Abstract
Acinetobacter baumannii is an important source of infections in intensive care units (ICUs) of our hospitals in Kerman, Iran and the most frequently isolated strains produce biofilm. There is a little information about role of iron (Fe) levels on acyl homoserine lactone (AHL) production and biofilm formation in this microorganism. In the present study, we investigated the influence of iron-III limitation on AHL, siderophore, catechol and virulence factors in the biofilm forming clinical strains of A. baumannii. A total of 65 non-duplicated multidrug resistance (MDR) strains of A. baumannii were isolated from patients in ICUs of 2 hospitals in Kerman, Iran. Antibiotic susceptibility, siderophore and other iron chelators, hemolysis, cell twitching motility, capsule, gelatinase and DNase were studied. Presence of quorum sensing, LuxI and LuxR genes was detected by multiplex-PCR. AHL activity quantified by colorimetric method and the functional groups were determined by Fourier Transform Infra-Red Spectroscopy (FT-IR). Biofilm formation was detected by microtiter plate technique. All of the isolates were resistant to third generation of cephalosporins, ciprofloxacin, levofloxacin, tetracycline, whereas, 78% and 81% were resistant to amikacin and carbapenems, respectively. The siderophore activity was highest at 20 μM Fe3+ (70%); however, it decreased to 45% as concentration of Fe3+ increased to 80 μM. Furthermore, screening of the isolates for LuxI and LuxR genes showed that presence of both genes required in the isolates with high AHL activity. FT-IR analysis indicated C=O bond of the lactone ring and primary amides. Significantly, a higher amount of AHL (70%) was detected in the presence of low concentration of iron-III (20 μM); as iron concentration increased to 80 μM, the AHL activity was reduced to 40% (P ≤ 0.05). All the isolates exhibited twitching motility and had a capsule. No any gelatinase or DNase activity was detected. Quantification of the biofilm formation introduced 23 isolates with efficient attachment to microplate wells and strong biofilm. We found that both the AHL production and biofilm formation were regulated by iron concentration in a dose dependent manner. These findings provide evidence that iron limitation plays an important regulatory role in AHL and siderophore production resulting in strong or weak biofilm, thereby helping the organism to persist in less available micronutrient environment.
Keywords: Acinetobacter baumannii, biofilm, iron, N-Acyl homoserine lactone, siderophore
Introduction
A. baumannii is a gram negative opportunistic pathogen causing life threatening infections in immuncompromised patients, elderly and people hospitalized in the hospitals for a long period of time.1,2 One of important therapeutic features of this bacterium is resistance to many antibiotics including third generation of cephalosporins, carbapenems, aminoglycosides, monobactams and fluoroquinolones.3-6 Its remarkable widespread resistance to different antibiotics and its ability to persist in nosocomial environments and medical devices makes A. baumannii a threat to the hospitals, as documented by recurring outbreaks both in highly developed countries and elsewhere.7-9
There are several reports indicating emergence of the multidrug resistant A. baumannii (MDR-AB) in different hospital units in Iran.10 The rate of Infections due to MDR-AB in Tehran (Iran) hospitals showed a drastic increase (30%) in resistance to different antibiotics within 5 years, while all the isolates remained susceptible to either minocycline or tobramycine.11A recent investigation of MDR-AB infection associated with respiratory tract specimens from one teaching hospital in the southern Tehran showed that among 101 isolates, 96% were resistant to third generation of cephalosporins, 93% to amikacin, and 85% to ciprofloxacin.12
Most of the A. baumannii strains can cause biofilm related infections due to contaminated catheter, ventilator, endotracheal tubes or internal prosthetic devices, and bring about very high mortality rate especially in the ICUs where patients do not respond to many antibiotics.13The development of a biofilm may allow for better protection from environmental conditions, less subjected to mutations, and to be increasingly antibiotic resistant as compare to planktonic cells.
Little research is carried out on how the bacterial cells communicate with each other and produce biofilm. Such communication used by the bacteria is chemical in nature and generally designated as quorum sensing (QS), small diffusible molecules produced by bacteria which can reach other cells and help to communicate.14,15
Acyl homoserine lactones (AHLs) are major class of autoinducer signals used by Gram negative bacteria to regulate diverse physiological functions such as virulence factors and biofilm.16In A. baumannii, 2 major genes, LuxI and LuxR, that encodes an AbaI synthase and AbaR receptor are involved in AHL production and regulation.17AHL has been found to be a major component for biofilm formation in A. baumannii that has been reported by various authors.18 In A. baumannii, N-(3-hydroxydodecanoyl)-l-homoserine lactone (3-hydroxy-C12-HSL) is the only quorum sensing molecule identified, though mass spectrometry suggested that other AHL molecules are also present at significantly lower amounts.19-21However,contribution of quorum sensors and iron concentration to the overall pathogenic potential in the genus is currently unknown.
In the present investigation, we assessed the influence of iron-III limitation on AHL, siderophore, other catechol groups and related virulence factors in biofilm forming strains of A. baumannii isolated from ICUs of 2 main hospitals in Kerman, Iran.
Results
Bacterial sources and antibiotic susceptibility
Previous results from our laboratory indicated A. baumannii is found almost exclusively in the hospital environment, particularly ICU.10 In present study, a total of 65 MDR A. baumannii were isolated from patients hospitalized in ICUs of 2 main university hospitals (AfzaliPoor, and Shahid-Bahonar with 250 and 170-bed) in the city of Kerman, southeast of Iran during 7 months. 42% (n = 27) of the isolates were recovered from patients admitted with pulmonary and lower respiratory tract infections. 27% (n = 18) of the isolates collected from patients admitted with signs of urinary tract infection (UTI) and remaining isolates were obtained from patients had surgical procedures. Majority of the patients (38%) in ICU had aged ≤50 years. 65% were male and 45% were female.
All of the isolates detected by the analytical profile index (API) 20NE assay (BioMérieux, Marcy l'Etoile, France) and showing the blaOXA-51 like gene PCR band at 324bp in agarose gel electrophoresis were considered as A. baumannii and selected for further study (Fig. S1).
The antibiotic resistance pattern of the MDR A. baumannii isolates to 16 antibiotics commonly used in our hospitals are shown in Table 1. All of the isolates were resistant to third generation of cephalosporins, ciprofloxacin, levofloxacin, tetracycline and piperacillin, whereas, 78% and 81% were resistant to amikacin and carbapenems, respectively. The MDR-AB isolates were further tested for tigecycline and colistin by microdilution method (Table 1). It was found that only 7% and 13% of the isolates were resistant to these 2 antibiotics.
Table 1.
Antibiotic susceptibility of A. baumannii isolated from ICU patients in 2 main hospitals in Kerman, Iran to 16 antibiotics
| Sensitivity |
|||
|---|---|---|---|
| Antibiotica | Resistant | Intermediate | Sensitive |
| CFM | 100% | 0% | 0% |
| GM | 92% | 5% | 7% |
| TOB | 48% | 28% | 24% |
| AN | 78% | 9% | 12% |
| PIP | 100% | 0% | 0% |
| CAZ | 100% | 0% | 0% |
| CTX | 100% | 0% | 0% |
| NA | 100% | 0% | 0% |
| RIF | 100% | 0% | 0% |
| IMP | 81% | 7% | 11% |
| MEM | 93% | 4% | 3% |
| CL | 13% | 0% | 87% |
| TE | 100% | 0% | 0% |
| TIG | 7% | 4% | 89% |
| LE | 100% | 0% | 0% |
| CIP | 100% | 0% | 0% |
CFM = Cefixime, GM = gentamicin, TOB = tobramycin, AN = amikacin, PIP = piperacillin, LE =levofloxacin, CAZ = ceftazidime, CTX = cefotaxime, NA = nalidixic acid, RIF = rifampin, IMP = imipenem, MEM = meropenem, CL = colistin, TIG = tigecycline. Muller-Hinton agar was used for susceptibility test. The inoculum was at concentration 1 × 108. The break point for each antibiotic was calculated according to the CLSI procedure.
Virulence factors
The maximum hemolytic activity was observed for horse red blood cells (12%), while in the case of sheep red blood cells no hemolysis activity was detected (P ≤ 0.05) (Fig. 1A). Positive control (Pseudomonas aeruginosa PAO1) showed the highest percentage of hemolysis. Furthermore, all exhibited twitching motility and had capsule. No any gelatinase or DNase activities were detected in the entire bacterial population.
Figure 1.
(A) Comparative hemolytic activity of A. baumannii isolates in the presence of defibrinated sheep and horse red blood cells. NC = negative control (medium without bacteria), P.aeruginosa PAO1 = positive control showing hemolysis. The percent hemolysis in supernatant was measured at OD620 nm. Values represent the mean (± SD) of 3 independent experiments. (B) Quantification of siderophore in A. baumannii isolates in 3 different Fe concentrations. Values are expressed as percentage of siderophore units (U) normalized to the cell density OD600nm of bacterial culture. † = values are the means for a representative assay performed in triplicate. (C) Quantification of catechol groups in 3 different Fe concentrations. Presence of catechol groups in the supernatants of the A. baumannii isolates was detected by the Arnow test. Iron-chelating activity in culture supernatants of A. baumannii strains grown in Fe-limited medium showed with different color. A P ≤ 0.05 was considered as statistically significant for 2-tailed tests. (D and E) Effect of temperature on siderophore activity and pH on siderophore activity.
Quantification of siderophore and other catechol groups
To determine whether iron concentration has any effect on siderophore activity, we measured the amount of siderophore at 3 different FeCl3 concentrations (20, 50 and 80 μM) as presented in Fig. 1B. One A. baumannii isolate had maximum siderophore activity (70%) at 20 μM FeCl3 concentration and 11 isolates had 60%. As FeCl3 concentration increased in the Fe-limited medium to 50 μM, the siderophore activity reduced considerably. The quantity of siderophore for AHL producing strains decreased further (5%) as FeCl3 concentration increased to 80 μM (P ≤ 0.05). Similar results were observed for catechol activity among the isolates at 3 different FeCl3 concentrations (20, 50, 80 μM) as illustrated in Fig. 1C. In 20 μM of FeCl3, maximum catechol was detected (40 μM); however, increase in FeCl3 concentration to 80 μM resulted in loss of most of the catechol activities (4 μM). The above results were compared with positive (A. baumannii ATCC 19606) and negative (without bacteria) controls. The mean value for each reading was calculated according to Fisher exact test.
Effect of temperature and pH on siderophore activity
The effects of temperature and pH on siderophore activity are shown in Figure 1D and E. We found that the siderophore activity in 3 different pH (5, 7 and 9) was more or less the same. All assays were performed in 50 μM FeCl3 concentration. At pH- 5, 7, and 9, no significant difference in siderophore activity was observed (SD = ± 0.1- 0.2%) suggesting that changing pH of the medium may not contribute to siderophore quantity. Furthermore, the Arnow assay showed that, 3 different temperatures (30, 37 and 40°C) had no any significant role in the amounts of siderophore when cultured in iron limited medium supplemented with appropriate carbon source.
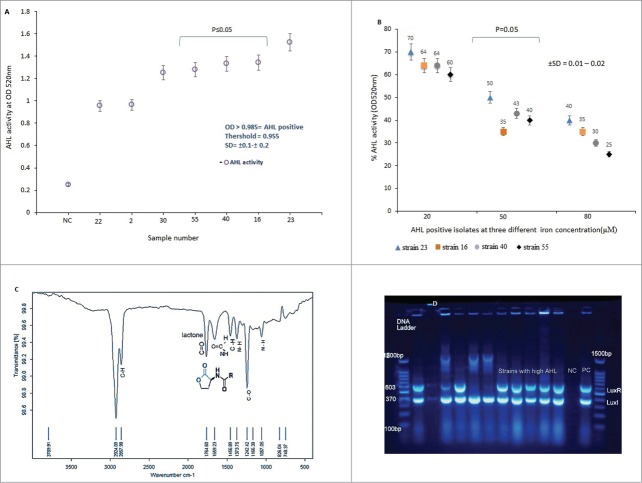
Effect of iron- III on AHL activity
The isolates were screened for AHL activity. It was found that, isolates number 30, 55, 40, 16 and 23 produced higher amount of AHL as compared to the other strains. Isolate number 23 exhibited the highest AHL activity (OD ≥ 1.524), while, isolate 22 showed minimum AHL productions (OD ≥ 0.955). Indeed, the average optical density (OD) for this strain was below the recommended absorbance at OD ≥ 0 .985, as shown in Fig. 2A. It was found that both the AHL and siderophore production was regulated by iron concentration in a dose dependent manner. Under iron limitation (Fe 20 μM) the production of AHL increased considerably; however, further activity was lost as iron concentration increased to 80 μM (Fig. 2B). We observed that growth rates of the test isolates were not significantly affected over the range of 20–80 μM iron-III (data not shown), indicating that iron does not simply interfere with CFU/mL and biofilm formation.
Figure 3.
(A) Biofilm formation of A. baumannii isolates investigated in this study. The above results are average of 3 OD reading at 570nm.The amount of biofilm remaining was determined by the absorbance of the crystal violet dye.39 Error bars represent the standard deviation from the mean of 3 observations. A standard culture of P. aeruginosa PAO1 was used as positive control. Growth was expressed as percentage relative to the untreated control.
Figure 2.
(A) N-acyl homoserine lactone (AHL) activities of A. baumannii isolates collected in this study. Threshold for AHL production is shown in the graph. NC = negative control. Intensity of color was measured at OD520nm.The results are mean of 3 simultaneous experiments. A P ≤ 0.05 was considered as statistically significant for 2-tailed tests. (B) Effect of iron-III concentrations on AHL activity in A. baumannii. Error bars represent the standard errors of the means for a representative assay performed in triplicate. X axis indicates A. baumannii isolates that showed a high and low AHL. (C) FT – IR spectra of AHL produced by A. baumannii isolates. The AHL was extract from organism by LLE- methods as described in the text. The pure compound was then subjected to FT-IR spectroscopy. The lactone ring and amide group were shown at 1764.69cm−1 and 1659.23cm−1 wave number. (D) Detection of LuxI (370 bp) and LuxR (603 bp) quorum sensing genes in A. baumannii isolates by multiplex-PCR. Electrophoresis was carried out in 1% agarose gel for 2 h at 80 V, the gel was stained with tracking dye (Syber green).
Detection of AHL functional group
FT-IR spectrum of AHL functional groups are presented in Fig. 2C. The existence of a peak at 1659 cm−1 was due to carbonyl group of amide (stretching bond). Similarly, analysis of the transmission peak at approximately 1764 cm−1 was correspond to the C=O stretching bond of the lactone ring. The peak at wave number 1373 cm−1 indicated the presence of N-H (bending bond) functional amide group. The IR spectrum of the AHL appeared at the same wave number of the reported literature and confirmed the existence of AHL obtained in this study.
Detection of LuxI and LuxR genes by multiplex-PCR
All isolates were screened for quorum sensing genes, LuxI and LuxR by M-PCR. It was found that 80% (n = 52) of the A. baumannii isolates harbored the LuxI gene, while, 61% (n = 40) of the strains carried both LuxR and LuxI genes (Fig. 2D), furthermore, in only 31% of the bacterial population, LuxR gene was not detected. In addition, PCR amplicon of 5 isolates showed high AHL activity and presence of both LuxI and LuxR QS genes, as shown in Figure 2D suggesting that for high AHL production, expression of both the genes were required.
Biofilm formation under static condition
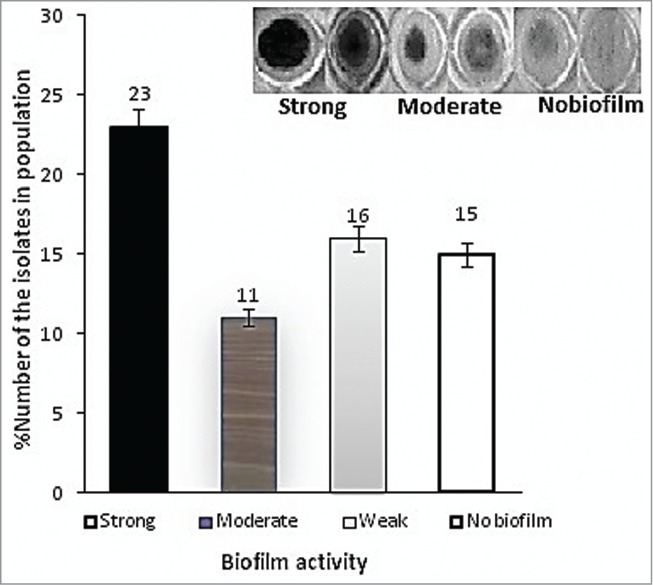
Using a defined Fe-limited minimal medium and Fe supplementation, we examined the specific impacts of various iron-III levels on biofilm formation. Primary attachment assays revealed a significant initial binding difference among those isolates with strong, moderate or weak biofilm to polystyrene surface (66.7 ± 0.2%, 47.3 ± 0.4 and 22.3 ± 0.1) compared with initial inoculums. Quantitative analysis of biofilm formed on polystyrene surfaces of the represented strains introduced 23 isolates with strong (severely adherent), 11 isolates with moderate (moderately adherent), 16 isolates with weak (weakly adherent), and 15 isolates (23%) with no biofilm formation, as shown in Fig. 3A.
To proof increase in optical density (OD) was due to increase in bacterial biofilm, we determined CFU/mL count of the each isolate at different iron concentrations. It was found that, increase in the biofilm formation was indeed due to increase in cell attachment to microtiter plate wells (data not shown). The results obtained by us also indicated that, those isolates producing high AHL at low iron concentration also formed strong biofilm. Similar observation was also made in the case of siderophore production. Table 2 shows direct relationship between the amount of iron, AHL, siderophore and biofilm formation in A. baumannii isolates in present study. As the iron concentration increased in the chemically defined medium, both the amount of AHL and siderophore decreased in dose dependent manner which resulted in a change in the biofilm activity from strong to weak.
Table 2.
Effect of iron-III concentrations on AHL, siderophore and biofilm formation by A.baumannii isolates investigated in this study
| Iron concentration (μM) | AHL* (μM) | Siderophore (U)C | Biofilm (adherence) |
|---|---|---|---|
| 20a | 74b | 40c | Strong |
| 50 | 64 | 35 | Moderate |
| 80 | 36 | 25 | Weak |
= N-Acyl homoserine lactone,a=μM, b= μM, c = U
The above results are average of 3 independent experiments. Numbers in the table indicate the % of activity.
Discussion
The role of iron limitation on AHL production and biofilm formation in the strains of A. baumannii is only shown in a few publications.21,22 In this study, we aimed to determine this phenomenon for the first time and confirm that indeed both the biofilm formation and AHL production are under regulation of iron concentration. For this reason, A. baumannii clinical isolates were selected for this study because their resistance to the majority of the antibiotics tested and their ability to form biofilms in- vitro.
It was observed that the majority of clinical isolates was significantly formed strong adherents to microplate wells and also produced high levels of AHL, siderophore and low molecular weight organic chelators with high affinity for Fe3+. The quorum sensing genes (LuxI) were detected in both high AHL producing and low AHL producing strains, while, both LuxR and LuxI genes were harbored mainly in the strain with strong biofilm indicating that for production of higher amount of AHL and strong biofilm, expression of both the genes were required. The presence of AHL was further supported by FT-IR spectroscopy, demonstrated presence of lactone ring attached to the amide group.
We found that, when the concentration of iron-III in the iron poor medium was restricted, the activity of both AHL and siderophore was increased which resulted in strong biofilm. However, the isolates lost ability to form strong biofilm in response to high concentration of iron. It is likely that iron plays an important regulatory role in production of AHL and other signal transduction factors resulting in strong or weak biofilm. Indeed, iron played an important role in regulation of this phenomenon in dose dependent manner. The environmental factors like pH and temperature did not influence siderophore production. We also could not find any relationship between formation of capsule, and hemolysis of horse red blood cells with iron concentrations and biofilm activity in this research.
Iron acquisition and quorum sensing system using N-acyl homoserine lactone signals enable bacterial cells to live under inappropriate environmental conditions (like low concentration of micronutrients) and form biofilm.23,24 QS was found to play a major role in biofilm maintenance and maturation in Acinetobacter.
Experiments by Gentile et al.25 revealed that neither planktonic nor biofilm growth of A. baumannii was affected by chelators (biofilm formation was either stimulated by iron or not responsive to iron in the majority of isolates tested, indicating that iron starvation had no effect on biofilm). In another report on P. aeruginosa, it was shown that, high iron concentration inhibited biofilm formation (as iron concentration decreased in the medium the biofilm formation was enhanced).26A study conducted on influence of iron on biofilm formation in Staphylococcus aureus indicated that all strains showed enhanced biofilm formation under low-iron conditions, whereas addition of iron resulted in a significant decrease in biofilm. (It was also shown that biofilm formation was not influenced by changes in pH resulting from addition of ferric sulfate to the medium).27In pathogens such as Escherichia coli, Vibrio cholerae, and Corynebacterium diphtheriae, iron levels dictate the expression of virulence-associated genes, and the production of those virulence factors reaches a maximal level when the concentration of iron is lower than that required for optimal growth.28
Further research is recommended be carried out to address the molecular mechanisms of the iron limitation on AHL and siderophore production in the biofilm forming A. baumannii.
Materials and Methods
Chemicals
Chromo-Azurol S (CAS), 2,2-bipyridine (iron-III), C8-HSL 3-hydroxy-C12-HSL fatty acids, Methylene chloride (CH2Cl2), Na2SO4, Hydroxyl ammonium chloride (ClH4NO), FeCl3, Acetonitryl (HPLC grade), Ethidium bromide (EB) [C21H20BrN3], Tris-HCl and Methanol were purchased from Sigma-Aldrich, Merck, Biomol and QIAGEN companies, respectively. All other chemicals were of analytical grades.
Patients and bacterial source
From February to August 2013, a total of 65 non-repeated MDR strains of A. baumannii were isolated from the specimens collected from 266 patients hospitalized in the ICUs of 2 main hospitals in the city of Kerman, south east of Iran. Only A. baumannii were included in this study and the other Acinetobacter species were excluded. Prior to collection of the samples, demographic criteria including sex, age, patient clinical condition such as chronic obstructive pulmonary diseases (COPD), hypotension, fever, tachypnea, tachycardia, ventilator associated pneumonia, catheter associated urinary tract infections (UTIs), length of hospitalization and previous antibiotic exposure were considered. Samples were collected by an expert laboratory technician, inoculated into 5 mL sterile Stuart Transport medium (Merck, Darmstadt- Germany) and broth to the Department of Microbiology laboratory within 24 h of the collections.
Bacterial identification
Primary bacterial identification was performed by biochemical tests.29 The overnight growth of the organisms on blood and Luria- Bertani (LB) agar were checked on the basis of Gram staining, colonial morphology on cysteine electrolyte deficient agar (CLED) (Hi-media, India), motility test, cytochrome oxidase reaction (Oxidase test), catalase test, and ability to ferment various sugars.
A species level was confirmed using the analytical profile index (API) 20NE assay (BioMérieux, Marcy l'Etoile, France) and blaOXA-51 gene amplification by polymerase chain reaction (PCR) method. For blaOXA-51 PCR, genomic DNA of A. baumannii isolates was extracted by DNA purification kit (Thermo SCIENTIFIC, Lithuania). The reactions for PCR were initiated in the solution containing 200 μM concentrations of dNTPs, 10 pM of forward primer (5′-TAA TGC TTT GAT CGG CCT TG-3′) and reversed primer (5′-TGG ATT GCA CTTCATCTT GG-3′). Primers used were manufactured by Thermo scientific, Lithuania. 0.8 mM MgCl2, 0.5 U Taq polymerase (Amplicon, Denmark) and 50 ng DNA template in a final volume of 25 μL. DNA ladder consisted of a plasmid double digest sized range of 100–1500 bp obtained from Cinnagen Co. (Tehran, Iran). The specificity of the candidate primers for blaOXA-51 sequence in the database was verified by blast analysis (http://www.ncbi.nlm.nih.gov/GeneBank). DNA amplification was conducted in gradient thermal cycler (Biometra-T gradient, Australia). PCR conditions were as follows: initial denaturation temperature was at 94°C for 5 min, 30 cycles of 94°C for 35 s, annealing 60°C for 35 s respectively followed by 72°C for 40 s with final, elongation step which was set at 72°C for 6 min. DNA of the reference strains of A.baumannii ATCC 19606 and E.coli DH5α [dlacZ Delta M15 Delta (lacZYA-argF) U169 recA1 endA1 hsdR17(rK-mK+) supE44 thi-1 gyrA96 relA1)] were run simultaneously as positive and negative controls, respectively. The identified isolates were mixed with 40% glycerol in DNase free True North TM Cryogenic Vials (TNC) containing 1 mL TSB medium and preserved at −70°C.
Antibiotic susceptibility
Antimicrobial susceptibility of all A. baumannii isolated in this study was carried out by the Kirby-Bauer (KB) disk diffusion breakpoint method according to Clinical Laboratory Standards Institute (CLSI 2012) guide lines.30Oxoid antibiotic disks (Hi-media, India) were used in the following concentrations (in μg/mL−1): tetracycline (TE) [30 μg], amikacin (AN) [30 μg], tigecycline (TIG) [15 μg], colistin (CL) [10 μg], gentamicin (GM) [10 μg], piperacillin (PIP) [100 μg],ciprofloxacin (CIP) [5 μg], ceftazidime (CAZ) [30 μg], cefotaxime (CTX) [30 μg], tobramycin (TOB) [10 μg], amoxicillin + clavulanic acid (AMC) [30/10 μg], rifampin (Rif) [30 μg], cefixime (CFM) [5 μg], nalidixic acid (NA) [30 μg], imipenem (IMP) [10 μg] and meropenem (MEM) [10 μg]. Susceptibility to tigecycline was classified based on EUCAST 2012 criteria [www.eucast.com] (susceptible, MIC of ≤0.5 μg/mL or zone around disk ≤17 mm). For susceptibility testing, freshly prepared 200 μL of inoculum at concentration 1 × 108 was inoculated and spread on Mueller-Hinton agar (MHA) (Merck-Germany). The antibiotic disks were placed at equal distances according to CLSI guidelines and incubated at 37°C for 24 h. The zone of inhibition surrounding each disk was measured and labeled as resistance, intermediate, and sensitive.
Screening for Virulence Factors
Hemolysis of horse and sheep red blood cells
For hemolysis, 2 types of red blood cells were used (sheep and horse), the bacterial cells grown in 100 mL Tryptic Soy Broth Dialysate (TSBD) medium (BioMérieux, Marcy l'Etoile, France) at 37°C for 14 h under shaking condition (150 rpm), optical density adjusted to 1 at OD600nm. The culture supernatant was separated from cell mass by filtration using sterile membrane filter size 0.25 μm (Sartorius-Germany). 0.9 mL of supernatant was then added into sterile 1.5 mL microfuge tube containing 0.1 mL (10%) horse and sheep defibrinated blood (EDTA 0.5 M) previously washed several times with sterile ice-cold phosphate buffered saline (PBS), pH- 7.4 and the suspension was incubated for 14 h at 37°C. Intact erythrocytes were harvested by centrifugation at 4000 rpm for 20 min at 4°C. The amount of hemoglobin released in the supernatant was evaluated by measuring OD545nm using UV/VIS spectrophotometer (Bausch and Lomb70-Belgium). The percentage of hemolysis (P) was calculated by employing the following equation P = (X−B) / (T−B) × 100 as described previously 23and then normalized to the cell density (OD600nm) of the bacterial culture. X is the OD545nm, while, B = negative control (DD/water) and T = total hemolysis. The experiment was repeated 3 times.
Detection of extracellular compounds with siderophore activity
The CAS assay by Chrome Azurol S (CAS) reagent was used to quantitate siderophore activity in culture supernatant extracts of A.baumannii by measuring the decrease in the absorbance of blue color at OD630nm.31 The presence of phenolic extracellular compounds was detected in culture supernatants with the Arnow colorimetric assay.32 Briefly, 1 × 106 CFU/mL of bacterial cells was inoculated into 10 mL volume of iron medium containing 20, 50, and 80 μM FeCl3 and incubated at 37°C for overnight. 0.1 mL of the growth was transferred into 10 mL of the freshly prepared same medium and incubated as described above, centrifuged at 10.000 rpm for 10 min. 0.5 mL supernatant was then mixed with 0.5 mL CAS assay solution and OD was measured at 630 nm. A flask with freshly prepared medium containing the above concentration of FeCl3 without bacteria was considered as negative control, while A. baumannii ATCC 19606 was used as positive siderophore producing control. The quantity of siderophore produced by the bacteria was reported in terms of iron-binding equivalents, expressed as micromoles of ligand per gram (dry weight) of bacteria. The experiment was performed in triplicate.
Presence of catechol groups
Presence of catechol groups in the supernatants of the A. baumannii isolates was detected by the Arnow test.32 To obtain the maximum amount; isolates were grown in LB broth medium overnight under shaking condition (15 rpm). The overnight cultures (0.5 mL) were collected by centrifugation and washed once with M9 medium without a carbon source. The washed bacteria were transferred to M9 medium (50 mL) supplemented with 5 mM sodium pyruvate plus 1% glucose and grown at 37°C with agitation. Growth was monitored by measuring the optical density at OD600nm; the isolates were then treated as the above section and centrifuged at 10.000 rpm (4°C). One mL of reagent A (0.5 N HCl), reagent B (10% Na nitrite, Na molybdate), and reagent C (1 N NaOH) were then successively added to an equal volume of culture supernatant. The reaction was measured by determining OD510nm after 10 min incubation at room temperature. Only FeCl3 and A.baumannii ATCC 19606 were used as negative and positive controls, respectively. Experiments were performed in duplicate.
Effect of pH and temperature on siderophore production
The procedures were the same as above but the pH of siderophore medium was adjusted to pH-5, 7 and 9 by the help of HCl (1 M) and NaOH (1 M) before the experiment. Similarly, for temperature 3 sets of the medium were prepared and inoculated with A. baumannii isolates. The tubes were incubated at 3 different temperatures (30, 37, and 40°C).
Cell twitching motility
Cell motility was studied as described previously.33 Twitching media were prepared containing 10 g tryptone, l.5 g NaCl and 0.3 % (W/V) agarose. The plates surfaces were inoculated with bacteria from an overnight LB agar culture either using sterile wooden sticks or by depositing 3 μL of freshly grown LB cultures (OD600nm = 0.3) and incubated at 24°C for 24 h in the dark. Twitching motility was assessed by removing the agarose layer, staining the plates with a 0.1% crystal violet solution for 30 min, and measuring the diameter of the motility disk.
Presence of capsule, gelatinase and DNase
Presence of capsule in all A. baumannii isolates was examined according the method described by Welch et al.34 Smears from 18 h culture of the above isolates grown in Litmus milk or Brain Hearth Infusion broth (BHI) (Hi-media, India) containing 2% of yeast extract were used for capsule staining. The smears were air dried and covered with 1% crystal violet for 2 min and rinse with a 20% solution of copper sulfate. All the slides were examined under an oil immersion lens.
The gelatinase test was performed on Tryptic agar (TA) containing 4% (W/V) gelatin; Frazier reagent (15% W/V) HgCl2 in 20% (V/V) HCl) was used for detection of gelatin hydrolysis. DNase Test Agar (Merck, Germany) was used for the determination of DNase activity and was prepared according to the manufacturer's description. The cultures were inoculated perpendicularly onto the surface test agar and were grown for 24 h at 37°C. HCl (1 N) solution was then poured onto the colonies and after 5 min the HCl solution was removed. The clear zones around the colonies indicated DNase activity.35
Detection of LuxI and LuxR genes by multiplex-PCR
Genomic DNA of the strains was extracted with DNA purification kit (Fermentas, Lithuania) as described by the manufacturer. PCR amplifications were performed with Taq DNA polymerase (Amplicon, Denmark) using primers for LuxI; PF (5′-GGTTGGGAGTTGAACTGTCC-3′) PR; (5′-AAACGTTCTACTCCAAGAGG 3′) and LuxR; PF (5′-TCGGATTTGATTATTGCGCTTATG-3′) PR; (5′-ACAGCTCGAATAGCTGCTG-3′). Amplification started with an initial denaturation step at 95°C for 2 min followed by 30 cycles of denaturation at 95°C, annealing at 58°C for 45 s and extension at 72°C for 1 min. The expected sizes of the PCR products for LuxI and LuxR were 370 bp and 603 bp, respectively.36 At the same time standard strain of A. baumannii ATCC 19606 was kept as positive control.
Detection of N-acylhomoserine lactone
The AHL activity of all A. baumannii isolates was detected by colorimetric method as described previously.37 1 mL of the bacterial isolates grown overnight in LB broth were transferred to Iron-medium and incubated at 37°C under constant shaking condition (150 rpm). Then, the whole inoculums were aseptically transferred into a 1.5 mL sterile centrifuge tubes (Eppendrof- Germany) and centrifuged at 10,000 rpm for 15 min and the cell pellets were discarded. The supernatant was filtered through 0.25 μm membrane filter (Sartorius-Germany) to remove the cell debris. Later, 60 mL of the filtrate was mixed with 30 mL of ethyl acetate (2: 1 ratio) and shaker incubated for 10 min. The mixture was allowed to stand for 5 min in a 200 mL separating Funnel Conical Pyrex to get 2 immiscible layers (organic layer and aqueous layer). The upper layer was organic and the bottom layer was aqueous. The organic layer was collected in a sterile tube and the remaining aqueous layer was extracted twice as described above. The entire organic layer was pooled and dried in oven at 40°C and concentrate sequentially taken to smaller vials. 400 μL of each sample was inoculated into 96 well plate polystyrene flat bottoms, tissue culture supplemented with 50 μL mixture of 2 M C1H4NO and 3.5 M NaOH. To this mixture, the same amounts of FeCl3 (10% in 4 M HCl) and ethanol (95%) (Merck-Germany) were added. A dark brown color (indicates production of AHL) suspension was measured at the optical density 520nm. Standard C8-HSL was used as positive control. Under alkaline conditions, AHLs were rapidly inactivated by pH-dependent lactonolysis in which the homoserine lactone ring was hydrolyzed to the ring open form corresponding N-acylhomoserine. The reaction can be reversed by acidification. Therefore, we checked the pH of the extract during extraction process and maintained it to acidic pH.
Determination of AHL functional groups
To assess the AHL functional groups, we performed Fourier transform infra- red (FT-IR), as suggested before.38 Briefly, a drop of the AHL previously extracted by LL- extraction method as described as the above section was placed on one of the KBr grid/ plates. Then, the second plate was placed on top and subjected to IR (Brauker Tensor 70 FT-IR Spector-photometer). Presence of various functional groups was detected in the range of 500–4000 Cm−1 wave numbers.
Primary attachment assay for biofilm formation
The initial attachment to surfaces is an important step in biofilm formation, as well as in the subsequent pathogenesis of A. baumannii biofilm associated infections. We therefore tested the capability of the isolates to attach to a polystyrene surface. Briefly, overnight cultures were adjusted to an OD600nm of 0.5 (1 × 108 CFU/mL) and diluted to 103 CFU/mL. Aliquots (100 μL) of the suspension were inoculated on microtiter plate wells. After 30 min of incubation at 37°C, the plate was gently rinsed 3 times with sterile phosphate-buffered saline (PBS) and then covered with 150 μL of Tryptic Soy Broth (TSB) (BioMérieux, Marcy l'Etoile, France). The primary attachment was expressed as the mean percentage of CFU (± SD) remaining on the microtiter plate compared to the initial inoculums.
Biofilm formation under static condition
Formation of the biofilm in each A. baumannii strain isolated in this study was quantified by microtiter method39 with some modifications. Briefly, one loopful from each A. baumannii colony was inoculated into a sterile TSB medium (2 mL) containing glucose (1% W/V) to optimize biofilm production. The optical density (OD650nm) was then adjusted to 0.13 to reach 0.5 McFarland standard (1.5 × 108 CFU/mL) followed by further dilution of prepared bacterial suspension to reach ∼106 CFU/mL. 100 μL of the standardized cell suspension was transferred to the wells of a round bottom 96-well microtiter plate (MTP). Negative controls contained 100 μL non-inoculated medium. Bacteria were allowed to adhere and grow without agitation for 24 h at 37°C under static condition. After incubation, non-adherent cell suspensions were aseptically aspirated, washed and replaced with 10 μL of sterile phosphate buffered solution (pH- 7.2) to remove any remaining suspended cells. Both cell viability and biofilm biomass were determined as described previously.40 In order to fix the biofilm, 150 μL of methanol was added to each well and kept at room temperature (25°C) for 20 min. The methanol was then removed and replaced with 200 μL of crystal violet solution (1% W/V). The wells containing biofilm matrix were washed slowly with sterile deionized water and kept at room temperature till dried. Thereafter, 200 μL of glacial acetic acid (33% V/V) was added to each well and the optical density was measured at OD570nm by using Synergy 2 multi-mode microplate reader (BioTek, USA). The isolates were classified into strongly adherent (strong biofilm), moderately adherent (moderate biofilm), weakly adherent (weak biofilm) and not adherent (no biofilm) based on the formula described by Stepanovic et al.39 Simultaneously, CFU/mL of each isolate in the wells was determined and subtracted from CFU/mL control. All the mentioned experiments were performed in triplicate and results were expressed as mean ± SD and the most potent biofilm producer isolates were selected for AHL activity.
Statistical analyses
Statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL, USA). A P ≤ 0.05 was considered as statistically significant for 2-tailed tests.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank the staff of Research Center for Infectious Diseases and Tropical Medicine, Department of Microbiology and Virology Kerman University of Medical Sciences (Kerman, Iran) for their help during this research.
Funding
This research was supported by research council in Kerman University of Medical Sciences as part of PhD thesis offered to Mr. Modarresi.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Cisneros JM, Rodriguez-Bano J. Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin Microbiol Infect 2002; 8:687-93; PMID:12445005 [DOI] [PubMed] [Google Scholar]
- 2. Giamarellou H, Antoniadou A, Kanellakopoulou K. Acinetobacter baumannii: a universal threat to public health? Int J Antimicrob Agents 2008; 32:106-19; PMID:18571905; http://dx.doi.org/ 10.1016/j.ijantimicag.2008.02.013 [DOI] [PubMed] [Google Scholar]
- 3. Frere JM, Galleni M, Bush K, Dideberg O. Is it necessary to change the classification of β-lactamases? J Antimicrob Chemother 2005; 55:1051-3; PMID:15886262; http://dx.doi.org/ 10.1093/jac/dki155 [DOI] [PubMed] [Google Scholar]
- 4. Navon-Venezia S, Ben-Ami R, Carmeli Y. Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the healthcare setting. Curr Opin Infect Dis 2005; 18:306-13; PMID:15985826 [DOI] [PubMed] [Google Scholar]
- 5. Kuo HY, Chang KC, Kuo JW, Yueh HW, Liou ML. Imipenem: a potent inducer of multidrug resistance in Acinetobacter baumannii. Int J Antimicrob Agents 2012;39:33-8; PMID:21996406; http://dx.doi.org/ 10.1016/j.ijantimicag.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 6. Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect 2006; 12:826-36; PMID:16882287; http://dx.doi.org/ 10.1111/j.1469-0691.2006.01456.x [DOI] [PubMed] [Google Scholar]
- 7. de Breij A, Dijkshoorn L, Lagendijk E, van der Meer J, Koster A, Bloemberg G, Wolterbeek R, van den Broek P, Nibbering P. Do biofilm formation and interactions with human cells explain the clinical success of Acinetobacter baumannii? PLOS One 2010; 5:e10732; PMID:20505779; http://dx.doi.org/ 10.1371/journal.pone.0010732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infect Immun 2000; 68:4839-49; PMID:10948095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 2001; 25:365-404; PMID:11524130 [DOI] [PubMed] [Google Scholar]
- 10. Shakibaie MR, Adeli S, Salehi MH. Antibiotic resistance patterns and extended-spectrum β-lactamase production among Acinetobacter spp. isolated from an intensive care Unit of a hospital in Kerman, Iran. Antimicrobial Resist Infect Control 2012. 1:1. PMID:22958725; http://dx.doi.org/ 10.1186/2047-2994-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bahador A, Raoofian R, Taheri M, Pourakbari B, Hashemzadeh Z, Hashemi F. Multidrug resistance among Acinetobacter baumannii isolated from Iran:Changes in antimicrobial susceptibility patterns and genotypic profile. Microbial Drug Resist 2014; 20(6):632-40; http://dx.doi.org/ 10.1089/mdr.2013.0146 [DOI] [PubMed] [Google Scholar]
- 12. Vahdani P, Yaghoubi T Z A. Hospital acquired antibiotic-resistant Acinetobacter baumannii infections in a 400-bed hospital in Tehran, Iran. Int J Prev Med 2011; 3:127-30 [PMC free article] [PubMed] [Google Scholar]
- 13. Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 2007; 5:939-51; PMID:18007677; http://dx.doi.org/ 10.1038/nrmicro1789 [DOI] [PubMed] [Google Scholar]
- 14. Anbazhagan D, Mansor M, Yan GO, Md Yusof MY, Hassan H, Sekaran SD. Detection of quorum sensing signal molecules and identification of an autoinducer synthase gene among biofilm forming clinical isolates of Acinetobacter spp. PLOS One 2012; 7:e36696; PMID:22815678; http://dx.doi.org/ 10.1371/journal.pone.0036696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet 2009; 43:197-222; PMID:19686078; http://dx.doi.org/ 10.1146/annurev-genet-102108-134304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antunes LC, Ferreira RB, Buckner MM, Finlay BB. Quorum sensing in bacterial virulence. Microbiology 2010; 156:2271-82; PMID:20488878; http://dx.doi.org/ 10.1099/mic.0.038794-0 [DOI] [PubMed] [Google Scholar]
- 17. Gaddy JA, Actis LA. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol 2009; 4:273-8; PMID:19327114; http://dx.doi.org/ 10.2217/fmb.09.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brelles-Marino G, Bedmar EJ. Detection, purification and characterisation of quorum-sensing signal molecules in plant-associated bacteria. J Biotechnol 2001; 91:197-209; PMID:11566391 [DOI] [PubMed] [Google Scholar]
- 19. Niu C, Clemmer KM, Bonomo RA, Rather PN. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol 2008; 190:3386-92; PMID:18281398; http://dx.doi.org/ 10.1128/jb.01929-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez RH, Nusblat A, Nudel BC. Detection and characterization of quorum sensing signal molecules in Acinetobacter strains. Microbiol Res 2001; 155:271-7; PMID:11297357; http://dx.doi.org/ 10.1016/s0944-5013(01)80004-5 [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez RH, Dijkshoorn L, Van den Barselaar M, Nudel C. Quorum sensing signal profile of Acinetobacter strains from nosocomial and environmental sources. Rev Argent Microbiol 2009; 41:73-8; PMID:19623895; http://dx.doi.org/ 10.1111/j.1600-0463.2007.apm_630.x [DOI] [PubMed] [Google Scholar]
- 22. Joly-Guillou ML. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect 2005; 11:868-73; PMID:16216100; http://dx.doi.org/ 10.1111/j.1469-0691.2005.01227.x [DOI] [PubMed] [Google Scholar]
- 23. Antunes LC, Imperi F, Carattoli A, Visca P. Deciphering the multifactorial nature of Acinetobacter baumannii pathogenicity. PLoS One 2011; 6:e22674; PMID:21829642; http://dx.doi.org/ 10.1371/journal.pone.0022674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asik G. Current approaches to explain the virulence of Acinetobacter baumannii. Mikrobiyol Bul 2011; 45:371-80; PMID:21644082 [PubMed] [Google Scholar]
- 25. Gentile V, Frangipani E, Bonchi C, Minandri F, Runci F, P V. Iron and Acinetobacter baumannii biofilm formation. Pathogens 2014; 3:704-19; http://dx.doi.org/ 10.3390/pathogens3030704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Musk DJ, Banko DA, PJ H. Iron salts perturb biofilm formation and disrupt existing biofilms of Pseudomonas aeruginosa. Chem & Biol 2005; 12:789-96; PMID:16039526; http://dx.doi.org/ 10.1016/j.chembiol.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 27. Johnson M, Cockayne A, Williams PH, Morrissey JA. Iron-responsive regulation of biofilm formation instaphylococcus aureus involves fur-dependent andfur-independent mechanisms. J Bacteriol 2005; 187(23): 8211-8215; PMCID: PMC1291266; http://dx.doi.org/ 10.1128/JB.187.23.8211-8215.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Payne SM. 2003. Regulation of bacterial toxin synthesis by iron, p 25-38. In Burns LD, Barbieri JT, Iglewski BH, Rappuoli R. (ed), Bacterial protein toxins. ASM Press, Washington, DC [Google Scholar]
- 29. Bouvet PJ, Grimont PA. Identification and biotyping of clinical isolates of Acinetobacter. Ann Inst Pasteur Microbiol 1987; 138:569-78; PMID:3440090 [DOI] [PubMed] [Google Scholar]
- 30. CLSI Performance standards for antimicrobial susceptibility testing: Twenty-second informational supplement. CLSI document M100-S22. Clinical and Laboratory Standard Institute: Wayne, PA; 2012. [Google Scholar]
- 31. Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem 1987; 160:47-56; PMID:2952030 [DOI] [PubMed] [Google Scholar]
- 32. Arnow LE. Colorimetric determination of the components of 3, 4-dihydroxyphenylalaninetyrosine mixtures. J Biol Chem 1937; 118:531-7 [Google Scholar]
- 33. Mussi MA, Gaddy JA, Cabruja M, Arivett BA, Viale AM, Rasia R, Actis LA. The opportunistic human pathogen Acinetobacter baumannii senses and responds to light. J Bacteriol 2010; 192:6336-45; PMID:20889755; http://dx.doi.org/ 10.1128/jb.00917-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Welch WH, Flexner S. Observations Concerning the Bacillus erogenes Capsulatus. J Exp Med 1896; 1:5-45; PMID:19866795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeffries CD, Holtman DF, Guse DG. Rapid method for determining the activity of microorganisms on nucleic acids. J Bacteriol 1957; 73:590-1; PMID:13428699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bitrian M, Solari CM, González RH, Nudel CB. Identification of virulence markers in clinically relevant strains of Acinetobacter genospecies. Int Microbiol 2012; 15:79-88; PMID:22847269; http://dx.doi.org/ 10.2436/20.1501.01.161 [DOI] [PubMed] [Google Scholar]
- 37. Yang YH, Lee TH, Kim JH, Kim EJ, Joo HS, Lee CS, Kim BG. High-throughput detection method of quorum-sensing molecules by colorimetry and its applications. Anal Biochem 2006; 356:297-9; PMID:16857158; http://dx.doi.org/ 10.1016/j.ab.2006.05.030 [DOI] [PubMed] [Google Scholar]
- 38. Glansdorp FG, Thomas GL, Lee JK, Dutton JM, Salmond GP, Welch M, Spring DR. Synthesis and stability of small molecule probes for Pseudomonas aeruginosa quorum sensing modulation. Org Biomol Chem 2004; 2:3329-36; PMID:15534711; http://dx.doi.org/ 10.1039/b412802h [DOI] [PubMed] [Google Scholar]
- 39. Stepanovic S, Vukovic D, Hola V, Di Bonaventura G, Djukic S, Cirkovic I, Ruzicka F. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. APMIS 2007; 115:891-9; PMID:17696944; http://dx.doi.org/ 10.1111/j.1600-0463.2007.apm_630.x [DOI] [PubMed] [Google Scholar]
- 40. Shakibaie M, Forootanfar H, Yaser Golkari Y, Mohammadi-Khorsand T, Shakibaie MR. Anti-biofilm activity of biogenic selenium nanoparticles and sele-nium dioxide against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. J Trace Elem Med Biol 2015; 29:235-41. http://dx.doi/ 10.1016/j.jtemb.2014.07.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.