ABSTRACT
Termination of transcription of short non-coding RNAs is carried out in yeast by the Nab3-Nrd1-Sen1 complex. Nab3 and Nrd1 are hnRNP-like proteins that dimerize and bind RNA with sequence specificity. We show here that an essential region of Nab3 that is predicted to be prion-like based upon its sequence bias, formed amyloid-like filaments. A similar region from Nrd1 also assembled into filaments in vitro. The purified Nab3 domain formed a macroscopic gel whose lattice organization was observed by X-ray fiber diffraction. Filaments were resistant to dissociation in anionic detergent, bound the fluorescent dye thioflavin T, and showed a β-sheet rich structure by circular dichroism spectroscopy, similar to human amyloid β which served as a reference amyloid. A version of the Nab3 domain with a mutation that impairs its termination function, also formed fibers as observed by electron microscopy. Using a protein fragment interaction assay, the purified Nab3 domain was seen to interact with itself in living yeast. A similar observation was made for full length Nab3. These results suggest that the Nab3 and Nrd1 RNA-binding proteins can attain a complex polymeric form and raise the possibility that this property is important for organizing their functional state during termination. These findings are congruent with recent work showing that RNA binding proteins with low complexity domains form a dynamic subcellular matrix in which RNA metabolism takes place but can also aberrantly yield pathological aggregated particles.
Keywords: hnRNP, amyloid, RNA binding protein, transcription termination, fibril
Abbreviations
- Aβ
amyloid beta
- BSA
bovine serum albumin
- CPEB
cytoplasmic polyadenylation element binding protein
- CTD
carboxy terminal domain
- DHFR
dihydrofolate reductase
- DMSO
dimethyl sulfoxide
- EDTA
ethylenediaminetetraacetic acid
- GFP
green fluorescent protein
- HFIP
hexafluoroisopropanol
- hnRNP
heterogeneous nuclear ribonucleoprotein
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- PCR
polymerase chain reaction
- RRM
RNA recognition motif
- SDD-AGE
semi-denaturing detergent agarose gel electrophoresis
- SDS
sodium dodecyl sulfate
- TEV
tobacco etch virus
Introduction
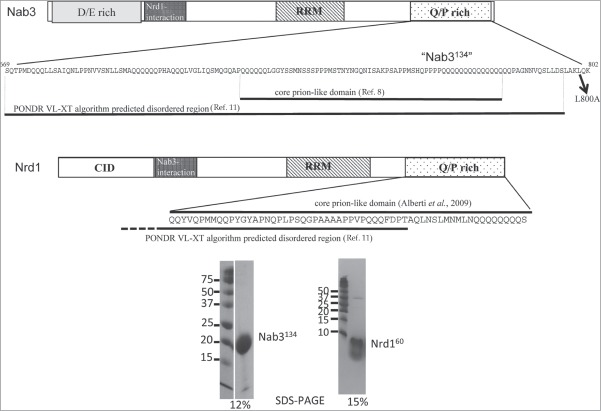
In yeast, a specialized reaction insures the correct termination of transcription by RNA polymerase II for short, non-coding RNAs.1 This machinery employs the Nrd1-Nab3-Sen1 complex. The former two proteins are hnRNP-like proteins and the third is a putative helicase.2,3 Nrd1 and Nab3 dimerize to bind RNA sequence elements through their RNA recognition motifs (RRMs).4 Using a genetic screen, we previously identified a 134 amino acid carboxy-terminal region in Nab3 ("Nab3134") that is essential for cell viability and important for termination at an intergenic terminator involved in the regulation of IMD2 .5-7 This domain of Nab3 is made up of a low complexity amino acid sequence that is intrinsically unstructured and prion-like based on prediction algorithms and its demonstrated biochemical properties.8,9 Nab3's very C-terminal residues also contain a short region of structural homology to human hnRNP-C that is predicted to be α-helical and can crosslink to itself (Fig. 1).10 This biochemical data, combined with genetic data indicating that 2 different mutant versions of Nab3 can complement cell viability in trans, suggests that multiple copies of this protein work together during termination.9 This extends a model postulating that multiple copies of Nab3 and Nrd1 operate together on a short nascent transcript bearing consensus sequences, while interacting with RNA polymerase II to provoke termination.4
Figure 1.
Schematic of the yeast Nab3 and Nrd1 proteins with an expanded view of the low complexity region studied here (not to scale). For Nab3 the Asp-Glu (D/E) rich domain, the Nrd1-interaction domain, the RRM domain, and the Q/P rich domains are indicated with patterned boxes. The computationally predicted core prion-like domain and disordered region are indicated on the expanded sequence. The L800A mutation is shown with an arrow. The Nrd1 schematic shows its CTD-interaction domain, Nab3-interaction domain, RRM, and Q/P rich region with patterned boxes. The expanded sequence shows the C-terminal 60 amino acids studied here and the portions that score as prion-like and intrinsically unstructured based on computer algorithms. The purity of purified Nab3134 and Nrd160 based upon SDS-PAGE analysis is shown with the acrylamide percentages shown below each image. Both lanes of the Nab3134 gel were run on 2 ends of the same slab. A trace of added thrombin is evident at 36kD in the Nrd160 lane.
Some proteins have regions that are considered intrinsically disordered, meaning the polypeptide backbone populates a dynamic set of positions in space; generally lacking fixed secondary structural elements.11,12 Intrinsically disordered domains contain amino acid signatures showing a bias toward repeated sequences that use only a few different amino acids. Low complexity sequences enriched in glutamine, proline, and serine, and poor in aromatic amino acids, have been observed as unstructured domains that can nevertheless interact with each other to form polymeric aggregates including highly ordered amyloid-like structures with a stereospecific packing of β-sheets.13 In some cases, this feature is associated with prion proteins in which the aggregated conformation can recruit monomers for assembly thereby self-perpetuating the state in vivo.14
A number of proteins in yeast display low complexity, intrinsically unstructured regions. Using a genome wide screen starting with a prediction algorithm, the Lindquist lab showed that many of these aggregate into amyloid-like fibers and some are prionogenic.8 This set of proteins is enriched for those involved in gene expression with a particular over-representation of RNA binding proteins. A number of mammalian proteins involved in RNA metabolism also possess low complexity regions that can spontaneously form fibers and hydrogels.15,16 Filamentation and gel formation are thought to reflect a natural function of these proteins and may be part of their ability to form dynamic cellular compartments in which RNA metabolism takes place.17 Some of the proteins with low complexity domains that self-assemble, associate with RNA polymerase II to impact transcriptional activation.18 This property also has consequences for human disease as exemplified by heritable mutations in hnRNPA1 and hnRNPA2B1 that result in amyloid-like deposits integral to the pathology of specific neurological diseases.19
Here we show that the low complexity, prion-like domain of the termination proteins Nab3 and Nrd1 formed amyloid-like filaments in vitro. Nab3 formed a hydrogel and bears many of the hallmarks of amyloid aggregates as gauged by biophysical measures. Both the Nab3 low complexity domain and full-length Nab3, made homotypic interactions with themselves in vivo. Taken together with prior genetic and biochemical evidence, the data raise the possibility that these proteins function in a polymeric form during transcription termination.
Materials and Methods
Plasmid construction. A PCR product encoding Nab3 amino acids 668–802 (Nab3134) was amplified using primers 5′-atatagatctatctcaaactccaatggaccagc-3′ and 5′-atatctcgagctatttttgtagttttgctaaac-3′, digested with BglII and XhoI, and inserted into similarly cut pET32a (Novagen) to yield pET32-Nab3–134aa. This yields a fusion protein with thioredoxin followed by a His6 tag, a thrombin cleavage site, and an S-tag to the amino terminal side of Nab3 sequence. A PCR product encoding Nrd1 amino acids 516–575 was amplified using primers 5′-atatagatctagaaaacctgtattttcagggccagcaatatgtgcaacctat-3′ and 5′-atatctcgagttagctttgttgttgttgc-3′, digested with BglII and XhoI, and inserted into similarly cut pET32a to generate pET32a60aaNrd1. This yields a fusion protein with thioredoxin followed by a His6 tag, a thrombin cleavage site, the S-tag, and a thrombin protease cleavage site to the amino terminal side of Nrd1 sequence. A PCR product encoding Nab3134 was made using oligos 5′-ggtctagaactagtaccatgtctcaaactccaatgg-3′ and 5′-ccatcgattttttgtagttttgctaaactatc-3′ and digested with ClaI and XbaI and inserted into similarly cut p416-ADH1-ZIP-DHFR[1,2] and p413-ADH1-ZIP-DHFR[3] which replaces the DNA encoding the bZIP domain of yeast Gcn4.20 The resulting plasmids (pBatman and pRobin, respectively) were confirmed by sequencing. Full length Nab3 was inserted similarly into each backbone using PCR primers 5′-atatcgattttttgtagttttgctaaactatctaatagact-3′ and 5′-attctagaatgtcagatgaaaaccataacagtg-3′ to yield the plasmids pAvengers and pAssemble, respectively.
Protein purification. Nrd160 and Nab3134 were purified from E. coli following transformation of plasmids into BL21(DE3) cells. Cultures were grown at 37°C to an optical density of 0.5 and induced with 1mM IPTG. Cells were resuspended in lysis buffer (50 mM Tris, pH 7.5, 500 mM NaCl, 10 mM imidazole) containing a protease inhibitor tablet (EasyPack Catalog No. 04693132001 Roche), lysed with 10 μg/ml lysozyme, sonicated, and centrifuged at 27,000 × g for 30 min. Supernatants were applied to 1 ml HisTrap HP nickel columns (GE Healthcare) equilibrated in lysis buffer, washed with 25 ml of lysis buffer, and eluted with lysis buffer containing 250 mM imidazole. Fractions were pooled and digested with 0.5 units thrombin (EMD/Novagen)/mg protein, for 16 hrs at 22°C. Digested proteins were exchanged into lysis buffer by centrifugal filtration in Vivaspin units (3 or 5 kDa molecular weight cutoff, GE Healthcare) and loaded onto HisTrap nickel columns as described above. Flow through was collected and the protein was concentrated by centrifugal filtration while exchanging into 20 mM Tris, pH 7.5, 0.2 M NaCl.
Yeast strains and growth. Yeast strain BY4742 was transformed with pBatman and pRobin to generate DY2214, p416-ADH1-ZIP-DHFR[1,2] and p413-ADH1-ZIP-DHFR[3] to generate DY2315 (positive control), pAvengers and pAssemble to generate DY2217, or p416-ADH1-ZIP-DHFR[1,2] and pAssemble to generate DY2219 (negative control). Where indicated, cells were grown in the presence of 200 μg/ml methotrexate and compared to DMSO (vehicle) on solid SC ura- his- dropout media.
X-ray fiber diffraction. Nab3 hydrogel was dialyzed against distilled water and harvested using a mounted cryoloop (Hampton Research), inserted in a CrystalCap Magnetic Vial (Hampton Research), and dried in an evacuated desiccator overnight. These loops were inserted into a nitrogen cryostream (100 K) and exposed to X-rays for 45 sec from a Rigaku Micromax 007HF X-ray generator with a copper anode running at maximum power with Varimax optics and 0.3 mm collimator. Diffraction images were recorded using a Saturn 944+ CCD detector approximately 100 mm from the specimen.
Semi-denaturing detergent agarose electrophoresis. Purified protein samples were adjusted to 2% SDS, 140 mM β-mercaptoethanol, 10% glycerol, 0.002% bromphenol blue, 80 mM Tris, pH 6.8 and separated by electrophoresis in agarose gels (1.5% w/v) in 40 mM Tris-acetate, pH 7.8; 1 mM EDTA, 0.1% SDS run at 4°C. Bio-Rad Precision Plus Protein Kaleidoscope (Cat. No. 161–0375) molecular weight markers were run as standards. Proteins were blotted to Protran nitrocellulose transfer membrane (Whatman) by capillary action for 18 hrs. Filters were blocked in 5% (w/v) nonfat dry milk in Tris-buffered saline with 0.1% Tween-20 and probed with rabbit anti-S tag antibody (catalog no. PM021; MBL International Corp., Woburn, MA) as a primary antibody.
Electron microscopy. Five μl of sample suspension were placed on a 400-mesh carbon coated copper grid that had been made hydrophilic by glow discharge. After 5 minutes, the grid was rinsed by briefly touching the sample side to a drop of distilled water. The residual water was then removed by blotting to filter paper. For negative staining, 5 μl 1% aqueous phosphotungstic acid (pH 6.5) was applied to the grid immediately after water removal, and excess liquid was removed by blotting after 30 seconds. The grid was air dried before viewing on a JEOL (Tokyo, Japan) IEM-1400 transmission electron microscope equipped with a Gatan (Pleasanton CA) 2k x 2k US1000 CCD camera. Images were captured digitally as .tif files.
Aβ peptide synthesis. The amyloid β peptide (1–40: DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVG-GVV) was synthesized on a Liberty CEM microwave automated peptide synthesizer as described previously21 with the following modifications. After ether precipitation, the peptide was dissolved in 40% acetonitrile and 0.1% formic acid. HPLC was performed with a water-acetonitrile-0.1% formic acid gradient. Trifluoroacetic acid was replaced with formic acid. Guanidinium hydrochloride was not used, and the peptide was purified twice by C18-reverse phase chromatography for improved purity. For filament formation, lyophilized powder was dissolved in minimal hexafluoroisopropanol (HFIP, Aldrich Chemical Co.) for 2 h, dried under argon gas to form a clear film which was dissolved in 10 mM phosphate buffer, pH 7.4, 0.02% sodium azide and incubated at 37°C for 25 d.
Thioflavin T binding. Assays were carried out as described by LeVine.22 Thioflavin T (Sigma Chemical, St. Louis, MO) was dissolved in 50 mM glycine-NaOH, pH 8.5 and diluted to 10μM for use. The fluorescence from the dye alone, or the dye mixed with protein (2 μM each, except for BSA which was 20 μM), was read in a Shimadzu RF-5301PC spectrofluorophotometer in spectrum mode, with the excitation filter at 450+/-5 nm and the emission filter at 485+/-5 nm. Triplicate readings were averaged and plotted.
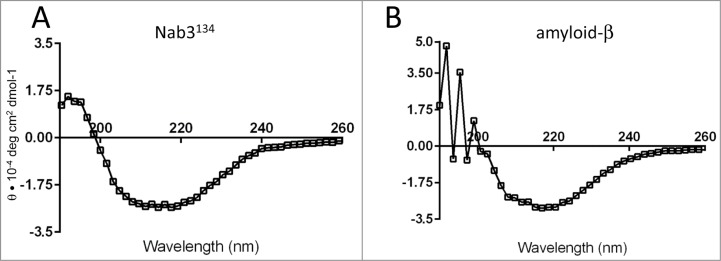
Circular dichroism. Proteins were diluted into 50 mM NaF, 17mM sodium phosphate, pH 7.25 and spectra were recorded on a JASCO J-810 Spectropolarimeter from 190 nm to 300 nm with a 100nm/min scan speed, 0.2nm scan pitch at room temperature with either a 1mm or 0.2mm path length quartz cuvette for amyloid β or Nab3134, respectively. Scans were repeated 3 times. Raw ellipticity data was transformed for each protein to obtain a mean residue ellipticity value at every 0.2nm of wavelength between 190 and 300nm.
Results
Purified Nab3 and Nrd1 low complexity domains form polymeric filaments in vitro. Nab3′s carboxy-terminal 134 residues (Nab3134) show a skewed sequence composition typical of intrinsically unstructured domains containing 32% glutamine (Q), 12% proline (P), and 12% serine (S) (Fig. 1). This segment is poor in aromatic residues, lacking phenylalanine and tryptophan and containing only a single tyrosine. These features, and the heat stability and protease sensitivity of the recombinant protein purified from E. coli, suggest it is intrinsically unstructured and prion-like.8,9 Although unstructured, prion-like proteins can change conformation and polymerize to form ordered aggregates in vitro and in vivo.23 Although essential, the function of this portion of the protein has remained obscure. The properties of Nab3's carboxy-terminal Q/P rich domain suggested it could form amyloid in a manner similar to yeast prions and aggregates containing Nab3 have been observed microscopically in yeast cells when expressed as a GFP-fusion.8 We previously showed that this region of Nab3, which is distant from the RNA binding portion of the protein (Fig. 1), can be robustly expressed in a soluble form in E. coli as a fusion to the carboxy-terminus of His6-tagged E. coli thioredoxin. Following affinity chromatography, the piece liberated by thrombin cleavage yielded the Nab3134 polypeptide with an S-tag at its amino terminus 9(Fig. 1). Purification and incubation of this piece of Nab3 at 4°C for a number of days at high concentration yielded a translucent macroscopic gel (Fig. 2). Our ability to detect polymerization may be due to the choice of boundaries of the domain, the absence of denaturants during the purification from E. coli, and/or the fusion of the domain to a distinct protein tag, as compared to a prior study.8 In any case, the protein polymerizes over a period of hours to days and the rate of formation of fibers increased with protein concentration, incubation at cold temperatures, and with agitation. The dimensions of the fibers seem to be characteristic of each protein. Whether this is an intrinsic feature of their polymerization geometry or a physical property such as their brittleness or flexibility during manipulation, remains to be determined. In any case, the assembled state appears essentially irreversible.
Figure 2.
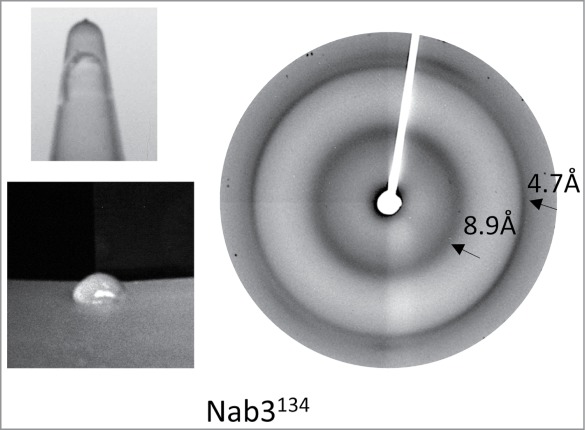
Gel formation and X-ray fiber diffraction analysis of Nab3134. Photographs of a hydrogel formed from Nab3134 in an upside-down tube and extruded onto a surface are shown at left. X-ray diffraction of dried fibers of Nab3134 is shown at right.
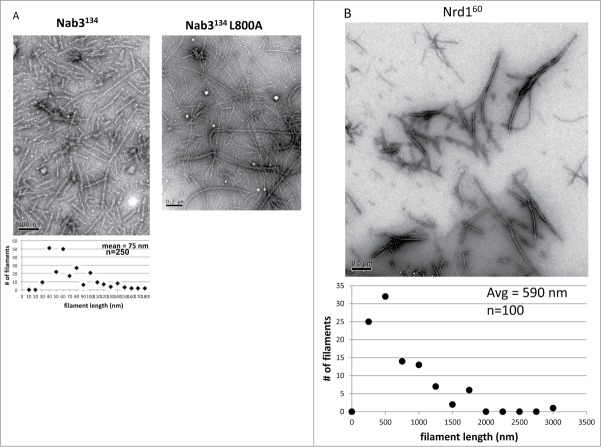
To learn if the Nab3134 gel was composed of amyloid-like fibers, we subjected it to X-ray fiber diffraction as described for the low complexity domains of Fus and hnRNPA2, for example.15 The pattern for Nab3134 revealed diffractions centered at 4.7 and 8.9 Å indicative of periodicity in the gel as seen for these 2 mammalian RNA binding proteins (Fig. 2). Examination of a sample of the hydrogel by negative staining and transmission electron microscopy, showed clearly that the gel is composed of filaments averaging 75 nm in length (Fig. 3A).
Figure 3.
Transmission electron microscopy on filaments formed from (A) purified Nab3134 and its L800A derivative or purified Nrd160 (B). Scale bars are shown at the lower left of each image. A quantification of the size distribution of Nab3134 and Nrd160 is plotted below their images.
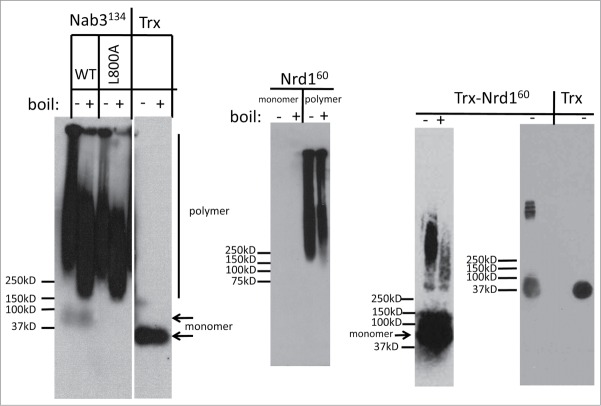
Many amyloid fibers are resistant to the ionic detergent sodium dodecyl sulfate (SDS).24 In contrast, amorphous, non-amyloid precipitates are solubilized by SDS.25,26 To examine Nab3134 aggregates for this property, we subjected them to semi-denaturing agarose gel electrophoresis (SDD-AGE) which separates monomers from large multimers following their exposure to SDS. Proteins were transferred to nitrocellulose and probed with antibodies against the S-tag epitope (KETAAAKFERQHMDSSTSAA). The Nab3134 gel was highly SDS-resistant, running as a complex high molecular weight species as observed for a number of yeast prion-like domains.8 In contrast, the control protein E. coli thioredoxin, runs as a single species, indicating this is not a property of all proteins fused to the S-tag (Fig. 4). Boiling in SDS resulted in a subtle decrease in the overall size of aggregated Nab3 but it was largely resistant to this treatment as well (Fig. 4). This observation is consistent with the idea that the Nab3 low complexity domain can assume an amyloid form.
Figure 4.
Semi-denaturing agarose gel electrophoresis on polymers formed in vitro. Purified Nab3134, the L800A mutant derivative of Nab3134, purified Nrd160 (unpolymerized monomer and polymerized), thioredoxin-Nrd160 fusion, and thioredoxin (TRX) were incubated with SDS at 22° (-) or 100°C (+), subjected to electrophoresis, and transferred to nitrocellulose. Membranes were probed with anti-S-tag antibody and developed with chemiluminescent reagents. The positions of monomeric (arrowheads) and polymeric (vertical bar) versions of the proteins are indicated, as are the size (kD) and migration positions of Precision Plus Protein Kaleidoscope molecular weight standards (Bio-Rad, Inc.). TRX and Nab3134 samples from the composite panel on the left were run on different ends of the same gel. Note that Nrd160 monomers were not detectable but the monomeric TRX-Nrd160 fusion protein was.
Like Nab3, Nrd1 is an essential protein that possesses an RRM and is important for termination of small non-coding RNAs. It dimerizes with Nab3 and also possesses a low complexity domain [32% Q, 17% P, 10% alanine (A)], that is predicted to be prion-like and intrinsically unstructured8,11 (Fig. 1). However, direct evidence supporting this has not yet been reported. Like the Nab3 domain, the carboxy-terminal 60 residues of Nrd1 could be expressed in E. coli and obtained in high yield as a thioredoxin-fusion. Soluble protein with an S-tag was purified following thrombin cleavage (Fig. 1). When incubated at 22° or 4°C, the domain formed filaments observable by electron microscopy (Fig. 3B), although they did not form a translucent hydrogel as Nab3134 did. Measurements indicated the Nrd160 filaments were on average 590 nm in length. Unexpectedly, monomeric Nrd160 could not be detected by SDD-AGE even though its polymeric form was readily detectable (Fig. 4, middle panel). Presumably, it binds poorly to filters (either nitrocellulose or polyvinylidene fluoride membranes). To aid in the detection of Nrd160 monomers, we studied the behavior of the purified Nrd160-thioredoxin fusion protein without separating the 2 by digestion with thrombin. In this case, both monomeric and polymerized forms were detectable and boiling in SDS reduced, but did not completely dissolve, the polymer (Fig. 4, right panels). Brief centrifugation of the high molecular weight Nrd160 yielded a more discrete and smaller polymeric fraction in which integral units of polymer could be seen in a ladder-like pattern (Fig. 4, right panel). Neither the Nab3 nor Nrd1 polymers could be dissolved by dilution, warming, or 8 M urea, although they were readily soluble in dimethyl sulfoxide, as described for human amyloid β1-40 peptide.27 We conclude that the low complexity domains of these 2 proteins form amyloid-like polymers in vitro.
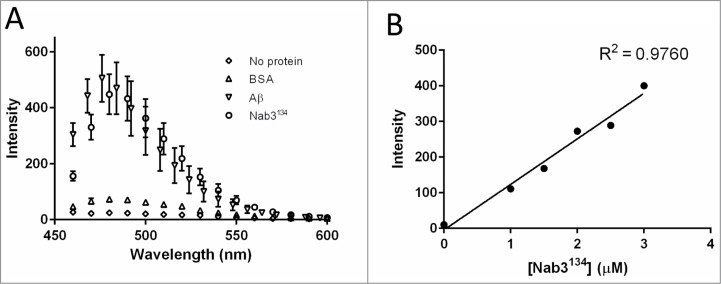
The Nab3134 polymer bears the physical hallmarks of amyloid. A characteristic of the β-sheet rich structure of amyloid polymers is their ability to bind thioflavin T which fluoresces with a characteristic emission maximum at 482 nm upon binding to the polymerized protein.28 To test if Nab3134 filaments possessed this property, we incubated the polymer with thioflavin T and scored for fluorescence using spectrofluorometry. We compared Nab3134 to a positive control, polymeric human amyloid β peptide extending from residues 1–40 (Aβ). Nab3134 demonstrated a strong response from thioflavin T with a profile comparable to that seen for Aβ (Fig. 5A), and distinct from the lack of fluorescence when 10-fold more bovine serum albumin was mixed with thioflavin T or when the dye was excited in the absence of any protein (Fig. 5A). Titration of Nab3134 showed that the fluorescent signal was linear with respect to protein concentration (Fig. 5B).
Figure 5.
Fluorescence spectroscopy of thioflavin T binding to Nab3134 and Aβ. (A) Thioflavin T was mixed with the indicated proteins (2 μmolar each, except BSA which was 20 μM) and the spectra of fluorescence was scored in a spectrofluorometer in triplicate and averaged. Error bars show the standard deviation around those averages. (B) Increasing amounts of Nab3134 protein were added to thioflavin and absorbance at 485 nm was quantified and plotted. The correlation coefficient (R2) was calculated with Prism 6.02 software (GraphPad).
The structure of the canonical amyloid, human amyloid β, has been examined by circular dichroism spectroscopy in which its β sheet content can be observed.29 We found that the far ultraviolet spectrum for Nab3134 fibers was characteristic of a β-rich structure with a profile similar to the Aβ control, consistent with the model that Nab3134 attains an amyloid form (compare Fig. 6A and 6B).
Figure 6.
Circular dichroism spectroscopy of Nab3134 and Aβ. Suspensions of Nab3134 and Aβ (1–40) were independently analyzed by CD spectroscopy as described in Materials and Methods. A representative spectrum is shown for each. θ was calculated and plotted at 1.6 nm intervals. Lines connect the average values. Nab3134 was read in a 0.2 mm path length cuvette and Aβ was read in a 1 cm path length cuvette.
A termination defective mutant of Nab3134 forms filaments. Mutations in the low complexity domain of Nab3 have been identified that compromise its ability to support termination of short, non-coding transcripts.5,9,10 One of these is a Leu to Ala substitution at position 800 (Fig. 1) that shows a strong terminator override phenotype.10 A recombinant version of Nab3134 bearing this substitution was expressed, cleaved off thioredoxin, and purified. The protein readily formed a semi-solid gel and more interestingly, presented as a distinct filamentous form when negative stained and analyzed by electron microscopy (Fig. 3A). The filaments were many microns in length and their extreme size made it difficult to measure them precisely. They also tended to form side-by-side bundles in this preparation. When examined by SDD-AGE, the Nab3134-L800A protein demonstrated resistance to boiling in SDS with no monomeric form detectable (Fig. 4).
In vivo analysis of the Nab3 low-complexity domains. As a test of Nab3's ability to interact with itself in vivo, we employed the protein fragment complementation assay developed by Michnick and co-workers.20,30 In this assay, a methotrexate resistant dihydrofolate reductase (DHFR) enzyme is recombinantly split into an amino and carboxy terminal portion each of which is fused to candidate proteins. If the candidate proteins interact, the pieces of DHFR are brought together generating active enzyme that renders the growth of yeast resistant to methotrexate. As a positive control, the bZIP domain of the yeast transcription factor GCN4 was shown to interact with itself when fused to both pieces of DHFR (DHFRN-term and DHFRC-term), as seen previously20 (Fig. 7, top quadrants). We adapted this assay to test if Nab3134 interacts with itself by fusing it independently to both the amino- and carboxy-terminal fragments of DHFR and introducing both plasmids into yeast. Growth of this strain in the presence of methotrexate indicated that the Nab3134-DHFRN-term and Nab3134-DHFRC-term fusion proteins interact in vivo (Fig. 7, right quadrants). Pairing a bZIP-DHFR plasmid with a Nab3134-DHFR plasmid served as a negative control showing that the mere coexistence of both halves of DHFR in the cell was insufficient to provide drug resistance unless they were brought together by fused binding partners. Finally, we extended this approach to full length Nab3 by fusing the full 802 amino acids of this protein to the DHFRN-term and DHFRC-term fragments and introducing both plasmids into cells. Again, an interaction was detectable as seen by methotrexate resistance (Fig. 7, bottom quadrant). Although this assay does not demonstrate filament formation, it does provide additional independent evidence that Nab3 interacts with itself in living yeast.
Figure 7.
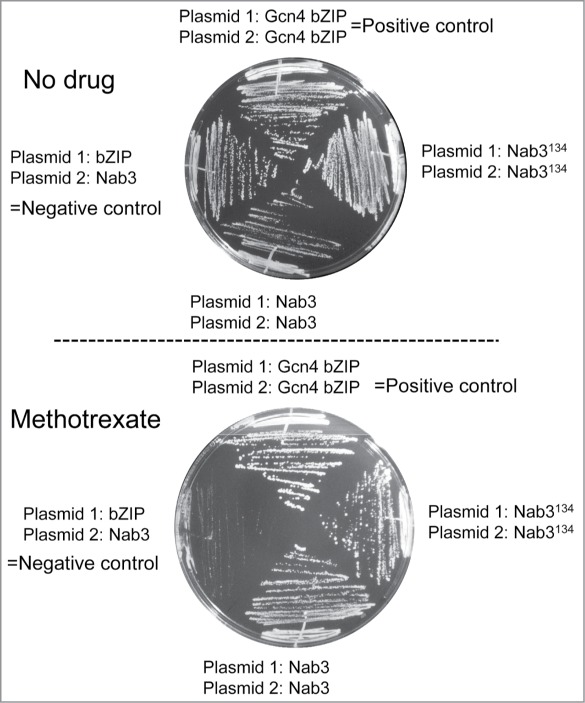
Yeast strains containing the indicated plasmids were struck to solid media containing 200 μg/ml methotrexate or its solvent, DMSO, as indicated, and were grown at 30°. The negative control strain was DY2219, the positive control strain was DY2315, the strain with two Nab3134-fusion proteins was DY2214, and that with two full-length Nab3-fusion proteins was DY2217.
Discussion
In this report we provide new evidence that the low complexity domains of two yeast hnRNP-like proteins, Nab3 and Nrd1, can polymerize into a fibrillar form. Nab3 in particular, readily assembles into filamentous structures which organize into a semi-solid gel in vitro. The evidence that the low complexity domain of Nab3 forms amyloid-like fibers includes: 1) its ability to form stable, regular filaments, 2) its ability to assemble into a high molecular weight form resistant to disruption by the anionic detergent SDS that is separable from unassembled monomer, similar to yeast prions, 3) its propensity to spontaneously gel and show a periodic organization of fibers as seen by X-ray fiber diffraction that is similar to that seen for other RNA binding proteins with low complexity sequence, 4) its capacity to bind the amyloid-specific fluorescent dye, thioflavin T, in a manner indistinguishable from amyloid-β, 5) its circular dichroism properties which closely parallel those of the well-studied amyloid-β fibrils. Together, we take this as compelling evidence that this domain of the yeast RNA binding protein and termination factor Nab3 attains an authentic amyloid form.
The obvious question is what is the biological role of this feature? The following findings make it likely that filament formation is important for Nab3s function as an RNA-binding protein in transcription termination: 1) prior genetic analysis has supported a model that the assembly of multiple copies of Nab3 onto transcripts in living cells is needed for cells to survive,9 2) evidence presented here using an independent assay indicates that the Nab3 low complexity domain interacts with itself in living cells, as does intact Nab3, 3) the region of Nab3 that assembles into filaments is essential for cell viability,9 the small size of the region limits the number of functions it can contain, increasing the likelihood that its essential function is related to its polymerization potential, and finally, 4) recent findings across species have revealed that RNA-binding proteins, many of which are hnRNPs and many of which contain low complexity regions, undergo reversible associations important for their functions and yield filaments and semi-solid gels in vitro.15,16 As well, amyloid formation by prion-like proteins is becoming recognized as a functionally important state and it forms the basis of regulatory switches in diverse organisms. Recent examples include neuronal CPEB,31 yeast Mot3,32 and yeast Mod5.33 Based upon these studies, we infer that the assembly of Nab3 we observe in vitro, represents the potential for this protein to reversibly cycle between protomers and assembled forms in vivo. The biological signals that induce and dissolve these structures remain to be identified. One good candidate, however, is phosphorylation of Nab3 and Nrd1 both of which have been shown to be phospho-proteins.34-36 Nrd1's phosphorylation state has been shown to change in conjunction with the availability of a carbon source.37 Interestingly, dephosphorylation during glucose starvation is concurrent with rearrangement of Nrd1 into nuclear punctae. This process could be related to the assembly potential of Nab3 and Nrd1 observed here. Furthermore, the low complexity domains of other transcriptionally important proteins, such as Taf15, form filaments and are recruited to the carboxy terminal repeat domain of the large subunit of RNA polymerase II.18 This part of pol II is an intrinsically disordered, simple repeated sequence, and it contains a signature [G/S]Y[G/S] amino acid motif shown to be important for polymerization of some low complexity domains. Filaments made from the Fus and Taf15 domains associate with the pol II repeat in its hypophosphorylated form, but not when it is phosphorylated.18 While these low complexity proteins are transcriptional activators, we propose that a similar situation may exist for termination-related proteins such as Nab3 and Nrd1 which may polymerize, perhaps reversibly, while transiently interacting with pol II. Association of filamentous Nrd1 or Nab3 with the CTD through their low complexity domains would be a second means of interaction with the CTD in addition to the well-characterized CTD-interaction domain of Nrd1.38
A feature of Nab3 and Nrd1 thought to be important for their function is the ability to co-assemble into a stable heterodimer.36 A careful study of dimerization was done with truncated recombinant Nab3 and Nrd1 derivatives that lacked the self-assembly domains described here. Thus, they can form a heterodimer without these low complexity regions.4 Are heterodimerization and the self-assembly property observed here two-different associations or can they co-exist? One possibility is that Nrd1-Nab3 heterodimerization is mutually exclusive with the filament assembly ability of each protein. Alternatively, the heterodimer may be a protomeric unit of polymerization, either constitutively or inducibly, which would result in a Nrd1-Nab3 heteropolymer. Further probing of this possibility must await the reconstitution of significant quantities of recombinant full-length Nrd1 and Nab3 that contain both their heterodimerization regions and their low complexity self-assembly domains. Yet a third possibility is that there are distinct pools of Nrd1-Nab3 dimers independent of a filamentous form of each protein.
The findings of filament formation for the low complexity domains of these RNA-binding proteins are in accordance with comparable data obtained for mammalian hnRNPs and other RNA-binding proteins, such as FUS and TDP-43, that have low complexity domains.15,16 A growing body of evidence suggests that RNA-binding proteins can assume a polymerized state which manifests itself as a hydrogel in vitro, and whose in vivo form is a subcellular, non-membranous compartment such as a P-body or stress granule.17 An emerging concept is that formation of amyloid-like fibers and hydrogels by RNA-interacting proteins reflects their capacity to dynamically and reversibly assemble and disassemble in vivo to form localized complexes that handle, metabolize, and modify RNA. The abnormal persistence of such aggregates can lead to a class of diseases known as 'proteinopathies', some of which are due to mutations in hnRNPs that potentiate their filamentation.19,39,40 We propose that the assembly of Nrd1 and Nab3 seen in vitro, and that of Nab3 seen in vivo, reflects a role for self-assembly in their function during termination of transcription in the nucleus. This would be consistent with prior genetic data indicating that multiple copies of Nab3 are important for function,9 and accumulating evidence that prion-like assemblages are functionally significant.31,41,42
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Drs. Graeme Conn, Tanya Chernova, Yury Chernoff, Richard Cummings, and Xiaodong Cheng for helpful discussions and sharing of reagents and equipment, and Alissandra K. Mowles, Noel X. Li, and Dr. David Lynn for advice on CD and fibrillized Aβ. We also thank Dr. Stephen Michnick for materials and advice. The authors also acknowledge the assistance of Hong Yi in the Emory Electron Microscopy Core.
Funding>
This work was supported, in whole or in part, by National Institutes of Health, NIGMS, Grant R01GM46331, NIH training grant T32GM008367, and NIH grant S10RR025679.
REFERENCES
- 1. Kuehner JN, Pearson EL, Moore C. Unravelling the means to an end: RNA polymerase II transcription termination. Nat Rev Mol Cell Biol 2011; 12:283-94; PMID:21487437; http://dx.doi.org/ 10.1038/nrm3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson SM, Datar KV, Paddy MR, Swedlow JR, Swanson MS. Characterization of nuclear polyadenylated RNA-binding proteins in Saccharomyces cerevisiae. J Cell Biol 1994; 127:1173-84; PMID:7962083; http://dx.doi.org/ 10.1083/jcb.127.5.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steinmetz EJ, Brow DA. Repression of gene expression by an exogenous sequence element acting in concert with a heterogeneous nuclear ribonucleoprotein-like protein, Nrd1, the putative helicase Sen1. Mol Cell Biol 1996; 16:6993-7003; PMID:8943355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carroll KL, Ghirlando R, Ames JM, Corden JL. Interaction of yeast RNA-binding proteins Nrd1 and Nab3 with RNA polymerase II terminator elements. Rna 2007; 13:361-73; PMID:17237360; http://dx.doi.org/ 10.1261/rna.338407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loya TJ, O'Rourke TW, Reines D. A genetic screen for terminator function in yeast identifies a role for a new functional domain in termination factor Nab3. Nucleic Acids Res 2012; 40:7476-91; PMID:22564898; http://dx.doi.org/ 10.1093/nar/gks377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hyle JW, Shaw RJ, Reines D. Functional distinctions between IMP dehydrogenase genes in providing mycophenolate resistance and guanine prototrophy to yeast. J Biol Chem 2003; 278:28470-8; PMID:12746440; http://dx.doi.org/ 10.1074/jbc.M303736200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jenks MH, O'Rourke TW, Reines D. Properties of an intergenic terminator and start site switch that regulate IMD2 transcription in yeast. Mol Cell Biol 2008; 28:3883-93; PMID:18426909; http://dx.doi.org/ 10.1128/MCB.00380-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alberti S, Halfmann R, King O, Kapila A, Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 2009; 137:146-58; PMID:19345193; http://dx.doi.org/ 10.1016/j.cell.2009.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loya TJ, O'Rourke TW, Degtyareva N, Reines D. A network of interdependent molecular interactions describes a higher order Nrd1-Nab3 complex involved in yeast transcription termination. J Biol Chem 2013; 288:34158-67; PMID:24100036; http://dx.doi.org/ 10.1074/jbc.M113.516765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loya TJ, O'Rourke TW, Reines D. Yeast Nab3 protein contains a self-assembly domain found in human heterogeneous nuclear ribonucleoprotein-C (hnRNP-C) that is necessary for transcription termination. J Biol Chem 2013; 288:2111-7; PMID:23192344; http://dx.doi.org/ 10.1074/jbc.M112.430678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Romero P, Obradovic Z, Li X, Garner EC, Brown CJ, Dunker AK. Sequence complexity of disordered protein. Proteins 2001; 42:38-48; PMID:11093259; http://dx.doi.org/ 10.1002/1097-0134(20010101)42:1%3c38::AID-PROT50%3e3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- 12. Radivojac P, Iakoucheva LM, Oldfield CJ, Obradovic Z, Uversky VN, Dunker AK. Intrinsic disorder and functional proteomics. Biophys J 2007; 92:1439-56; PMID:17158572; http://dx.doi.org/ 10.1529/biophysj.106.094045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frieden C. Protein aggregation processes: In search of the mechanism. Protein Sci 2007; 16:2334-44; PMID: 17962399; http://dx.doi.org/ 10.1110/ps.073164107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liebman SW, Chernoff YO. Prions in yeast. Genetics 2012; 191:1041-72; PMID:22879407; http://dx.doi.org/ 10.1534/genetics.111.137760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012; 149:753-67; PMID:22579281; http://dx.doi.org/ 10.1016/j.cell.2012.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, Allen J, Xiao G, et al. Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell 2012; 149:768-79; PMID:22579282; http://dx.doi.org/ 10.1016/j.cell.2012.04.016 [DOI] [PubMed] [Google Scholar]
- 17. Weber SC, Brangwynne CP. Getting RNA and protein in phase. Cell 2012; 149:1188-91; PMID:22682242; http://dx.doi.org/ 10.1016/j.cell.2012.05.022 [DOI] [PubMed] [Google Scholar]
- 18. Kwon I, Kato M, Xiang S, Wu L, Theodoropoulos P, Mirzaei H, Han T, Xie S, Corden JL, McKnight SL. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell 2013; 155:1049-60; PMID:24267890; http://dx.doi.org/ 10.1016/j.cell.2013.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 2013; 495:467-73; PMID:23455423; http://dx.doi.org/ 10.1038/nature11922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Remy I, Michnick SW. Clonal selection and in vivo quantitation of protein interactions with protein-fragment complementation assays. Proc Natl Acad Sci U S A 1999; 96:5394-9; PMID:10318894; http://dx.doi.org/ 10.1073/pnas.96.10.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehta AK, Rosen RF, Childers WS, Gehman JD, Walker LC, Lynn DG. Context dependence of protein misfolding and structural strains in neurodegenerative diseases. Biopolymers 2013; 100:722-30; PMID:23893572; http://dx.doi.org/ 10.1002/bip.22283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. LeVine H, 3rd. Quantification of β-sheet amyloid fibril structures with thioflavin T. Methods Enzymol 1999; 309:274-84; PMID:10507030; http://dx.doi.org/ 10.1016/S0076-6879(99)09020-5 [DOI] [PubMed] [Google Scholar]
- 23. Halfmann R, Alberti S, Krishnan R, Lyle N, O'Donnell CW, King OD, Berger B, Pappu RV, Lindquist S. Opposing effects of glutamine and asparagine govern prion formation by intrinsically disordered proteins. Mol Cell 2011; 43:72-84; PMID:21726811; http://dx.doi.org/ 10.1016/j.molcel.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chernoff YO, Uptain SM, Lindquist SL. Analysis of prion factors in yeast. Methods Enzymol 2002; 351:499-538; PMID:12073366; http://dx.doi.org/ 10.1016/S0076-6879(02)51867-X [DOI] [PubMed] [Google Scholar]
- 25. Bagriantsev SN, Kushnirov VV, Liebman SW. Analysis of amyloid aggregates using agarose gel electrophoresis. Methods Enzymol 2006; 412:33-48; PMID:17046650; http://dx.doi.org/ 10.1016/S0076-6879(06)12003-0 [DOI] [PubMed] [Google Scholar]
- 26. Bagriantsev S, Liebman SW. Specificity of prion assembly in vivo. [PSI+] and [PIN+] form separate structures in yeast. J Biol Chem 2004; 279:51042-8; PMID:15465809; http://dx.doi.org/ 10.1074/jbc.M410611200 [DOI] [PubMed] [Google Scholar]
- 27. Shen CL, Murphy RM. Solvent effects on self-assembly of β-amyloid peptide. Biophys J 1995; 69:640-51; PMID:8527678; http://dx.doi.org/ 10.1016/S0006-3495(95)79940-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Biancalana M, Koide S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta 2010; 1804:1405-12; PMID:20399286; http://dx.doi.org/ 10.1016/j.bbapap.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB. Amyloid β-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem 1999; 274:25945-52; PMID: 10464339; http://dx.doi.org/ 10.1074/jbc.274.36.25945 [DOI] [PubMed] [Google Scholar]
- 30. Tarassov K, Messier V, Landry CR, Radinovic S, Serna Molina MM, Shames I, Malitskaya Y, Vogel J, Bussey H, Michnick SW. An in vivo map of the yeast protein interactome. Science 2008; 320:1465-70; PMID:18467557; http://dx.doi.org/ 10.1126/science.1153878 [DOI] [PubMed] [Google Scholar]
- 31.Heinrich SU, Lindquist S. Protein-only mechanism induces self-perpetuating changes in the activity of neuronal Aplysia cytoplasmic polyadenylation element binding protein (CPEB). Proc Natl Acad Sci USA 2011; 108:2999-3004; PMID:21270333; http://dx.doi.org/22517861 10.1073/pnas.1019368108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes DL, Lancaster AK, Lindquist S, Halfmann R. Heritable remodeling of yeast multicellularity by an environmentally responsive prion. Cell 2013; 153:153-65; PMID:23540696; http://dx.doi.org/22517861 10.1016/j.cell.2013.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suzuki G, Shimazu N, Tanaka M. A yeast prion, Mod5, promotes acquired drug resistance and cell survival under environmental stress. Science 2012; 336:355-9; PMID: 22517861; http://dx.doi.org/ 10.1126/science.1219491 [DOI] [PubMed] [Google Scholar]
- 34. Chi A, Huttenhower C, Geer LY, Coon JJ, Syka JE, Bai DL, Shabanowitz J, Burke DJ, Troyanskaya OG, Hunt DF. Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry. Proc Natl Acad Sci U S A 2007; 104:2193-8; PMID:17287358; http://dx.doi.org/ 10.1073/pnas.0607084104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, Ross MM, Shabanowitz J, Hunt DF, White FM. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat Biotechnol 2002; 20:301-5; PMID:11875433; http://dx.doi.org/ 10.1038/nbt0302-301 [DOI] [PubMed] [Google Scholar]
- 36. Conrad NK, Wilson SM, Steinmetz EJ, Patturajan M, Brow DA, Swanson MS, Corden JL. A yeast heterogeneous nuclear ribonucleoprotein complex associated with RNA polymerase II. Genetics 2000; 154:557-71; PMID:10655211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Darby MM, Serebreni L, Pan X, Boeke JD, Corden JL. The Saccharomyces cerevisiae Nrd1-Nab3 transcription termination pathway acts in opposition to Ras signaling and mediates response to nutrient depletion. Mol Cell Biol 2012; 32:1762-75; PMID:22431520; http://dx.doi.org/ 10.1128/MCB.00050-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vasiljeva L, Kim M, Mutschler H, Buratowski S, Meinhart A. The Nrd1-Nab3-Sen1 termination complex interacts with the Ser5-phosphorylated RNA polymerase II C-terminal domain. Nat Struct Mol Biol 2008; 15:795-804; PMID:18660819; http://dx.doi.org/ 10.1038/nsmb.1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 2013; 79:416-38; PMID:23931993; http://dx.doi.org/ 10.1016/j.neuron.2013.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramaswami M, Taylor JP, Parker R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell 2013; 154:727-36; PMID:23953108; http://dx.doi.org/ 10.1016/j.cell.2013.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pezza JA, Villali J, Sindi SS, Serio TR. Amyloid-associated activity contributes to the severity and toxicity of a prion phenotype. Nature Commun 2014; 5:4384; PMID: 25023996; http://dx.doi.org/ 10.1038/ncomms5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raveendra BL, Siemer AB, Puthanveettil SV, Hendrickson WA, Kandel ER, McDermott AE. Characterization of prion-like conformational changes of the neuronal isoform of Aplysia CPEB. Nat Struct Mol Biol 2013; 20:495-501; PMID:23435382; http://dx.doi.org/ 10.1038/nsmb.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]