Abstract
Background:
Psoriasis is a chronic inflammatory disease driven by exaggerated production of pro-inflammatory cytokines and interleukins. Various genetic polymorphisms including IL-1 are implicated in pathogenesis of psoriasis. The exact role of IL-1 gene polymorphisms and their interaction with NFκB is not yet determined. We aimed to study various genetic polymorphisms of IL-1 in psoriasis and their influence on NFκB and histopathological features.
Materials and Methods:
112 newly diagnosed cases of psoriasis vulgaris were included in this prospective study. Histology was done on sections and genotyping was done for the IL-1β and IL-1 receptor antagonist (IL-1RA) genetic polymorphisms. In addition, NFκB immunostaining was performed on 89 sections and the intensity of staining was evaluated in the epidermis, basal cells, and the lymphocytes.
Results:
A strong association of IL-1β 511 C/T polymorphism was found with both genotypes and alleles in psoriasis. A strong correlation was also detected between the IL-1β genotype and the grade of NFκB immunostaining in the epidermis (P = 0.012). The grade of NFκB lymphocyte staining showed a strong correlation with the IL-1RA genotype (P = 0.025) but not with the IL-1β genotype (P = 0.226). The genetic polymorphisms did not show any correlation with the histological features.
Conclusions:
IL-1 genetic polymorphisms may not play a very direct role in pathogenesis of psoriasis. However, their interaction with NFκB appears to be a significant factor in this direction as NFκB is activated by pro-inflammatory genetic polymorphisms and therefore may influence the severity of psoriasis.
Keywords: Genetic polymorphisms, histopathology, IL-1, psoriasis
What was known?
IL-1and NFκB are known to play important roles in pathogenesis of psoriasis.
IL-1 gene polymorphisms have important roles in various inflammatory diseases.
Introduction
Psoriasis is a chronic inflammatory disease affecting about 1–3% of Caucasian population.[1] Tissue alterations seen in psoriasis are driven by exaggerated production of pro-inflammatory cytokines such as TNFα, IFNγ, IL-1β, IL-8, and IL-12.[2] Anti-inflammatory factors such as IL-10 and 1L-1RA are relatively decreased, thus, creating an imbalance between pro and anti-inflammatory mediators.[3,4,5,6,7]
Cytokine gene polymorphisms alter gene transcription and thereby influence inflammatory processes in response to various diseases.[8,9,10,11] Some important cytokines that are known to harbor polymorphic regions are genes of IL-1 cluster and TNF-α. Three di-allelic polymorphisms in IL-1β have been reported, all representing C–T base transitions at positions -511, -31, and + 3954 (base pairs) from the transcription initiation site. Of these, the 511 C–T transition is considered the most important.
NFκB regulates the expression of an exceptionally large number of genes and has a well-characterized role in immune and inflammatory responses as well as in protection against apoptotic cell death.[12,13,14,15,16] NFκB is one of the most important regulators of pro-inflammatory gene expression by synthesizing cytokines such as TNF-α, IL-1β, IL-6, and IL-8 and is one of the key factors involved in pathogenesis of the disease.
Knowing the role of IL-1 polymorphisms in various inflammatory diseases, we aimed to study how polymorphisms in these genes influence the course of psoriasis.[17,18,19,20] Considering the central role of NFκB in regulating cytokine gene expression, we also looked for the expression of NFκB in the tissues by immunohistochemistry. We correlated these two factors with various histopathological parameters.
Materials and Methods
This study included 112 freshly detected patients with psoriasis vulgaris presenting to the hospital over duration of 5 years between 2007 and 2012. The patients had not received any kind of systemic therapy before biopsy procedure. Five milliliter of peripheral blood was collected to study IL-1β and IL-1 RA genetic polymorphisms. The genetic polymorphisms in these 112 patients (224 alleles) were compared with 243 healthy blood donors (486 alleles) who were age and sex matched. The study was conducted in accordance with principles of the Helsinki Declaration and was approved by the institutional ethics committee.
Biopsies were taken from the youngest lesion and sections were stained with Hematoxylin and eosin stain (H and E). Histopathological analysis of the biopsies was done and included seven criteria (epithelial hyperplasia, parakeratosis, Munro's, and Kogoj's microabscesses, suprapapillary thinning, inflammatory infiltrate and widened rete ridges). Grading was done using a visual analog scale and the biopsies were graded as 0 to 3 (nil to marked) for all these characteristics.
Eighty-nine patients were analyzed for NFκB expression. Serial 4 mm thick sections were mounted on poly-L lysine coated slides. Paraffin sections were immersed in xylene for 5 min and hydrated using a gradient series of alcohol. Antigen retrieval was performed by standard techniques using citric acid buffer (pH 6.0) in a microwave oven for 15 min. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min and then incubated with a primary antibody in a humidified chamber overnight. Primary antibody used was monoclonal mouse anti- NFκB antibody (Santa Cruz Biotechnology, Inc, Dallas USA) at 1:200 dilution. Localization of immunohistochemical staining was classified as epidermal (suprabasal), basal cell, and lymphocyte staining.
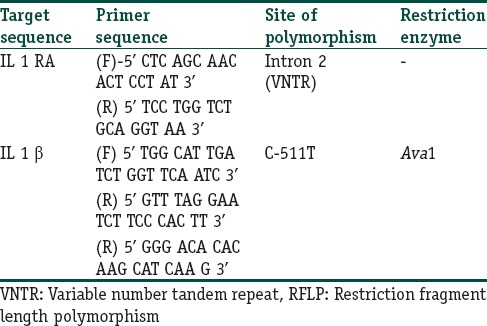
Genomic DNA was retrieved from the peripheral blood by using a standard kit (Quiagen GmBH, Netherlands). The IL-1 RA intron 2 polymorphism was analyzed by Polymerase chain reaction (PCR). The procedure for all the PCR reactions was standardized in the laboratory. Five alleles were characterized based upon the number of repeats. Allele 1 (four repeats) was 410 bp, allele 2 (two repeats) was 240 bp, allele 3 (five repeats) was 500 bp, allele 4 (three repeats) was 325 bp and allele 5 (six repeats) was 595 bp, in length. Allele 2 was the pro-inflammatory allele.
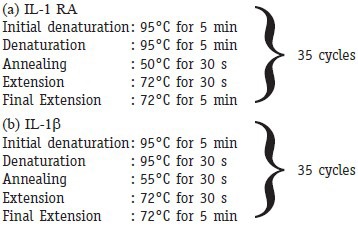
For Restriction fragment length (RFLP) analysis of the -511 position IL-1β polymorphism, the PCR products were first amplified (PCR standardised in laboratory). The confirmation of a successful PCR was done by running the products on a 2% agarose gel. If the products were present, they were digested with 10U of Ava1 at 37°C for 3 hrs. Fragments were analyzed by electrophoresis on 3% agarose gel and stained with ethidium bromide. This yielded products of 304 bp (TT allele), 190 bp and 104 bp (CC allele) and 304, 190, and 104 bp (CT allele). The primer sequences are given in Table 1. All PCR reactions were performed in an Eppendorf Thermal Cycler (MJ Research Inc, Waltham MA).
Table 1.
The primer sequences, site of polymorphism and the restriction enzymes used in the RFLP
PCR reaction conditions
Statistical analysis
The results of study were statistically analyzed using SPSS 14.0 (Statistical Package for Social Sciences, Lead Technologies Inc, USA). The descriptive data for quantitative variables were given as mean and standard deviation. Chi square test was used for detecting association. For analyzing the correlation between the gene polymorphisms and histopathology/immunohistochemistry results, Spearman's rho test was calculated. A value of P < 0.05 was considered to be statistically significant.
Results
One hundred and twelve cases of psoriasis were analyzed in this study. The mean age was 38.9 years with a range of 13 to 76 years. Sixty three males and 49 females comprised the study population.
IL-1 RA polymorphism
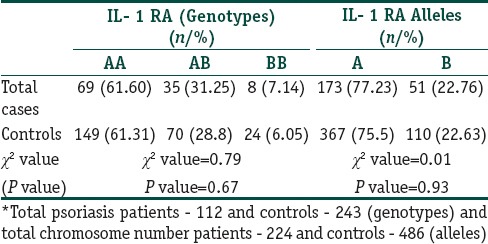
Association of genetic polymorphisms: The distribution of alleles A, pro- inflammatory allele B, genotypes AA, BB, and AB in both cases and controls were as given in Table 2. In controls, all the frequencies of tested genotypes and alleles were in Hardy–Weinberg equilibrium. There was no association between different IL-1 RA alleles among cases and controls (P = 0.93) as also between IL-1 RA genotypes (P = 0.67).
Table 2.
Frequency of IL- 1 RA genotypes and allelic polymorphisms among psoriasis
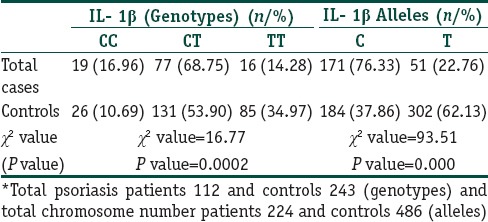
IL-1β polymorphism: Association of genetic polymorphisms
The distribution of allele C, pro- inflammatory allele T, genotypes CC, CT, and TT in both cases and controls are as given in Table 3. In controls, all the frequencies of tested genotypes and alleles were in Hardy–Weinberg equilibrium. There was a strong association of IL-1β -511C/T polymorphism with psoriasis of both genotypes and alleles as the frequencies of genotypes were significantly different between patients and controls.
Table 3.
Frequency of IL 1β genotypes and allelic polymorphisms among psoriasis
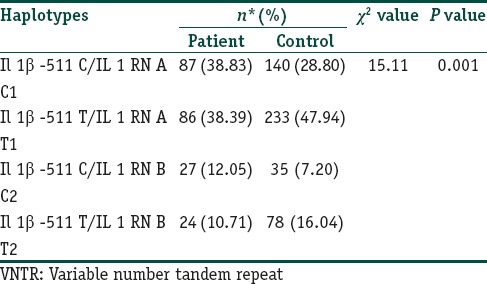
Haplotype estimation
Four different haplotypes C1, C2, T1, and T2 were assessed based on the genotyping results. Odds ratio for risk estimation was calculated only for those haplotypes that had frequency > 10%. All the controls were in Hardy Weinberg equilibrium. IL-1β-511*C-IL-1RN*1 (C1) and the IL-1β -511*C-IL-1RN*2 (C2) haplotype were associated with a predisposition for psoriasis [Table 4]. The IL-1B -511*T-IL-1RN*1 (T1) and the IL-1B -511*T-IL-1RN*2 (T2) were associated with a protection against the development of psoriasis.
Table 4.
Haplotype frequency distribution of IL-1B (-511 C/T) and IL-1RA (VNTR) in patients and controls
Association of Genetic polymorphisms with NFκB immunostaining
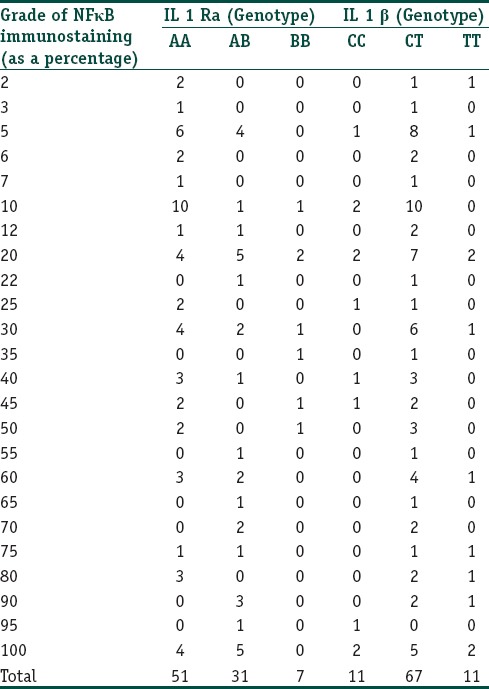
Epidermal nuclear positivity for NFκB and correlation with genetic polymorphisms: The percentage of epidermal cells (suprabasal) showing a nuclear staining for NFκB ranged from 0% to 90% with an average of 16.95%. There was a strong correlation between the IL-1β genotype and the grade of NFκB epidermal immunostaining (P = 0.012, Spearmans rho 0.050). There was no correlation between the IL-1RA genotype and the grade of epidermal nuclear immunostaining (P = 0.970, Spearmans rho - 0.211). Data are presented in Table 5.
Basal cell positivity for NFκB and correlation with genetic polymorphisms: The percentage of basal cells showing a nuclear staining for NFκB ranged from 0 to 100 with an average of 37.95%. There was no correlation between the IL-1 RA and IL-1β genotypes with the grade of basal cell immunostaining (Spearmans rho - 0.320 and 0.857 respectively). Data are presented in Table 6.
Lymphocyte staining: The percentage of lymphocytes in the dermis showing a cytoplasmic staining for NFκB ranged from 2 to 100 with an average of 38.39%. The grade of lymphocyte staining showed a strong correlation with the IL-1 RA genotype (P = 0.025, Spearmans rho - 0.037) but not with the IL-1β genotype (P = 0.226, Spearmans rho - 0.622). Data are presented in Table 7.
Table 5.
Strength of association has already been done in the previous paragraph with the Pearsons and Spearmans tests
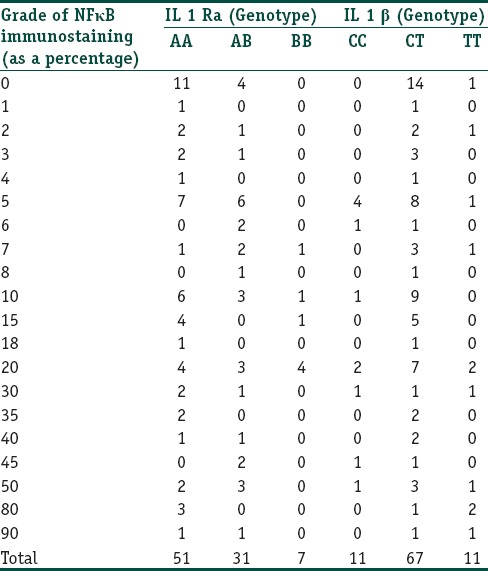
Table 6.
The correlation between the IL-1RA and IL-1β genotypes and the grade of basal cell positivity for NFκB
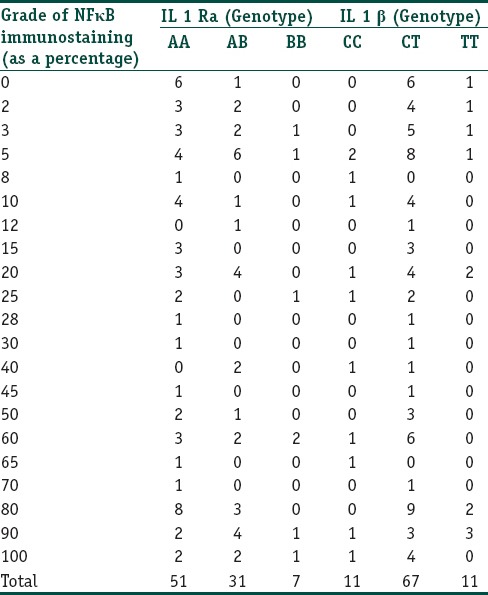
Table 7.
The correlation between the IL-1RA and IL-1β genotypes and the grade of lymphocyte positivity for NFκB
Association of gene polymorphisms with histological features
Biopsies taken from the lesional skin were evaluated using seven criteria as outlined previously. Of the seven factors, epidermal hyperplasia, inflammatory infiltrate, and capillary dilatation were considered the most relevant in the pathogenesis of psoriasis. There was no association seen between the IL-1 RA and IL-1β genotypes with the histopathological severity of psoriasis [Tables 8 and 9].
Table 8.
The association between the IL-1RA genotypes and the histopathological indicators of severity
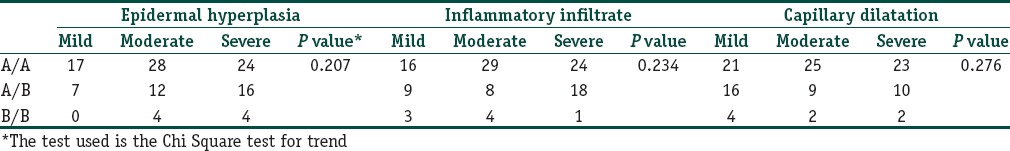
Table 9.
The association between the IL-1β genotypes and the histopathological indicators of severity
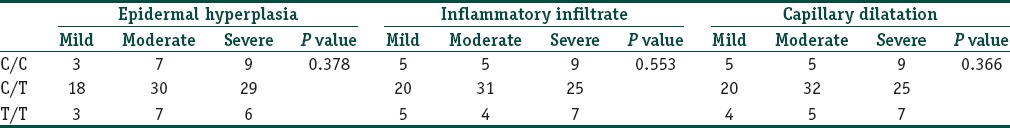
Discussion
The IL-1 gene cluster on chromosome 2q contains three related genes within a 430 kb region. The genes are IL-1α, IL-1β and the IL-1 RN gene, which code for the proinflammatory cytokines IL-1α, IL-1β as well as their endogenous receptor antagonist, IL-1 RA, respectively. Three di-allelic polymorphisms in IL-1β have been reported, all representing C–T base transitions at positions –511, –31, and + 3954 base pairs from the transcriptional start site.[21,22] The IL-1RA gene has a penta-allelic polymorphic site in intron two containing variable numbers of an 86 bp tandem repeat sequence.[23] There are conflicting data regarding the functional effect of these polymorphisms on IL-1β production.[10,22,24]
In this study, we found that there was no association between the IL-1 RA alleles among cases and controls. We also found that there was a strong association between the IL-1β C/C genotype and psoriasis. These findings were replicated when we performed a haplotype analysis and found that the anti inflammatory haplotypes (IL-1β -511 C/IL-1 RA ‘A’) were associated with psoriasis and the pro inflammatory haplotypes (IL-1β -511 T/IL-1 RA ‘B’) was protective for psoriasis. Similarly, in a study addressing genetic polymorphisms in early and late onset psoriasis, Reich et al. found that the C/C genotype was associated with late onset psoriasis.[7] This is an unusual finding since the C/C genotype is supposed to be anti inflammatory and therefore it should logically not be associated with psoriasis. Other studies evaluating IL-1 RA genetic polymorphisms in psoriasis found no association (25) or a decreased frequency of IL-1RN allele 2 in patients with late onset psoriasis (age of onset > 40 y), but the finding was only of borderline significance.[25,26] Essentially, all these studies (including the present study) have demonstrated that an anti-inflammatory IL-1β genotype is more likely to be associated with psoriasis. There are several explanations for this apparent paradox. It would be attractive to hypothesize that the IL- 1β -511 C/T polymorphism is a nonfunctional polymorphism as suggested.[10,22] However, Reich et al. have shown that the IL-1β -511 C/T is functional with the IL-1β -511 C/C genotype producing more IL-1 RA which is an anti-inflammatory cytokine. It has been shown that for some reason, the IL-1β present in the psoriatic skin, though increased in amount in experimental models is functionally inactive.[27] In contrast, other studies have emphasized the importance of IL-1β in psoriatic skin.[28] With the present findings, we would tend to agree with the opinion of most workers who have stated that although the IL-1β -511 C/T genetic polymorphism is a functional polymorphism, it is not a critical gene involved in the pathogenesis of psoriasis.[7,29]
There was a strong correlation between the IL-1β T/T genotype (pro-inflammatory) and the grade of NFκB epidermal nuclear staining. There was also a significant correlation between the IL-1 RA ‘B’ allele (proinflammatory) with the grade of NFκB staining in the lymphocytes. To the best of our knowledge, such a finding has not been reported in the literature. It is known that NFκB is one of the most important regulators of proinflammatory gene expression. It is also known that the synthesis of cytokines, such as TNF-α, IL-1β, IL-6, and IL-8 is mediated by NFκB, as is the expression of cyclooxygenase 2 (Cox-2).[30] The NFκB pathway is well known to be a central pathway in the pathogenesis of psoriasis.[31,32,33] Both the IL-1β -511 T/T genotype and the IL-1 RA ‘B’ allele are pro inflammatory alleles. The fact that there was a correlation between the pro inflammatory genetic polymorphisms and an increased degree of NFκB translocation in the nucleus of the epidermal cells and lymphocytes suggests that the translocation of NFκB occurs more in patients who have pro-inflammatory genotypes. This would mean that patients with pro-inflammatory genotypes would secrete more IL-1β and therefore present with a more severe form of psoriasis. We confirmed this finding by analyzing the grade of NFκB translocation and the inflammatory infiltrate. There was a strong correlation between the NFκB translocation in the epidermis and the inflammatory infiltrate (P = 0.040). This finding again underlies the roles that NFκB and IL-1 gene polymorphisms play in the pathogenesis of psoriasis.
There was no correlation between the genetic polymorphisms and the histopathological indicators of severity. In a previous study Kingo et al. have found a relationship between IL-10 genetic polymorphisms and disease activity.[34] In studies correlating cytokine gene polymorphisms with the severity of gastritis, it has been seen that little or no correlation exists between the gene polymorphisms and the histopathological indicators of severity.[35,36,37,38] The present finding showing a lack of correlation between genetic polymorphisms and histopathology is not surprising considering the fact that the genetic polymorphisms do not necessarily correlate with the transcription rate or translational product of the gene. Our study suggests that NFκB expression is a better indicator of disease activity since it is the prominent molecule which influences cytokine secretion and therefore disease activity.
In conclusion, our study suggests that the IL-1 genetic polymorphisms may not directly be a critical factor in the pathogenesis of psoriasis. However, their interaction with NFκB seems to be a significant factor in the pathogenesis as NFκB appears to be more activated in cases showing pro-inflammatory genetic polymorphisms. This study has attempted to define the roles played by NFκB and IL-1 genetic polymorphisms in the pathogenesis of psoriasis.
What is new?
There is no correlation between IL-1 gene polymorphisms and histopathological indicators of severity in psoriasis.
IL-1 gene polymorphisms do not have a major role in pathogenesis of psoriasis.
NFκB expression is a better indicator of disease activity.
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
References
- 1.Reich K, Westphal G, Schulz T, Müller M, Zipprich S, Fuchs T, et al. Combined analysis of polymorphisms of the tumor necrosis factor-alpha and interleukin-10 promoter regions and polymorphic xenobiotic metabolizing enzymes in psoriasis. J Invest Dermatol. 1999;113:214–20. doi: 10.1046/j.1523-1747.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- 2.Bos JD, De Rie MA. The pathogenesis of psoriasis: Immunological facts and speculations. Immunol Today. 1999;20:40–6. doi: 10.1016/s0167-5699(98)01381-4. [DOI] [PubMed] [Google Scholar]
- 3.Gruaz-Chatellard D, Baumberger C, Saurat JH, Dayer JM. Interleukin 1 receptor antagonist in human epidermis and cultured keratinocytes. FEBS Lett. 1991;294:137–40. doi: 10.1016/0014-5793(91)81360-k. [DOI] [PubMed] [Google Scholar]
- 4.Bigler CF, Norris DA, Weston WL, Arend WP. Interleukin-1 receptor antagonist production by human keratinocytes. J Invest Dermatol. 1992;98:38–44. doi: 10.1111/1523-1747.ep12494196. [DOI] [PubMed] [Google Scholar]
- 5.Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, et al. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: A new therapeutic approach. J Clin Invest. 1998;101:783–94. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nickoloff BJ, Fivenson DP, Kunkel SL, Strieter RM, Turka LA. Keratinocyte interleukin-10 expression is upregulated in tape-stripped skin, poison ivy dermatitis, and Sezary syndrome, but not in psoriatic plaques. Clin Immunol Immunopathol. 1994;73:63–8. doi: 10.1006/clin.1994.1170. [DOI] [PubMed] [Google Scholar]
- 7.Reich K, Mössner R, König IR, Westphal G, Ziegler A, Neumann C. Promoter Polymorphisms of the Genes encoding Tumor Necrosis Factor-a and Interleukin-1b are associated with different subtypes of Psoriasis characterized by early and late disease onset. J Invest Dermatol. 2002;118:155–63. doi: 10.1046/j.0022-202x.2001.01642.x. [DOI] [PubMed] [Google Scholar]
- 8.Mansfield JC, Holden H, Tarlow JK, Di Giovine FS, McDowell TL, Wilson AG, et al. Novel genetic association between ulcerative colitis and the anti inflammatory cytokine interleukin 1 receptor antagonist. Gastroenterology. 1994;106:637–42. doi: 10.1016/0016-5085(94)90696-3. [DOI] [PubMed] [Google Scholar]
- 9.Clay FE, Cork MJ, Tarlow JK, Blakemore AI, Harrington CI, Lewis F, et al. Interleukin 1 receptor antagonist gene polymorphism associated with lichen sclerosus. Hum Genet. 1994;94:407–10. doi: 10.1007/BF00201602. [DOI] [PubMed] [Google Scholar]
- 10.Bidwell J, Keen L, Gallagher G, Kimberly R, Huizinga T, McDermott MF, et al. Cytokine gene polymorphisms in human disease: On line databases. Genes Immun. 1999;1:3–19. doi: 10.1038/sj.gene.6363645. [DOI] [PubMed] [Google Scholar]
- 11.Hurme M, Lahdenpohja N, Santilla S. Gene polymophisms of interleukins 1 and 10 in infectious and autoimmune disease. Ann Med. 1998;30:469–73. doi: 10.3109/07853899809002488. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 13.Barnes PJ, Karin M. Nuclear factor-kappa B: A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 14.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF kappa B. Annu Rev Cell Biol. 1994;10:405–55. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 15.Sonenshein GE. Rel/NF-kappa B transcription factors and the control of apoptosis. Semin Cancer Biol. 1997;8:113–9. doi: 10.1006/scbi.1997.0062. [DOI] [PubMed] [Google Scholar]
- 16.Tak PP, Firestein GS. NF-KB: A key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawashima K, Doi H, Ito Y, Shibata MA, Yoshinaka R, Otsuki Y. Evaluation of cell death and proliferation in psoriatic epidermis. J Dermatol Sci. 2004;35:207. doi: 10.1016/j.jdermsci.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Prens E, t Hooft-Benne K, Tank B, Van Damme J, van Joost T, Benner R. Adhesion molecules and IL-1 costimulate T lymphocytes in the autologous MECLR in psoriasis. Arch Dermatol Res. 1996;288:68–73. doi: 10.1007/BF02505046. [DOI] [PubMed] [Google Scholar]
- 19.Wei L, Debets R, Hegmans JJ, Benner R, Prens EP. IL-1 beta and IFN-gamma induce the regenerative epidermal phenotype of psoriasis in the transwell skin organ culture system. IFN-gamma up-regulates the expression of keratin 17 and keratinocyte transglutaminase via endogenous IL-1 production. J Pathol. 1999;187:358–64. doi: 10.1002/(SICI)1096-9896(199902)187:3<358::AID-PATH253>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Schon M, Behmenburg C, Denzer D, Schon MP. Pathogenic function of IL-1 beta in psoriasiform skin lesions of flaky skin (fsn/fsn) mice. Clin Exp Immunol. 2001;123:505–10. doi: 10.1046/j.1365-2249.2001.01421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.di Giovine FS, Takhsh E, Blakemore AI, Duff GW. Single base polymorphism at -511 in the human interleukin-1 beta gene (IL1 beta) Hum Mol Genet. 1992;1:450. doi: 10.1093/hmg/1.6.450. [DOI] [PubMed] [Google Scholar]
- 22.Pociot E, Molvig J, Wogensen L, Worsaae H, Nerup J. A Taq1 polymorphisms in the human interleukins 1 beta gene (IL 1B) correlates with IL 1 beta secretion in vitro. Eur J Clin Invest. 1992;22:396–402. doi: 10.1111/j.1365-2362.1992.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 23.Tarlow JK, Blakemore AI, Lennard A, Solari R, Hughes HN, Steinkasserer A, et al. Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Hum Genet. 1993;91:403–4. doi: 10.1007/BF00217368. [DOI] [PubMed] [Google Scholar]
- 24.Santilla S, Savinainen K, Hurme M. Presence of the IL-1RA allele 2 (IL-1RN*2) is associated with enhanced IL-1b production in vitro. Scand J Immunol. 1998;47:195–8. doi: 10.1046/j.1365-3083.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- 25.Settin AA, Hassan HA, El Baz RA, Hasan TA. Association of Cytokine Gene polymorphisms with psoriasis from the Nile delta in Egypt. Ind J Dermatol. 2011;56:272–7. doi: 10.4103/0019-5154.82479. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Tarlow JK, Cork MJ, Clay FE, Schmitt-Egenolf M, Crane AM, Stierle C, et al. Association between interleukin-1 receptor antagonist (IL-1ra) gene polymorphism and early and late-onset psoriasis. Br J Dermatol. 1997;36:147–8. doi: 10.1111/j.1365-2133.1997.tb08779.x. [DOI] [PubMed] [Google Scholar]
- 27.Cooper KD, Hammerberg C, Baadsgaard O, Elder JT, Chan LS, Taylor RS, et al. Interleukin 1 in Human Skin: Dysregulation in Psoriasis. J Invest Dermatol. 1990;95:S24–6. doi: 10.1111/1523-1747.ep12505698. [DOI] [PubMed] [Google Scholar]
- 28.Bonifati C, Carducci M, Mussi A, D’auria L, Ameglio F. IL-1 alpha, IL-1 beta and psoriasis: Conflicting results in the literature. Opposite behaviour of the two cytokines in lesional or non-lesional extracts of whole skin. J Biol Regul Homeost Agents. 1997;11:133–6. [PubMed] [Google Scholar]
- 29.Chang YT, Chou CT, Yu CW, Lin MW, Shiao YM, Chen CC, et al. Cytokine gene polymorphisms in Chinese patients with psoriasis. Br J Dermatol. 2007;156:899–905. doi: 10.1111/j.1365-2133.2007.07820.x. [DOI] [PubMed] [Google Scholar]
- 30.Tak PP, Firestein GS. NF-KB: A key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lizzul PF, Aphale A, Malaviya R, Sun Y, Masud S, Dombrovskiy V, et al. Differential Expression of Phosphorylated NF-jB/RelA in Normal and Psoriatic Epidermis and Downregulation of NF-κB in Response to Treatment with Etanercept. J Invest Dermatol. 2005;124:1275–83. doi: 10.1111/j.0022-202X.2005.23735.x. [DOI] [PubMed] [Google Scholar]
- 32.Abdou AG, Hanout HM. Evaluation of survivin and NF-kB in psoriasis, an immunohistochemical study. J Cutan Pathol. 2008;35:445–51. doi: 10.1111/j.1600-0560.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 33.Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genomewide Scan Reveals Association of Psoriasis with IL-23 and NF-κB Pathways. Nat Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kingo K, Koks S, Silm H, Vasar E. IL-10 promoter polymorphisms influence disease severity and course in psoriasis. Genes Immun. 2003;4:455–7. doi: 10.1038/sj.gene.6364004. [DOI] [PubMed] [Google Scholar]
- 35.Moorchung N, Srivastava AN, Gupta NK, Ghoshal UC, Achyut BR, Mittal B. Cytokine Gene Polymorphisms in the Pathogenesis of Chronic Gastritis. Singapore Med J. 2007;48:447–54. [PubMed] [Google Scholar]
- 36.Achyut BR, Tripathi P, Ghoshal U, Moorchung N, Mittal B. Interleukin 10 (-819 C/T) and Tumor Necrosis Factor alpha (-308 G/A) gene variants influence gastritis and lymphoid follicle development. Dig Dis Sci. 2007;24:622–9. doi: 10.1007/s10620-007-9925-y. [DOI] [PubMed] [Google Scholar]
- 37.Achyut BR, Ghoshal U, Moorchung N, Mittal B. Association of Toll Like Receptors 4 (Asp 299Gly and Thr399Lue) gene polymorphisms with gastritis and precancerous lesions. Hum Immunol. 2007;68:901–7. doi: 10.1016/j.humimm.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Achyut BR, Moorchung N, Mittal B. Genetic association of Interleukin 1 haplotypes with Gastritis and Precancerous lesions in North Indians. Clin Exp Med. 2008;8:23–9. doi: 10.1007/s10238-008-0152-4. [DOI] [PubMed] [Google Scholar]


