Abstract
Background:
Lichen planus (LP), a T-cell-mediated inflammatory disorder, wherein inflammation produces lipid metabolism disturbances, is linked to increase in cardiovascular (CV) risk with dyslipidemia. Increased reactive oxygen species and lipid peroxides have also been implicated in its pathogenesis.
Aim and Objective:
The aim of the study was to evaluate the status on lipid disturbances, oxidative stress, and inflammation in LP patients.
Materials and Methods:
The study was initiated after obtaining Institutional Ethics Committee permission and written informed consent from participants. The study included 125 patients (74 LP patients and 51 age and sex-matched controls) visiting the outpatient clinic in the dermatology department of our hospital. Variables analyzed included lipid profile, C-reactive protein (CRP), malondialdehyde (MDA), and catalase (CAT) activity.
Results:
Analysis of lipid parameters revealed significantly higher levels of total cholesterol (TC), triglycerides, and low-density lipoprotein cholesterol (LDL-C) along with decreased levels of high-density lipoprotein cholesterol (HDL-C) in LP patients as compared to their respective controls. LP patients also presented with a significantly higher atherogenic index that is, (TC/HDL-C) and LDL-C/HDL-C ratios than the controls. A significant increase in CRP levels was observed among the LP patients. There was a statistically significant increase in the serum levels of the lipid peroxidation product, MDA and a statistically significant decrease in CAT activity in LP patients as compared to their respective controls. A statistically significant positive correlation (r = 0.96) was observed between serum MDA levels and duration of LP whereas a significantly negative correlation (r = −0.76) was seen between CAT activity and LP duration.
Conclusion:
Chronic inflammation in patients with LP may explain the association with dyslipidemia and CV risk. Our findings also suggest that an increase in oxidative stress and imbalance in the antioxidant defense mechanisms in LP may play a role in the pathogenesis of LP.
Keywords: Cardiovascular risk, dyslipidemia, inflammation, lichen planus, oxidative stress
What was known?
Lichen planus is associated with increased cardiovascular risk especially in co-morbid conditions like dyslipidemia, diabetes mellitus, and oxidative stress.
Introduction
Lichen planus (LP) is an inflammatory papulosquamous disorder affecting the skin, mucous membranes, hair, and nails. LP is clinically characterized by small, flat-topped, shiny, polygonal violaceous papules that may coalesce into plaques. It affects part or the entire skin, genitalia, mucous membranes, and appendages. The clinical presentation of LP has several forms, including the classic plaque, oral, hypertrophic, follicular, linear, actinic, and bullous types. It is worldwide in distribution with a variable incidence. It usually develops at middle age (between 30 and 60 years) and shows a slight female preponderance (female: male = 3:2).[1,2] Although its precise etiology and pathogenesis remain unclear despite intensive investigation, it is believed that LP represents a T-cell-mediated inflammatory disorder. Inflammation produces lipid metabolism disturbances, such as serum increases in triglycerides (TGs) and low-density lipoprotein cholesterol (LDL-C) and decreases in high-density lipoprotein cholesterol (HDL-C).[3] These lipid disturbances linked to chronic inflammation participate in the increase of cardiovascular (CV) risk associated with dyslipidemia. Recent evidence suggests that various dermatological diseases including LP are associated with metabolic syndrome and/or its components.[4]
Recently, it has been suggested that increased reactive oxygen species (ROS) and lipid peroxides may play a part in the pathogenesis of various skin diseases, such as atopic dermatitis, psoriasis, vitiligo, and LP.[5,6,7,8] Activated T-cells release cytokines leading to the attraction of inflammatory cells and the destruction of keratinocytes by cell-mediated cytotoxicity.[9] The presence of different cells like keratinocytes, fibroblasts, and inflammatory cells may inflict cellular damage by overwhelming the antioxidant defense mechanisms leading to excessive production of ROS. We hypothesized that the antioxidant defense mechanisms are overwhelmed in LP resulting in an increase of oxidative damage to lipids, protein and DNA, which may be involved in the inflammatory processes of the disease.
Thus, the present study was conducted to evaluate the status of lipid metabolism, oxidative stress, and inflammation in patients with LP and assess their role in predicting the risk of CV disorders.
Materials and Methods
Study participants
This case-control study was initiated after obtaining permission from the Institutional Ethics Committee of our hospital. The sample size was calculated using MedCalc. Precision (α) was set at 0.05 with a 95% confidence interval (CI), and power 80%. The estimated sample size per group came to 51.
Participants were enrolled in the study following written informed consent. Patients of either sex aged between 18 and 70 years attending the outpatient dermatology department of our hospital and consenting to participate in the study were grouped into two groups. One group of patients had those inflicted with LP while the other group had patients suffering from other skin disorders (scabies). This group of patients acted as age and sex-matched controls for the LP patient group.
The diagnosis of LP was based on clinical findings and confirmed by histopathology. Different types of LP (classic, oral, hypertrophic, pigmentosus, planopilaris, eruptive, genital, and nail) were diagnosed. Patients with known history of co-morbid disorders that are associated with dyslipidemia like thyroid disorders and diabetes mellitus were excluded. Patients with lichenoid drug eruption, those consuming drugs such as lipid-lowering agents viz., statins and fibrates, systemic corticosteroids, retinoic acid, methotrexate, other immunosuppressive drugs, as well as nonsteroidal anti-inflammatory drugs, for the past 4 weeks, and applying topical medications for the last 2 weeks prior to blood sample collection were also excluded from the study. Patients were requested to come in a 12 h fasting state for collection of blood for the various study related investigations within 7 days following their consent to participate in the study. The study was carried out over a period of 6 months from July to December 2011.
Blood samples and variables for assessment
Ten milliliter of blood was obtained aseptically by venipuncture after a 12 h fasting state from all patients. Of 10 ml, 2 ml of blood was collected in an ethylenediaminetetraacetic acid (EDTA) tube for the estimation of erythrocytes catalase (CAT) activity and remaining 8 ml of blood was collected in the clot activator tube for the estimation of lipid profile, malondialdehyde (MDA), and C-reactive protein (CRP). Blood samples collected in the clot activator tube was allowed to clot for 30–40 min and was then centrifuged at 2000 rpm for 10 min to obtain the serum for analysis of lipid profile, MDA, and CRP estimation. In addition, total cholesterol (TC)/HDL-C and LDL-C/HDL-C ratios were calculated. The presence of dyslipidemia was defined as per the National Cholesterol Education Program adult treatment plan III criteria, wherein serum TG >150 mg/dl, TC >200 mg/dl, and/or LDL-C >130 mg/dl are considered as cut-offs for dyslipidemia.[10]
Preparation of erythrocyte hemolysate
The hemolysate was prepared by centrifuging 1 ml EDTA blood at 2000 rpm for 10 min. After removal of plasma and buffy coat erythrocytes were washed 3 times with two volumes of isotonic saline. Erythrocytes were lysed with EDTA stabilizing reagent for CAT activity and were stored in a deep freezer at −20°C until estimation.
Measurement of serum lipid profile levels and C-reactive protein
Cholesterol, TGs, HDL-C, and LDL-D were measured using standardized kit procured from AMP diagnostic kits, Ameda Labordiagnostik, GmbH on fully automated biochemistry analyzer. The atherogenic index (AI) was calculated using the formula TC/HDL-C. Inflammation was measured in terms of CRP by sandwich ELISA technique using NycoCard reader.
Measurement of malondialdehyde levels
Lipid peroxidation was measured in terms of MDA by using thiobarbituric acid (TBA) reactive substances method, most common and widely used which is an indicator of peroxidative free radical damage.[11] MDA is formed as a result of lipid peroxidation and reacts with TBA under high temperature (90–100°C) and acidic condition. The reaction yields a pink MDA-TBA adduct and was measured at 530 nm spectrophotometrically. The results were expressed as nmol/ml.
Measurement of catalase activity
Catalase is an important cellular antioxidant enzyme that defends against oxidative stress and considered as first line of enzymatic antioxidant defense. It is a tetrameric enzyme, consisting of four identical subunits that contain a single ferriprotoporphoryn group per subunit. CAT reacts efficiently in peroxisomes with hydrogen peroxide (H2O2) to form water and molecular oxygen. The Catalase (CAT, EC 1.11.1.6) activity was measured by using Beutler method.[12] This rate of hydrogen peroxide decomposition by CAT is measured spectrophotometrically at 230 nm. Enzyme activity was expressed as U/g Hb.
Random samples were tested for both MDA levels and CAT activity in duplicate (12 per group) to assess the reproducibility of the tests.
Statistical analysis
The statistical analyses were performed with the SPSS/PC software (version 15.0 for Windows; SPSS Inc., Chicago, Illinois, USA). Two-sample Student's t-test was used to compare mean values of quantitative variables in case of normally distributed data and Mann–Whitney U-test in cases where the data did not pass the KS test for normality. Qualitative variables were analyzed with Chi-square test. The association of CV risk (using Castelli's AI) with age, sex, and presence of LP was estimated using logistic regression model. Spearman's correlation coefficient (r) was used to determine the relationship between different variables. P < 0.05 was considered significant in all analyses.
Results
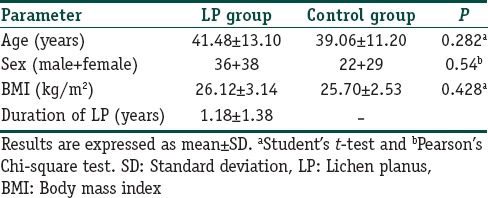
A total of 125 participants were enrolled in the study, of which 74 patients were from the LP group (36 males and 38 females) while 51 patients were from the control group (22 males and 29 females). The mean age was 41.18 (standard deviation [SD] 13.30) years for LP patients and 39.06 (SD 11.20) years was for the controls. The mean age, body mass index (BMI), and duration of LP is summarized in Table 1 for patients with LP and their respective controls. No significant difference was found between the age and BMI between the two groups. The tests for homogeneity also demonstrated that the two groups were homogenous with respect to age, sex, and BMI.
Table 1.
Homogeneity of the study groups in terms of the demographic characteristics
All the patients had cutaneous involvement; 30 patients (40.54%) presented with classic LP, 10 patients (13.51%) had oral LP (OLP), 10 patients (13.51%) presented with the hypertrophic type, 8 patients (10.81%) with the pigmentosus type, 7 patients (9.46%) with planopilaris type, 5 patients (6.76%) with eruptive type, 2 patients (2.70%) had both oral and classic LP, nail involvement was observed in one patient (1.35%) and genital LP type was observed in one of the patients (1.35%).
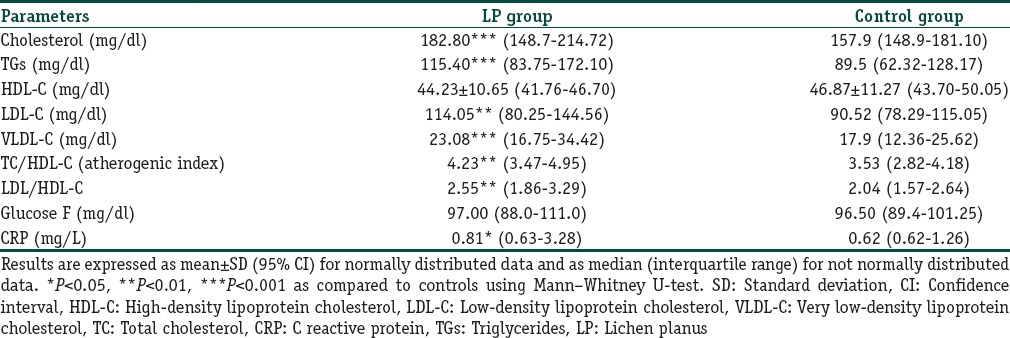
In case of the lipid profile, the serum levels of TC, TGs, HDL-C, LDL-C and very LDL-C (VLDL-C) was estimated in both groups and the results are summarized in Table 2. As seen from the table, a statistically significant increase in the serum levels of TC, TGs, LDL-C, and VLDL-C was observed in LP patients as compared to their controls whereas HDL-C level was lower in LP patients although not statistically significant. LP patients also presented with a significantly higher TC/HDL and LDL-C/HDL-C ratio than the controls. The prevalence of dyslipidemia seen in patients with LP was 30% while it was 6% for controls (Chi-square test: P < 0.0001; odds ratio [OR] 0.18; 95% CI: 0.07297–0.4257). A multivariate binary regression model also demonstrated that LP was associated with dyslipidemia, even after controlling for confounders like age and sex. Serum CRP level, a marker for inflammation, was found to be significantly raised in LP patients compared to controls.
Table 2.
Lipid profile, blood sugar, and CRP in patients with LP and their respective controls
As seen from Table 3, logistic regression model was also performed to look at the association of CV risk using Castelli's AI (values ≥5.1 for men and ≥4.5 for women) and age, sex and presence or absence of LP. A significant association was seen between the AI and LP (OR 2.66, 95% CI 1.04–6.81, P = 0.03).
Table 3.
Logistic regression model for association of LP with cardiovascular risk (using Castelli's atherogenic index)
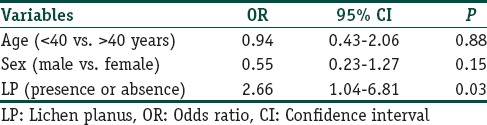
Table 4 depicts the results of the oxidant status in both groups of patients. Serum level of erythrocyte CAT, a free radical scavenger, was found to be significantly lower in LP patients as compared to the controls while significantly higher level of serum MDA, the end product of lipid peroxidation, was seen in LP patients as compared to controls. Moreover, a statistically significant positive correlation between MDA (r = 0.96, P < 0.0001) and the duration of LP, and a statistically negative correlation between erythrocyte CAT activity (r = −0.76, P < 0.0001) and the duration of LP was observed as shown in Figure 1.
Table 4.
Erythrocyte catalase and serum MDA in patients with LP and their respective controls
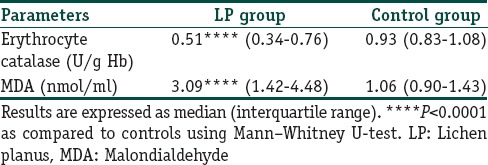
Figure 1.
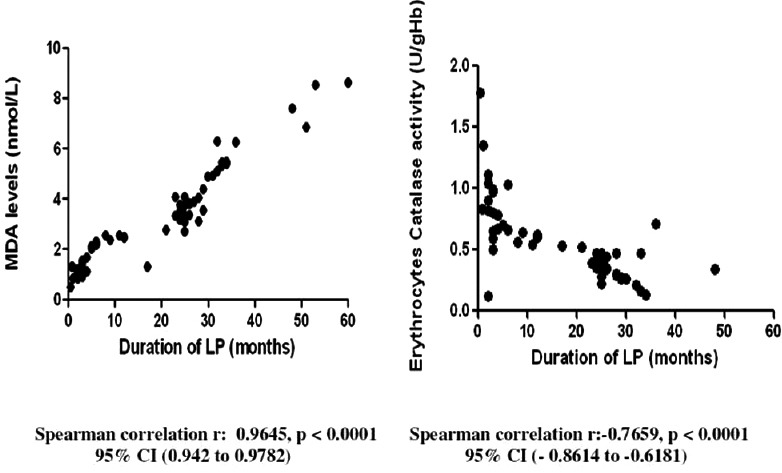
Correlation between antioxidant markers and duration of lichen planus
Discussion
Lichen planus is a chronic autoimmune mucocutaneous disease mainly affecting the middle-aged and women in particular. Reports suggest that ROS and lipid peroxides affect the pathogenesis of LP.[8] Studies have shown that LP is associated with CV risks, such as dyslipidemia,[3,13] diabetes mellitus,[14] and increased oxidative stress.[15]
Lichen planus has been reported to be associated with dyslipidemia in some studies.[3,13,16] Arias-Santiago et al.[3] documented higher serum lipid levels in the LP group as compared to the controls. Moreover, Dreiher et al.[13] reported a higher prevalence of dyslipidemia in the LP group in comparison to the control group. Our study too demonstrated statistically significant higher levels of TC, TGs, and LDL-C together with a decrease in the levels of HDL-C in LP patients as compared to their controls. Our findings are thus in accordance with reported literature and confirm the association between dyslipidemia and LP as reported in the previous studies.[3,13,16] An increased LDL-C/HDL-C ratio has already been considered as a sensitive predictor of CV risk, and recently, TC/HDL-C ratio (AI) has been found to be an even better predictor of atherosclerosis.[17] Arias-Santiago et al.[3] have reported higher values of both ratios in patients with LP indicating higher CV risk. Our study too reports similar higher LDL-C/HDL-C and AI values. Numerous genetic and environmental factors influence CV risk. Smoking, dyslipidemia, diabetes, and hypertension are classic risk factors with multiple interactions between themselves and with other factors viz., family history of premature disease, obesity, and sedentary lifestyles, that determine the extent and evolution of the disease.[3] The statistically significant increased atherogenic risk factor seen in the present study is hence associated with the presence of LP. However, we did not consider other risk factors like smoking and blood pressure in our study, which also play a critical role in predicting CV risk.
The frequent presence of chronic inflammation parameters in patients with psoriasis has been used to suggest a relationship with CV disease. Several cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL-2), and IL-6, have been implicated in the increased lipid levels in these patients.[3] LP too is an immune-mediated disease; antigens are processed by Langerhans cells and then presented to T-lymphocytes. This stimulated lymphocytic infiltrate is epidermotropic and attacks keratinocytes, resulting in the generation of ROS. During this lymphocytotoxic process, keratinocytes release more cytokines that, in turn, attract more lymphocytes. The cytokines involved in LP pathogenesis such as TNF-α, IL-6, IL-10, and IL-4 could explain the association with dyslipidemia.[3]
Some studies have hypothesized that measurement of inflammatory markers, like high-sensitivity-CRP, may provide an adjunctive method to globally assess CV risk.[18,19] Ridker et al. had demonstrated higher levels of CRP and the strongest and most significant predictor of the risk of future CV events.[18] The raised CRP levels were also observed in our study indicates the presence of chronic inflammation which may explain the CV risk associated with dyslipidemia and similar results are reported by other authors.[3,19]
It has also been reported that LP is associated with hyperglycemia[20,21] and a higher prevalence of diabetes than among the general population. Grinspan et al.[22] first described an association between OLP and diabetes. Later on, this relationship between OLP and diabetes has been extensively studied by various researchers.[23,24,25] However, in our study, only a marginal rise in blood glucose values was observed in the LP group as compared to controls and the effect statistically insignificant.
Oxidative stress is caused by an imbalance between the production of reactive oxygen and the ability of the biological system to readily detoxify the reactive intermediates or easily repair the resulting damage. This usually results in the production of free radicals that can damage cell membranes through the production of lipid peroxides as well as numerous cellular molecules such as proteins, nucleic acids, amino acids, carbohydrates, and vitamins.[26] Recently, it has been proposed that oxidative stress may have a role to play in the pathogenesis of LP. Lipid peroxidation, which results from the oxidation of membrane-associated polyunsaturated fatty acids of phospholipids, has been considered a major presentation of oxidative stress.[27] MDA, the end product of lipid peroxidation, is a known marker of free radical mediated damage and oxidative stress.[28] Sezer et al. and Sander et al. have reported that the serum levels of MDA were higher in the LP patient group than the control group. Moreover, Rai et al. also reported high MDA levels in LP, leukoplakia, and cancer. In our study, we observed a significantly higher level of serum MDA in LP patients as compared to controls. This suggests that oxidative stress may lead to an increased production of ROS, thus leading to increased lipid peroxidation. This is in agreement with other studies which have also demonstrated increased MDA levels in patients with LP.[29,30,31,32]
The skin possesses an array of defense mechanisms that interact with ROS to obviate their deleterious effect. CAT is considered as the main enzyme involved in removing H2O2 by breaking it down to water and oxygen. We found significantly reduced activity of erythrocyte CAT activity in LP patients as compared to the control group. Similar findings have been reported by other researchers suggesting an imbalance in the antioxidant status, resulting in accumulation of H2O2, and leading to vacuolization of the basal layer seen in LP.[8,16]
Our study, thus highlights that, chronic inflammation in patients with LP might explain the association with dyslipidemia. Hence, screening of LP patients for their lipid profile might be useful in detecting individuals at risk and initiating preventive measures to protect against the development of CV disorders. Furthermore, the significantly altered status of the antioxidant defense mechanisms observed in our study highlighted the role of oxidative stress in pathogenesis of LP.
The present study has some limitations. This was a single-center study and although planned as a case-control study, the sample size in the two groups was not equal. However, this did not appear to affect the study outcomes. Its cross-sectional nature did not allow us to infer causality from the results; these should be a considered as a hypothesis-generating study that needs confirmation by larger prospective studies. It would also be worthwhile to investigate other parameters of CV risk (smoking, abdominal circumference, insulin levels, and blood pressure) which would confirm the association. Nevertheless, our study highlights the fact that alterations in lipid and antioxidant markers play an important role in LP.
What is new?
There are different studies exploring the impact of lipid profile and the antioxidant status in lichen planus (LP). However, no study has evaluated these metabolic parameters together in these patients. Our study attempts to identify the impact of alteration in both these parameters simultaneously on patients with LP.
Acknowledgments
We would like to thank Miss. Roopa Dharmatti and Miss. Manjushree C. Khendkar for their technical assistance.
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
References
- 1.Neville BW, Damm DD, Allen CM, Bouquot JE. Dermatological diseases. In: Neville BW, Damm DD, Allen CM, Bouquot JE, editors. Oral and Maxillofacial Pathology. 2nd ed. Philadelphia: Saunders; 2002. pp. 680–5. [Google Scholar]
- 2.Jontell M, Holmstrup P. Red and white lesions of the oral mucosa. In: Green Berg MS, Glick M, Ship JA, editors. Burket's Oral Medicine. 11th ed. Hamilton: BC Decker Inc; 2008. pp. 90–1. [Google Scholar]
- 3.Arias-Santiago S, Buendía-Eisman A, Aneiros-Fernández J, Girón-Prieto MS, Gutiérrez-Salmerón MT, Mellado VG, et al. Cardiovascular risk factors in patients with lichen planus. Am J Med. 2011;124:543–8. doi: 10.1016/j.amjmed.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 4.Padhi T, Garima Metabolic syndrome and skin: Psoriasis and beyond. Indian J Dermatol. 2013;58:299–305. doi: 10.4103/0019-5154.113950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omata N, Tsukahara H, Ito S, Ohshima Y, Yasutomi M, Yamada A, et al. Increased oxidative stress in childhood atopic dermatitis. Life Sci. 2001;69:223–8. doi: 10.1016/s0024-3205(01)01124-9. [DOI] [PubMed] [Google Scholar]
- 6.Relhan V, Gupta SK, Dayal S, Pandey R, Lal H. Blood thiols and malondialdehyde levels in psoriasis. J Dermatol. 2002;29:399–403. doi: 10.1111/j.1346-8138.2002.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 7.Yildirim M, Baysal V, Inaloz HS, Kesici D, Delibas N. The role of oxidants and antioxidants in generalized vitiligo. J Dermatol. 2003;30:104–8. doi: 10.1111/j.1346-8138.2003.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 8.Sezer E, Ozugurlu F, Ozyurt H, Sahin S, Etikan I. Lipid peroxidation and antioxidant status in lichen planus. Clin Exp Dermatol. 2007;32:430–4. doi: 10.1111/j.1365-2230.2007.02436.x. [DOI] [PubMed] [Google Scholar]
- 9.Middel P, Lippert U, Hummel KM, Bertsch HP, Artuc M, Schweyer S, et al. Expression of lymphotoxin-alpha by keratinocytes: A further mediator for the lichenoid reaction. Pathobiology. 2000;68:291–300. doi: 10.1159/000055940. [DOI] [PubMed] [Google Scholar]
- 10.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 11.Devasagayam TP, Boloor KK, Ramasarma T. Methods for estimating lipid peroxidation: An analysis of merits and demerits. Indian J Biochem Biophys. 2003;40:300–8. [PubMed] [Google Scholar]
- 12.Beutler's E. 3rd ed. New York: Grune and Stratton; 1984. Red Cell Metabolism; p. 72. [Google Scholar]
- 13.Dreiher J, Shapiro J, Cohen AD. Lichen planus and dyslipidaemia: A case-control study. Br J Dermatol. 2009;161:626–9. doi: 10.1111/j.1365-2133.2009.09235.x. [DOI] [PubMed] [Google Scholar]
- 14.Seyhan M, Ozcan H, Sahin I, Bayram N, Karincaoglu Y. High prevalence of glucose metabolism disturbance in patients with lichen planus. Diabetes Res Clin Pract. 2007;77:198–202. doi: 10.1016/j.diabres.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Aly DG, Shahin RS. Oxidative stress in lichen planus. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19:3–11. [PubMed] [Google Scholar]
- 16.Kurgansky D, Burnett JW. Widespread lichen planus in association with Turner's syndrome and multiple endocrinopathies. Cutis. 1994;54:108–10. [PubMed] [Google Scholar]
- 17.Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J, et al. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: The Quebec Cardiovascular Study. Arch Intern Med. 2001;161:2685–92. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 19.Lagrand WK, Visser CA, Hermens WT, Niessen HW, Verheugt FW, Wolbink GJ, et al. C-reactive protein as a cardiovascular risk factor: More than an epiphenomenon? Circulation. 1999;100:96–102. doi: 10.1161/01.cir.100.1.96. [DOI] [PubMed] [Google Scholar]
- 20.Lowe NJ, Cudworth AG, Clough SA, Bullen MF. Carbohydrate metabolism in lichen planus. Br J Dermatol. 1976;95:9–13. doi: 10.1111/j.1365-2133.1976.tb15530.x. [DOI] [PubMed] [Google Scholar]
- 21.Romero MA, Seoane J, Varela-Centelles P, Diz-Dios P, Garcia-Pola MJ. Prevalence of diabetes mellitus amongst oral lichen planus patients. Clinical and pathological characteristics. Med Oral. 2002;7:121–9. [PubMed] [Google Scholar]
- 22.Grinspan D, Diaz J, Villapol LO, Schneiderman J, Berdichesky R, Palèse D, et al. Lichen ruber planus of the buccal mucosa. Its association with diabetes. Bull Soc Fr Dermatol Syphiligr. 1966;73:898–9. [PubMed] [Google Scholar]
- 23.Albrecht M, Bánóczy J, Dinya E, Tamás G., Jr Occurrence of oral leukoplakia and lichen planus in diabetes mellitus. J Oral Pathol Med. 1992;21:364–6. doi: 10.1111/j.1600-0714.1992.tb01366.x. [DOI] [PubMed] [Google Scholar]
- 24.Sousa MG, Costa Ade L, Roncalli AG. Clinical study of the oral manifestations and related factors in type 2 diabetics patients. Braz J Otorhinolaryngol. 2011;77:145–52. doi: 10.1590/S1808-86942011000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ara SA, Mamatha GP, Rao B. Incidence of diabetes mellitus in patients with diabetes mellitus. J Dent Clin. 2011;3:29–33. [Google Scholar]
- 26.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 27.Picardo M, Passi S, Morrone A, Grandinetti M, Di Carlo A, Ippolito F. Antioxidant status in the blood of patients with active vitiligo. Pigment Cell Res. 1994;7:110–5. doi: 10.1111/j.1600-0749.1994.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 28.Kasperska-Zajac A, Brzoza Z, Rogala B, Polaniak R, Birkner E. Antioxidant enzyme activity and malondialdehyde concentration in the plasma and erythrocytes of patients with urticaria induced by nonsteroidal anti-inflammatory drugs. J Investig Allergol Clin Immunol. 2008;18:372–5. [PubMed] [Google Scholar]
- 29.Sezer E, Ozugurlu F, Ozyurt H, Sahin S, Etikan I. Lipid peroxidation and antioxidant status in lichen planus. Clin Exp Dermatol. 2007;32:430–4. doi: 10.1111/j.1365-2230.2007.02436.x. [DOI] [PubMed] [Google Scholar]
- 30.Sander CS, Chang H, Hamm F, Elsner P, Thiele JJ. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int J Dermatol. 2004;43:326–35. doi: 10.1111/j.1365-4632.2004.02222.x. [DOI] [PubMed] [Google Scholar]
- 31.Sander CS, Ali I, Dean D, Thiele JJ, Wojnarowska F. Oxidative stress is implicated in the pathogenesis of lichen sclerosus. Br J Dermatol. 2004;151:627–35. doi: 10.1111/j.1365-2133.2004.06142.x. [DOI] [PubMed] [Google Scholar]
- 32.Rai B, Kharb S, Jain R, Anand SC. Salivary lipid peroxidation product malonaldehyde in pre-cancer and cancer. Adv Med Dent Sci. 2008;2:7–8. [Google Scholar]


