Abstract
Background:
Growth factors have long been known as an effective treatment for facial wrinkles. We developed growth factor concentrate (GFC) from the platelets and evaluated their clinical outcome in nasolabial folds.
Aims and Objectives:
We evaluated safety and efficacy of autologous GFC on patients with nasolabial folds.
Materials and Methods:
Study was conducted on 80 patients for nasolabial folds in two groups. Group I (20) received bilateral single injection of GFC and group II (60) received single injection of GFC on the right side of the face and platelet-rich plasma (PRP) on the left side of the face. Severity of nasolabial folds was determined at the baseline and 3 months of follow-up visits based on wrinkle severity rating scale (WSRS), Global aesthetic improvement scale (GAIS) and atlas photographic grading at rest and at full smile. Objective clinical assessment and subjective satisfaction scale was determined for overall improvement at the end of the study.
Results:
In group I, 2 subjects showed improvement after GFC treatment with the score of 3.1–4 (76–100%), 3 subjects with the score of 2.1–3 (51–75%), 14 with the score of 1.1–2 (26–50%) and 1 subject with the score of 0–1 (<25%) at the end of study. In group II, 51 subjects were evaluated at the end of study where, 34 (66%) showed superior improvements after GFC, 6 (11%) patients showed similar improvement on both side of the face, 10 (19.6%) patients showed no noticeable improvement on the either side of the face and only 1 patient (1.96%) showed superior improvement for PRP at the end of the study. Overall improvement score analysis showed that GFC was significantly superior to PRP (P < 0.001).
Conclusion:
Present study is a strong evidence to support the use of GFC for nasolabial folds. The results showed that the single application of GFC is highly effective and safe.
Keywords: Acne scar, autologous, growth factor concentrates, nasolabial folds, platelets
What was known?
Platelet-rich plasma (PRP) is defined as a portion of the plasma that has platelet concentrated above baseline level. PRP contains growth factors, chemokine factors, cytokine and adhesive proteins that have both mitogenic and chemotactic properties. However, evidence for the effectiveness of PRP therapy is not very conclusive and results are inconsistent. Several other approaches have numerous side effects. GFC contain more or less consistent level of natural growth factors and hence provide consistent results without any side effect.
Introduction
Facial folds are the results of aging process. Studies showed that in aged and wrinkled skin, there is an accumulation of altered elastic fibres and degradation and degeneration of collagen in the dermis.[1] Nasolabial folds and crow feet are the first sign of facial aging. Age related changes on the face are like facial bone loss, facial soft tissue loss, dermal dystrophy, loss of subcutaneous tissue, redistribution of fat and dermal thickening that contribute to the facial folds.[2,3] There are several factors that causes age-related changes such as exposure to sunlight, nutritional habits, and hereditary factors that result in degeneration of elastic fibres, degradation of collagen and collapse of fibroblast that loses skin elasticity and resilience.[4,5,6,7]
Correction of facial folds and restoration to its original shape is a key approach to rejuvenation and enhancement of facial appearance. Several surgical and non-surgical treatment modalities have been reported and currently being used regularly in the aesthetic clinics with numerous side effects. It is therefore necessary that soft tissue augmentation need safe, reliable and long-lasting alternative.
Recently, autologous platelet-rich plasma (PRP) is being used in a wide variety of clinical application.[8] However, evidence for the effectiveness of PRP therapy is not very conclusive. Several factors like platelet concentration, growth factor levels, activation process, and time of application are severely affecting the results.[9] It has been observed that growth factor derived from platelets has a dose–response relationship in proliferation of fibroblast and the production of Type-I collagen. Higher concentration of platelets inhibits the proliferation, whereas lower concentration did not show optimum results, hence different growth factor concentration may have an impact on the results that can be obtained in vivo.[10]
Here we have developed autologous growth factor concentrate (GFC) from the fixed number of platelets and evaluated for its safety and efficacy for nasolabial folds and compared with conventional PRP in a split face clinical study.
Materials and Methods
For this study, two clinical sites in Mumbai, India and one clinical site in Madrid, Spain were selected. This study was designed and performed according to the Declaration of Helsinki and approved by the local institutional ethics committee (IEC). Written informed consent from all the patients was obtained before participation in this study and they were informed about experimental protocol and being advised about the risks of treatment. Clinical study in India was registered with clinical trial registry of India (CTRI) no CTRI/2012/05/002627 and US clinical trial registry NCT 01644461.
Study design
This study was divided into two groups. In group I, total 20 subjects were enrolled with nasolabial folds and all of them received 2.5 ml of single dose of GFC on each side of the face, whereas in group II, 60 subjects were enrolled with nasolabial folds and received 2.5 ml of single dose of GFC on the right side of the face and 2.5 ml of PRP on the left side of the face as a comparator.
All subjects with nasolabial wrinkle with grade of 3–4 were recruited during April–June 2012 and followed them for 3 months till October 2012 at two clinical centers of Mumbai and one clinical center of Madrid, Spain. Extended post study follow-up of 6 and 12 months was carried out in January 2013 and June 2013, respectively.
Inclusion criteria were that the patient with either sex and should be aged between 35 and 65 years with grade 3 or 4 on folds severity rating scale (WSRS) (1–5) on clinical evaluation. They must be willing to refrain from any other treatment of facial folds during the entire study.
Patients not included in the study were already undergoing aesthetic facial treatment 6 weeks prior to enrolment, patient with hematopoietic disorders or known bleeding disorders such as platelet dysfunction syndrome, thrombocytopenia or hypo-fibrinogenemia.
Isolation of GFC
Thirty-four milliliters of whole blood from each patient was drawn in vacutainer (Hindustan Syringe and Medical Devices, Faridabad, India) containing acid citrate dextrose (ACD) as anti-coagulant. Along with this 5 ml of blood was drawn in EDTA vacutainer and 5 ml was drawn in plain vacutainer for serum for patient screening. Entire collection kit with collected whole blood is kept in transport box maintained at a temperature of 20–22°C and transported to the central processing laboratory within 24 hours of collection. Tubes were barcoded and sent for quality testing. Blood samples were tested for complete blood count (CBC) and infectious disease markers like human immunodeficiency virus 1, 2 (HIV1), hepatitis B virus (HBV), hepatitis C virus (HCV) and venereal disease research laboratory test (VDRL) by rapid tests before processing for GFC.
Entire process was carried out in centralized Good Manufacturing Practices (GMP)-approved clean room. ACD vacutainer containing whole blood was centrifuged at 380 gravitational forces (g) for 15 minutes. The upper layer of plasma that is rich in platelets was removed in another sterile centrifuge tube leaving behind buffy coat in the middle and packed red blood cells at the bottom. PRP was centrifuged again at 2700 g for 10 minutes. Supernatant containing platelet-poor plasma (PPP) was then removed and collected in other sterile centrifuge tube for subsequent use. Platelet-rich pellet at the bottom was then suspended in 5 ml of plasma to make platelet-rich suspension. Total platelets in the platelet-rich suspension was adjusted to 625 × 106 platelets/ml as a therapeutic dose for lateral epicondylitis using an automated cell counting machine (Coulter) and diluted accordingly using PPP.
The platelet-rich suspension containing 625 × 106 platelets/ml was then processed in GMP clean suits for isolation of growth factors. Final product was developed in 5 ml of plasma. The final product enriched with growth factors was then sterile filtered through 0.22 μm filter and stored at –20°C until use.
Fibroblast proliferation assay
In order to identify optimal therapeutic dose, the dermal fibroblast proliferation assay was performed using different concentrations of GFC derived from voluntary donor blood samples as described previously.[11] Dermal fibroblasts were seeded at a density of 400 cells/well in a 96-well plate in DMEM-KO medium supplemented with 10% GFC at concentration derived from 2500, 1250, 625, 250 and 0 million platelets per ml and were allowed to proliferate up to day 9. The proliferation was assayed using the cell-counting Kit-8 (Dojindo Molecular Technologies, Inc., Gaithersburg; MD) according to manufacturer's instructions. The absorbance was measured at 450 nm.
Study procedure
Subjects were enrolled with Fitzpatrick skin type II–V and predominantly of Asian and Caucasian origin. Prior to the treatment affected skin was cleaned with antiseptic and followed by sterile saline. In order to carry out comparative analysis, digital photographs were collected for each patient. A topical anesthetic cream was applied on the skin for 45 minutes. Face was again cleaned with sterile saline. The patient was seated in the inclined position in dental chair. A 28–30 gauge needle attached to 1 ml hypodermic syringe was used. Skin was stretched tightly before injecting the needle in the dermis. The needle was inserted into the dermis at an approximately 30° angle, parallel to the length of nasolabial folds or other folds. GFC was injected either by linear threading or fanning at the base of folds. 2.5 ml of GFC was injected at each side of nasolabial folds in group I patients, whereas 2.5 ml GFC and 2.5 ml of PRP was distributed on the right and left side of the face for facial folds in group II patients.
Patients were followed up for every month for the improvement up to 3 months. Post-trial follow-up at 6 and 12 month was done with telephonic interview for any side effects and further improvements or any deterioration.
Efficacy endpoints
Primary efficacy end points were changes in the global aesthetic improvement scale (GAIS) score from screening to end of the study. Secondary efficacy end points were photographic assessments, Physician's assessment and patient's assessment score.
Objective clinical assessment
Improvement score for facial folds were assessed by the investigators and two different dermatologists. Scores were assigned from 0 to 4, where 0–1 (<25% improvement), 1.1–2 (26-50%), 2.1–3 (51–75%), 3.1–4 (76–100%).
Subjective satisfaction scale
Patient completed self-assessment questionnaires and rated their overall improvement and satisfaction on a scale from 0 (unsatisfied) 1, (Partially satisfied), 2 (Moderately satisfied) and 3 (completely satisfied).
Statistics
Statistical analyses were performed using SPSS-16 for Windows (SPSS Inc., Chicago, IL). Data were assessed for normality with Shapiro-Wilks normality tests and all variables had normal distribution and hence parametric tests including t-test and GLM: repeated measure analysis of variance was run to compare these variables before and after treatment with α = 0.05. Comparison of aesthetic grading and objective assessment by physician, blinded investigators and patients between GFC and PRP was done using Mann Whitney U test and Wilcoxon signed ranked test.
Results
GFC isolation and analysis
Multiplex ELISA analysis of GFC showed that levels of major growth factors, cytokines and other signaling proteins were found to be linearly proportional to the platelet numbers. On comparison with PRP, levels of major growth factors in GFC were 3–10 times higher than PRP (data not shown). In order to identify optimum therapeutic dose, in vitro proliferation and MTT assay on normal skin fibroblast was carried out with different concentration of GFC. Data demonstrated that 250 and 625 × 106 platelet/ml concentration was most optimal for cell proliferation and hence we considered 625 × 106 platelets/ml as a therapeutic dose for facial skin regeneration and used for clinical study. Cell proliferation was significantly inhibited at higher concentration of growth factor (P < 0.001) [Figure 1].
Figure 1.
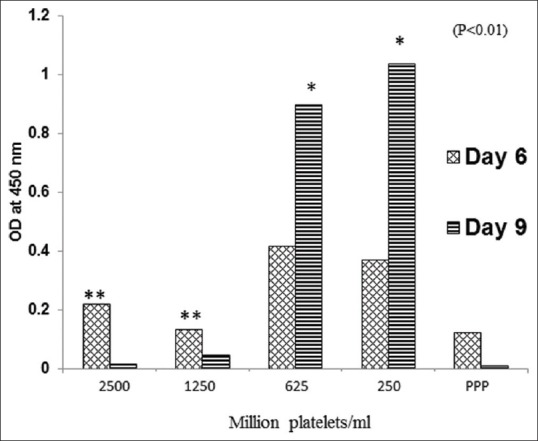
Dermal fibroblast proliferation assay using 2500, 1250, 625 and 250 × 106 platelets/ml and platelet poor plasma (PPP). Proliferation was assayed using cell counting kit-8. Absorbance was measured at 450 nm. Proliferation was considered linearly proportional to the cell number
Subjects
Total 80 subjects were enrolled in two groups. In group I, total 20 subjects were enrolled of which 17 were females and 3 were males of Asian origin with an average (SD) age of 34.95 + 5.8 years. In group II, total 60 subjects were enrolled of which 55 were female and 5 were males of Caucasian origin with an average age of 49.2 + 4.8 years.
Patients received single injection of 5 ml of GFC in group I distributed 2.5 ml on each side, whereas in group II, patients received 2.5 ml of GFC on the right side and 2.5 ml of PRP on the left side of the face. Efficacy results were evaluated for 3 months following GFC or PRP application. Efficacy endpoints were improvement on wrinkle severity rating scale (WSRS) and global aesthetic improvement scale (GAIS) assessment of nasolabial folds, objective clinician assessment score, subjective patient satisfaction score and photographic evidence.
During the study, subjects did not show any adverse reaction after injection of autologous GFC.
In group I, out of 20 subjects, 2 subjects showed improvement with a score of 3.1–4 (improvement in between 76% and 100%) and 3 subjects with score of 2.1–3 (51–75%), 14 subjects with score of 1.1–2 (26–50%) and 1 subject improved with the score of 0–1 (<25%) in objective clinician assessment score [Table 1]. The subjective satisfaction assessment showed that 1 patient was satisfied, 1 patient was partially satisfied and 18 patients were completely satisfied with GFC treatment in the improvement in nasolabial folds over screening [Table 2]. The mean WSRS as per atlas grading showed a reduction in the nasolabial folds from screening (3.45 + 0.51) to the end of study (2.15 + 0.61) (P < 0.001). This was seen uniformly in almost all the patients [Figure 2].
Table 1.
Objective assessment of improvement after GFC injection for nasolabial wrinkle in group I at the end of study by two blind investigators and treating physician. Results are the average of these three observations
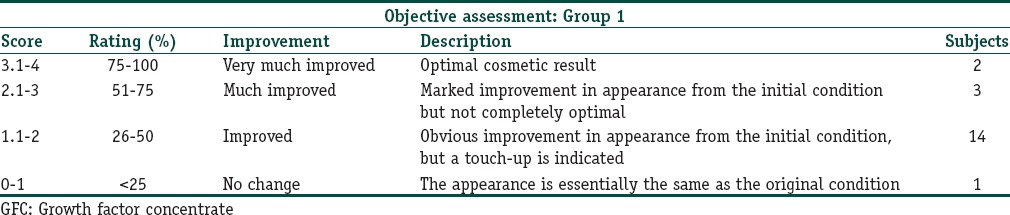
Table 2.
Subjective satisfaction assessment of the patients after GFC injection for nasolabial folds in group I at the end of study

Figure 2.
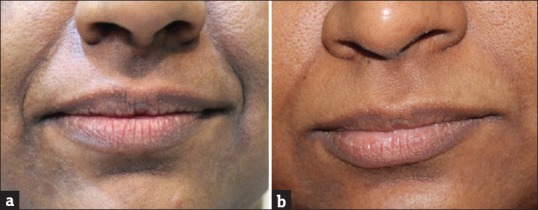
Subject from group I demonstrating the effect of GFC in nasolabial folds before screening (a) and at the end of the study (b). GFC showed significant improvement in nasolabial folds
In group II, global aesthetic improvement scale for nasolabial folds showed mean severity index 2.02 (range 2.8–1.4) at baseline that significantly improved to the mean GAIS of 1.5 (range 2.1–0.7) for GFC (P < 0.0001). Whereas mean severity score for the PRP group was 2.14 (range 2.9–1.4) at the baseline which improved to the mean GAIS of 2.0 (range 2.7–1.4) (P < 0.01). Between both the groups, GFC showed highly significant improvement which was prominently visible within 1 month of injection than PRP (P < 0.001). Results were continuously improved until 3 months and persistent till the last telephonic interview at 12 months of injection [Figures 3–8].
Figure 3.
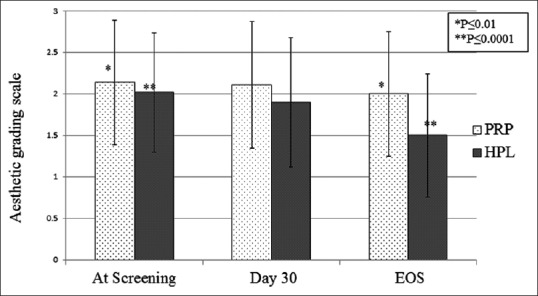
Mean global aesthetic grading scale in Nasolabial folds of subjects at screening, day 30 follow-up and at the end of study in GFC and PRP groups. Results showed significant improvement in GAIS in GFC group (P < 0.0001) than PRP group (P < 0.01)
Figure 8.
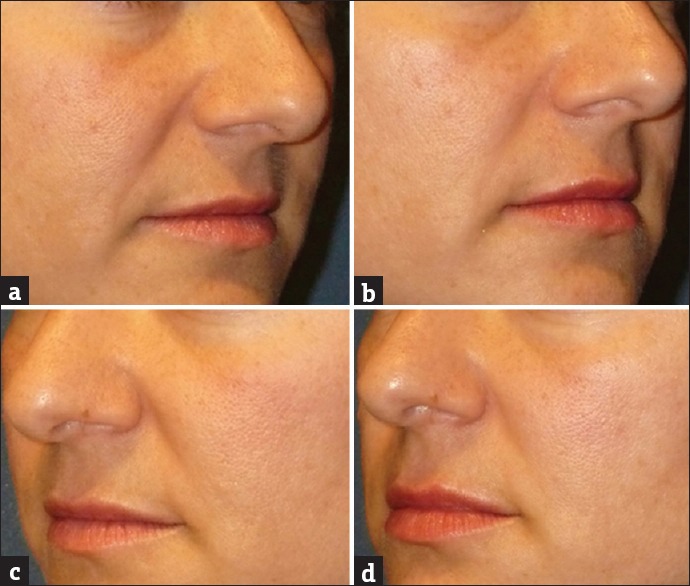
A 46-year-old subject demonstrating the effect of GFC in nasolabial folds before screening (a) and at the end of the study (b). Results showed significant improvement in nasolabial folds in 90 days. Similarly, the effect of PRP in nasolabial folds before screening (c) and at the end of the study (d) showed minor visible improvement in nasolabial folds in 90 days
Figure 4.
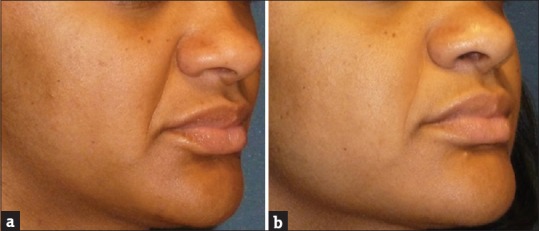
A 42-year-old subject demonstrating the effect of GFC in nasolabial folds before screening (a) and at the end of the study (b). Results showed significant improvement in nasolabial folds in 90 days
Figure 5.
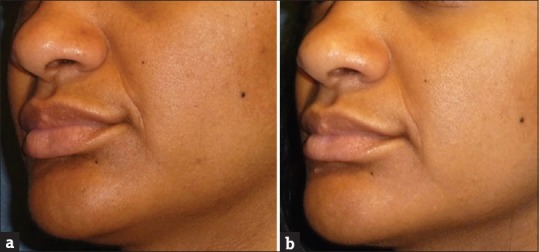
A 42-year-old subject demonstrating the effect of PRP in nasolabial folds before screening (a) and at the end of the study (b). Results showed minor visible improvement in nasolabial folds in 90 days
Figure 6.
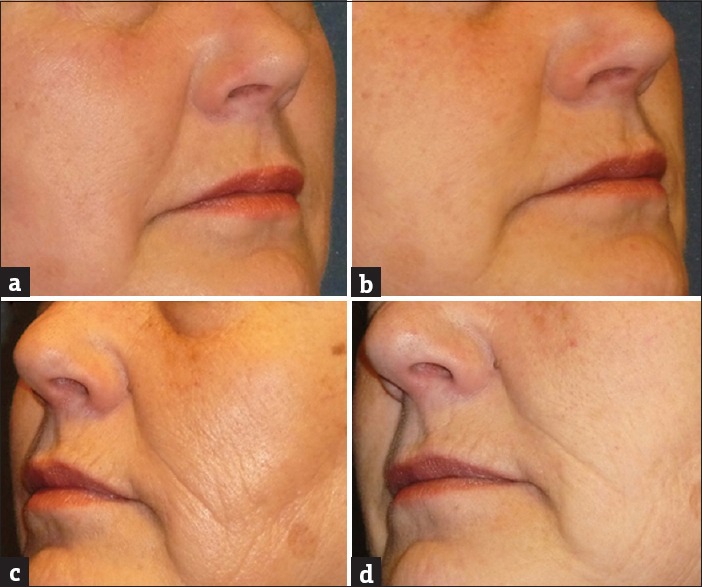
A 61-year-old subject demonstrating the effect of GFC in nasolabial folds before screening (a) and at the end of the study (b). Results showed significant improvement in nasolabial folds in 90 days. Similarly, the effect of PRP in nasolabial folds before screening (c) and at the end of the study (d) showed minor visible improvement in nasolabial folds in 90 days
Figure 7.
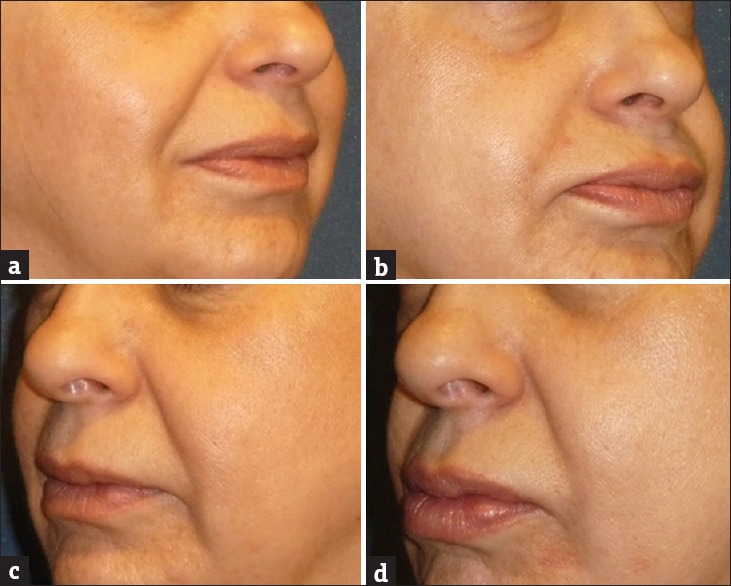
A 54-year-old subject demonstrating the effect of GFC in nasolabial folds before screening (a) and at the end of the study (b). Results showed significant improvement in nasolabial folds in 90 days. Similarly, the effect of PRP in nasolabial folds before screening (c) and at the end of the study (d) showed minor visible improvement in nasolabial folds in 90 days
At the end of study, assessment was made by treating physician, blinded investigator and patients. In the objective, Physician assessment in the PRP was ranging from 0 to 1.65 with an average of 0.84 (improvement <25%), whereas in GFC score was in the range of 0.5 to 2.5 with average of 1.49 (26–50%) that is higher than PRP (P < 0.001). Similarly, objective assessment score by the blinded investigators was significantly better in GFC than PRP (P < 0.001). Whereas in patient assessment score, PRP was 0.5 to 2.5 with mean of 1.53 (26–50%) and GFC was 1.5 to 3.5 with an average score of 2.47 (improvement 51–75%), which was significantly higher than PRP (P < 0.0001) [Figure 9].
Figure 9.
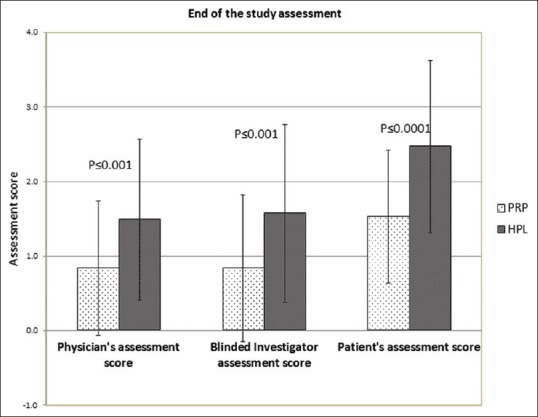
Objective assessment by treating physician, blinded investigators and patient assessment score at the end of the study. Assessment score in GFC treated subjects was significantly higher than PRP treated subjects
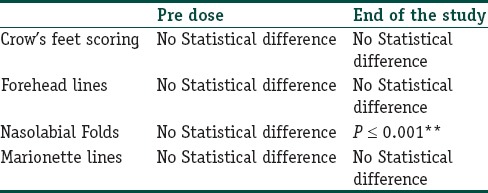
Comparison of pre and post treatment (between groups) for GAIS was done using Mann–Whitney U test and Wilcoxon signed ranked test and is represented in Table 3. Improvement in GAIS score between GFC and PRP for nasolabial folds was highly significant (P < 0.001). In group II, out of 60 subjects, 34 showed superior improvements for GFC (66%) and 6 patients showed similar improvement on both side of the face (11%). Ten patients showed no noticeable improvement on the either side of the patient's face (19.6%). Only one patient showed superior improvement for PRP at the end of the study (1.9%). Nine subjects lost to follow-up.
Table 3.
Improvement score analysis between HPL and PRP in group-II study by Wilcoxon Signed Ranked test
DISCUSSION
Correction of facial folds and restoration to its original shape is a key approach to rejuvenation and enhancement of facial appearance. Several surgical and non-surgical treatment modalities have been reported and currently being used regularly in the aesthetic clinics.[12,13,14]
Autologous PRP is now being used in a wide variety of clinical application. There are several reports of using PRP in facial folds.[8,15,16,17]
Purpose of this split face study was to clinically analyze GFC for its safety and efficacy in facial wrinkle condition in comparison with PRP. Both PRP and GFC showed significant improvement in GAIS. However, improvement was highly significant in GFC than PRP. This showed that both the treatments modalities are good in its way, but GFC was superior in clinical outcome compared to PRP.
Multiplex ELISA analysis demonstrated that autologous GFC contains several signaling proteins like non-inflammatory cytokines, growth factors, chemokines, adhesive proteins and basal proteins. The presence of such signaling proteins was reported to have favorable effects on fibroblast proliferation, cell migration and regulation of MMP and neo-collagen synthesis. We have shown that level of prominent growth factors from 625 million platelets/ml was significantly higher than calcium-activated PRP when compared with earlier reported literature.[18]
We have observed that GFC or PRP was found to be less effective if given to the patient above 60 years of age. It may be because of inheriting low level of growth factors in the platelets derived from the older patients (data not shown). Studies showed that GFC derived from older donors increased activity of senescence-associated β-galactosidase and hence supported the notion that aging is associated with systemic feedback mechanism that conversely influences cell intrinsic aging programs. Perhaps, cell intrinsic age-associated epigenetic modifications of the fibroblasts might be influenced by systemic factors and may be ineffective or less effective when treated with their own growth factors.[19] Perhaps in older patients higher concentration of GFC may be required for effective results.
Mechanism of action of GFC is not well studied, however, we hypothesized that TGF-β, EGF and bFGF are the most important growth factors and affect most aspect of tissue repair like initiation, proliferation and differentiation of dermal cells.[20] GFC increases level of MMP-1, MMP-3 and MMP-9 and corrects the ratio of MMP and TIMP that helps in removal of damaged collagen and stimulation of de novo collagen synthesis that may compensate for the defect that arises due to nasolabial folds and other facial folds.[21] Newly synthesize collagen may improve the structural integrity of the dermal ECM and stimulate fibroblast to produce more collagen.[22] We have also seen very high level of chemokine factors in GFC like growth-regulated oncogene-α (GRO-α) and regulated on activation normal T cell expressed and secreted (RANTES). These factors might be helping in mobilizing endogenous stem cells for regeneration and remodeling the tissue.
For optimum clinical results in facial wrinkle, it is likely a function of many variables that includes platelet concentration, volume of injection and severity of condition. There is no clear definition and recommendation for the fold increase of platelet concentration over baseline for optimum clinical outcome. In PRP, platelet concentration is significantly variable from patient to patient. Lower concentration of platelets may not yield optimal clinical outcome whereas higher concentration may be inhibitory for cell proliferation. The GFC prepared in this study was derived from fixed number of platelets (625 × 106 per ml) and therefore variation in the platelet concentration was eliminated in a larger extent. This platelet concentration was identified as a therapeutic dose after analyzing various platelet concentrations under in vitro conditions using the skin fibroblast proliferation assay.
There are several current therapies for facial folds in practice including topical retinoic acid, Botulinum toxic type-A, fillers, collagen injection, and laser resurfacing and PRP. Each therapy has their limitations and side effects. We are presenting for the first time the effect of growth factors derived from the autologous platelets in the form of GFC for facial rejuvenation. In the present study, we have shown that the single injection of GFC significantly improves facial folds. These observations proved that GFC can be used as a standalone treatment for facial folds.
In conclusion, this study shows that a single application of GFC improves facial folds which touted it as a possible alternative as a standalone treatment. The treatment is safe and simple and there is no down time. Hence as a standalone treatment GFC should be studied for long term implications as well as on a larger population.
What is new?
Growth factor concentrate (GFC) contains consistent level of growth factors derived from the known number of the platelets for therapeutic purpose. GFC is free from cells, cell membranes and exogenous reagents and chemicals. Single application of GFC improves nasolabial fold correction which touted it as a possible alternative as a standalone treatment.
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
References
- 1.Beer K, Beer J. Overview of facial aging. Facial Plast Surg. 2009;5:281–4. doi: 10.1055/s-0029-1243075. [DOI] [PubMed] [Google Scholar]
- 2.Glogau RG. Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg. 1996;5:134–8. doi: 10.1016/s1085-5629(96)80003-4. [DOI] [PubMed] [Google Scholar]
- 3.Jacovella PF. Calcium hydroxylapatite facial filler (Radiesse): Indications, technique and results. Clin Plast Surg. 2006;33:511–3. doi: 10.1016/j.cps.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Imayama S, Nakamura K, Takeuchi M, Hori Y, Takema Y, Sakaino Y, et al. Ultraviolet-B irradiation deforms the configuration of elastic fibres during the induction of actinic elastosis in rats. J Dermatol Sci. 1994;7:32–8. doi: 10.1016/0923-1811(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 5.Imokawa G, Takema Y, Yorimoto Y, Tsukahara K, Kawai M, Imayama S. Degree of ultraviolet-induced tortuosity of elastic fibres in rat skin is age dependent. J Invest Dermatol. 1995;105:254–8. doi: 10.1111/1523-1747.ep12317607. [DOI] [PubMed] [Google Scholar]
- 6.Takema Y, Imokawa G. The effect of UVA and UVB irradiation on the viscoelastic properties of hairless mouse skin in vivo . Dermatology. 1998;196:397–400. doi: 10.1159/000017931. [DOI] [PubMed] [Google Scholar]
- 7.Jacovella PF. Use of calcium hydroxylapatite (Radiesse) for facial augmentation. Clin Interv Aging. 2008;3:161–74. doi: 10.2147/cia.s2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang BK, Shin MK, Lee JH, Kim NI. Effects of platelet-rich plasma on folds and skin tone on Asian lower eye lid skin: Preliminary results from a prospective, randomized split face trial. Eur J Dermatol. 2014:100–1. doi: 10.1684/ejd.2014.2267. [DOI] [PubMed] [Google Scholar]
- 9.Wang HL, Avila G. Platelet rich plasma: Myth or reality? Eur J Dent. 2007;1:192–4. [PMC free article] [PubMed] [Google Scholar]
- 10.Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Impl Res. 2006;17:212–9. doi: 10.1111/j.1600-0501.2005.01203.x. [DOI] [PubMed] [Google Scholar]
- 11.Kukudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation promoting effect of platelet-rich plasma on human adipose derived stem cells and human dermal fibroblast. Plast Reconstrsurg. 2008;122:1352–60. doi: 10.1097/PRS.0b013e3181882046. [DOI] [PubMed] [Google Scholar]
- 12.Knapp TR, Kaplan EN, Daniels JR. Injectable collagen for soft tissue augmentation. Plast Reconstr Surg. 1997;60:398–405. [PubMed] [Google Scholar]
- 13.Stegman SJ, Chu S, Armstrong RC. Adverse reactions to bovine collagen implant: Clinical and histologic features. J Dermatol Surg Oncol. 1988;14:39–48. [Google Scholar]
- 14.Baumann LS, Shamban AT, Lupo MP, Monheit GD, Thomas JA, Murphy DK. Comparison of smooth-gel hyaluronic acid dermal fillers with cross-linked bovine collagen: A multicenter, double-masked, randomized, within-subject study. Dermatol Surg. 2007;33(Suppl 2):S128–35. doi: 10.1111/j.1524-4725.2007.33352.x. [DOI] [PubMed] [Google Scholar]
- 15.Sclafani AP, Saman M. Platelet-rich fibrin matrix for facial plastic surgery. Facial Plast Surg Clin North Am. 2012;20:177–86. doi: 10.1016/j.fsc.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Redaelli A, Romano D, Marcianó A. Face and neck revitalization with platelet rich plasma (PRP): Clinical outcome in a series of 23 consecutively treated patients. J Drugs Dermatol. 2010;9:466–72. [PubMed] [Google Scholar]
- 17.Sclafani AP. Platelet rich fibrin matrix for improvement of deep nasolabial folds. J Cosmet Dermatol. 2010;9:66–71. doi: 10.1111/j.1473-2165.2010.00486.x. [DOI] [PubMed] [Google Scholar]
- 18.Park HB, Yang JH, Chung KH. Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Up-regulation of matrix metalloproteinase-1 and -9 in HaCaT cells. Korean J Hematol. 2011;46:265–73. doi: 10.5045/kjh.2011.46.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohmann M, Walenda G, Hemeda H, Joussen S, Drescher W, Jockenhoevel S, et al. Donor Age of Human Platelet Lysate affects proliferation and differentiation of Mesenchymal Stem Cells. PLoS One. 2012;7:e37839. doi: 10.1371/journal.pone.0037839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi Y, Fuchs E. TGF-beta and retinoic acid: Regulators of growth and modifiers of differentiation in human epidermal cells. Cell Regul. 1990;1:791–809. doi: 10.1091/mbc.1.11.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DH, Je YJ, Kim CD, Lee YH, Seo YJ, Lee JH, et al. Can Platelet-rich Plasma Be Used for Skin Rejuvenation? Evaluation of Effects of Platelet-rich Plasma on Human Dermal Fibroblast. Ann Dermatol. 2011;23:424–31. doi: 10.5021/ad.2011.23.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almeida Issa MC, Piñeiro-Maceira J, Farias RE, Pureza M, Raggio Luiz R, Manela-Azulay M. Immunohistochemical expression of matrix metalloproteinases in photodamaged skin by photodynamic therapy. Br J Dermatol. 2009;161:647–53. doi: 10.1111/j.1365-2133.2009.09326.x. [DOI] [PubMed] [Google Scholar]


