Abstract
Candida albicans is a major cause of bloodstream infection which may present as sepsis and septic shock - major causes of morbidity and mortality world-wide. After invasion of the pathogen, innate mechanisms govern the early response. Here, we outline the models used to study these mechanisms and summarize our current understanding of innate immune responses during Candida bloodstream infection. This includes protective immunity as well as harmful responses resulting in Candida induced sepsis. Neutrophilic granulocytes are considered principal effector cells conferring protection and recognize C. albicans mainly via complement receptor 3. They possess a range of effector mechanisms, contributing to elimination of the pathogen. Neutrophil activation is closely linked to complement and modulated by activated mononuclear cells. A thorough understanding of these mechanisms will help in creating an individualized approach to patients suffering from systemic candidiasis and aid in optimizing clinical management.
Keywords: bloodsteam infection, Candida albicans, innate immune response, sepsis
Candida Bloodstream Infection and Sepsis
Severe sepsis and septic shock are major causes of death and morbidity world wide, 1 and several studies have suggested that the problem is increasing due to growing numbers of patients at risk.1,2 Epidemiological analyses show a shift in the classes of microorganisms causing sepsis. The incidence of Gram-positive organisms has increased for several years, and drawn equal with Gram-negative bacteria in some studies.1 However, with the global spread of Gram-negative multi-resistance, Gram-negative pathogens continue to pose a major threat. In addition to bacteria, fungi—mainly Candida albicans and other Candida spp.—can cause sepsis and this entity has increased over the last decades, now causing significant impact and health care-associated costs.2,3 In addition, fungal sepsis is associated with a higher mortality than bacterial sepsis.2,4-8 Candida bloodstream infection frequently arises from either gastrointestinal colonization and transmigration of the pathogen through the mucosal barrier, or from colonization of foreign material for example, intravenous (i.v.) catheters.3 Colonized i.v. catheters may account for as much as 25–40% of cases of candidemia.9-11 In the EPIC-II study, a 1-day point prevalence study involving 13,796 analyzed patients in 1,265 intensive care units, fungi accounted for 19% of all infections.12 A retrospective analysis of this patient cohort revealed that 12.6% of all positive blood cultures were either positive for Candida spp. alone or detected mixed bacterial and fungal infection.13 This is in line with other data showing that in the United States, Candida spp. account for 8–10% of all positive blood cultures.14,15 However, despite being a frequent cause of nosocomial infection, Candida spp. generally account for only ∼5% of sepsis cases.16 This is related to the fact that Candida bloodstream infections—although showing a high mortality—do not fulfill classical diagnostic criteria for sepsis and septic shock in most cases.5,17,18 (Table 1). This suggests that classical diagnostic criteria for sepsis may be inadequate to fully account for the clinical implication of systemic fungal infection.19 In addition to primary Candida sepsis, invasive Candida infection frequently occurs as a complication of bacterial sepsis due to concomitant immune paralysis. These secondary Candida infections have been shown to prolong ICU stay, increase mortality and generate additional costs.20
Table 1.
Candida BSI and sepsis
| Candida BSI | Candida Sepsis | |
|---|---|---|
| Frequency | 5–15% of all BSI | 2–5% of sepsis cases, only a minority of Candida BSI proceed to severe forms of sepsis (see below) |
| Diagnostic criteria | Positive blood-culture for Candidaa | Systemic inflammatory response syndrom (SIRS) with 2 or more of the following symptoms: temperature <36°C or >38°C; heart rate >90/min; respiratory rate >20/min or PaCO2<32 mmHg; WBC <4×109/L or >12×109/L or ≥10% bands due to an infection with Candidac |
| Pathology | Dissemination of Candida in the bloodstream with/without affection of (multiple) organs presenting as “acute disseminated candidiasis” or “chronic disseminated candidiasis” with the latter mainly occurring in neutropenic patients. | Clinical presentation is dominated by severe dysregulation of immunity, coagulation and circulation. In progressive disease this results in organ failure (“severe sepsis”) and cardial decompensation (“septic shock”). |
| Associated mortality | < 30–40%b, 6–8 | ∼70%5 septic shock complicating Candida BSI is “a near fatal condition”18 |
Note: aOther diagnostic tests may also be indicative, e.g., PCR based detection in blood, β-glucan testing. bThese are mortality rates from case series of Candida BSI including patients with sepsis, severe sepsis or septic shock; so fatality rates for Candida BSI without sepsis will be lower. cCurrently, several authors suggest to rephrase sepsis definitions and restrict sepsis to cases with resulting organ failure.19
In recent years, our understanding of early immune activation processes during systemic Candida infections has advanced considerably. On the one hand, this has been achieved by combining insights from different infection models. Most importantly however, modern genomic technologies have allowed researchers to elucidate mechanisms of immune activation and response based on the analysis of genetic variation in human patients.21 In this review, we summarize our current understanding of early immune response to Candida during bloodstream infections which includes mechanisms that govern protective immune reaction to C. albicans invasion as well as harmful immune responses resulting in Candida induced sepsis and septic shock.
Analyzing Systemic Candida Infections
Various model systems have been employed in C. albicans research to date, including the fruit fly, nematode, wax moth and zebrafish. The latter has been particularly useful due to the presence of innate and adaptive immune systems, transparent tissues and comparative intra-species transcriptional responses of C. albicans.22-24 Notably, the roles of NADPH oxidase in response to C. albicans hyphae have been expanded upon with the help of non-invasive imaging of spacio-temporal macrophage responses in this model.24 The most commonly used infection model is the mouse, and murine models have been developed to mimic both major routes of C. albicans dissemination.25-27 In the i.v. murine infection model, fungal cells are administered directly into the bloodstream and rapidly disseminate, evoking a strong inflammatory reaction.28 The major target organs are the kidneys, and both systemic inflammation as well as rapid deterioration of the animals resembles hyper-inflammatory sepsis. However, exclusive kidney involvement is rare in human systemic candidiasis and kidney manifestation typically only occurs in disseminated candidiasis affecting multiple organs.29,30 Despite this, the murine infection model enables the analysis of rapid immune activation induced by systemic Candida dissemination and has undoubtedly revealed important insights into host responses to fungal infection.31,32
Unlike humans, mice are intrinsically Candida naïve and establishing colonization of the gastrointestinal tract in adult mice requires anti-microbial therapy and oral application of Candida.32 A murine model of C. albicans gastrointestinal colonization and systemic spread has been described by Koh et al.25,32,33 Concomitant introduction of immuno-suppression and mucosal damage after colonization resulted in translocation and dissemination by C. albicans. This model is particularly useful for studying virulence factors and immune mechanisms involved in translocation and dissemination. A major advance in our technological portfolio to study host-pathogen interaction during systemic infection is the development of in vivo imaging systems.34,35 Recently, these tools have been used for in vivo imaging of Candida infection.36 This allows monitoring of dissemination and systemic infection over time in living animals with considerable sensitivity. Furthermore, it reduces animal tolls and offers the possibility to shift from end-point data toward kinetic analyses. Initial experiments already revealed the gall-bladder as an unexpected site of C. albicans persistence during anti-microbial therapy.36
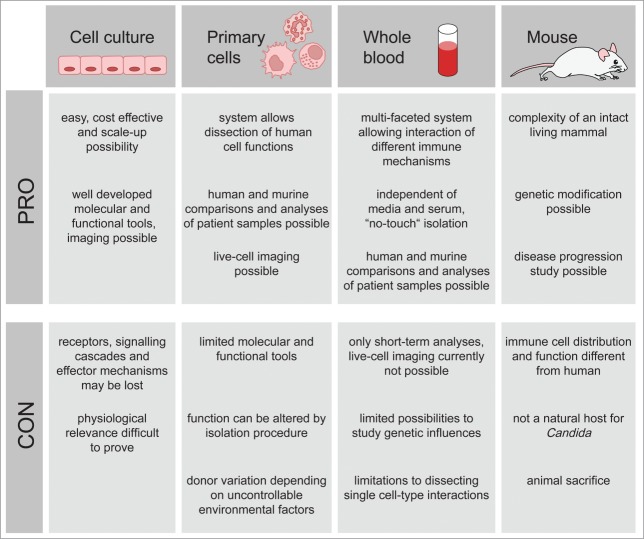
As for all murine infection models, it has to be kept in mind that peripheral blood components in mice differ, both in numbers and function, from their human counterparts37,38 and conclusions from defined animal models are not necessarily transferable to human patients. To overcome some of these limitations, human whole blood infection models can be used to analyze host-pathogen interactions in a situation which closely mirrors that in vivo.39 Such infection models have successfully been used to identify microbial virulence factors,40 to analyze early immune responses,41 to determine the influence of genetic polymorphisms on immune response42 or to test potential therapeutic approaches or vaccine efficacy.43-46 With regard to activation of host immunity, whole blood infection assays can provide time-resolved data on cell activation, localization and physiological state of the pathogen. Most importantly whole blood infection assays require minimal pre-analytical handling of the cells. Therefore these assays avoid modulation of immune cell function by the isolation procedure that inevitably occurs when using purified primary human immune cells47-49 (see Fig. 1). However, purified primary cells provide an important tool to analyze specific contributions of receptors and signaling pathways in defined cell populations50,51 and patterns of activation observed in the whole blood model do not necessarily reflect those observed in organ tissue. Furthermore, immune cell activation in blood in vivo is also determined by tissue derived mediators which are absent in ex vivo blood. In contrast, in the whole blood model, many parameters of immune cell function remain inaccessible to direct quantification due to experimental limitations. We have shown recently that bio-mathematical modeling can provide tools to partially overcome these limitations. Using such a virtual infection model, it was for the first time possible to prove the dominant role of neutrophils in the immune response to Candida in human blood.39
Figure 1.
Advantages and disadvantages of C. albicans infection models. The most commonly employed C. albicans infection models are immortalized cell culture, primary immune cells, whole blood and mice. Each method bears both limitations and advantages, a thorough knowledge of which can be applied to determining the most suitable model.
Finally—with all models being but models—it is encouraging to see that modern technologies allow the analysis of molecular pathways determining the outcome of host–pathogen interaction directly in human infection. Genetic analyses in patients suffering from chronic mucocutaneous candidiasis have generated unprecedented insight into the role of STAT1 signaling and Th17 response in anti-fungal immunity.3,52,53 These findings have been extended to other fungal infections and significantly advanced our knowledge of antifungal immunology.54 By integrating transcriptional analysis and functional genomics, Smeekens et al. identified a prominent role of the type I interferon pathway in anti-Candida host defense. They confirmed these analyses by showing that polymorphisms in type I interferon genes modulated Candida-induced cytokine production and were correlated with susceptibility to systemic candidiasis.55 Genetic analyses of patients at risk for non-Candida fungal infections have also identified other important regulators of anti-fungal immunity.3,21,56 Together, these model systems have generated important insight into mechanisms governing immune responses against Candida and established a repertoire of receptors and signaling cascades relevant for fungal recognition.57 In the next sections, we will put a focus on immune effector mechanisms that are relevant for systemic Candida infections.
Complement in Candida Sepsis
Considerable evidence shows that complement activation plays a central role in systemic infection and sepsis.58,59 The interaction of C. albicans with complement has recently been reviewed in detail and we refer to the review of Luo et al.60 Although patients suffering from genetic defects in complement do not show increased risk for fungal infections, evidence from both murine and in vitro experiments indicates an important role of complement in antifungal responses. However, even in patients with chronic granulomatous disease – a severe functional defect of neutrophils - numbers of invasive Candida infections are surprisingly low, (Winkelstein et al.60b and Falcone and Holland60c). This may reflect both redundancy of immune effector functions or the fact that intestinal barrier integrity may be at large protective against Candida invasion.25,60a
Aside from multiple functions in the immune response against invading pathogens, complement activation also modulates other signaling events during systemic infection. Several studies have shown that Toll-like receptor (TLR) activation can occur by way of complement, and multiple nodes of interaction between complement and coagulation have been identified.61,62 The surface of C. albicans is a strong trigger inducing all 3 pathways of complement activation63 (Fig. 2). This results in rapid formation of C3 convertase, generation of chemotactic cleavage fragments and subsequent fungal opsonization by C3b, which facilitates phagocytosis.64-66 Of major importance during sepsis is the generation of high levels of the complement activation products C3a and C5a, which act as anaphylatoxins.59 Mice lacking the C5a precursor molecule C5 or the C3a precursor C3 are highly susceptible to invasive C. albicans infection.67-69 Moreover, C5 deficiency is associated with increased levels of pro-inflammatory cytokines, including TNF-α and IL-6, and rapid fungal replication in many organs.60a,70,71 The prominent effects of C5 deletion are most likely related to the lack of its activation product C5a, which has been shown to be critical for activation of human monocytes by C. albicans and which significantly enhances the release of pro-inflammatory cytokines, e.g., IL-6 and IL-1β.72 Furthermore, C5a can influence neutrophil function during sepsis and even induce paralysis of neutrophils.73,74 Thus, early and pronounced activation of complement is also a critical determinant in the activation of cellular responses toward Candida and directly triggers activation of major innate immune cell populations involved in anti-fungal immunity while at the same time potentially contributing to adverse effects of fulminant immune activation.
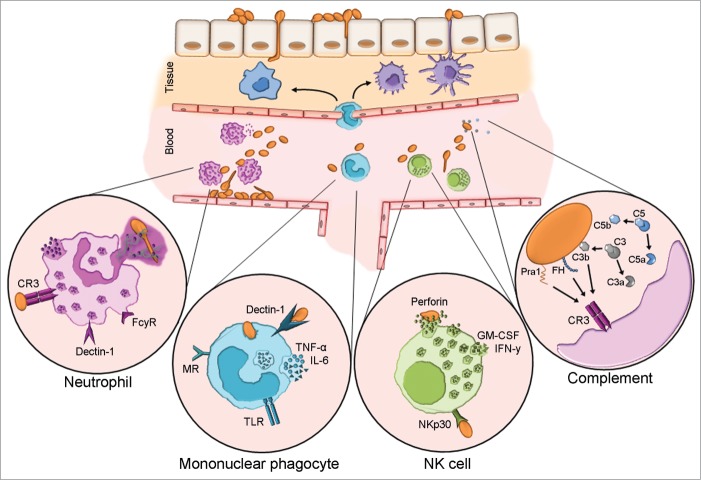
Figure 2.
Host innate immune responses to C. albicans blood stream infection. Upon transmigration of skin skin/mucosal barrier and entry to the bloodstream, C. albicans will activate the complement system and encounter circulating and resident leukocytes. Neutrophils are considered the forerunners of innate responses to C. albicans due to their efficient recognition and clearance of the fungus. Complement receptor 3 (CR3) and FCγR are the paramount human neutrophil receptors capable of recognizing C. albicans. Contact to the fungus initiates various signaling cascades, which in turn instigate effector mechanisms e.g. phagocytosis, oxidative burst and neutrophil extracellular trap (NET) formation. Mononuclear phagocytes include circulating monocytes as well as macrophages and dendritic cells residing in various tissues. These cells recognize C. albicans principally via dectin-1 which acts in concert with other pattern recognition receptors. They are a dominant source of IL-6 and TNF-α, both of which can exert direct effects on the fungus and also influence other immune cells. Although NK cells harbor many PRR capable of C. albicans recognition, NKp30 is the principal mediator of NK cell anti-Candida activity. NK cell-released perforin is directly candidacidal. Additionally, NK cells secrete GM-CSF and IFN-γ which both potently modulate other immune cells. Candida is a potent activator of human complement. Complement activation results in opsonization by deposition of C3b and release of anaphylatoxins C5a and C3a which influence immune cell recruitment and effector mechanisms. In addition to C3b, recognition of the fungal protein Pra1 and surface-recruited Factor H, a major regulator of complement activation, mediate recognition by immune cell CR3.
The Various Roles of Neutrophils in C. albicans Sepsis
Polymorphonuclear leukocytes (PMN) represent the majority of circulating leukocytes in humans. The sheer number of this cell type in circulation, as well as their aggressive and successful elimination of invading pathogens,75 advocates them as forerunners of innate defense. In the murine system, early availability of neutrophils has been shown to be essential for protection.76 Both clinical and experimental evidence has confirmed that neutrophils are integral components of the innate immune system during C. albicans infection. Most importantly, human neutrophils are the only immune cell which can prevent the transition from yeast to filamentous growth—a key virulence trait of C. albicans,37,77 and dominate the transcriptional response of C. albicans in whole blood.78 PMN control the elimination of C. albicans from the bloodstream39 and as such, these professional phagocytes are considered primary effector cells in C. albicans infection prevention and neutropenia is a clear risk factor for mortality in human systemic candidiasis.79,80 However, it must be noted that in systemic Candida infection, PMN can also exert adverse effects which are linked to their potent pro-inflammatory activity and bystander damage to host tissues inflicted by anti-microbial effector mechanisms. In line with this, neutropenic patients with invasive candidiasis may require corticosteroid therapy after neutrophil reconstitution to avoid adverse effects of hyper-inflammation.81 Circulating PMN are recruited rapidly to sites of Candida infection and upon activation IL-8 is the major cytokine released by C. albicans activated PMN which promotes the further recruitment of PMN.77 Consequently, the IL-8 – IL-8R signaling axis is essential for protective immunity.82 However, several other cytokines contribute to PMN recruitment and function. In the murine model system for example type 1 interferon (INF-1) signaling mediates neutrophil recruitment by stimulating early release of inflammatory cytokines, e.g., IL-6.83 IL-17 can be produced by T cells, but also neutrophils during Candida sepsis, when it promotes early and sustained recruitment of neutrophils into the C. albicans infected kidney.84 While IFN-1 and IL-17 are important at early stages, chemokine receptor CCR1 is necessary for PMN trafficking from the blood to the kidney during later stages of infection,85 which is correlated with neutrophil-mediated immunopathology and mortality. While other myeloid cells constantly expressed CCR1, neutrophils were found not to express the receptor until days after C. albicans infection. Independent of CCR1 expression, neutrophils were able to mount normal effector mechanisms, demonstrating that the immunopathology related to the quantity of infiltrating neutrophils and not their activity.85 Aside from recruiting PMN, cytokines and chemokines are involved in activating these cells during Candida infection. Murine knock-out strains of several cytokines display a decrease in PMN anti-C. albicans activity due to an impaired intrinsic pre-stimulation of PMN.86 Cell types that secrete factors modulating PMN anti-fungal activity include antigen-presenting cells, epithelial and endothelial cells as well as antigen-specific T cells.86-88 In addition to this, NK cell–PMN cross-talk may be immunologically relevant89,90 (see later).
Neutrophil receptors involved in Candida recognition
PMN express various pattern recognition receptors as well as receptors for opsonizing antibodies and complement components.91 Thus, interaction with Candida as well as concomitant activation is mediated by a set of closely interlinked interactions and signaling events and cannot be contributed to a single receptor. However, several lines of evidence suggest that complement receptor 3 (CR3; also known as αmβ2 integrin; Mac-1, CD11b/CD18) is a major receptor for C. albicans yeast and hyphae on human neutrophils.50,92,93 CR3 is expressed on circulating neutrophils and may be rapidly recruited from intracellular compartments to the cell surface upon activation.94 Van Bruggen and co-workers found that phagocytosis of unopsonized C. albicans by human PMN was mainly mediated by CR3, while no explicit role for neutrophil expressed dectin-1 was observed95(Fig. 2). Multiple possibilities for the interaction of C. albicans with this receptor have been described: CR3 is the major receptor for C3b and its cleavage product iC3b and can therefore recognize C. albicans after complement mediated opsonization.96 Furthermore, the C. albicans surface protein Pra1 as well as the cell-wall component β-glucan can directly bind to CR3.97 Finally, C. albicans harbors a set of proteins known as CRASPs (complement regulator surface acquiring proteins) that can recruit the complement regulator factor H (CFH) and related complement regulators to its surface.60a After recruitment to the surface of Candida, CFH family proteins CFH, CFH-like protein 1 (CFHL1) and CFH-related protein (CFHR) 1 can bind to CR3 and increase attachment of neutrophils to C. albicans.98 Thus, CR3 is able to mediate both uptake of both (C3b-)opsonized and non-opsonized C. albicans. In contrast to CR3, human dectin-1, a major human receptor for β-glucan,99 seems to play a minor role in phagocytosis of Saccharomyces cerevisiae or zymosan by human PMN.95 In addition, neither generation of reactive oxygen intermediates (ROI) nor secretion of IL-8 in response to zymosan required dectin-1 signaling in human PMN.95 These data may indicate a less pronounced role for dectin-1 in PMN-Candida interaction, contrary to the dominant role of dectin-1 signaling in other cell types (see below). This is further confirmed by recent findings showing that killing of C. albicans by human PMN occurs independently of dectin-150. In contrast, loss of caspase-associated recruitment domain 9 (CARD9), the intracellular adapter molecule downstream of dectin-1 signaling,100,101 has been shown to significantly impair unopsonized anti-Candida immunity in human neutrophils.51 However, this function seems to be independent of dectin-1 and is known to act downstream of several receptors, including other C-type lectin receptors.50 It should be noted that dectin-1 may be more important for the activation of murine PMN by Candida.102 In the murine system, dectin-1 has been shown to induce and activate CR3 after ligand binding to also recognize fungal components.103,104 This cross-activation was found to be required for murine neutrophil cytotoxic responses.104 CR3 activation and neutrophil effector functions in murine neutrophils also required exchange factors for RhoGTPases Vav1 and Vav3.104
Neutrophils also express a range of Toll-like receptors (TLR). In mice, TLR2 expression is required for optimal neutrophil chemotaxis, pro-inflammatory cytokine production and MPO activity in response to murine C. albicans infection.105 However, TLR signaling is not essential for anti-Candida activity of human PMN as shown by testing PMN from patients with IRAK4 deficiency, a central component in TLR signaling.106 Finally, neutrophils constitutively express FcγR; specifically, FcγRIII (CD16) activation can initiate characteristic neutrophil activation mechanisms, e.g., degranulation and respiratory burst.107,108 In summary, while CR3 seems to play a central role multiple receptors may contribute to the interaction of PMN with C. albicans.
Anti-candida effector mechanisms of neutrophils
Once they recognize the pathogen, PMN have a range of weapons they can unleash against C. albicans. Among the most prominent mechanisms is the rapid formation of reactive oxygen intermediates (ROI) termed ‘oxidative burst’. Upon activation, the neutrophilic NADPH oxidase-complex is assembled on the cytoplasmic membrane to release superoxide into the extracellular space, or on the phagosomal membrane to release oxidants into phagosome.109,110 PMN isolated from NADPH (and MPO) deficient mice show reduced C. albicans killing ex vivo.111,112 Aside from inducing oxidative stress,113,114 ROI are required for the formation of the so-called neutrophil extracellular traps (NETs).115–117 However, this may only be the case in the blood stream as NET formation seems to be CR3 dependent and ROI independent in tissues93 NETs provide a barrier past which a pathogen cannot easily pass, and instead becomes entangled in a mesh of cytotoxic compounds. These are structures formed of released neutrophil chromatin decorated with anti-microbial substances, principally calprotectin,117 which are normally stored within neutrophilic granules and can be formed within 10 minutes of activation.118 NETs have been shown to entrap free bacteria in the bloodstream and therefore prevent dissemination in an Escherichia coli model of sepsis.119 They may form simply from the plasma of septic patients,120 as well as upon direct contact with a pathogen. C. albicans induces NET formation, after which both filamentous and yeast forms are trapped and killed.116 The relevance of NETs to Candida sepsis may be suggested by increased susceptibility of mice deficient in calprotectin, a key component of NETs, to systemic candidiasis. However, with additional immunomodulatory effects of calprotectin well established in the literature, this is not formal proof for a role of NET-formation in anti-Candida immunity.117 In addition to oxidative burst and NET formation, neutrophils contain an arsenal of anti-microbial peptides and proteins, many of which also have anti-fungal activity. Furthermore, they can release cytokines, which recruit other immune cells and potentially induce damage in Candida and induce carbohydrate and nitrogen starvation.113,121 However, it is still relatively unclear exactly how PMN kill C. albicans. Most likely, a combination of different stresses forms the basis for their fungicidal activity.122,123 In a recent study, 2 distinct mechanisms for killing of C. albicans dependent on how PMN recognize either opsonized or unopsonized fungus have been described.50 Unopsonized C. albicans is recognized via CR3 and killing is CR3 and CARD9 dependent, whereas dectin-1 was not required. In contrast, opsonized C. albicans was recognized via FcγR, and PKC and NADPH oxidase activity were the principal killing machinery.50 The latter studies demonstrate that in the complex environment of the host, combinations of killing mechanisms are in play which occur independent of pattern recognition receptors like TLR and dectin-1 dominating the activation of monocytic cells and can compensate for each other under deficiency conditions. Thus, redundancy of anti-fungal mechanisms is most likely a major contributor to the potent fungicidal activity of PMN.
Linking Innate and Adaptive Immune Responses: Monocytes, Macrophages and Dendritic Cells
Relative to neutrophils, monocytes—the second most abundant innate immune cell population in human blood—may play a smaller role in the initial response to C. albicans blood stream infection and are in fact less effective in C. albicans killing in whole blood.39 Nevertheless, monocytes as well as macrophages and dendritic cells (DC) are crucial in establishing protective immunity and monocyte deficient mice suffer quick dissemination into organs and higher mortality following C. albicans infection,124 although monocytopenia alone does not confer susceptibility to candidiasis.125,126 Monocytes may also play an integral role in anti-Candida defense in locations of dissemination, e.g., the kidney, where early and organ specific innate responses have recently been demonstrated in the murine model.127 Abrogation of inflammatory monocyte trafficking into the kidneys impaired fungal clearance and decreased survival. Migration of these cells was mainly mediated by CCR2 and depletion of CCR2-expressing cells led to uncontrolled fungal growth in the kidneys and brain.128 Similarly, the promotion of macrophage survival and accumulation in tissues by CX3CR1-dependent mononuclear cells is a critical mechanism by which the early innate response can protect against candidiasis.129 DC are the most potent antigen presenting cells in the human body and play a crucial role in inducing and modulating adaptive immune responses. Recently the role of DC in anti-fungal immune responses has been reviewed (see ref130). The spectrum of receptors used for recognition of Candida in these cells is broad and includes C-type lectin receptors (CLR) including dectin-1, dectin-2, Macrophage Mannose Receptor (MMR) and DC-SIGN, as well as TLRs, namely TLR2, TLR4, TLR7 and TLR9.91,130 Aside from these, several other receptors can contribute to recognition of Candida, including complement receptors CR3, CR4 and Fc-receptors. Despite this plethora of potential receptors, dectin-1 seems to play a prominent role in the recognition of C. albicans and activation of DC by C. albicans occurs via dectin-1 recognition of β-glucan, and involving, to a lesser extent, recognition of other surface structures by TLR.131 Similarly, dectin-1 is central for recognition of A. fumigatus.132 Dectin-1-triggered CARD9 signaling then drives cytokine production, through an NF-kB and NFAT-dependent pathway.133 The central importance of CARD9 signaling to the DC response to C. albicans is highlighted by the finding that mice deficient in the dectin-1-CARD9 pathway are unable to mount normal DC cytokine secretion, for example IL-6 and TNF-α, and neither are they able to generate Th17 cells upon confrontation with C. albicans.101,134 In addition, IFN-β production by DC induced by C. albicans is largely dependent on dectin-1 and dectin-2 mediated signaling and plays a crucial role in the defense against C. albicans infection.83,135,136 The prominent role of CLR signaling in murine Candida infection has recently been confirmed by a study showing that the selective loss of spleen tyrosine kinase Syk but not the TLR adaptor protein MyD88 in DC abrogates innate resistance to systemic C. albicans infection in mice. Syk is recruited by dectin-1 and other CLR and can trigger NF-kB activation via CARD9101 as well as other signaling cascades e.g., NFAT, MAPK and PI3K.137,138 Engagement of dectin-1 with C. albicans leads to Syk expression and CARD9 complex assembly. This was found to be essential for C. albicans induced IL-23p19 release, which in turn mediates GM-CSF secretion by natural killer (NK) cells at the site of infection. As NK cell-derived GM-CSF sustains the anti-Candida activity of neutrophils, the authors conclude that DC mediated an innate response to Candida sepsis, dependent on SYK signaling.139,140
Natural Killer Cells
Although traditionally studied in the context of anti-viral and anti-tumor immunity, NK cells have recently gained prominence as key players in various fungal infections. These cells form a population of innate lymphocytes, accounting for 5–10% of circulating blood lymphocytes. Most of the blood NK cells express high levels of CD56 (CD56bright) and produce high levels of perforin.141 Early on, activity of NK cells was reported against Cryptococcus neoformans.142,143 Anti-fungal roles for NK cells in aspergillosis and cryptococcosis are attributed to cytokine and perforin release, respectively.144–146 Whereas patients with inherited NK cell deficiencies are generally not more susceptible to candidiasis than the healthy population, in a murine model of invasive oropharyngeal candidiasis, combined T and NK cell deficiencies were detrimental to outcome, while T cell deficiency alone exerts no discernible phenotype.147 Recently, several studies have addressed the role of NK cells in systemic Candida infection. NK cells are activated by C. albicans and can wield direct perforin mediated cytotoxic effects on the fungus.90 Interestingly, human NK cells have been found to ingest C. albicans by phagocytosis and elicit pro-inflammatory responses.90 NK cells harbor a range of receptors capable of recognizing C. albicans such as TLR, mannose, scavenger, FCγ receptor and NK cell activating receptors.148,149 However, the principal C. albicans recognition receptor was recently shown to be NKp30.150 NKp30 was responsible for recognition and killing of C. albicans and also C. neoformans. Recognition of fungi via NKp30 resulted in PI3K signaling and perforin release, which has been shown to exert anti-fungal activity. Using NK cells from HIV infected patients, which exhibit a diminished expression of NKp30, the authors showed that reduced levels of NKp30 are associated with defective anti-fungal activity.150 NK cells can also indirectly affect C. albicans via modulation of other immune cells.90,140 Several cytokines released by Candida-activated NK cells, including GM-CSF and IFN-γ, may directly trigger anti-fungal effector mechanisms in other immune cells.140,151,152 NK cells have been shown to exert immuno-modulatory functions,128,153-155 influence PMN survival156 and expression of neutrophil activation markers.157 In a murine model for C. albicans sepsis in immuno-competent mice, NK cells have a detrimental influence on the course of disease by promoting hyper-inflammation, which resulted in reduced survival time.158 In contrast, in immuno-compromised animals deficient in B and T cells, NK cells were found to be beneficial in recruiting and activating other immune cells, aiding in eventual clearance of the fungus.158
Conclusion and Outlook
Immune responses in systemic Candida infection and sepsis are complex and involve several rapidly acting players. More importantly, the balance between protective immunity and harmful hyper-inflammation is hard to define and several protective inflammatory reactions have been shown to also contribute to sepsis pathology. A future thorough understanding of these mechanisms may offer new insight into the pathophysiology of these infections, as well as open new avenues for tests allowing early discrimination of bacterial and fungal sepsis and targeted anti-microbial therapy. With individualized approaches to clinical management of infections rapidly developing and a pressing need for stratification of the broad clinical entity sepsis being increasingly recognized, this research forms the basis for translational approaches to fungal sepsis.159 To get meaningful insight into the underlying mechanisms, a combination of models has to be used, taking into account the strengths and weaknesses of each of them. Thus, although the murine system clearly provides the model of the highest complexity, it is not necessarily always superior. Finally, the field of infection genetics has provided major advances to our understanding of anti-Candida immunity. With molecular tools rapidly evolving and sequencing approaches becoming more and more feasible, it is likely that new findings will arise from in-depth studies of individuals suffering from well characterized diseases. Clearly, these studies will pave the way toward optimized and individualized clinical management of infectious diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The German National Reference Center for Invasive Fungal Infections (NRZMyk, head: OK) is supported by the Robert-Koch-Institute from funds provided by the German Ministry of Health (grant-No. 1369–240). Work in the Kurzai lab is supported by the Deutsche Forschungsgemeinschaft [KU1540–3/1 and SFB/TR124 FungiNet, project C3] and the German Ministry for Education and Science in the program Unternehmen Region (BMBF 03Z2JN21). SD was supported by the Excellence graduate school, Jena School for Microbial Communication (JSMC, Jena).
References
- 1.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 2014; 5:4-11; PMID:24335434; http://dx.doi.org/10.4161/viru.27372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546-54; PMID:12700374; http://dx.doi.org/10.1056/NEJMoa022139 [DOI] [PubMed] [Google Scholar]
- 3.Lionakis MS. New insights into innate immune control of systemic candidiasis. Med Mycol 2014; 52:555-64; PMID:25023483; http://dx.doi.org/10.1093/mmy/myu029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leleu C, Gloria E, Renault G, Barrey E. Analysis of trotter gait on the track by accelerometry and image analysis. Equine Vet J Suppl 2002:344-8; PMID:12405713 [DOI] [PubMed] [Google Scholar]
- 5.Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis 2012; 54:1739-46; PMID:22423135; http://dx.doi.org/10.1093/cid/cis305 [DOI] [PubMed] [Google Scholar]
- 6.Yang ZT, Wu L, Liu XY, Zhou M, Li J, Wu JY, Cai Y, Mao EQ, Chen EZ, Lortholary O. Epidemiology, species distribution and outcome of nosocomial Candida spp. bloodstream infection in Shanghai. BMC Infect Dis 2014; 14:241; PMID:24886130; http://dx.doi.org/10.1186/1471-2334-14-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 2005; 49:3640-5; PMID:16127033; http://dx.doi.org/10.1128/AAC.49.9.3640-3645.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labelle AJ, Micek ST, Roubinian N, Kollef MH. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med 2008; 36:2967-72; PMID:18824910; http://dx.doi.org/10.1097/CCM.0b013e31818b3477 [DOI] [PubMed] [Google Scholar]
- 9.Komshian SV, Uwaydah AK, Sobel JD, Crane LR. Fungemia caused by Candida species and Torulopsis glabrata in the hospitalized patient: frequency, characteristics, and evaluation of factors influencing outcome. Rev infect Dis 1989; 11:379-90; PMID:2749102; http://dx.doi.org/10.1093/clinids/11.3.379 [DOI] [PubMed] [Google Scholar]
- 10.Richet H, Roux P, Des Champs C, Esnault Y, Andremont A, French Candidemia Study G. Candidemia in French hospitals: incidence rates and characteristics. Clin Microbiol Infect 2002; 8:405-12; PMID:12199850; http://dx.doi.org/10.1046/j.1469-0691.2002.00446.x [DOI] [PubMed] [Google Scholar]
- 11.Miranda LN, van der Heijden IM, Costa SF, Sousa AP, Sienra RA, Gobara S, Santos CR, Lobo RD, Pessoa VP, Jr, Levin AS. Candida colonisation as a source for candidaemia. J Hosp Infect 2009; 72:9-16; PMID:19303662; http://dx.doi.org/10.1016/j.jhin.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 12.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302:2323-9; PMID:19952319; http://dx.doi.org/10.1001/jama.2009.1754 [DOI] [PubMed] [Google Scholar]
- 13.Kett DH, Azoulay E, Echeverria PM, Vincent JL. Extended Prevalence of Infection in ICUSGoI. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med 2011; 39:665-70; PMID:21169817; http://dx.doi.org/10.1097/CCM.0b013e318206c1ca [DOI] [PubMed] [Google Scholar]
- 14.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309-17; PMID:15306996; http://dx.doi.org/10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 15.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK; National Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilities. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol 2008; 29:996-1011; PMID:18947320; http://dx.doi.org/10.1086/591861 [DOI] [PubMed]
- 16.Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2014; 5:161-9; PMID:24157707; http://dx.doi.org/10.4161/viru.26187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 2006; 43:25-31; PMID:16758414; http://dx.doi.org/10.1086/504810 [DOI] [PubMed] [Google Scholar]
- 18.Guzman JA, Tchokonte R, Sobel JD. Septic shock due to candidemia: outcomes and predictors of shock development. J Clin Med Res 2011; 3:65-71; PMID:21811532; http://dx.doi.org/10.4021/jocmr536w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet 2013; 381:774-5; PMID:23472921; http://dx.doi.org/10.1016/S0140-6736(12)61815-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie GH, Fang XM, Fang Q, Wu XM, Jin YH, Wang JL, Guo QL, Gu MN, Xu QP, Wang DX, et al. Impact of invasive fungal infection on outcomes of severe sepsis: a multicenter matched cohort study in critically ill surgical patients. Crit Care 2008; 12:R5; PMID:18199317; http://dx.doi.org/10.1186/cc6766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunha C, Aversa F, Lacerda JF, Busca A, Kurzai O, Grube M, Löffler J, Maertens JA, Bell AS, Inforzato A, et al. Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N Engl J Med 2014; 370:421-32; PMID:24476432; http://dx.doi.org/10.1056/NEJMoa1211161 [DOI] [PubMed] [Google Scholar]
- 22.Chao CC, Hsu PC, Jen CF, Chen IH, Wang CH, Chan HC, Tsai PW, Tung KC, Wang CH, Lan CY, et al. Zebrafish as a model host for Candida albicans infection. Infect Immun 2010; 78:2512-21; PMID:20308295; http://dx.doi.org/10.1128/IAI.01293-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen YY, Chao CC, Liu FC, Hsu PC, Chen HF, Peng SC, Chuang YJ, Lan CY, Hsieh WP, Wong DS. Dynamic transcript profiling of Candida albicans infection in zebrafish: a pathogen-host interaction study. PLOS One 2013; 8:e72483; PMID:24019870; http://dx.doi.org/10.1371/journal.pone.0072483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brothers KM, Gratacap RL, Barker SE, Newman ZR, Norum A, Wheeler RT. NADPH oxidase-driven phagocyte recruitment controls Candida albicans filamentous growth and prevents mortality. PLOS Pathog 2013; 9:e1003634; PMID:24098114; http://dx.doi.org/10.1371/journal.ppat.1003634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh AY, Kohler JR, Coggshall KT, Van Rooijen N, Pier GB. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLOS Pathog 2008; 4:e35; PMID:18282097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spellberg B, Ibrahim AS, Edwards JE, Jr., Filler SG. Mice with disseminated candidiasis die of progressive sepsis. J Infect Dis 2005; 192:336-43; PMID:15962230; http://dx.doi.org/10.1086/430952 [DOI] [PubMed] [Google Scholar]
- 27.Lionakis MS, Lim JK, Lee CC, Murphy PM. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun 2011; 3:180-99; PMID:21063074; http://dx.doi.org/10.1159/000321157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maccallum DM. Hosting infection: experimental models to assay Candida virulence. Int J Microbiol 2012; 2012:363764; PMID:22235206; http://dx.doi.org/10.1155/2012/363764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groll AH, Shah PM, Mentzel C, Schneider M, Just-Nuebling G, Huebner K. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect 1996; 33:23-32; PMID:8842991; http://dx.doi.org/10.1016/S0163-4453(96)92700-0 [DOI] [PubMed] [Google Scholar]
- 30.Lehrnbecher T, Frank C, Engels K, Kriener S, Groll AH, Schwabe D. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect 2010; 61:259-65; PMID:20624423; http://dx.doi.org/10.1016/j.jinf.2010.06.018 [DOI] [PubMed] [Google Scholar]
- 31.Marakalala MJ, Vautier S, Potrykus J, Walker LA, Shepardson KM, Hopke A, Mora-Montes HM, Kerrigan A, Netea MG, Murray GI, et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLOS Pathog 2013; 9:e1003315; PMID:23637604; http://dx.doi.org/10.1371/journal.ppat.1003315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabo EK, MacCallum DM. The contribution of mouse models to our understanding of systemic candidiasis. FEMS Microbiol Lett 2011; 320:1-8; PMID:21395661; http://dx.doi.org/10.1111/j.1574-6968.2011.02262.x [DOI] [PubMed] [Google Scholar]
- 33.Koh AY. Murine models of Candida gastrointestinal colonization and dissemination. Eukaryot Cell 2013; 12:1416-22; PMID:24036344; http://dx.doi.org/10.1128/EC.00196-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brock M. Application of bioluminescence imaging for in vivo monitoring of fungal infections. Int J Microbiol 2012; 2012:956794; PMID:22121368; http://dx.doi.org/10.1155/2012/956794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papon N, Courdavault V, Lanoue A, Clastre M, Brock M. Illuminating fungal infections with bioluminescence. PLOS Pathog 2014; 10:e1004179; PMID:25010008; http://dx.doi.org/10.1371/journal.ppat.1004179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobsen ID, Luttich A, Kurzai O, Hube B, Brock M. In vivo imaging of disseminated murine Candida albicans infection reveals unexpected host sites of fungal persistence during antifungal therapy. J Antimicrob Chemother 2014; 69(1):1785-96; PMID:24951534; http://dx.doi.org/10.1093/jac/dku198 [DOI] [PubMed] [Google Scholar]
- 37.Ermert D, Niemiec MJ, Rohm M, Glenthoj A, Borregaard N, Urban CF. Candida albicans escapes from mouse neutrophils. J Leukoc Biol 2013; 94:223-36; PMID:23650619; http://dx.doi.org/10.1189/jlb.0213063 [DOI] [PubMed] [Google Scholar]
- 38.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Nat Acad Sci U S A 2013; 110:3507-12; PMID:23401516; http://dx.doi.org/10.1073/pnas.1222878110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hünniger K, Lehnert T, Bieber K, Martin R, Figge MT, Kurzai O. A virtual infection model quantifies innate effector mechanisms and candida albicans immune escape in human blood. PLOS Comput Biol 2014; 10:e1003479; PMID:24586131; http://dx.doi.org/10.1371/journal.pcbi.1003479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Echenique-Rivera H, Muzzi A, Del Tordello E, Seib KL, Francois P, Rappuoli R, Pizza M , Serruto D. Transcriptome analysis of Neisseria meningitidis in human whole blood and mutagenesis studies identify virulence factors involved in blood survival. PLOS Pathog 2011; 7:e1002027; PMID:21589640; http://dx.doi.org/10.1371/journal.ppat.1002027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tena GN, Young DB, Eley B, Henderson H, Nicol MP, Levin M, Kampmann B. Failure to control growth of mycobacteria in blood from children infected with human immunodeficiency virus and its relationship to T cell function. J Infect Dis 2003; 187:1544-51; PMID:12721934; http://dx.doi.org/10.1086/374799 [DOI] [PubMed] [Google Scholar]
- 42.Lin TS, Huang HH, Fan YH, Chiou SH, Chow KC. Genetic polymorphism and gene expression of microsomal epoxide hydrolase in non-small cell lung cancer. Oncology Rep 2007; 17:565-72; PMID:17273734 [PubMed] [Google Scholar]
- 43.Plested JS, Welsch JA, Granoff DM. Ex vivo model of meningococcal bacteremia using human blood for measuring vaccine-induced serum passive protective activity. Clin Vaccine Immunol 2009; 16:785-91; PMID:19339487; http://dx.doi.org/10.1128/CVI.00007-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jemmett K, Macagno A, Molteni M, Heckels JE, Rossetti C, Christodoulides M. A cyanobacterial lipopolysaccharide antagonist inhibits cytokine production induced by Neisseria meningitidis in a human whole-blood model of septicemia. Infect Immun 2008; 76:3156-63; PMID:18443097; http://dx.doi.org/10.1128/IAI.00110-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deslouches B, Islam K, Craigo JK, Paranjape SM, Montelaro RC, Mietzner TA. Activity of the de novo engineered antimicrobial peptide WLBU2 against Pseudomonas aeruginosa in human serum and whole blood: implications for systemic applications. Antimicrob Agents Chemother 2005; 49:3208-16; PMID:16048927; http://dx.doi.org/10.1128/AAC.49.8.3208-3216.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sprong T, Brandtzaeg P, Fung M, Pharo AM, Hoiby EA, Michaelsen TE, Aase A, van der Meer JW, van Deuren M, Mollnes TE. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood 2003; 102:3702-10; PMID:12881318; http://dx.doi.org/10.1182/blood-2003-03-0703 [DOI] [PubMed] [Google Scholar]
- 47.Glasser L, Fiederlein RL. The effect of various cell separation procedures on assays of neutrophil function. A critical appraisal. Am J Clin Pathol 1990; 93:662-9; PMID:2327366 [DOI] [PubMed] [Google Scholar]
- 48.Watson F, Robinson JJ, Edwards SW. Neutrophil function in whole blood and after purification: changes in receptor expression, oxidase activity and responsiveness to cytokines. Biosci Rep 1992; 12:123-33; PMID:1421055; http://dx.doi.org/10.1007/BF02351217 [DOI] [PubMed] [Google Scholar]
- 49.Hasenberg M, Kohler A, Bonifatius S, Borucki K, Riek-Burchardt M, Achilles J, Männ L, Baumgart K, Schraven B, Gunzer M. Rapid immunomagnetic negative enrichment of neutrophil granulocytes from murine bone marrow for functional studies in vitro and in vivo. PLOS One 2011; 6:e17314; PMID:21383835; http://dx.doi.org/10.1371/journal.pone.0017314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gazendam RP, van Hamme JL, Tool AT, van Houdt M, Verkuijlen PJ, Herbst M, Liese JG, van de Veerdonk FL, Roos D, van den Berg TK, et al. Two independent killing mechanisms of Candida albicans by human neutrophils: evidence from innate immunity defects. Blood 2014; 124:590-7; PMID:24948657; http://dx.doi.org/10.1182/blood-2014-01-551473 [DOI] [PubMed] [Google Scholar]
- 51.Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, van Hamme JL, van Leeuwen EM, Roos D, Scalais E, de Beaufort C, et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 2013; 121:2385-92; PMID:23335372; http://dx.doi.org/10.1182/blood-2012-08-450551 [DOI] [PubMed] [Google Scholar]
- 52.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, Arts P, Rosentul DC, Carmichael AJ, Smits-van der Graaf CA, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med 2011; 365:54-61; PMID:21714643; http://dx.doi.org/10.1056/NEJMoa1100102 [DOI] [PubMed] [Google Scholar]
- 53.Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol 2012; 12:616-22; PMID:23026768; http://dx.doi.org/10.1097/ACI.0b013e328358cc0b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sampaio EP, Hsu AP, Pechacek J, Bax HI, Dias DL, Paulson ML, Chandrasekaran P, Rosen LB, Carvalho DS, Ding L, et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol 2013; 131:1624-34; PMID:23541320; http://dx.doi.org/10.1016/j.jaci.2013.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smeekens SP, Ng A, Kumar V, Johnson MD, Plantinga TS, van Diemen C, Arts P, Verwiel ET, Gresnigt MS, Fransen K, et al. Functional genomics identifies type I interferon pathway as central for host defense against Candida albicans. Nat Commun 2013; 4:1342; PMID:23299892; http://dx.doi.org/10.1038/ncomms2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cunha C, Aversa F, Romani L, Carvalho A. Human genetic susceptibility to invasive aspergillosis. PLOS Pathog 2013; 9:e1003434; PMID:23950708; http://dx.doi.org/10.1371/journal.ppat.1003434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Netea MG, Marodi L. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol 2010; 31:346-53; PMID:20705510; http://dx.doi.org/10.1016/j.it.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 58.Ward PA, Guo RF, Riedemann NC. Manipulation of the complement system for benefit in sepsis. Crit Care Res Pract 2012; 2012:427607; PMID:22482043; http://dx.doi.org/10.1155/2012/427607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward PA, Gao H. Sepsis, complement and the dysregulated inflammatory response. J Cell Mol Med 2009; 13:4154-60; PMID:19725914; http://dx.doi.org/10.1111/j.1582-4934.2009.00893.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.(a)Luo S, Skerka C, Kurzai O, Zipfel PF. Complement and innate immune evasion strategies of the human pathogenic fungus Candida albicans. Mol Immunol 2013; 56:161-9; PMID:23809232; http://dx.doi.org/10.1016/j.molimm.2013.05.218; [DOI] [PubMed] [Google Scholar]
- 61.Oikonomopoulou K, Ricklin D, Ward PA, Lambris JD. Interactions between coagulation and complement–their role in inflammation. Semin Immunopathol 2012; 34:151-65; PMID:21811895; http://dx.doi.org/10.1007/s00281-011-0280-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, Lambris JD, Huber-Lang M. Interaction between the coagulation and complement system. Adv Exp Med Biol 2008; 632:71-9; PMID:19025115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kozel TR. Activation of the complement system by pathogenic fungi. Clin Microbiol Rev 1996; 9:34-46; PMID:8665475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kozel TR, Weinhold LC, Lupan DM. Distinct characteristics of initiation of the classical and alternative complement pathways by Candida albicans. Infect Immun 1996; 64:3360-8; PMID:8757876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thong YH, Ferrante A. Alternative pathway of complement activation by Candida albicans. Aust N Zeal J Med 1978; 8:620-2; PMID:373736; http://dx.doi.org/10.1111/j.1445-5994.1978.tb04850.x [DOI] [PubMed] [Google Scholar]
- 66.Morelli R, Rosenberg LT. The role of complement in the phagocytosis of Candida albicans by mouse peripheral blood leukocytes. J Immunol 1971; 107:476-80; PMID:5568774 [PubMed] [Google Scholar]
- 67.Ashman RB, Bolitho EM, Papadimitriou JM. Patterns of resistance to Candida albicans in inbred mouse strains. Immunol Cell Biol 1993; 71(Pt 3):221-5; PMID:8349305; http://dx.doi.org/10.1038/icb.1993.25 [DOI] [PubMed] [Google Scholar]
- 68.Radovanovic I, Mullick A, Gros P. Genetic control of susceptibility to infection with Candida albicans in mice. PLOS One 2011; 6:e18957; PMID:21533108; http://dx.doi.org/10.1371/journal.pone.0018957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsoni SV, Kerrigan AM, Marakalala MJ, Srinivasan N, Duffield M, Taylor PR, Botto M, Steele C, Brown GD. Complement C3 plays an essential role in the control of opportunistic fungal infections. Infect Immun 2009; 77:3679-85; PMID:19581397; http://dx.doi.org/10.1128/IAI.00233-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mullick A, Elias M, Picard S, Bourget L, Jovcevski O, Gauthier S, Tuite A, Harakidas P, Bihun C, Massie B, et al. Dysregulated inflammatory response to Candida albicans in a C5-deficient mouse strain. Infect Immun 2004; 72:5868-76; PMID:15385488; http://dx.doi.org/10.1128/IAI.72.10.5868-5876.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mullick A, Leon Z, Min-Oo G, Berghout J, Lo R, Daniels E, Gros P. Cardiac failure in C5-deficient A/J mice after Candida albicans infection. Infect Immun 2006; 74:4439-51; PMID:16861630; http://dx.doi.org/10.1128/IAI.00159-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng SC, Sprong T, Joosten LA, van der Meer JW, Kullberg BJ, Hube B, Schejbel L, Garred P, van Deuren M, Netea MG. Complement plays a central role in Candida albicans-induced cytokine production by human PBMCs. Eur J Immunol 2012; 42:993-1004; PMID:22531923; http://dx.doi.org/10.1002/eji.201142057 [DOI] [PubMed] [Google Scholar]
- 73.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol 2005; 23:821-52; PMID:15771587; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115835 [DOI] [PubMed] [Google Scholar]
- 74.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol 2004; 4:133-42; PMID:15040586; http://dx.doi.org/10.1038/nri1269 [DOI] [PubMed] [Google Scholar]
- 75.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol 2012; 30:459-89; PMID:22224774; http://dx.doi.org/10.1146/annurev-immunol-020711-074942 [DOI] [PubMed] [Google Scholar]
- 76.Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol 1997; 158:2356-62; PMID:9036985 [PubMed] [Google Scholar]
- 77.Wozniok I, Hornbach A, Schmitt C, Frosch M, Einsele H, Hube B, Löffler J, Kurzai O. Induction of ERK-kinase signalling triggers morphotype-specific killing of Candida albicans filaments by human neutrophils. Cell Microbiol 2008; 10:807-20; PMID:18034864; http://dx.doi.org/10.1111/j.1462-5822.2007.01086.x [DOI] [PubMed] [Google Scholar]
- 78.Fradin C, De Groot P, MacCallum D, Schaller M, Klis F, Odds FC, Hube B. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol Microbiol 2005; 56:397-415; PMID:15813733; http://dx.doi.org/10.1111/j.1365-2958.2005.04557.x [DOI] [PubMed] [Google Scholar]
- 79.Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 2009; 48:1695-703; PMID:19441981; http://dx.doi.org/10.1086/599039 [DOI] [PubMed] [Google Scholar]
- 80.Uzun O, Ascioglu S, Anaissie EJ, Rex JH. Risk factors and predictors of outcome in patients with cancer and breakthrough candidemia. Clin Infect Dis 2001; 32:1713-7; PMID:11360213; http://dx.doi.org/10.1086/320757 [DOI] [PubMed] [Google Scholar]
- 81.Legrand F, Lecuit M, Dupont B, Bellaton E, Huerre M, Rohrlich PS, Lortholary O. Adjuvant corticosteroid therapy for chronic disseminated candidiasis. Clin Infect Dis 2008; 46:696-702; PMID:18230039; http://dx.doi.org/10.1086/527390 [DOI] [PubMed] [Google Scholar]
- 82.Balish E, Wagner RD, Vazquez-Torres A, Jones-Carson J, Pierson C, Warner T. Mucosal and systemic candidiasis in IL-8Rh-/- BALB/c mice. J Leukoc Biol 1999; 66:144-50; PMID:10411002 [DOI] [PubMed] [Google Scholar]
- 83.Majer O, Bourgeois C, Zwolanek F, Lassnig C, Kerjaschki D, Mack M, Müller M, Kuchler K. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLOS Pathog 2012; 8:e1002811; PMID:22911155; http://dx.doi.org/10.1371/journal.ppat.1002811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 2004; 190:624-31; PMID:15243941; http://dx.doi.org/10.1086/422329 [DOI] [PubMed] [Google Scholar]
- 85.Lionakis MS, Fischer BG, Lim JK, Swamydas M, Wan W, Richard Lee CC, Cohen JI, Scheinberg P, Gao JL, Murphy PM. Chemokine receptor Ccr1 drives neutrophil-mediated kidney immunopathology and mortality in invasive candidiasis. PLOS Pathog 2012; 8:e1002865; PMID:22916017; http://dx.doi.org/10.1371/journal.ppat.1002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Basu S, Quilici C, Zhang HH, Grail D, Dunn AR. Mice lacking both G-CSF and IL-6 are more susceptible to Candida albicans infection: critical role of neutrophils in defense against Candida albicans. Growth Factors 2008; 26:23-34; PMID:18365876; http://dx.doi.org/10.1080/08977190801987513 [DOI] [PubMed] [Google Scholar]
- 87.Netea MG, van Tits LJ, Curfs JH, Amiot F, Meis JF, van der Meer JW, Kullberg BJ. Increased susceptibility of TNF-alpha lymphotoxin-alpha double knockout mice to systemic candidiasis through impaired recruitment of neutrophils and phagocytosis of Candida albicans. J Immunol 1999; 163:1498-505; PMID:10415052 [PubMed] [Google Scholar]
- 88.Farah CS, Elahi S, Pang G, Gotjamanos T, Seymour GJ, Clancy RL, Ashman RB. T cells augment monocyte and neutrophil function in host resistance against oropharyngeal candidiasis. Infect Immun 2001; 69:6110-8; PMID:11553549; http://dx.doi.org/10.1128/IAI.69.10.6110-6118.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hall LJ, Clare S, Dougan G. NK cells influence both innate and adaptive immune responses after mucosal immunization with antigen and mucosal adjuvant. J Immunol 2010; 184:4327-37; PMID:20220095; http://dx.doi.org/10.4049/jimmunol.0903357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Voigt J, Hunniger K, Bouzani M, Jacobsen ID, Barz D, Hube B, Löffler J, Kurzai O. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J Infect Dis 2014; 209:616-26; PMID:24163416; http://dx.doi.org/10.1093/infdis/jit574 [DOI] [PubMed] [Google Scholar]
- 91.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 2008; 6:67-78; PMID:18079743; http://dx.doi.org/10.1038/nrmicro1815 [DOI] [PubMed] [Google Scholar]
- 92.Rubin-Bejerano I, Abeijon C, Magnelli P, Grisafi P, Fink GR. Phagocytosis by human neutrophils is stimulated by a unique fungal cell wall component. Cell Host Microbe 2007; 2:55-67; PMID:18005717; http://dx.doi.org/10.1016/j.chom.2007.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Byrd AS, O'Brien XM, Johnson CM, Lavigne LM, Reichner JS. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J Immunol 2013; 190:4136-48; PMID:23509360; http://dx.doi.org/10.4049/jimmunol.1202671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegård KT, Köhl J, Lambris JDVidem V. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 2002; 100:1869-77; PMID:12176911 [PubMed] [Google Scholar]
- 95.van Bruggen R, Drewniak A, Jansen M, van Houdt M, Roos D, Chapel H, Verhoeven AJ, Kuijpers TW. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles. Mol Immunol 2009; 47:575-81; PMID:19811837; http://dx.doi.org/10.1016/j.molimm.2009.09.018 [DOI] [PubMed] [Google Scholar]
- 96.van Lookeren Campagne M, Wiesmann C, Brown EJ. Macrophage complement receptors and pathogen clearance. Cell Microbiol 2007; 9:2095-102; PMID:17590164; http://dx.doi.org/10.1111/j.1462-5822.2007.00981.x [DOI] [PubMed] [Google Scholar]
- 97.Soloviev DA, Jawhara S, Fonzi WA. Regulation of innate immune response to Candida albicans infections by alphaMbeta2-Pra1p interaction. Infect Immun 2011; 79:1546-58; PMID:21245270; http://dx.doi.org/10.1128/IAI.00650-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Losse J, Zipfel PF, Jozsi M. Factor H and factor H-related protein 1 bind to human neutrophils via complement receptor 3, mediate attachment to Candida albicans, and enhance neutrophil antimicrobial activity. J Immunol 2010; 184:912-21; PMID:20008295; http://dx.doi.org/10.4049/jimmunol.0901702 [DOI] [PubMed] [Google Scholar]
- 99.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Verhoeven AJ, Kuijpers TW. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 2007; 8:31-8; PMID:17159984; http://dx.doi.org/10.1038/ni1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hara H, Ishihara C, Takeuchi A, Imanishi T, Xue L, Morris SW, Inui M, Takai T, Shibuya A, Saijo S, et al. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat Immunol 2007; 8:619-29; PMID:17486093; http://dx.doi.org/10.1038/ni1466 [DOI] [PubMed] [Google Scholar]
- 101.Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Inui M, Takai T, Shibuya A, Saijo S, et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 2006; 442:651-6; PMID:16862125; http://dx.doi.org/10.1038/nature04926 [DOI] [PubMed] [Google Scholar]
- 102.McDonald JU, Rosas M, Brown GD, Jones SA, Taylor PR. Differential dependencies of monocytes and neutrophils on dectin-1, dectin-2 and complement for the recognition of fungal particles in inflammation. PLOS One 2012; 7:e45781; PMID:23049859; http://dx.doi.org/10.1371/journal.pone.0045781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Le HT, Tran VG, Kim W, Kim J, Cho HR, Kwon B. IL-33 priming regulates multiple steps of the neutrophil-mediated anti-Candida albicans response by modulating TLR and dectin-1 signals. J Immunol 2012; 189:287-95; PMID:22661085; http://dx.doi.org/10.4049/jimmunol.1103564 [DOI] [PubMed] [Google Scholar]
- 104.Li X, Utomo A, Cullere X, Choi MM, Milner DA, Jr., Venkatesh D, Yun SH, Mayadas TN. The beta-glucan receptor Dectin-1 activates the integrin Mac-1 in neutrophils via Vav protein signaling to promote Candida albicans clearance. Cell host & microbe 2011; 10:603-15; PMID:22177564; http://dx.doi.org/10.1016/j.chom.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tessarolli V, Gasparoto TH, Lima HR, Figueira EA, Garlet TP, Torres SA, Garlet GP, Da Silva JS, Campanelli AP. Absence of TLR2 influences survival of neutrophils after infection with Candida albicans. Med Mycol 2010; 48:129-40; PMID:19468929; http://dx.doi.org/10.3109/13693780902964339 [DOI] [PubMed] [Google Scholar]
- 106.van Bruggen R, Drewniak A, Tool AT, Jansen M, van Houdt M, Geissler J, van den Berg TK, Chapel H, Kuijpers TW. Toll-like receptor responses in IRAK-4-deficient neutrophils. J Innate Immun 2010; 2:280-7; PMID:20375545; http://dx.doi.org/10.1159/000268288 [DOI] [PubMed] [Google Scholar]
- 107.Huizinga TW, Dolman KM, van der Linden NJ, Kleijer M, Nuijens JH, von dem Borne AE, Roos D. Phosphatidylinositol-linked FcRIII mediates exocytosis of neutrophil granule proteins, but does not mediate initiation of the respiratory burst. J Immunol 1990; 144:1432-7; PMID:2137491 [PubMed] [Google Scholar]
- 108.Huizinga TW, van Kemenade F, Koenderman L, Dolman KM, von dem Borne AE, Tetteroo PA, Roos D. The 40-kDa Fc gamma receptor (FcRII) on human neutrophils is essential for the IgG-induced respiratory burst and IgG-induced phagocytosis. J Immunol 1989; 142:2365-9; PMID:2538508 [PubMed] [Google Scholar]
- 109.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods 1999; 232:3-14; PMID:10618505; http://dx.doi.org/10.1016/S0022-1759(99)00146-5 [DOI] [PubMed] [Google Scholar]
- 110.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol 2004; 76:760-81; PMID:15240752; http://dx.doi.org/10.1189/jlb.0404216 [DOI] [PubMed] [Google Scholar]
- 111.Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun 1999; 67:1828-36; PMID:10085024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lehrer RI, Cline MJ. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest 1969; 48:1478-88; PMID:5796360; http://dx.doi.org/10.1172/JCI106114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miramon P, Dunker C, Windecker H, Bohovych IM, Brown AJ, Kurzai O, Hube B. Cellular responses of Candida albicans to phagocytosis and the extracellular activities of neutrophils are critical to counteract carbohydrate starvation, oxidative and nitrosative stress. PLOS One 2012; 7:e52850; PMID:23285201; http://dx.doi.org/10.1371/journal.pone.0052850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miramon P, Dunker C, Kasper L, Jacobsen ID, Barz D, Kurzai O, Hube B. A family of glutathione peroxidases contributes to oxidative stress resistance in Candida albicans. Med Mycol 2014; 52:223-39; PMID:24625675; http://dx.doi.org/10.1093/mmy/myt021 [DOI] [PubMed] [Google Scholar]
- 115.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007; 176:231-41; PMID:17210947; http://dx.doi.org/10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol 2006; 8:668-76; PMID:16548892; http://dx.doi.org/10.1111/j.1462-5822.2005.00659.x [DOI] [PubMed] [Google Scholar]
- 117.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLOS Pathog 2009; 5:e1000639; PMID:19876394; http://dx.doi.org/10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532-5; PMID:15001782; http://dx.doi.org/10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 119.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell host & microbe 2012; 12:324-33; PMID:22980329; http://dx.doi.org/10.1016/j.chom.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 120.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007; 13:463-9; PMID:17384648; http://dx.doi.org/10.1038/nm1565 [DOI] [PubMed] [Google Scholar]
- 121.Fradin C, Kretschmar M, Nichterlein T, Gaillardin C, d'Enfert C, Hube B. Stage-specific gene expression of Candida albicans in human blood. Mol Microbiol 2003; 47:1523-43; PMID:12622810; http://dx.doi.org/10.1046/j.1365-2958.2003.03396.x [DOI] [PubMed] [Google Scholar]
- 122.Kaloriti D, Tillmann A, Cook E, Jacobsen M, You T, Lenardon M, Ames L, Barahona M, Chandrasekaran K, Coghill G, et al. Combinatorial stresses kill pathogenic Candida species. Med Mycol 2012; 50:699-709; PMID:22463109; http://dx.doi.org/10.3109/13693786.2012.672770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaloriti D, Jacobsen M, Yin Z, Patterson M, Tillmann A, Smith DA, Cook E, You T, Grimm MJ, Bohovych I, et al. Mechanisms underlying the exquisite sensitivity of Candida albicans to combinatorial cationic and oxidative stress that enhances the potent fungicidal activity of phagocytes. Mbio 2014; 5:e01334-14; PMID:25028425; http://dx.doi.org/10.1128/mBio.01334-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qian Q, Jutila MA, Van Rooijen N, Cutler JE. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol 1994; 152:5000-8; PMID:8176217 [PubMed] [Google Scholar]
- 125.van 't Wout JW, Linde I, Leijh PC, van Furth R. Contribution of granulocytes and monocytes to resistance against experimental disseminated Candida albicans infection. Eur J Clin Microbiol Infect Dis 1988; 7:736-41PMID:3145854; http://dx.doi.org/10.1007/BF01975039 [DOI] [PubMed] [Google Scholar]
- 126.Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, Spalding C, Hughes S, Pittaluga S, Raffeld M, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood 2010; 115:1519-29; PMID:20040766; http://dx.doi.org/10.1182/blood-2009-03-208629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. J Infect Dis 2014; 209:109-19; PMID:23922372; http://dx.doi.org/10.1093/infdis/jit413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Blanca IR, Bere EW, Young HA, Ortaldo JR. Human B cell activation by autologous NK cells is regulated by CD40-CD40 ligand interaction: role of memory B cells and CD5+ B cells. J Immunol 2001; 167:6132-9; PMID:11714772; http://dx.doi.org/10.4049/jimmunol.167.11.6132 [DOI] [PubMed] [Google Scholar]
- 129.Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A, Weigert R, Mikelis C, et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest 2013; 123:5035-51; PMID:24177428; http://dx.doi.org/10.1172/JCI71307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ramirez-Ortiz ZG, Means TK. The role of dendritic cells in the innate recognition of pathogenic fungi (A. fumigatus, C. neoformans and C. albicans). Virulence 2012; 3:635-46; PMID:23076328; http://dx.doi.org/10.4161/viru.22295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romani L. Immunity to fungal infections. Nat Rev Immunol 2011; 11:275-88; PMID:21394104; http://dx.doi.org/10.1038/nri2939 [DOI] [PubMed] [Google Scholar]
- 132.Mezger M, Wozniok I, Blockhaus C, Kurzai O, Hebart H, Einsele H, Loeffler J. Impact of mycophenolic acid on the functionality of human polymorphonuclear neutrophils and dendritic cells during interaction with Aspergillus fumigatus. Antimicrob Agents Chemother 2008; 52:2644-6; PMID:18426895; http://dx.doi.org/10.1128/AAC.01618-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Ann Rev Immunol 2012; 30:491-529; PMID:22224766; http://dx.doi.org/10.1146/annurev-immunol-031210-101352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 2007; 8:630-8; PMID:17450144; http://dx.doi.org/10.1038/ni1460 [DOI] [PubMed] [Google Scholar]
- 135.Bourgeois C, Majer O, Frohner IE, Lesiak-Markowicz I, Hildering KS, Glaser W, et al. Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-beta signaling. J Immunol 2011; 186:3104-12; PMID:21282509; http://dx.doi.org/10.4049/jimmunol.1002599 [DOI] [PubMed] [Google Scholar]
- 136.del Fresno C, Soulat D, Roth S, Blazek K, Udalova I, Sancho D, Ruland J, Ardavín C. Interferon-beta production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans. Immunity 2013; 38:1176-86; PMID:23770228; http://dx.doi.org/10.1016/j.immuni.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 137.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol 2007; 178:3107-15; PMID:17312158; http://dx.doi.org/10.4049/jimmunol.178.5.3107 [DOI] [PubMed] [Google Scholar]
- 138.Slack EC, Robinson MJ, Hernanz-Falcon P, Brown GD, Williams DL, Schweighoffer E, Tybulewicz VL, Reis e Sousa C. Syk-dependent ERK activation regulates IL-2 and IL-10 production by DC stimulated with zymosan. Eur J Immunol 2007; 37:1600-12; PMID:17458858; http://dx.doi.org/10.1002/eji.200636830 [DOI] [PubMed] [Google Scholar]
- 139.Whitney PG, Bar E, Osorio F, Rogers NC, Schraml BU, Deddouche S, LeibundGut-Landmann S, Reis e Sousa C. Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLOS Pathog 2014; 10:e1004276; PMID:25033445; http://dx.doi.org/10.1371/journal.ppat.1004276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 2014; 40:117-27; PMID:24412614; http://dx.doi.org/10.1016/j.immuni.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 141.Chiche L, Forel JM, Thomas G, Farnarier C, Vely F, Blery M, Papazian L, Vivier E. The role of natural killer cells in sepsis. J Biomed Biotechnol 2011; 2011:986491; PMID:21629707; http://dx.doi.org/10.1155/2011/986491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murphy JW, McDaniel DO. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol 1982; 128:1577-83; PMID:6120974 [PubMed] [Google Scholar]
- 143.Levitz SM, Dupont MP, Smail EH. Direct activity of human T lymphocytes and natural killer cells against Cryptococcus neoformans. Infect Immun 1994; 62:194-202; PMID:8262627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Marr KJ, Jones GJ, Zheng C, Huston SM, Timm-McCann M, Islam A, Berenger BM, Ma LL, Wiseman JC, Mody CH. Cryptococcus neoformans directly stimulates perforin production and rearms NK cells for enhanced anticryptococcal microbicidal activity. Infect Immun 2009; 77:2436-46; PMID:19307209; http://dx.doi.org/10.1128/IAI.01232-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schmidt S, Tramsen L, Hanisch M, Latge JP, Huenecke S, Koehl U, Lehrnbecher T. Human natural killer cells exhibit direct activity against Aspergillus fumigatus hyphae, but not against resting conidia. J Infect Dis 2011; 203:430-5; PMID:21208932; http://dx.doi.org/10.1093/infdis/jiq062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bouzani M, Ok M, McCormick A, Ebel F, Kurzai O, Morton CO, Einsele H, Loeffler J. Human NK cells display important antifungal activity against Aspergillus fumigatus, which is directly mediated by IFN-gamma release. J Immunol 2011; 187:1369-76; PMID:21697457; http://dx.doi.org/10.4049/jimmunol.1003593 [DOI] [PubMed] [Google Scholar]
- 147.Balish E, Warner T, Pierson CJ, Bock DM, Wagner RD. Oroesophageal candidiasis is lethal for transgenic mice with combined natural killer and T-cell defects. Med Mycol 2001; 39:261-8; PMID:11446529; http://dx.doi.org/10.1080/mmy.39.3.261.268 [DOI] [PubMed] [Google Scholar]
- 148.Magor BG, Magor KE. Evolution of effectors and receptors of innate immunity. Dev Comp Immunol 2001; 25:651-82; PMID:11602189; http://dx.doi.org/10.1016/S0145-305X(01)00029-5 [DOI] [PubMed] [Google Scholar]
- 149.Blach-Olszewska Z. Innate immunity: cells, receptors, and signaling pathways. Arch Immunol Ther Exp 2005; 53:245-53; PMID:15995585 [PubMed] [Google Scholar]
- 150.Li SS, Kyei SK, Timm-McCann M, Ogbomo H, Jones GJ, Shi M, Xiang RF, Oykhman P, Huston SM, Islam A, et al. The NK receptor NKp30 mediates direct fungal recognition and killing and is diminished in NK cells from HIV-infected patients. Cell Host Microbe 2013; 14:387-97; PMID:24139398; http://dx.doi.org/10.1016/j.chom.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 151.Mathews HL, Witek-Janusek L. Antifungal activity of interleukin-2-activated natural killer (NK1.1+) lymphocytes against Candida albicans. J Med Microbiol 1998; 47:1007-14; PMID:9822300; http://dx.doi.org/10.1099/00222615-47-11-1007 [DOI] [PubMed] [Google Scholar]
- 152.Marodi L, Schreiber S, Anderson DC, MacDermott RP, Korchak HM, Johnston RB., Jr Enhancement of macrophage candidacidal activity by interferon-gamma. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J Clin Invest 1993; 91:2596-601; PMID:8390485; http://dx.doi.org/10.1172/JCI116498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol 2004; 25:47-52; PMID:14698284; http://dx.doi.org/10.1016/j.it.2003.10.012 [DOI] [PubMed] [Google Scholar]
- 154.Dalbeth N, Gundle R, Davies RJ, Lee YC, McMichael AJ, Callan MF. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol 2004; 173:6418-26; PMID:15528382; http://dx.doi.org/10.4049/jimmunol.173.10.6418 [DOI] [PubMed] [Google Scholar]
- 155.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008; 9:503-10; PMID:18425107; http://dx.doi.org/10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- 156.Bhatnagar N, Hong HS, Krishnaswamy JK, Haghikia A, Behrens GM, Schmidt RE, Jacobs R. Cytokine-activated NK cells inhibit PMN apoptosis and preserve their functional capacity. Blood 2010; 116:1308-16; PMID:20501895; http://dx.doi.org/10.1182/blood-2010-01-264903 [DOI] [PubMed] [Google Scholar]
- 157.Costantini C, Micheletti A, Calzetti F, Perbellini O, Pizzolo G, Cassatella MA. Neutrophil activation and survival are modulated by interaction with NK cells. Int Immunol 2010; 22:827-38; PMID:20739460; http://dx.doi.org/10.1093/intimm/dxq434 [DOI] [PubMed] [Google Scholar]
- 158.Quintin J, Voigt J, van der Voort R, Jacobsen ID, Verschueren I, Hube B, Giamarellos-Bourboulis EJ, van der Meer JW, Joosten LA, Kurzai O, et al. Differential role of NK cells against Candida albicans infection in immunocompetent or immunocompromised mice. Eur J Immunol 2014; 44(8):2405-14; PMID:24802993 [DOI] [PubMed] [Google Scholar]
- 159.Boomer JS, Green JM, Hotchkiss RS. The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence 2014; 5:45-56; PMID:24067565; http://dx.doi.org/10.4161/viru.26516 [DOI] [PMC free article] [PubMed] [Google Scholar]