Abstract
Streptococcus tigurinus is a novel species of viridans streptococci, shown to cause severe invasive infections such as infective endocarditis, spondylodiscitis and meningitis. S. tigurinus belongs to the Streptococcus mitis group and is most closely related to Streptococcus mitis, Streptococcus oralis, Streptococcus pneumoniae, Streptococcus pseudopneumoniae and Streptococcus infantis. The presence of S. tigurinus in the human oral cavity has been documented, including in patients with periodontal disease. This review addresses the available scientific knowledge on S. tigurinus and its association with closely related streptococci, and discusses its putative involvement in common oral infections. While there is as yet no strong evidence on the involvement of S. tigurinus with oral infections, its presence in the oral cavity and its association with endocarditis warrants special attention for a link between oral and systemic infection.
Keywords: endocarditis, oral infection, periodontal disease, Streptococcus mitis group, Streptococcus tigurinus
Introduction
The human oral microbiome consists of a number of bacteria; most of them are non-pathogenic commensals or act as opportunistic pathogens.1 Some oral bacteria are implicated in oral diseases such as dental caries and periodontitis, which are among the most common infections in humans.2,3 Several oral bacteria have the capacity to form biofilms, which are built by complex polymicrobial mechanisms on surfaces of teeth or soft oral mucosa.4 Viridans streptococci form a major part of the human oral microbiome, comprising 4 species groups: namely salivarius, anginosus, mutans and mitis group, respectively.5 Streptococcus mutans is a key player in the development of dental caries,6 and Streptococcus anginosus is detected in periapical odontogenic abscesses,7 while the other species groups are less known as causative agents of oral diseases. Some bacterial species are rather associated with oral health than disease, e.g., Streptococcus salivarius, which is commonly found on mucosal surfaces and in the saliva.8 Interestingly, the filamentation capacity of S. salivarius is markedly increased compared to other viridans streptococci, a property which may be required for a more efficient adhesion onto continuously shedding oral mucosal surfaces.9 Other more virulent bacteria potentially enter the bloodstream, which increases the risk for invasive infections, e.g., infective endocarditis.10 Streptococcus mitis, a prominent representative of viridans streptococci, is a leading cause of infective endocarditis.11
Recently, a novel pathogen associated with severe invasive infections such as infective endocarditis was described: Streptococcus tigurinus belonging to viridans streptococci.12-14 S. tigurinus initially was detected in an elderly patient with infective endocarditis.12 First, a viridans streptococcal organism was isolated in multiple blood cultures of the patient. Then, the bacterial strain was analyzed by 16S rRNA gene sequencing for accurate species identification. Analysis of the aortic valve specimen of the patient by direct 16S rRNA gene broad-range PCR15 revealed an identical sequence compared to that of the isolate from the blood. 16S rRNA gene sequence comparison to validated sequences in the public database showed highest sequence similarity to the strain S. mitis ATCC 15914 with 99.9% identity (GenBank accession number AY281076). The next best match in the database was the type strain of the species S. mitis ATCC 49456T (AY485601) but only with 98.6% sequence identity. Obviously, this was a novel species because of the high sequence demarcation of 1.3%. Additionally, the strain ATCC 15914, which was typed in 1977 based solely on phenotypic characteristics, must have been erroneously assigned to the species S. mitis.16 Other authors have questioned the correct species assignment of strain ATCC 15914 on basis of analyses of the housekeeping genes zwf and gki17 and the ribosomal 16S-23S intergenic spacer region.18 Deeper analyses by different methods such as conventional biochemical testing, molecular analyses and DNA-DNA hybridization techniques proofed the presence of a novel species for which the name S. tigurinus was assigned.12
S. tigurinus is a member of the Streptococcus mitis group
S. tigurinus belongs to the S. mitis group, which consists of different species, i.e., Streptococcus pneumoniae, Streptococcus pseudopneumoniae, S. mitis, Streptococcus oralis, Streptococcus infantis, Streptococcus sanguinis, Streptococcus parasanguinis, Streptococcus cristatus, Streptococcus gordonii, Streptococcus peroris, Streptococcus australis, Streptococcus oligofermentans and Streptococcus sinensis.19,20 Recently, 3 novel species were assigned to the S. mitis group, i.e., Streptococcus dentisani,21 Streptococcus rubneri22 and Streptococcus lactarius.23 S. dentisani was isolated from supragingival dental plaque from adult individuals who had never suffered from dental caries21; S. rubneri was isolated from throat swab samples of healthy humans22 and S. lactarius from breast milk of healthy women.23 Members of the S. mitis group are known as commensal bacteria of the human oral cavity, gastrointestinal tract and the female genital tract, however, invasive infections might occur when entering the bloodstream.10 S. pneumoniae is associated with bacteremia, meningitis, otitis media and sinusitis; and is the most common cause of community-acquired pneumonia.10 Commensals such as S. oralis and S. mitis are major pathogens for infective endocarditis in native or prosthetic heart valves.10, 24
Proper identification of species within the S. mitis group consisting of phenotypically very closely related species still remains a challenge, in particular for S. pneumoniae, S. pseudopneumoniae, S. mitis, and S. oralis. Identification by conventional phenotypic methods including commercial kits as VITEK 2 GP colorimetric card or API rapid 20 Strep strip (bioMérieux, Marcy-l’Etoile, France) are limited25,26 and newer rapid technologies such as matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) are useful for initial assessment to the S. mitis group but frequently do not allow for accurate identification.27-29 However, there are promising reports elaborating on the differentiation of S. pneumoniae from other S. mitis group bacteria by MALDI-TOF MS.30,31 Molecular analysis by the 5′-part of the 16S rRNA gene, which is the gold standard for bacterial identification,26,32-34 is not sufficiently discriminative to differentiate S. pneumoniae, S. pseudopneumoniae, S. mitis and S. oralis due to a >99% sequence homology.25,35 In the past, several other target genes like sodA,36,37 rpoB38 and groEL39 were proposed for species differentiation. The recA gene was recently demonstrated as alternative target to differentiate S. pneumoniae from other viridans streptococci.40 An interspecies homology of less than 95.8% was shown within a 313-bp part of recA, representing a hypervariable region. Additionally, 6 signature nucleotides specific for S. pneumoniae were identified within the 313-bp recA fragment.
Difficulties in identifying bacteria of the S. mitis group to the species level might explain why the novel species S. tigurinus was unrecognized and underreported in the past. However, proper identification of those bacteria is important regarding species-specific pathogenicity, efficient patient management and guidance of appropriate antimicrobial therapy where needed.
Microbiological characteristics of S. tigurinus.
S. tigurinus belongs to Gram-positive cocci arranged in chains and is a non-motile, non-spore-forming and catalase-negative bacterium. Colonies on sheep blood agar are smooth, white to grayish, α-hemolytic and 0.5 to 1 mm in diameter after 24 h incubation at 37°C in aerobic atmosphere. The type strain of the species is AZ_3aT and is deposited in 2 different culture collections: the Culture Collection of Switzerland, CCOS, Wädenswil, Switzerland, under accession number CCOS 600T; and the Deutsche Sammlung für Mikroorganismen und Zellkulturen, DSMZ, Braunschweig, Germany, under accession number DSM 24864T. The type strain AZ_3aT has a G+C content of the DNA of 40 mol%.12
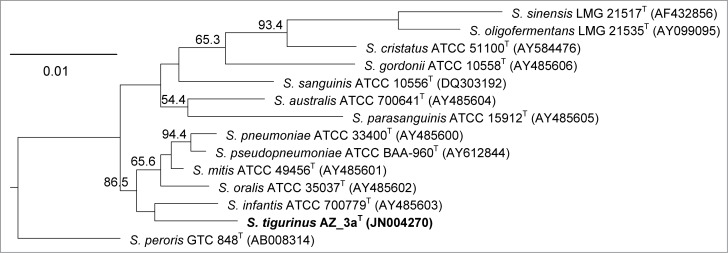
Phylogenetic analysis by the 16S rRNA gene of species of the S. mitis group demonstrated that S. tigurinus type strain AZ_3aT is clearly distinguished from the other species (Fig. 1). Within the S. mitis group, S. tigurinus forms a subcluster with S. pseudopneumoniae, S. pneumoniae, S. mitis, S. oralis and S. infantis (Fig. 1). The species most closely related to the S. tigurinus type strain AZ_3aT is S. mitis AY485601 with a sequence similarity of 98.6%; the next related species are S. infantis AY485603 (98.5%), S. pseudopneumoniae AY612844 (98.3%), S. pneumoniae AY485600 (98.2%) and S. oralis AY485602 (98.1%).12
Figure 1.
Phylogenetic analysis of the Streptococcus mitis group. The neighbor-joining phylogenetic tree based on partial 16S rRNA gene sequences (>1300 bp) shows the relationships among S. tigurinus strain AZ_3aT and related species within the S. mitis group. Bootstrap percentages (based on 1000 replications) > 50% are shown at branching points. Published sequences used were from the public GenBank database. Bar, 0.01 substitutions per nucleotide position.
For laboratory diagnostic means, commercial systems such as VITEK 2 colorimetric card (bioMérieux) or MALDI-TOF MS are useful as screening method for assignment of the unknown organism into the S. mitis group but do not allow for accurate identification of S. tigurinus. Because of the limited database, analysis of the S. tigurinus strains by the VITEK 2 colorimetric card (bioMérieux) revealed identification as S. mitis / S. oralis; whereas MALDI-TOF MS analysis yielded scores of ≥ 2.2 with S. pneumoniae, which suggests an identification on species level.12 Thus, for accurate identification of S. tigurinus, sequence analysis of the partial 16S rRNA gene is mandatory. Recently, a S. tigurinus specific real-time TaqMan PCR was developed for highly sensitive and specific detection of S. tigurinus directly in clinical samples.41
Ecological niche of S. tigurinus in the human oral microbial flora
The human oral microbiome consists of diverse bacterial phyla, e.g., Firmicutes, Actinobacteria, Proteobacteria, Bacteroidetes, Synergistetes and Proteobacteria.42,43 Viridans streptococci, e.g., S. mitis, are known to be the predominant bacterial species in the human oral cavity and were detected in various dental sites.42 S. oralis is an early colonizing species of the tooth surface and can mediate the first events of biofilm formation. Thus, S. tigurinus was also expected to be present in the human oral microbial flora. In a previous report, S. tigurinus was not detected in saliva samples, however, the method applied was only based on culturing methods followed by analyses by MALDI-TOF MS.13 As described before, these methods are limited in accurate identification of S. tigurinus thus an underestimation of S. tigurinus in the oral cavity seemed likely. By using a more sensitive method, i.e., a S. tigurinus specific real-time TaqMan PCR, S. tigurinus was detected in half of the individuals investigated.41 Zbinden et al. analyzed 51 saliva samples and 51 subgingival plaque samples obtained from 51 individuals by the S. tigurinus specific real-time TaqMan PCR.41 S. tigurinus was detected both in saliva (n = 22, 43%) and in subgingival plaque samples (n = 18, 35%). Overall, in 27 (53%) out of 51 individuals, S. tigurinus was detected in the saliva samples and / or in the plaque samples. Saliva consists of bacteria from different oral sites including the subgingival area. Thus it is not surprising that S. tigurinus was found in the saliva in a higher frequency than in individually selected subgingival sites. Saliva has been shown to be a suitable biological fluid as alternative to pooled subgingival plaque samples for detection of oral bacteria such as newly identified Synergistetes.44 The presence of S. tigurinus in the oral cavity was neither influenced by age nor nicotine consumption.41
Clinical manifestation of S. tigurinus
Members of the microbial flora originating from the oral cavity may be involved in the pathogenesis of systemic infections.45 Biofilm formation, complex mechanisms with other bacteria or underlying diseases might play a crucial role in the development of invasive infections. To date, all S. tigurinus isolates detected from clinical human patient samples were causing severe invasive infections. S. tigurinus most frequently caused infective endocarditis (n = 7).13 Over a period of 10 years, 15% of all infective endocarditis cases caused by viridans streptococci were caused by S. tigurinus.13 Other patients developed spondylodiscitis (n = 3), bacteremia (n = 3), prosthetic joint infection (n = 2) and meningitis (n = 1) caused by S. tigurinus.12-14 S. tigurinus was isolated from normally sterile human body sites, e.g., blood, heart valves, cerebrospinal fluid and periarticular joint biopsy specimens. All patients recovered after appropriate antimicrobial therapy, however, 8 out of 16 patients required surgical interventions. S. tigurinus affected not only immunocompromised patients or patients with underlying conditions such as preexisting cardiac morbidities but also healthy young patients without any comorbidities.12-14 To date, a specific risk factor profile for the development of invasive infections with S. tigurinus could not be established yet due to the limited number of patients.
To note, S. tigurinus still might be underreported as causative agent of invasive infections for laboratory diagnostic reasons. Most microbiological diagnostic laboratories rely on identification methods by mass spectrometry, which usually allows rapid identification of bacteria but has a limited discriminative power when analyzing closely related species. Molecular techniques facilitating accurate identification of S. tigurinus are not applicable in every routine microbiological laboratory. Yet, the occurrence of S. tigurinus in causing invasive infections seems geographically unlimited, since in a recent report of Japan S. tigurinus was demonstrated to be the causative agent in 2 cases of infective endocarditis.46
S. tigurinus is mostly fully susceptible to different antimicrobial classes. Susceptibility to penicillin, gentamicin, vancomycin, levofloxacin, erythromycin and clindamycin was demonstrated.13 Some S. tigurinus strains displayed reduced susceptibility or resistance to tetracycline.13 Nevertheless, the antibiotic resistance profile should be determined in each invasive isolate as S. mitis group species are known for bacterial transformation and can acquire multiple genetic elements containing resistance genes.47
Association of S. tigurinus with oral infections
Although more than 700 species were shown to colonize the oral cavity,42 evidence suggests that only a few of them, e.g., Aggregatibacter actinomycetemcomitans or Porphyromonas gingivalis, are associated with the pathogenesis of periodontitis or systemic complications.48,49 Streptococci were found to be more prevalent in healthy individuals, however, S. parasanguinis was proposed to be involved in localized aggressive periodontitis by interaction with the major pathogen A. actinomycetemcomitans.50 Furthermore, S. mitis is overrepresented in endodontic infections,51 the etiology of which likely is polymicrobial.52
To date, it is not yet fully understood whether or not S. tigurinus is more prevalent in individuals with periodontitis or if it is involved in the development of other oral infections. S. tigurinus not only was detected in patients with periodontitis but also in individuals without periodontal diseases.41,53 Earlier studies have demonstrated that S. mitis, which is the closest related species to S. tigurinus, is a predominant early colonizing species of dental biofilms.54 Although S. mitis is not a potent inducer of immune responses, it can antagonize the capacity of A. actinomycetemcomitans (a key pathogen associated with the localized aggressive form of periodontitis occurring in younger individuals) to stimulate IL-8.55 Interaction of S. tigurinus with A. actinomycetemcomitans might be of interest.56 Since its recent identification, it is not clear whether modifying factors are associated with the presence of S. tigurinus in the human oral microbiome and if its detection in the oral cavity has direct clinical implications in systemic diseases.
Pathogenicity of S. tigurinus
Although S. tigurinus is a commensal of the human oral cavity as other members of the S. mitis group, specific virulence factors which allow for entering the bloodstream and causing severe invasive infections, e.g., infective endocarditis, must be present. In a rat model of experimental endocarditis, S. tigurinus was demonstrated to be highly virulent, however, a natural intraspecies variability of different S. tigurinus isolates regarding its pathogenicity potential was observed.57 Most S. tigurinus strains had a more than 10-times higher capacity rate to induce aortic infection in rats than S. gordonii, a well-known endocarditis pathogen.58 Moreover, the infectivity rate was similar to the most aggressive infective endocarditis pathogens, i.e., Staphylococcus aureus and enterococci.57 Several virulence determinants were shown to be present in S. tigurinus. Phagocytosis of S. tigurinus by macrophages significantly was reduced compared to S. gordonii. The ability to resist to phagocytosis is a key attribute of invasive streptococci such as Streptococcus pyogenes.59 Adherence and invasion to endothelial cells, a prerequisite for effective host cell internalization, was enhanced in some S. tigurinus strains as well as induction of platelet aggregation.57 Whole-genome analyses of S. tigurinus were performed to unravel the genetic background of the pathogenic phenotype; genes of known virulence factors such as exfoliative toxin and fibronectin-binding protein, as well as several prophages were identified.60
Small-colony variants of S. tigurinus
The occurrence of small-colony variants (SCVs) in oral bacteria, e.g., viridans streptococci has been rather unknown. There are only a few reports describing S. pneumoniae mucoid variants and SCVs in biofilms.61,62 Recently, a prosthetic joint infection caused by S. tigurinus SCVs was described.14 SCVs of bacteria are a pathogenic life form promoting persistent and recurrent infections.63 SCVs frequently cause infections associated with foreign-body material such as cardiac devices63,64 or prosthetic joints.65 Infection by bacterial SCVs may lead to therapy failure and often makes a definite eradication very difficult.65-67 Therefore, timely diagnosis of these bacterial life variants has a major clinical impact regarding patient management and antibiotic therapy. Morphological and phenotypic characteristics of SCVs are small colony size, atypical colony morphology and reduced growth.63,68 SCVs of viridans streptococci might have been overlooked in the past as even the wild-type (WT) phenotype of viridans streptococci displays tiny colonies. Moreover, overgrowth by the WT phenotype might complicate the isolation of the SCVs. SCVs often display auxotrophy for hemin, menadione or thymidine due to deficiency in electron transport or thymidine biosynthesis.63,68 Morphological and biochemical characteristics of SCVs are extensively studied in staphylococci,63 however, SCVs are found in various genera and species, e.g., enterococci,69 Escherichia coli70 and Pseudomonas aeruginosa.63 The S. tigurinus SCVs displayed very small, pinpointed colonies, had a considerably reduced exponential growth phase compared to the WT and showed either a very stable or a fluctuating SCV phenotype.14 The unstable SCV phenotype characterized by a switch from SCVs to revertant normal colonies after a few passages was first recognized in staphylococci.64 In the S. tigurinus SCVs, no auxotrophy for hemin, menadione or thymidine was detected. The ultrastructure of the S. tigurinus SCVs were characterized in-depth by transmission electron microscopy analyses showing major alterations in cell separation and morphological abnormalities.14 Autolysis of S. tigurinus SCVs was impaired in such the SCVs displayed an increased spontaneous autolysis compared to the WT but an unexpectedly reduced autolysis under induction with Triton X-100, which is a potent autolysis inducer.14 Whole-genome sequencing revealed mutations in genes involved in general cell metabolism, cell division, stringent response and virulence, which might partially explain the S. tigurinus SCV phenotype.14
S. pneumoniae SCVs were identified in biofilms,61 however, whether or not S. tigurinus is able to develop a SCV phenotype under such circumstances is not yet known. Biofilm formation of SCV bacteria with specialized functions has been suggested to be a survival strategy, enabling tolerance to a wide variety of environmental conditions.71 Oral bacteria forming subgingival biofilm communities may lead to development of periodontitis. In the absence of the red complex (Treponema denticola, P. gingivalis and Tannerella forsythia), S. oralis was found to dominate the biofilm composition in in-vitro models.72 Hence in the absence of the red complex, S. oralis may display more decisive virulence characteristics within a mature biofilm community. S. oralis is a very closely related species of S. tigurinus, however, future investigations are warranted to prove if S. tigurinus has a similar behavior in subgingival biofilm formation.
Conclusions
S. tigurinus is detected as part of the oral microbiota, including patients with periodontal infection. Still, a cause-effect relationship in oral infections cannot be established by the limited data available. It further needs to be established if S. tigurinus can be part of pathogenic oral biofilms, how it associates with other members of the oral microbiota, and whether it is a potent inducer of pathogenic host responses. Whichever the case, however, its presence in the oral cavity and its association with endocarditis warrants special attention for a link between oral and systemic infection.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiol 2003; 149(Pt 2):279-94; http://dx.doi.org/ 10.1099/mic.0.26082-0 [DOI] [PubMed] [Google Scholar]
- 2. Albandar JM. Underestimation of periodontitis in NHANES surveys. J Periodontol 2011; 82(3):337-41; PMID:21214340; http://dx.doi.org/ 10.1902/jop.2011.100638 [DOI] [PubMed] [Google Scholar]
- 3. Konig J, Holtfreter B, Kocher T. Periodontal health in Europe: future trends based on treatment needs and the provision of periodontal services–position paper 1. Eur J Dent Educ 2010; 14(Suppl 1):4-24; PMID:20415972; http://dx.doi.org/ 10.1111/j.1600-0579.2010.00620.x [DOI] [PubMed] [Google Scholar]
- 4. Schaudinn C, Gorur A, Keller D, Sedghizadeh PP, Costerton JW. Periodontitis: an archetypical biofilm disease. Journal of the American Dental Association 2009; 140(8):978-86; PMID:19654249; http://dx.doi.org/ 10.14219/jada.archive.2009.0307 [DOI] [PubMed] [Google Scholar]
- 5. Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev 2002; 15(4):613-30; PMID:12364372; http://dx.doi.org/ 10.1128/CMR.15.4.613-630.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith EG, Spatafora GA. Gene regulation in S. mutans: complex control in a complex environment. J Dent Res 2012; 91(2):133-41; PMID:21743034; http://dx.doi.org/ 10.1177/0022034511415415 [DOI] [PubMed] [Google Scholar]
- 7. Fisher LE, Russell RR. The isolation and characterization of milleri group streptococci from dental periapical abscesses. J Dent Res 1993; 72(8):1191-3; PMID:8360361; http://dx.doi.org/ 10.1177/00220345930720080501 [DOI] [PubMed] [Google Scholar]
- 8. Burton JP, Drummond BK, Chilcott CN, Tagg JR, Thomson WM, Hale JD, Wescombe PA. Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J Med Microbiol 2013; 62(Pt 6):875-84; PMID:23449874; http://dx.doi.org/ 10.1099/jmm.0.056663-0 [DOI] [PubMed] [Google Scholar]
- 9. Rossetti V, Ammann TW, Thurnheer T, Bagheri HC, Belibasakis GN. Phenotypic diversity of multicellular filamentation in oral streptococci. PLoS One 2013; 8(9):e76221; PMID:24086713; http://dx.doi.org/ 10.1371/journal.pone.0076221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spellerberg B, Brandt C. Streptococcus. In: Versalovic J, Carroll KC, Jorgensen JH, Funke G, Landry ML, Warnock DW, eds. Manual of clinical microbiology. Washington DC: American Society for Microbiology; 2011:331-49. [Google Scholar]
- 11. Hoen B, Alla F, Selton-Suty C, Beguinot I, Bouvet A, Briancon S, Casalta JP, Danchin N, Delahaye F, Etienne J, et al. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA 2002; 288(1):75-81; PMID:12090865; http://dx.doi.org/ 10.1001/jama.288.1.75 [DOI] [PubMed] [Google Scholar]
- 12. Zbinden A, Mueller NJ, Tarr PE, Sproer C, Keller PM, Bloemberg GV. Streptococcus tigurinus sp. nov., isolated from blood of patients with endocarditis, meningitis and spondylodiscitis. Int J Syst Evol Microbiol 2012; 62:2941-5; PMID:22357776; http://dx.doi.org/ 10.1099/ijs.0.038299-0 [DOI] [PubMed] [Google Scholar]
- 13. Zbinden A, Mueller NJ, Tarr PE, Eich G, Schulthess B, Bahlmann AS, Keller PM, Bloemberg GV. Streptococcus tigurinus, a novel member of the Streptococcus mitis group, causes invasive infections. J Clin Microbiol 2012; 50(9):2969-73; PMID:22760039; http://dx.doi.org/ 10.1128/JCM.00849-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zbinden A, Quiblier C, Hernandez D, Herzog K, Bodler P, Senn MM, Gizard Y, Schrenzel J, Francois P. Characterization of Streptococcus tigurinus small-colony variants causing prosthetic joint infection by comparative whole-genome analyses. J Clin Microbiol 2014; 52(2):467-74; PMID:24478475; http://dx.doi.org/ 10.1128/JCM.02801-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bosshard PP, Kronenberg A, Zbinden R, Ruef C, Boettger EC, Altwegg M. Etiologic diagnosis of infective endocarditis by broad-range polymerase chain reaction: a 3-year experience. Clin Infect Dis 2003; 37(2):167-72; PMID:12856207; http://dx.doi.org/ 10.1086/375592 [DOI] [PubMed] [Google Scholar]
- 16. Facklam RR. Physiological differentiation of viridans streptococci. J Clin Microbiol 1977; 5(2):184-201; PMID:845245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kiratisin P, Li L, Murray PR, Fischer SH. Use of housekeeping gene sequencing for species identification of viridans streptococci. Diagn Microbiol Infect Dis 2005; 51(4):297-301; PMID:15808322; http://dx.doi.org/ 10.1016/j.diagmicrobio.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 18. Tung SK, Teng LJ, Vaneechoutte M, Chen HM, Chang TC. Identification of species of Abiotrophia, Enterococcus, Granulicatella and Streptococcus by sequence analysis of the ribosomal 16S-23S intergenic spacer region. J Med Microbiol 2007; 56(Pt 4):504-13; PMID:17374892; http://dx.doi.org/ 10.1099/jmm.0.47027-0 [DOI] [PubMed] [Google Scholar]
- 19. Hoshino T, Fujiwara T, Kilian M. Use of phylogenetic and phenotypic analyses to identify nonhemolytic streptococci isolated from bacteremic patients. J Clin Microbiol 2005; 43(12):6073-85; PMID:16333101; http://dx.doi.org/ 10.1128/JCM.43.12.6073-6085.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tong H, Gao X, Dong X. Streptococcus oligofermentans sp. nov., a novel oral isolate from caries-free humans. Int J Syst Evol Microbiol 2003; 53(Pt 4):1101-4; PMID:12892133; http://dx.doi.org/ 10.1099/ijs.0.02493-0 [DOI] [PubMed] [Google Scholar]
- 21. Camelo-Castillo A, Benitez-Paez A, Belda-Ferre P, Cabrera-Rubio R, Mira A. Streptococcus dentisani sp. nov., a novel member of the mitis group. Int J Syst Evol Microbiol 2014; 64(Pt 1):60-5; PMID:24006481; http://dx.doi.org/ 10.1099/ijs.0.054098-0 [DOI] [PubMed] [Google Scholar]
- 22. Huch M, De Bruyne K, Cleenwerck I, Bub A, Cho GS, Watzl B, Snauwaert I, Franz CM, Vandamme P. Streptococcus rubneri sp. nov., isolated from the human throat. Int J Syst Evol Microbiol 2013; 63(Pt 11):4026-32; PMID:23749274; http://dx.doi.org/ 10.1099/ijs.0.048538-0 [DOI] [PubMed] [Google Scholar]
- 23. Martin V, Manes-Lazaro R, Rodriguez JM, Maldonado-Barragan A. Streptococcus lactarius sp. nov., isolated from breast milk of healthy women. Int J Syst Evol Microbiol 2011; 61(Pt 5):1048-1052; PMID:20511454; http://dx.doi.org/ 10.1099/ijs.0.021642-0 [DOI] [PubMed] [Google Scholar]
- 24. Douglas CW, Heath J, Hampton KK, Preston FE. Identity of viridans streptococci isolated from cases of infective endocarditis. J Med Microbiol 1993; 39(3):179-82; PMID:8366515; http://dx.doi.org/ 10.1099/00222615-39-3-179 [DOI] [PubMed] [Google Scholar]
- 25. Arbique JC, Poyart C, Trieu-Cuot P, Quesne G, Carvalho Mda G, Steigerwalt AG, Morey RE, Jackson D, Davidson RJ, Facklam RR. Accuracy of phenotypic and genotypic testing for identification of Streptococcus pneumoniae and description of Streptococcus pseudopneumoniae sp. nov. J Clin Microbiol 2004; 42(10):4686-96; PMID:15472328; http://dx.doi.org/ 10.1128/JCM.42.10.4686-4696.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bosshard PP, Abels S, Altwegg M, Boettger EC, Zbinden R. Comparison of conventional and molecular methods for identification of aerobic catalase-negative Gram-positive cocci in the clinical laboratory. J Clin Microbiol 2004; 42(5):2065-73; PMID:15131171; http://dx.doi.org/ 10.1128/JCM.42.5.2065-2073.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Veen SQ, Claas EC, Kuijper EJ. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J Clin Microbiol 2010: 48(3):900-7; PMID:20053859; http://dx.doi.org/ 10.1128/JCM.02071-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferroni A, Suarez S, Beretti JL, Dauphin B, Bille E, Meyer J, Bougnoux ME, Alanio A, Berche P, Nassif X. Real-time identification of bacteria and Candida species in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 2010; 48(5):1542-8; PMID:20237092; http://dx.doi.org/ 10.1128/JCM.02485-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schulthess B, Brodner K, Bloemberg GV, Zbinden R, Boettger EC, Hombach M. Identification of Gram-positive cocci by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry: comparison of different preparation methods and implementation of a practical algorithm for routine diagnostics. J Clin Microbiol 2013; 51(6):1834-40; PMID:23554198; http://dx.doi.org/ 10.1128/JCM.02654-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Werno AM, Christner M, Anderson TP, Murdoch DR. Differentiation of Streptococcus pneumoniae from nonpneumococcal streptococci of the Streptococcus mitis group by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 2012; 50(9):2863-7; PMID:22718935; http://dx.doi.org/ 10.1128/JCM.00508-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Branda JA, Markham RP, Garner CD, Rychert JA, Ferraro MJ. Performance of the Vitek MS v2.0 system in distinguishing Streptococcus pneumoniae from nonpneumococcal species of the Streptococcus mitis group. J Clin Microbiol 2013; 51(9):3079-82; PMID:23784130; http://dx.doi.org/ 10.1128/JCM.00824-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boettger EC. Approaches for identification of microorganism. ASM news 1996; 61:247-50. [Google Scholar]
- 33. Clarridge JE, 3rd. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 2004; 17(4):840-62, table of contents; PMID:15489351; http://dx.doi.org/ 10.1128/CMR.17.4.840-862.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rogall T, Flohr T, Boettger EC. Differentiation of Mycobacterium species by direct sequencing of amplified DNA. J Gen Microbiol 1990; 136(9):1915-20; PMID:2283506; http://dx.doi.org/ 10.1099/00221287-136-9-1915 [DOI] [PubMed] [Google Scholar]
- 35. Kawamura Y, Hou XG, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol 1995; 45(2):406-8; PMID:7537076; http://dx.doi.org/ 10.1099/00207713-45-2-406 [DOI] [PubMed] [Google Scholar]
- 36. Kawamura Y, Whiley RA, Shu SE, Ezaki T, Hardie JM. Genetic approaches to the identification of the mitis group within the genus Streptococcus. Microbiology 1999; 145(Pt 9):2605-13; PMID:10517614. [DOI] [PubMed] [Google Scholar]
- 37. Poyart C, Quesne G, Coulon S, Berche P, Trieu-Cuot P. Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J Clin Microbiol 1998; 36(1):41-7; PMID:9431917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Drancourt M, Roux V, Fournier PE, Raoult D. rpoB gene sequence-based identification of aerobic Gram-positive cocci of the genera Streptococcus, Enterococcus, Gemella, Abiotrophia, and Granulicatella. J Clin Microbiol 2004; 42(2):497-504; PMID:14766807; http://dx.doi.org/ 10.1128/JCM.42.2.497-504.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glazunova OO, Raoult D, Roux V. Partial sequence comparison of the rpoB, sodA, groEL and gyrB genes within the genus Streptococcus. Int J Syst Evol Microbiol 2009; 59(Pt 9):2317-22; PMID:19620365; http://dx.doi.org/ 10.1099/ijs.0.005488-0 [DOI] [PubMed] [Google Scholar]
- 40. Zbinden A, Kohler N, Bloemberg GV. A recA based PCR assay for accurate differentiation of Streptococcus pneumoniae from other viridans streptococci. J Clin Microbiol 2011; 49:523-7; PMID:21147955; http://dx.doi.org/ 10.1128/JCM.01450-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zbinden A, Aras F, Zbinden R, Mouttet F, Schmidlin PR, Bloemberg GV, Bostanci N. Frequent detection of Streptococcus tigurinus in the human oral microbial flora by a specific 16S rRNA gene real-time TaqMan PCR. BMC Microbiol 2014; 14(1):231. [Epub ahead of print]; PMID:25170686; http://dx.doi.org/ 10.1186/s12866-014-0231-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005; 43(11):5721-32; PMID:16272510; http://dx.doi.org/ 10.1128/JCM.43.11.5721-5732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol 2010; 192(19):5002-17; PMID:20656903; http://dx.doi.org/ 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Belibasakis GN, Ozturk VO, Emingil G, Bostanci N. Synergistetes cluster A in saliva is associated with periodontitis. J Periodontal Res 2013; 48(6):727-32; PMID:23441995. [DOI] [PubMed] [Google Scholar]
- 45. Deshpande RG, Khan M, Genco CA. Invasion strategies of the oral pathogen Porphyromonas gingivalis: implications for cardiovascular disease. Invasion Metastasis 1998; 18(2):57-69; PMID:10364686; http://dx.doi.org/ 10.1159/000024499 [DOI] [PubMed] [Google Scholar]
- 46. Miyazato A, Ohkusu K, Tachi Y, Hashikita G, Ezaki T, Mitsutake K. Two cases of infective endocarditis caused by Streptococcus tigurinus. Kansenshogaku Zasshi 2014; 88(3):304-6; PMID:24974456 [DOI] [PubMed] [Google Scholar]
- 47. Bek-Thomsen M, Poulsen K, Kilian M. Occurrence and evolution of the paralogous zinc metalloproteases IgA1 protease, ZmpB, ZmpC, and ZmpD in Streptococcus pneumoniae and related commensal species. MBio 2012; 3(5):e00303-12; PMID:23033471; http://dx.doi.org/ 10.1128/mBio.00303-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Papapanou PN, Behle JH, Kebschull M, Celenti R, Wolf DL, Handfield M, Pavlidis P, Demmer RT. Subgingival bacterial colonization profiles correlate with gingival tissue gene expression. BMC Microbiol 2009; 9:221; PMID:19835625; http://dx.doi.org/ 10.1186/1471-2180-9-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Socransky SS, Haffajee AD. Implications of periodontal microbiology for the treatment of periodontal infections. Compend Suppl 1994; (18):S684-685, 688-693; quiz S714-687. [PubMed] [Google Scholar]
- 50. Fine DH, Markowitz K, Fairlie K, Tischio-Bereski D, Ferrendiz J, Furgang D, Paster BJ, Dewhirst FE. A consortium of Aggregatibacter actinomycetemcomitans, Streptococcus parasanguinis, and Filifactor alocis is present in sites prior to bone loss in a longitudinal study of localized aggressive periodontitis. J Clin Microbiol 2013; 51(9):2850-61; PMID:23784124; http://dx.doi.org/ 10.1128/JCM.00729-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hsiao WW, Li KL, Liu Z, Jones C, Fraser-Liggett CM, Fouad AF. Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics 2012; 13:345; PMID:22839737; http://dx.doi.org/ 10.1186/1471-2164-13-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. do Cabo Fernandes C, Rechenberg DK, Zehnder M, Belibasakis GN. Identification of Synergistetes in endodontic infections. Microb Pathogenesis 2014; 73C:1-6; http://dx.doi.org/ 10.1016/j.micpath.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 53. Dhotre SV, Mehetre GT, Dharne MS, Suryawanshi NM, Nagoba BS. Isolation of Streptococcus tigurinus - a novel member of Streptococcus mitis group from a case of periodontitis. FEMS Microbiol Lett 2014; 357(2):131-5; PMID:24974898. [DOI] [PubMed] [Google Scholar]
- 54. Li J, Helmerhorst EJ, Leone CW, Troxler RF, Yaskell T, Haffajee AD, Socransky SS, Oppenheim FG. Identification of early microbial colonizers in human dental biofilm. J Appl Microbiol 2004; 97(6):1311-8; PMID:15546422; http://dx.doi.org/ 10.1111/j.1365-2672.2004.02420.x [DOI] [PubMed] [Google Scholar]
- 55. Sliepen I, Van Damme J, Van Essche M, Loozen G, Quirynen M, Teughels W. Microbial interactions influence inflammatory host cell responses. J Dent Res 2009; 88(11):1026-30; PMID:19828891; http://dx.doi.org/ 10.1177/0022034509347296 [DOI] [PubMed] [Google Scholar]
- 56. Ennibi OK, Benrachadi L, Bouziane A, Haubek D, Poulsen K. The highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans in localized and generalized forms of aggressive periodontitis. Acta Odontol Scand 2012; 70(4):318-22; PMID:22251014; http://dx.doi.org/ 10.3109/00016357.2011.642002 [DOI] [PubMed] [Google Scholar]
- 57. Veloso TR, Zbinden A, Andreoni F, Giddey M, Vouillamoz J, Moreillon P, Zinkernagel AS, Entenza JM. Streptococcus tigurinus is highly virulent in a rat model of experimental endocarditis. Int J Med Microbiol 2013; 303(8):498-504; PMID:23856340; http://dx.doi.org/ 10.1016/j.ijmm.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 58. Bizzini A, Beggah-Moller S, Moreillon P, Entenza JM. Lack of in vitro biofilm formation does not attenuate the virulence of Streptococcus gordonii in experimental endocarditis. FEMS Immunol Med Microbiol 2006; 48(3):419-23; PMID:17087816; http://dx.doi.org/ 10.1111/j.1574-695X.2006.00168.x [DOI] [PubMed] [Google Scholar]
- 59. Courtney HS, Hasty DL, Dale JB. Anti-phagocytic mechanisms of Streptococcus pyogenes: binding of fibrinogen to M-related protein. Mol Microbiol 2006; 59(3):936-47; PMID:16420362; http://dx.doi.org/ 10.1111/j.1365-2958.2005.04977.x [DOI] [PubMed] [Google Scholar]
- 60. Gizard Y, Zbinden A, Schrenzel J, Francois P. Whole-genome sequences of Streptococcus tigurinus type strain AZ_3a and S. tigurinus 1366, a strain causing prosthetic joint infection. Genome Announcements 2013; 1(2):e00210-2; PMID:23640198; http://dx.doi.org/ 10.1128/genomeA.00210-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Allegrucci M, Sauer K. Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms. J Bacteriol 2007; 189(5):2030-8; PMID:17189375; http://dx.doi.org/ 10.1128/JB.01369-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Allegrucci M, Sauer K. Formation of Streptococcus pneumoniae non-phase-variable colony variants is due to increased mutation frequency present under biofilm growth conditions. J Bacteriol 2008; 190(19):6330-9; PMID:18658260; http://dx.doi.org/ 10.1128/JB.00707-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 2006; 4(4):295-305; PMID:16541137; http://dx.doi.org/ 10.1038/nrmicro1384 [DOI] [PubMed] [Google Scholar]
- 64. Maduka-Ezeh A, Seville MT, Kusne S, Vikram HR, Blair JE, Greenwood-Quaintance K, Arabia F, Patel R. Thymidine auxotrophic Staphylococcus aureus small-colony variant endocarditis and left ventricular assist device infection. J Clin Microbiol 2012; 50(3):1102-5; PMID:22205806; http://dx.doi.org/ 10.1128/JCM.01170-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sendi P, Rohrbach M, Graber P, Frei R, Ochsner PE, Zimmerli W. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin Infect Dis 2006; 43(8):961-7; PMID:16983605; http://dx.doi.org/ 10.1086/507633 [DOI] [PubMed] [Google Scholar]
- 66. Vaudaux P, Kelley WL, Lew DP. Staphylococcus aureus small colony variants: difficult to diagnose and difficult to treat. Clin Infect Dis 2006; 43(8):968-70; PMID:16983606; http://dx.doi.org/ 10.1086/507643 [DOI] [PubMed] [Google Scholar]
- 67. von Eiff C, Becker K, Metze D, Lubritz G, Hockmann J, Schwarz T, Peters G. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with darier's disease. Clin Infect Dis 2001; 32(11):1643-7; PMID:11340539; http://dx.doi.org/ 10.1086/320519 [DOI] [PubMed] [Google Scholar]
- 68. Kahl BC, Belling G, Reichelt R, Herrmann M, Proctor RA, Peters G. Thymidine-dependent small-colony variants of Staphylococcus aureus exhibit gross morphological and ultrastructural changes consistent with impaired cell separation. J Clin Microbiol 2003; 41(1):410-3; PMID:12517881; http://dx.doi.org/ 10.1128/JCM.41.1.410-413.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Groebner S, Beck J, Schaller M, Autenrieth IB, Schulte B. Characterization of an Enterococcus faecium small-colony variant isolated from blood culture. Int J Med Microbiol 2012; 302(1):40-4; PMID:21968291; http://dx.doi.org/ 10.1016/j.ijmm.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 70. Sendi P, Frei R, Maurer TB, Trampuz A, Zimmerli W, Graber P. Escherichia coli variants in periprosthetic joint infection: diagnostic challenges with sessile bacteria and sonication. J Clin Microbiol 2010; 48(5):1720-5; PMID:20335421; http://dx.doi.org/ 10.1128/JCM.01562-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Boles BR, Thoendel M, Singh PK. Self-generated diversity produces "insurance effects" in biofilm communities. Proc Natl Acad Sci USA 2004; 101(47):16630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Thurnheer T, Belibasakis GN, Bostanci N. Colonisation of gingival epithelia by subgingival biofilms in vitro: Role of "red complex" bacteria. Arch Oral Biol 2014; 59(9):977-86; PMID:24949828; http://dx.doi.org/ 10.1016/j.archoralbio.2014.05.023 [DOI] [PubMed] [Google Scholar]