://dx.doi.org/ 10.1002/bies.20558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 2008; 9:846-59; PMID:18946474; http://dx.doi.org/ 10.1038/nrm2521 [DOI] [PubMed] [Google Scholar]
- 28. Fanger GR, Johnson NL, Johnson GL. MEK kinases are regulated by EGF and selectively interact with rac/cdc42. EMBO J 1997; 16:4961-72; PMID:9305638; http://dx.doi.org/ 10.1093/emboj/16.16.4961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by rac1 and nck. Nature 2002; 418:790-3; PMID:12181570; http://dx.doi.org/ 10.1038/nature00859 [DOI] [PubMed] [Google Scholar]
- 30. Brill S, Li S, Lyman CW, Church DM, Wasmuth JJ, Weissbach L, Bernards A, Snijders AJ. The ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin binding domain and interacts with calmodulin and rho family GTPases. Mol Cell Biol 1996; 16:4869-78; PMID:8756646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Williams R, Berndt A, Miller S, Hon WC, Zhang X. Form and flexibility in phosphoinositide 3-kinases. Biochem Soc Trans 2009; 37:615-26; PMID:19614567; http://dx.doi.org/ 10.1042/BST0370615 [DOI] [PubMed] [Google Scholar]
- 32. Vadas O, Burke JE, Zhang X, Berndt A, Williams RL. Structural basis for activation and inhibition of class I phosphoinositide 3-kinases. Sci Signal 2011; 4:re2; PMID:22009150; http://dx.doi.org/ 10.1126/scisignal.2002165 [DOI] [PubMed] [Google Scholar]
- 33. Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol Cell Biol 2005; 25:1596-607; PMID:15713620; http://dx.doi.org/ 10.1128/MCB.25.5.1596-1607.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao JJ, Cheng H, Jia S, Wang L, Gjoerup OV, Mikami A, Roberts TM. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc Natl Acad Sci U S A 2006; 103:16296-300; PMID:17060635; http://dx.doi.org/ 10.1073/pnas.0607899103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geering B, Cutillas PR, Nock G, Gharbi SI, Vanhaesebroeck B. Class IA phosphoinositide 3-kinases are obligate p85-p110 heterodimers. Proc Natl Acad Sci U S A 2007; 104:7809-14; PMID:17470792; http://dx.doi.org/ 10.1073/pnas.0700373104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schauder C, Ma LC, Krug RM, Montelione GT, Guan R. Structure of the iSH2 domain of human phosphatidylinositol 3-kinase p85beta subunit reveals conformational plasticity in the interhelical turn region. Acta Crystallogr Sect F Struct Biol Cryst Commun 2010; 66:1567-71; PMID:21139197; http://dx.doi.org/ 10.1107/S1744309110041333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fruman DA. Regulatory subunits of class IA PI3K. Curr Top Microbiol Immunol 2010; 346:225-44; PMID:20563711 [DOI] [PubMed] [Google Scholar]
- 38. Amzel LM, Huang CH, Mandelker D, Lengauer C, Gabelli SB, Vogelstein B. Structural comparisons of class I phosphoinositide 3-kinases. Nat Rev Cancer 2008; 8:665-9; PMID:18633356; http://dx.doi.org/ 10.1038/nrc2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Backer JM. The regulation of class IA PI 3-kinases by inter-subunit interactions. Curr Top Microbiol Immunol 2010; 346:87-114; PMID:20544340; http://dx.doi.org/ 10.1007/82_2010_52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu H, et al. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal 2008; 1:ra3; PMID:18780892; http://dx.doi.org/ 10.1126/scisignal.1161577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 2008; 454:776-9; PMID:18594509; http://dx.doi.org/ 10.1038/nature07091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dbouk HA, Vadas O, Shymanets A, Burke JE, Salamon RS, Khalil BD, Barrett MO, Waldo GL, Surve C, Hsueh C, et al. G protein-coupled receptor-mediated activation of p110beta by Gbetagamma is required for cellular transformation and invasiveness. Sci Signal 2012; 5:ra89; PMID:23211529; http://dx.doi.org/ 10.1126/scisignal.2003264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, Schultz G, Nurnberg B. Roles of G β gamma in membrane recruitment and activation of p110 gamma/p101 phosphoinositide 3-kinase gamma. J Cell Biol 2003; 160:89-99; PMID:12507995; http://dx.doi.org/ 10.1083/jcb.200210115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krugmann S, Cooper MA, Williams DH, Hawkins PT, Stephens LR. Mechanism of the regulation of type IB phosphoinositide 3OH-kinase byG-protein betagamma subunits. Biochem J 2002; 362:725-31; PMID:11879201; http://dx.doi.org/ 10.1042/0264-6021:3620725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tannert A, Voigt P, Burgold S, Tannert S, Schaefer M. Signal amplification between Gbetagamma release and PI3Kgamma-mediated PI(3,4,5)P3 formation monitored by a fluorescent Gbetagamma biosensor protein and repetitive two component total internal reflection/fluorescence redistribution after photobleaching analysis. Biochemistry 2008; 47:11239-50; PMID:18831540; http://dx.doi.org/ 10.1021/bi800596b [DOI] [PubMed] [Google Scholar]
- 46. Maier U, Babich A, Nurnberg B. Roles of non-catalytic subunits in gbetagamma-induced activation of class I phosphoinositide 3-kinase isoforms β and gamma. J Biol Chem 1999; 274:29311-7; PMID:10506190; http://dx.doi.org/ 10.1074/jbc.274.41.29311 [DOI] [PubMed] [Google Scholar]
- 47. Vadas O, Dbouk HA, Shymanets A, Perisic O, Burke JE, Abi Saab WF, Khalil BD, Harteneck C, Bresnick AR, Nurnberg B, et al. Molecular determinants of PI3Kgamma-mediated activation downstream of G-protein-coupled receptors (GPCRs). Proc Natl Acad Sci U S A 2013; 110:18862-7; PMID:24190998; http://dx.doi.org/ 10.1073/pnas.1304801110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suire S, Condliffe AM, Ferguson GJ, Ellson CD, Guillou H, Davidson K, Welch H, Coadwell J, Turner M, Chilvers ER, et al. Gbetagammas and the ras binding domain of p110gamma are both important regulators of PI(3)Kgamma signalling in neutrophils. Nat Cell Biol 2006; 8:1303-9; PMID:17041586; http://dx.doi.org/ 10.1038/ncb1494 [DOI] [PubMed] [Google Scholar]
- 49. Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, Nye E, Stamp G, Alitalo K, Downward J. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell 2007; 129:957-68; PMID:17540175; http://dx.doi.org/ 10.1016/j.cell.2007.03.051 [DOI] [PubMed] [Google Scholar]
- 50. Rodriguez-Viciana P, Warne PH, Vanhaesebroeck B, Waterfield MD, Downward J. Activation of phosphoinositide 3-kinase by interaction with ras and by point mutation. EMBO J 1996; 15:2442-51; PMID:8665852 [PMC free article] [PubMed] [Google Scholar]
- 51. Pacold ME, Suire S, Perisic O, Lara-Gonzalez S, Davis CT, Walker EH, Hawkins PT, Stephens L, Eccleston JF, Williams RL. Crystal structure and functional analysis of ras binding to its effector phosphoinositide 3-kinase gamma. Cell 2000; 103:931-43; PMID:11136978; http://dx.doi.org/ 10.1016/S0092-8674(00)00196-3 [DOI] [PubMed] [Google Scholar]
- 52. Yoshioka K, Yoshida K, Cui H, Wakayama T, Takuwa N, Okamoto Y, Du W, Qi X, Asanuma K, Sugihara K, et al. Endothelial PI3K-C2alpha, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat Med 2012; 18:1560-9; PMID:22983395; http://dx.doi.org/ 10.1038/nm.2928 [DOI] [PubMed] [Google Scholar]
- 53. Biswas K, Yoshioka K, Asanuma K, Okamoto Y, Takuwa N, Sasaki T, Takuwa Y. Essential role of class II phosphatidylinositol-3-kinase-C2alpha in sphingosine 1-phosphate receptor-1-mediated signaling and migration in endothelial cells. J Biol Chem 2013; 288:2325-39; PMID:23192342; http://dx.doi.org/ 10.1074/jbc.M112.409656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Katso RM, Pardo OE, Palamidessi A, Franz CM, Marinov M, De Laurentiis A, Downward J, Scita G, Ridley AJ, Waterfield MD, et al. Phosphoinositide 3-kinase C2beta regulates cytoskeletal organization and cell migration via rac-dependent mechanisms. Mol Biol Cell 2006; 17:3729-44; PMID:16775008; http://dx.doi.org/ 10.1091/mbc.E05-11-1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Blajecka K, Marinov M, Leitner L, Uth K, Posern G, Arcaro A. Phosphoinositide 3-kinase C2beta regulates rhoA and the actin cytoskeleton through an interaction with dbl. PLoS One 2012; 7:e44945; PMID:22984590; http://dx.doi.org/ 10.1371/journal.pone.0044945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J 2008; 410:1-17; PMID:18215151; http://dx.doi.org/ 10.1042/BJ20071427 [DOI] [PubMed] [Google Scholar]
- 57. Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol 1996; 16:1722-33; PMID:8657148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wennstrom S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol 1994; 4:385-93; PMID:7922352; http://dx.doi.org/ 10.1016/S0960-9822(00)00087-7 [DOI] [PubMed] [Google Scholar]
- 59. Hawkins PT, Eguinoa A, Qiu RG, Stokoe D, Cooke FT, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M, et al. PDGF stimulates an increase in GTP-rac via activation of phosphoinositide 3-kinase. Curr Biol 1995; 5:393-403; PMID:7627555; http://dx.doi.org/ 10.1016/S0960-9822(95)00080-7 [DOI] [PubMed] [Google Scholar]
- 60. Eva A, Aaronson SA. Isolation of a new human oncogene from a diffuse B-cell lymphoma. Nature 1985; 316:273-5; PMID:3875039; http://dx.doi.org/ 10.1038/316273a0 [DOI] [PubMed] [Google Scholar]
- 61. Hart MJ, Eva A, Evans T, Aaronson SA, Cerione RA. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature 1991; 354:311-4; PMID:1956381; http://dx.doi.org/ 10.1038/354311a0 [DOI] [PubMed] [Google Scholar]
- 62. Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci 2001; 26:724-32; PMID:11738596; http://dx.doi.org/ 10.1016/S0968-0004(01)01973-9 [DOI] [PubMed] [Google Scholar]
- 63. Soisson SM, Nimnual AS, Uy M, Bar-Sagi D, Kuriyan J. Crystal structure of the dbl and pleckstrin homology domains from the human son of sevenless protein. Cell 1998; 95:259-68; PMID:9790532; http://dx.doi.org/ 10.1016/S0092-8674(00)81756-0 [DOI] [PubMed] [Google Scholar]
- 64. Schmidt A, Hall A. Guanine nucleotide exchange factors for rho GTPases: turning on the switch. Genes Dev 2002; 16:1587-609; PMID:12101119; http://dx.doi.org/ 10.1101/gad.1003302 [DOI] [PubMed] [Google Scholar]
- 65. Lemmon MA, Ferguson KM, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell 1996; 85:621-4; PMID:8646770; http://dx.doi.org/ 10.1016/S0092-8674(00)81022-3 [DOI] [PubMed] [Google Scholar]
- 66. Fleming IN, Gray A, Downes CP. Regulation of the rac1-specific exchange factor tiam1 involves both phosphoinositide 3-kinase-dependent and -independent components. Biochem J 2000; 351:173-82; PMID:10998360; http://dx.doi.org/ 10.1042/0264-6021:3510173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of rac is required for the spatial restriction of signaling in cell migration. Cell 2008; 134:135-47; PMID:18614017; http://dx.doi.org/ 10.1016/j.cell.2008.05.034 [DOI] [PubMed] [Google Scholar]
- 68. Ziemba BP, Pilling C, Calleja V, Larijani B, Falke JJ. The PH domain of phosphoinositide-dependent kinase-1 exhibits a novel, phospho-regulated monomer-dimer equilibrium with important implications for kinase domain activation: single-molecule and ensemble studies. Biochemistry 2013; 52:4820-9; PMID:23745598; http://dx.doi.org/ 10.1021/bi400488f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rossman KL, Cheng L, Mahon GM, Rojas RJ, Snyder JT, Whitehead IP, Sondek J. Multifunctional roles for the PH domain of dbs in regulating rho GTPase activation. J Biol Chem 2003; 278:18393-400; PMID:12637522; http://dx.doi.org/ 10.1074/jbc.M300127200 [DOI] [PubMed] [Google Scholar]
- 70. Barber MA, Donald S, Thelen S, Anderson KE, Thelen M, Welch HC. Membrane translocation of P-Rex1 is mediated by G protein betagamma subunits and phosphoinositide 3-kinase. J Biol Chem 2007; 282:29967-76; PMID:17698854; http://dx.doi.org/ 10.1074/jbc.M701877200 [DOI] [PubMed] [Google Scholar]
- 71. Worthylake DK, Rossman KL, Sondek J. Crystal structure of rac1 in complex with the guanine nucleotide exchange region of tiam1. Nature 2000; 408:682-8; PMID:11130063; http://dx.doi.org/ 10.1038/35047014 [DOI] [PubMed] [Google Scholar]
- 72. Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for rac. Cell 2002; 108:809-21; PMID:11955434; http://dx.doi.org/ 10.1016/S0092-8674(02)00663-3 [DOI] [PubMed] [Google Scholar]
- 73. Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature 1997; 385:169-72; PMID:8990121; http://dx.doi.org/ 10.1038/385169a0 [DOI] [PubMed] [Google Scholar]
- 74. Aghazadeh B, Lowry WE, Huang XY, Rosen MK. Structural basis for relief of autoinhibition of the dbl homology domain of proto-oncogene vav by tyrosine phosphorylation. Cell 2000; 102:625-33; PMID:11007481; http://dx.doi.org/ 10.1016/S0092-8674(00)00085-4 [DOI] [PubMed] [Google Scholar]
- 75. Innocenti M, Frittoli E, Ponzanelli I, Falck JR, Brachmann SM, Di Fiore PP, Scita G. Phosphoinositide 3-kinase activates rac by entering in a complex with eps8, abi1, and sos-1. J Cell Biol 2003; 160:17-23; PMID:12515821; http://dx.doi.org/ 10.1083/jcb.200206079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nimnual AS, Yatsula BA, Bar-Sagi D. Coupling of ras and rac guanosine triphosphatases through the ras exchanger sos. Science 1998; 279:560-3; PMID:9438849; http://dx.doi.org/ 10.1126/science.279.5350.560 [DOI] [PubMed] [Google Scholar]
- 77. Zhao YN, Cao J, Wu FX, Ou C, Yuan WP, Mo QG, Wei W, Li Y, Su JJ, Liang AM. [Expression and significance of EGF mRNA and EGFR mRNA in hepatocellular carcinoma]. Ai Zheng 2004; 23:762-6; PMID:15248908 [PubMed] [Google Scholar]
- 78. Premkumar L, Bobkov AA, Patel M, Jaroszewski L, Bankston LA, Stec B, Vuori K, Cote JF, Liddington RC. Structural basis of membrane targeting by the dock180 family of rho family guanine exchange factors (rho-GEFs). J Biol Chem 2010; 285:13211-22; PMID:20167601; http://dx.doi.org/ 10.1074/jbc.M110.102517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shinohara M, Terada Y, Iwamatsu A, Shinohara A, Mochizuki N, Higuchi M, Gotoh Y, Ihara S, Nagata S, Itoh H, et al. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature 2002; 416:759-63; PMID:11961559; http://dx.doi.org/ 10.1038/416759a [DOI] [PubMed] [Google Scholar]
- 80. Hirsch E, Pozzato M, Vercelli A, Barberis L, Azzolino O, Russo C, Vanni C, Silengo L, Eva A, Altruda F. Defective dendrite elongation but normal fertility in mice lacking the rho-like GTPase activator dbl. Mol Cell Biol 2002; 22:3140-8; PMID:11940671; http://dx.doi.org/ 10.1128/MCB.22.9.3140-3148.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Turner M, Mee PJ, Walters AE, Quinn ME, Mellor AL, Zamoyska R, Tybulewicz VL. A requirement for the rho-family GTP exchange factor vav in positive and negative selection of thymocytes. Immunity 1997; 7:451-60; PMID:9354466; http://dx.doi.org/ 10.1016/S1074-7613(00)80367-2 [DOI] [PubMed] [Google Scholar]
- 82. Doody GM, Bell SE, Vigorito E, Clayton E, McAdam S, Tooze R, Fernandez C, Lee IJ, Turner M. Signal transduction through vav-2 participates in humoral immune responses and B cell maturation. Nat Immunol 2001; 2:542-7; PMID:11376342; http://dx.doi.org/ 10.1038/88748 [DOI] [PubMed] [Google Scholar]
- 83. Fujikawa K, Miletic AV, Alt FW, Faccio R, Brown T, Hoog J, Fredericks J, Nishi S, Mildiner S, Moores SL, et al. Vav1/2/3-null mice define an essential role for vav family proteins in lymphocyte development and activation but a differential requirement in MAPK signaling in T and B cells. J Exp Med 2003; 198:1595-608; PMID:14623913; http://dx.doi.org/ 10.1084/jem.20030874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Malliri A, van der Kammen RA, Clark K, van der Valk M, Michiels F, Collard JG. Mice deficient in the rac activator tiam1 are resistant to ras-induced skin tumours. Nature 2002; 417:867-71; PMID:12075356; http://dx.doi.org/ 10.1038/nature00848 [DOI] [PubMed] [Google Scholar]
- 85. Dong X, Mo Z, Bokoch G, Guo C, Li Z, Wu D. P-Rex1 is a primary rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr Biol 2005; 15:1874-9; PMID:16243036; http://dx.doi.org/ 10.1016/j.cub.2005.09.014 [DOI] [PubMed] [Google Scholar]
- 86. Tedford K, Nitschke L, Girkontaite I, Charlesworth A, Chan G, Sakk V, Barbacid M, Fischer KD. Compensation between vav-1 and vav-2 in B cell development and antigen receptor signaling. Nat Immunol 2001; 2:548-55; PMID:11376343; http://dx.doi.org/ 10.1038/88756 [DOI] [PubMed] [Google Scholar]
- 87. Lamarche-Vane N, Hall A. CdGAP, a novel proline-rich GTPase-activating protein for cdc42 and rac. J Biol Chem 1998; 273:29172-7; PMID:9786927; http://dx.doi.org/ 10.1074/jbc.273.44.29172 [DOI] [PubMed] [Google Scholar]
- 88. Karimzadeh F, Primeau M, Mountassif D, Rouiller I, Lamarche-Vane N. A stretch of polybasic residues mediates cdc42 GTPase-activating protein (CdGAP) binding to phosphatidylinositol 3,4,5-trisphosphate and regulates its GAP activity. J Biol Chem 2012; 287:19610-21; PMID:22518840; http://dx.doi.org/ 10.1074/jbc.M112.344606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lancaster CA, Taylor-Harris PM, Self AJ, Brill S, van Erp HE, Hall A. Characterization of rhoGAP. A GTPase-activating protein for rho-related small GTPases. J Biol Chem 1994; 269:1137-42; PMID:8288572 [PubMed] [Google Scholar]
- 90. Levay M, Bartos B, Ligeti E. p190RhoGAP has cellular racGAP activity regulated by a polybasic region. Cell Signal 2013; 25:1388-94; PMID:23499677; http://dx.doi.org/ 10.1016/j.cellsig.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 91. Tcherkezian J, Lamarche-Vane N. Current knowledge of the large rhoGAP family of proteins. Biol Cell 2007; 99:67-86; PMID:17222083; http://dx.doi.org/ 10.1042/BC20060086 [DOI] [PubMed] [Google Scholar]
- 92. Costa C, Barberis L, Ambrogio C, Manazza AD, Patrucco E, Azzolino O, Neilsen PO, Ciraolo E, Altruda F, Prestwich GD, et al. Negative feedback regulation of rac in leukocytes from mice expressing a constitutively active phosphatidylinositol 3-kinase gamma. Proc Natl Acad Sci U S A 2007; 104:14354-9; PMID:17720808; http://dx.doi.org/ 10.1073/pnas.0703175104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zheng Y, Bagrodia S, Cerione RA. Activation of phosphoinositide 3-kinase activity by cdc42Hs binding to p85. J Biol Chem 1994; 269:18727-30; PMID:8034624 [PubMed] [Google Scholar]
- 94. Bokoch GM, Vlahos CJ, Wang Y, Knaus UG, Traynor-Kaplan AE. Rac GTPase interacts specifically with phosphatidylinositol 3-kinase. Biochem J 1996; 315 (Pt 3):775-9; PMID:8645157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cain RJ, Ridley AJ. Phosphoinositide 3-kinases in cell migration. Biol Cell 2009; 101:13-29; PMID:19055486; http://dx.doi.org/ 10.1042/BC20080079 [DOI] [PubMed] [Google Scholar]
- 96. Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol 2003; 160:375-85; PMID:12551955; http://dx.doi.org/ 10.1083/jcb.200208179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hannigan M, Zhan L, Li Z, Ai Y, Wu D, Huang CK. Neutrophils lacking phosphoinositide 3-kinase gamma show loss of directionality during N-formyl-Met-Leu-Phe-induced chemotaxis. Proc Natl Acad Sci U S A 2002; 99:3603-8; PMID:11904423; http://dx.doi.org/ 10.1073/pnas.052010699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J Immunol 2003; 170:2647-54; PMID:12594293; http://dx.doi.org/ 10.4049/jimmunol.170.5.2647 [DOI] [PubMed] [Google Scholar]
- 99. Heit B, Liu L, Colarusso P, Puri KD, Kubes P. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J Cell Sci 2008; 121:205-14; PMID:18187452; http://dx.doi.org/ 10.1242/jcs.020412 [DOI] [PubMed] [Google Scholar]
- 100. Yang HW, Shin MG, Lee S, Kim JR, Park WS, Cho KH, Meyer T, Heo WD. Cooperative activation of PI3K by ras and rho family small GTPases. Mol Cell 2012; 47:281-90; PMID:22683270; http://dx.doi.org/ 10.1016/j.molcel.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell 2010; 18:226-36; PMID:20159593; http://dx.doi.org/ 10.1016/j.devcel.2009.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Peyrollier K, Hajduch E, Gray A, Litherland GJ, Prescott AR, Leslie NR, Hundal HS. A role for the actin cytoskeleton in the hormonal and growth-factor-mediated activation of protein kinase B. Biochem J 2000; 352 Pt 3:617-22; PMID:11104665; http://dx.doi.org/ 10.1042/0264-6021:3520617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang F, Herzmark P, Weiner OD, Srinivasan S, Servant G, Bourne HR. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol 2002; 4:513-8; PMID:12080345; http://dx.doi.org/ 10.1038/ncb810 [DOI] [PubMed] [Google Scholar]
- 104. Peng GE, Wilson SR, Weiner OD. A pharmacological cocktail for arresting actin dynamics in living cells. Mol Biol Cell 2011; 22:3986-94; PMID:21880897; http://dx.doi.org/ 10.1091/mbc.E11-04-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS, Diefenbacher M, Stamp G, Downward J. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell 2013; 153:1050-63; PMID:23706742; http://dx.doi.org/ 10.1016/j.cell.2013.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Stephens L, Milne L, Hawkins P. Moving towards a better understanding of chemotaxis. Curr Biol 2008; 18:R485-94; PMID:18522824; http://dx.doi.org/ 10.1016/j.cub.2008.04.048 [DOI] [PubMed] [Google Scholar]
- 107. Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol 2009; 10:538-49; PMID:19603038; http://dx.doi.org/ 10.1038/nrm2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol 2010; 26:315-33; PMID:19575647; http://dx.doi.org/ 10.1146/annurev.cellbio.011209.122036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Thelen M. Dancing to the tune of chemokines. Nat Immunol 2001; 2:129-34; PMID:11175805; http://dx.doi.org/ 10.1038/84224 [DOI] [PubMed] [Google Scholar]
- 110. Kamakura S, Nomura M, Hayase J, Iwakiri Y, Nishikimi A, Takayanagi R, Fukui Y, Sumimoto H. The cell polarity protein minsc regulates neutrophil chemotaxis via a noncanonical G protein signaling pathway. Dev Cell 2013; 26:292-302; PMID:23891662; http://dx.doi.org/ 10.1016/j.devcel.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 111. Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 2003; 114:201-14; PMID:12887922; http://dx.doi.org/ 10.1016/S0092-8674(03)00555-5 [DOI] [PubMed] [Google Scholar]
- 112. Yan J, Mihaylov V, Xu X, Brzostowski JA, Li H, Liu L, Veenstra TD, Parent CA, Jin T. A Gbetagamma effector, ElmoE, transduces GPCR signaling to the actin network during chemotaxis. Dev Cell 2012; 22:92-103; PMID:22264729; http://dx.doi.org/ 10.1016/j.devcel.2011.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, Cao Q, Sanematsu F, Kanai M, Hasegawa H, et al. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science 2009; 324:384-7; PMID:19325080; http://dx.doi.org/ 10.1126/science.1170179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Stephens LR, Hughes KT, Irvine RF. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature 1991; 351:33-9; PMID:1851250; http://dx.doi.org/ 10.1038/351033a0 [DOI] [PubMed] [Google Scholar]
- 115. Wymann M, Arcaro A. Platelet-derived growth factor-induced phosphatidylinositol 3-kinase activation mediates actin rearrangements in fibroblasts. Biochem J 1994; 298 Pt 3:517-20; PMID:8141762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J 1993; 296 (Pt 2):297-301; PMID:8257416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Niggli V. A membrane-permeant ester of phosphatidylinositol 3,4, 5-trisphosphate (PIP(3)) is an activator of human neutrophil migration. FEBS Lett 2000; 473:217-21; PMID:10812078; http://dx.doi.org/ 10.1016/S0014-5793(00)01534-9 [DOI] [PubMed] [Google Scholar]
- 118. Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 2000; 287:1037-40; PMID:10669415; http://dx.doi.org/ 10.1126/science.287.5455.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wong K, Pertz O, Hahn K, Bourne H. Neutrophil polarization: spatiotemporal dynamics of rhoA activity support a self-organizing mechanism. Proc Natl Acad Sci U S A 2006; 103:3639-44; PMID:16537448; http://dx.doi.org/ 10.1073/pnas.0600092103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Karunarathne WK, Giri L, Patel AK, Venkatesh KV, Gautam N. Optical control demonstrates switch-like PtdIns(3,4,5)P3 dynamics underlying the initiation of immune cell migration. Proc Natl Acad Sci U S A 2013; 110:E1575-83; PMID:23569254; http://dx.doi.org/ 10.1073/pnas.1220755110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science 2000; 287:1040-6; PMID:10669416; http://dx.doi.org/ 10.1126/science.287.5455.1040 [DOI] [PubMed] [Google Scholar]
- 122. Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 2000; 287:1049-53; PMID:10669418; http://dx.doi.org/ 10.1126/science.287.5455.1049 [DOI] [PubMed] [Google Scholar]
- 123. Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science 2000; 287:1046-9; PMID:10669417; http://dx.doi.org/ 10.1126/science.287.5455.1046 [DOI] [PubMed] [Google Scholar]
- 124. Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, Crabbe T, Finan P, Jones G, Jackson S, et al. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol 2007; 9:86-91; PMID:17173040; http://dx.doi.org/ 10.1038/ncb1517 [DOI] [PubMed] [Google Scholar]
- 125. Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR. To stabilize neutrophil polarity, PtdIns(3,4,5)P3 and cdc42 augment rhoA activity at the back as well as signals at the front. J Cell Biol 2006; 174:437-45; PMID:16864657; http://dx.doi.org/ 10.1083/jcb.200604113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 1998; 273:13375-8; PMID:9593664; http://dx.doi.org/ 10.1074/jbc.273.22.13375 [DOI] [PubMed] [Google Scholar]
- 127. Damen JE, Liu L, Rosten P, Humphries RK, Jefferson AB, Majerus PW, Krystal G. The 145-kDa protein induced to associate with shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci U S A 1996; 93:1689-93; PMID:8643691; http://dx.doi.org/ 10.1073/pnas.93.4.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Heit B, Robbins SM, Downey CM, Guan Z, Colarusso P, Miller BJ, Jirik FR, Kubes P. PTEN functions to 'prioritize' chemotactic cues and prevent 'distraction' in migrating neutrophils. Nat Immunol 2008; 9:743-52; PMID:18536720; http://dx.doi.org/ 10.1038/ni.1623 [DOI] [PubMed] [Google Scholar]
- 129. Nishio M, Watanabe K, Sasaki J, Taya C, Takasuga S, Iizuka R, Balla T, Yamazaki M, Watanabe H, Itoh R, et al. Control of cell polarity and motility by the PtdIns(3,4,5)P3 phosphatase SHIP1. Nat Cell Biol 2007; 9:36-44; PMID:17173042; http://dx.doi.org/ 10.1038/ncb1515 [DOI] [PubMed] [Google Scholar]
- 130. Mondal S, Subramanian KK, Sakai J, Bajrami B, Luo HR. Phosphoinositide lipid phosphatase SHIP1 and PTEN coordinate to regulate cell migration and adhesion. Mol Biol Cell 2012; 23:1219-30; PMID:22323291; http://dx.doi.org/ 10.1091/mbc.E11-10-0889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004; 4:181-9; PMID:15039755; http://dx.doi.org/ 10.1038/nri1312 [DOI] [PubMed] [Google Scholar]
- 132. Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of rac-related guanosine triphosphatases by Vav. Science 1998; 279:558-60; PMID:9438848; http://dx.doi.org/ 10.1126/science.279.5350.558 [DOI] [PubMed] [Google Scholar]
- 133. Han CH, Lee MH. Activation domain in P67phox regulates the steady state reduction of FAD in gp91phox. J Vet Sci 2000; 1:27-31; PMID:14612617 [PubMed] [Google Scholar]
- 134. Wilson MI, Gill DJ, Perisic O, Quinn MT, Williams RL. PB1 domain-mediated heterodimerization in NADPH oxidase and signaling complexes of atypical protein kinase C with par6 and p62. Mol Cell 2003; 12:39-50; PMID:12887891; http://dx.doi.org/ 10.1016/S1097-2765(03)00246-6 [DOI] [PubMed] [Google Scholar]
- 135. Lapouge K, Smith SJ, Groemping Y, Rittinger K. Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase. a central role for p67phox. J Biol Chem 2002; 277:10121-8; PMID:11796733; http://dx.doi.org/ 10.1074/jbc.M112065200 [DOI] [PubMed] [Google Scholar]
- 136. Chessa TA, Anderson KE, Hu Y, Xu Q, Rausch O, Stephens LR, Hawkins PT. Phosphorylation of threonine 154 in p40phox is an important physiological signal for activation of the neutrophil NADPH oxidase. Blood 2010; 116:6027-36; PMID:20861461; http://dx.doi.org/ 10.1182/blood-2010-08-300889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Rotrosen D, Leto TL. Phosphorylation of neutrophil 47-kDa cytosolic oxidase factor. translocation to membrane is associated with distinct phosphorylation events. J Biol Chem 1990; 265:19910-5; PMID:2246268 [PubMed] [Google Scholar]
- 138. Dewas C, Fay M, Gougerot-Pocidalo MA, El-Benna J. The mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway is involved in formyl-methionyl-leucyl-phenylalanine-induced p47phox phosphorylation in human neutrophils. J Immunol 2000; 165:5238-44; PMID:11046057; http://dx.doi.org/ 10.4049/jimmunol.165.9.5238 [DOI] [PubMed] [Google Scholar]
- 139. Kulkarni S, Sitaru C, Jakus Z, Anderson KE, Damoulakis G, Davidson K, Hirose M, Juss J, Oxley D, Chessa TA, et al. PI3Kbeta plays a critical role in neutrophil activation by immune complexes. Sci Signal 2011; 4:ra23; PMID:21487106; http://dx.doi.org/ 10.1126/scisignal.2001617 [DOI] [PubMed] [Google Scholar]
- 140. Ellson C, Davidson K, Anderson K, Stephens LR, Hawkins PT. PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation. EMBO J 2006; 25:4468-78; PMID:16990793; http://dx.doi.org/ 10.1038/sj.emboj.7601346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Anderson KE, Chessa TA, Davidson K, Henderson RB, Walker S, Tolmachova T, Grys K, Rausch O, Seabra MC, Tybulewicz VL, et al. PtdIns3P and rac direct the assembly of the NADPH oxidase on a novel, pre-phagosomal compartment during FcR-mediated phagocytosis in primary mouse neutrophils. Blood 2010; 116:4978-89; PMID:20813901; http://dx.doi.org/ 10.1182/blood-2010-03-275602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Diebold BA, Bokoch GM. Molecular basis for rac2 regulation of phagocyte NADPH oxidase. Nat Immunol 2001; 2:211-5; PMID:11224519; http://dx.doi.org/ 10.1038/85259 [DOI] [PubMed] [Google Scholar]
- 143. Debidda M, Williams DA, Zheng Y. Rac1 GTPase regulates cell genomic stability and senescence. J Biol Chem 2006; 281:38519-28; PMID:17032649; http://dx.doi.org/ 10.1074/jbc.M604607200 [DOI] [PubMed] [Google Scholar]
- 144. Ceccarelli DF, Blasutig IM, Goudreault M, Li Z, Ruston J, Pawson T, Sicheri F. Non-canonical interaction of phosphoinositides with pleckstrin homology domains of tiam1 and arhGAP9. J Biol Chem 2007; 282:13864-74; PMID:17339315; http://dx.doi.org/ 10.1074/jbc.M700505200 [DOI] [PubMed] [Google Scholar]
- 145. Snyder JT, Rossman KL, Baumeister MA, Pruitt WM, Siderovski DP, Der CJ, Lemmon MA, Sondek J. Quantitative analysis of the effect of phosphoinositide interactions on the function of dbl family proteins. J Biol Chem 2001; 276:45868-75; PMID:11577097; http://dx.doi.org/ 10.1074/jbc.M106731200 [DOI] [PubMed] [Google Scholar]
- 146. Baumeister MA, Martinu L, Rossman KL, Sondek J, Lemmon MA, Chou MM. Loss of phosphatidylinositol 3-phosphate binding by the C-terminal tiam-1 pleckstrin homology domain prevents in vivo rac1 activation without affecting membrane targeting. J Biol Chem 2003; 278:11457-64; PMID:12525493; http://dx.doi.org/ 10.1074/jbc.M211901200 [DOI] [PubMed] [Google Scholar]
- 147. Fleming IN, Batty IH, Prescott AR, Gray A, Kular GS, Stewart H, Downes CP. Inositol phospholipids regulate the guanine-nucleotide-exchange factor tiam1 by facilitating its binding to the plasma membrane and regulating GDP/GTP exchange on rac1. Biochem J 2004; 382:857-65; PMID:15242348; http://dx.doi.org/ 10.1042/BJ20040916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Viaud J, Gaits-Iacovoni F, Payrastre B. Regulation of the DH-PH tandem of guanine nucleotide exchange factor for rho GTPases by phosphoinositides. Adv Biol Regul 2012; 52:303-14; PMID:22781744; http://dx.doi.org/ 10.1016/j.jbior.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 149. Kostenko EV, Mahon GM, Cheng L, Whitehead IP. The sec14 homology domain regulates the cellular distribution and transforming activity of the rho-specific guanine nucleotide exchange factor dbs. J Biol Chem 2005; 280:2807-17; PMID:15531584; http://dx.doi.org/ 10.1074/jbc.M411139200 [DOI] [PubMed] [Google Scholar]
- 150. Rossman KL, Worthylake DK, Snyder JT, Siderovski DP, Campbell SL, Sondek J. A crystallographic view of interactions between dbs and cdc42: PH domain-assisted guanine nucleotide exchange. EMBO J 2002; 21:1315-26; PMID:11889037; http://dx.doi.org/ 10.1093/emboj/21.6.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Baumeister MA, Rossman KL, Sondek J, Lemmon MA. The dbs PH domain contributes independently to membrane targeting and regulation of guanine nucleotide-exchange activity. Biochem J 2006; 400:563-72; PMID:17007612; http://dx.doi.org/ 10.1042/BJ20061020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Vedham V, Phee H, Coggeshall KM. Vav activation and function as a rac guanine nucleotide exchange factor in macrophage colony-stimulating factor-induced macrophage chemotaxis. Mol Cell Biol 2005; 25:4211-20; PMID:15870290; http://dx.doi.org/ 10.1128/MCB.25.10.4211-4220.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hill K, Krugmann S, Andrews SR, Coadwell WJ, Finan P, Welch HC, Hawkins PT, Stephens LR. Regulation of P-Rex1 by phosphatidylinositol (3,4,5)-trisphosphate and Gbetagamma subunits. J Biol Chem 2005; 280:4166-73; PMID:15545267; http://dx.doi.org/ 10.1074/jbc.M411262200 [DOI] [PubMed] [Google Scholar]
- 154. Russo C, Gao Y, Mancini P, Vanni C, Porotto M, Falasca M, Torrisi MR, Zheng Y, Eva A. Modulation of oncogenic DBL activity by phosphoinositol phosphate binding to pleckstrin homology domain. J Biol Chem 2001; 276:19524-31; PMID:11278560; http://dx.doi.org/ 10.1074/jbc.M009742200 [DOI] [PubMed] [Google Scholar]


