Abstract
Rabs exist in two forms: the inactive GDP- and the active GTP-bound form. GEF proteins mediate the exchange of GDP for GTP and thereby activate Rabs. Although GEFs share a common action, which involves the opening of the Rab nucleotide binding site, they do not contain a conserved catalytic domain. Longin domains have been either found in several GEFs (TRAPP, DENN) or predicted by sequence analyses (Mon1-Ccz1, BLOC-3). At least in TRAPP, they serve as a platform for interaction with a GTPase. We recently generated a model of the predicted longin domains of the Mon1-Ccz1 complex based upon the structure of the respective TRAPP subunits. This allowed us to identify activity-related important regions of the complex. Moreover, we analyzed the GEF activity of Mon1-Ccz1 in the presence of membranes and uncovered that certain acidic phospholipids support the recruitment of the GEF complex. In this commentary, we will discuss our findings in a broader context.
Keywords: Rab GEFs, Rab7/Ypt7, Mon1-Ccz1 complex, TRAPP complex, longin domains
Rab GTPases orchestrate cargo trafficking along the exocytic and endocytic pathways. Rabs localize to specific intracellular membranes and thus provide organelles with a unique identity.
Just like other small GTPases, Rab family proteins cycle between an inactive GDP-bound and the active GTP-bound form.1 The key factors that drive Rab cycling are GEFs (guanine nucleotide exchange factors) and GAPs (GTPase activating proteins) (Fig. 1).2 GEFs promote GDP dissociation whereas GAPs facilitate GTP-hydrolysis by complementing the Rab's active site, resulting in Rab inactivation. In yeast, six Rab GEF and seven Rab GAP proteins have been identified until now. All Rab GAP proteins belong to the Gyp family and share a catalytic TBC (Tre-2/Bub2/Cdc16) domain.2,3 By contrast, GEFs can be part of protein complexes as in TRAPP or single proteins like Vps9 and exhibit distinct catalytic domains.4-6 We recently uncovered two new GEFs in yeast, Muk1 and the Mon1-Ccz1 complex.7,8 Muk1 contains a Vps9-domain9 and cooperates with Vps9 in Rab5/Vps21 activation,8,10 whereas the conserved Mon1-Ccz1 complex serves as a GEF for Ypt7.7,11 A first link between Mon1-Ccz1 and Ypt7 activation was established using Ypt7 suppressors of CCZ1 deletion phenotype.12 This GEF activity has been confirmed for the metazoan Rab7 with the corresponding Mon1a-Ccz1 complex.13 Active Ypt7 is able to recognize the tethering complex HOPS responsible for endosome-vacuole fusion (Fig. 2).14,15
Figure 1.
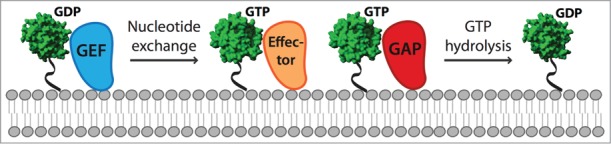
Rab cycle on membranes. After Rab delivery to the membranes, a GEF (guanine nucleotide exchange factor) stimulates the exchange of GDP for GTP. The effector can then recognize the GTP-bound Rab and be anchored to the membranes. Rab inactivation is triggered by a GAP (GTPase activating protein), which promotes GTP hydrolysis and the conversion from GTP-bound to GDP bound state.
Figure 2.
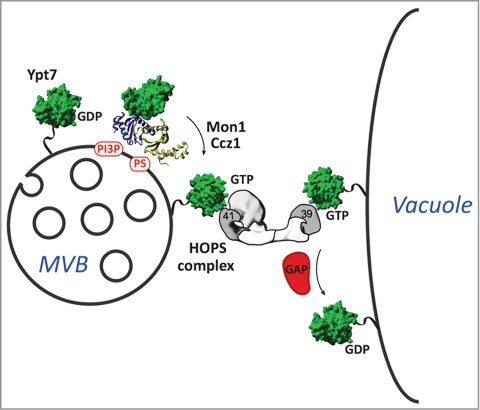
Rab regulators involved in endosome-vacuole tethering. The activation of the Rab Ypt7 is catalyzed by Mon1-Ccz1 complex. The longin domains of Mon1 (blue) and Ccz1 (yellow) participate in the interaction with Ypt7. PS and PI3P lipids mediate the recruitment of the GEF complex to endosomes and thereby facilitate its interaction with the Rab. The effector complex HOPS binds to Ypt7-GTP to promote endosome-vacuole tethering.30 Once the tethering is complete, a GAP protein (Gyp7) converts Ypt7 in the inactive GDP-bound state.31
Sequence analysis of Mon1 and Ccz1 proteins has revealed the presence of longin folds, which are also found in the TRAPP subunits Bet5 and Trs23.16,17 The prediction of these domains in Mon1/Ccz1 suggests a similar GEF mechanism for this complex. The longin domains of TRAPP subunits participate in the recognition of Rab1/Ypt1 and significantly contribute to the GEF activity of the holo complex.18 Likewise, Mon1 and Ccz1 may use their predicted longin domains to interact with the Rab Ypt7.7 To identify critical residues for the GEF activity of Mon1-Ccz1, we built a homology model of the predicted longin domains with the Bet5-Trs23-Ypt1 structure serving as a template (Fig. 2). In accordance with the model, we found that mutations in the S1-S2 region of Mon1 and the helixes H1 of both proteins disrupted the association with Ypt7.11 The mutants showed abnormal vacuole morphology and the purified mutant complexes could not counteract the GAP activity in the in vitro vacuole fusion assays. The GEF mutants also caused a mislocalization of Ypt7, which supports the essential role of the GEF proteins in Rab targeting.19 It must be kept in mind, however, that the longin domains of Bet5 and Trs23 are just part of the TRAPP interface responsible for its GEF activity. For this reason and in view of its considerably larger size, it is expected that additional portions of the dimeric complex are required for Mon1-Ccz1's GEF activity.
Once the main components involved in the Rab cycle are known the most challenging aspect is the reconstitution of GEF and GAP activities on membranes. To this end we included multilamellar vesicles (MLVs) in the fluorescent GEF assays.7 MLVs with the desired lipid composition were obtained easily from a lipids mixture by several freeze-thaw cycles. In addition, we used Ni-NTA lipids to allow for the membrane binding of the Rab Ypt7 with a C-terminal hexahistidine tag (6XHis). We then monitored nucleotide dissociation upon addition of Mon1-Ccz1 as before (Fig. 3). This assay was originally established in a study of Ras GAP activity on membranes.20 In our set-up, the recombinant Rab Ypt7–6XHis was loaded with a fluorescent version of GDP (Mant-GDP) and incubated with MLVs to allow the Rab binding to the membranes. The addition of the purified Mon1-Ccz1 complex and GTP triggered the nucleotide release, which was accompanied by a decrease in fluorescence (Fig. 3). It turned out that the GEF activity of Mon1-Ccz1 toward Ypt7 is enhanced thousand-fold in the presence of membranes (MLVs). PI3P (phosphatidylinositol-3-phosphate) and PS (phosphatidylserine) contributed to this stimulation and probably are required in vivo for full activity of the GEF (Fig. 2). Both lipids are present in the cytosolic face of endocytic organelles, and PI3P is enriched on endosomes,21,22 where Mon1 and Ccz1 localize. The initial lipid binding experiments using a lipid array showed that both PI3P and PS might be involved in Mon1 and Ccz1 recognition. Moreover, both lipids supported the recruitment of Mon1-Ccz1 complex to artificial membranes explaining their positive effect in the GEF activity of the complex. It has been shown that C. elegans Mon1 also recognizes PI3P,23 although in Drosophila PI3P is not required for the association of Rab7 with endosomes.24 Whether these lipids activate the GEF complex directly remains to be answered. When we examined the role of other players in Mon1-Ccz1 localization, we observed that the excess of the HOPS complex stabilizes the GEF at the vacuole membrane through positive-feedback. HOPS, the effector complex that binds exclusively to Rab-GTP maintains the corresponding GEF anchoring to membranes and thus prevents premature Rab inactivation. Similar results have been described for the GTPase Cdc42 and its effector Bem1.25 Although HOPS may regulate Mon1-Ccz1 localization, its effect in the GEF activity was minor.
Figure 3.
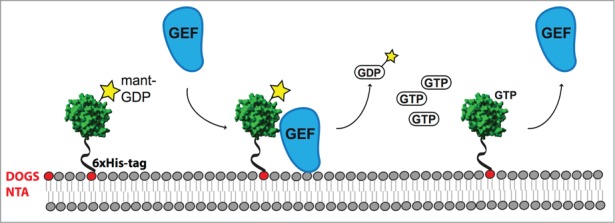
Fluorescent GEF assay on vesicles. The purified 6x(His)-Rab is loaded with the fluorescent analog of GDP (Mant-GDP) and incubated with vesicles containing Ni-NTA lipids (DOGS-NTA). After incubation with the GEF, nucleotide exchange is triggered by addition of GTP. The decrease of Mant-GDP fluorescence due to the nucleotide release is measured (modified from ref. 28).
It has been suggested that Mon1 and Ccz1 are recruited to membranes by Rab5/Vps21 and subunits of the effector complex CORVET.7,26 The role of these factors acting upstream of Ypt7 can be now analyzed using this version of the GEF assay.
Another crucial element in the Rab cycle is GDI (GDP dissociation inhibitor), which mediates the transfer of cytosolic Rabs to membranes.27 The analysis of GDI function in vitro implies the purification of prenylated Rab-GDI complexes, which is one important limiting factor in the assay. This has been solved successfully by the attachment of a fluorescent prenyl group to the recombinant Rab prior the incubation with GDI and isolation of the Rab-GDI complexes.28 An alternative approach is the purification of the prenylated Rab-GDI complexes, which could be used in GTP uptake assays.29 These future experiments will be critical to complete the in vitro reconstitution of the Rab cycle.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Work in the authors laboratory was supported by the DFG (SFB 944, project P11) and the Hans-Mühlenhoff foundation (to C.U.).
References
- 1. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; http://dx.doi.org/ 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- 2. Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol 2010; 22:461-70; PMID:20466531; http://dx.doi.org/ 10.1016/j.ceb.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barr FA. Review series: Rab GTPases and membrane identity: causal or inconsequential? J Cell Biol 2013; 202:191-9; PMID:23878272; http://dx.doi.org/ 10.1083/jcb.201306010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim Y-G, Raunser S, Munger C, Wagner J, Song Y-L, Cygler M, Walz T, Oh B-H, Sacher M. The architecture of the multisubunit TRAPP I complex suggests a model for vesicle tethering. Cell 2006; 127:817-30; PMID:17110339; http://dx.doi.org/ 10.1016/j.cell.2006.09.029 [DOI] [PubMed] [Google Scholar]
- 5. Hama H, Tall GG, Horazdovsky BF. Vps9p is a guanine nucleotide exchange factor involved in vesicle-mediated vacuolar protein transport. J Biol Chem 1999; 274:15284-91; PMID:10329739; http://dx.doi.org/ 10.1074/jbc.274.21.15284 [DOI] [PubMed] [Google Scholar]
- 6. Walch-Solimena C, Collins RN, Novick PJ. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol 1997; 137:1495-509; PMID:9199166; http://dx.doi.org/ 10.1083/jcb.137.7.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nordmann M, Cabrera M, Perz A, Bröcker C, Ostrowicz C, Engelbrecht-Vandré S, Ungermann C. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol 2010; 20:1654-9; PMID:20797862; http://dx.doi.org/ 10.1016/j.cub.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 8. Cabrera M, Arlt H, Epp N, Lachmann J, Griffith J, Perz A, Reggiori F, Ungermann C. Functional separation of endosomal fusion factors and the class C core vacuole/endosome tethering (CORVET) complex in endosome biogenesis. J Biol Chem 2013; 288:5166-75; PMID:23264632; http://dx.doi.org/ 10.1074/jbc.M112.431536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carney DS, Davies BA, Horazdovsky BF. Vps9 domain-containing proteins: activators of Rab5 GTPases from yeast to neurons. Trends Cell Biol 2006; 16:27-35; PMID:16330212; http://dx.doi.org/ 10.1016/j.tcb.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 10. Paulsel AL, Merz AJ, Nickerson DP. Vps9 family protein Muk1 is the second Rab5 guanosine nucleotide exchange factor in budding yeast. J Biol Chem 2013; 288:18162-71; PMID:23612966; http://dx.doi.org/ 10.1074/jbc.M113.457069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cabrera M, Nordmann M, Perz A, Schmedt D, Gerondopoulos A, Barr F, Piehler J, Engelbrecht-Vandré S, Ungermann C. The Mon1-Ccz1 GEF activates the Rab7 GTPase Ypt7 via a longin-fold-Rab interface and association with PI3P-positive membranes. J Cell Sci 2014; 127:1043-51; PMID:24413168; http://dx.doi.org/ 10.1242/jcs.140921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kucharczyk R, Kierzek AM, Slonimski PP, Rytka J. The Ccz1 protein interacts with Ypt7 GTPase during fusion of multiple transport intermediates with the vacuole in S. cerevisiae. J Cell Sci 2001; 114:3137-45; PMID:11590240 [DOI] [PubMed] [Google Scholar]
- 13. Gerondopoulos A, Langemeyer L, Liang J-R, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol 2012; 22:2135-9; PMID:23084991; http://dx.doi.org/ 10.1016/j.cub.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci U S A 2000; 97:9402-7; PMID:10944212; http://dx.doi.org/ 10.1073/pnas.97.17.9402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cabrera M, Ostrowicz CW, Mari M, LaGrassa TJ, Reggiori F, Ungermann C. Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites. Mol Biol Cell 2009; 20:1937-48; PMID:19193765; http://dx.doi.org/ 10.1091/mbc.E08-09-0943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kinch LN, Grishin NV. Longin-like folds identified in CHiPS and DUF254 proteins: vesicle trafficking complexes conserved in eukaryotic evolution. Protein Sci 2006; 2669-74; PMID:17075139; http://dx.doi.org/ 10.1110/ps.062419006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levine TP, Daniels RD, Wong LH, Gatta AT, Gerondopoulos A, Barr FA. Discovery of new Longin and Roadblock domains that form platforms for small GTPases in Ragulator and TRAPP-II. Small GTPases 2013; 4:62-9; PMID:23511850; http://dx.doi.org/ 10.4161/sgtp.24262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cai Y, Chin HF, Lazarova D, Menon S, Fu C, Cai H, Sclafani A, Rodgers DW, De La Cruz EM, Ferro-Novick S, et al. The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes. Cell 2008; 133:1202-13; PMID:18585354; http://dx.doi.org/ 10.1016/j.cell.2008.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cabrera M, Ungermann C. Guanine nucleotide exchange factors (GEFs) have a critical but not exclusive role in organelle localization of Rab GTPases. J Biol Chem 2013; 288:28704-12; PMID:23979137; http://dx.doi.org/ 10.1074/jbc.M113.488213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sot B, Behrmann E, Raunser S, Wittinghofer A. Ras GTPase activating (RasGAP) activity of the dual specificity GAP protein Rasal requires colocalization and C2 domain binding to lipid membranes. Proc Natl Acad Sci U S A 2013; 110:111-6; PMID:23251034; http://dx.doi.org/ 10.1073/pnas.1201658110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wurmser AE, Emr SD. Phosphoinositide signaling and turnover: PtdIns(3)P, a regulator of membrane traffic, is transported to the vacuole and degraded by a process that requires lumenal vacuolar hydrolase activities. EMBO J 1998; 17:4930-42; PMID:9724630; http://dx.doi.org/ 10.1093/emboj/17.17.4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng J, Fujita A, Yamamoto H, Tatematsu T, Kakuta S, Obara K, Ohsumi Y, Fujimoto T. Yeast and mammalian autophagosomes exhibit distinct phosphatidylinositol 3-phosphate asymmetries. Nat Commun 2014; 5:3207; PMID:24492518; http://dx.doi.org/ 10.1038/ncomms4207 [DOI] [PubMed] [Google Scholar]
- 23. Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell 2010; 141:497-508; PMID:20434987; http://dx.doi.org/ 10.1016/j.cell.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 24. Yousefian J, Troost T, Grawe F, Sasamura T, Fortini M, Klein T. Dmon1 controls recruitment of Rab7 to maturing endosomes in Drosophila. J Cell Sci 2013; 126:1583-94; PMID:23418349; http://dx.doi.org/ 10.1242/jcs.114934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Butty A-C, Perrinjaquet N, Petit A, Jaquenoud M, Segall JE, Hofmann K, Zwahlen C, Peter M. A positive feedback loop stabilizes the guanine-nucleotide exchange factor Cdc24 at sites of polarization. EMBO J 2002; 21:1565-76; PMID:11927541; http://dx.doi.org/ 10.1093/emboj/21.7.1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kinchen JM, Ravichandran KS. Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature 2010; 464:778-82; PMID:20305638; http://dx.doi.org/ 10.1038/nature08853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seabra MC, Wasmeier C. Controlling the location and activation of Rab GTPases. Curr Opin Cell Biol 2004; 16:451-7; PMID:15261679; http://dx.doi.org/ 10.1016/j.ceb.2004.06.014 [DOI] [PubMed] [Google Scholar]
- 28. Schoebel S, Oesterlin LK, Blankenfeldt W, Goody RS, Itzen A. RabGDI displacement by DrrA from Legionella is a consequence of its guanine nucleotide exchange activity. Mol Cell 2009; 36:1060-72; PMID:20064470; http://dx.doi.org/ 10.1016/j.molcel.2009.11.014 [DOI] [PubMed] [Google Scholar]
- 29. Machner MP, Isberg RR. A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science 2007; 318:974-7; PMID:17947549; http://dx.doi.org/ 10.1126/science.1149121 [DOI] [PubMed] [Google Scholar]
- 30. Bröcker C, Kuhlee A, Gatsogiannis C, Balderhaar HJ, Hönscher C, Engelbrecht-Vandré S, Ungermann C, Raunser S. Molecular architecture of the multisubunit homotypic fusion and vacuole protein sorting (HOPS) tethering complex. Proc Natl Acad Sci U S A 2012; 109:1991-6; PMID:22308417; http://dx.doi.org/ 10.1073/pnas.1117797109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brett CL, Plemel RL, Lobingier BT, Vignali M, Fields S, Merz AJ. Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase. J Cell Biol 2008; 182:1141-51; PMID:18809726; http://dx.doi.org/ 10.1083/jcb.200801001 [DOI] [PMC free article] [PubMed] [Google Scholar]


