Abstract
Infections of the root canal space and their sequelae can be extremely painful and potentially dangerous, yet they do not necessarily have to be. Chronic, asymptomatic inflammatory lesions around the apex of a tooth with a necrotic dental pulp or an insufficient root canal treatment can develop unnoticed by the patient, and remain so for years. The course of disease is modulated by both the virulence of the microbiota established in the root canal space and the capacity of the immune system to curb the infection. To both ends, highly convincing investigations to help us understand when and why the tissues around an endodontically involved tooth become acutely inflamed are missing. We will discuss how recent advances in molecular identification of microorganisms have altered our understanding of root canal infections, and which information is currently missing to link clinical experience with observations from experimental research.
Keywords: ecological plaque hypothesis, immunity, immune response, metagenomics, sequencing
Since the groundbreaking observations in the 1970s in humans and the preceding controlled experiments in gnotobiotic rats, it has become clear that inflammatory conditions of the dental pulp and, later in the disease process, the periapical tissues are caused by microorganisms.1-4 These infections and their concurrent inflammations are the main causes for patients to seek emergency dental care.5,6 The conditions can be excruciatingly painful; associated with sleep disturbance, limited ability to work, and difficulty in performing everyday tasks.7 Left untreated, exacerbating root canal infections can be an immediate threat to systemic health, with nearly 7000 hospitalizations per annum reported in the US alone.8 Root canal infections thus pose a problem not only on the individual, but also on the community level.9,10 The intriguing fact in this context, however, is that that pulpal and periapical disease can also develop unnoticed by the patient,11 and, because of a lack in accurate diagnostic tools, evade detection by the dentist as well.12 Obviously, whether a root canal infection becomes acute or not must have something to do with the virulence of the invading microbiota and the capacity of the immune system to curb the process.13,14 But when, how, and why root canal infections become acute remains unclear. We evaluate and discuss these questions in the current communication with a focus on infection and the factors that modulate the virulence of the microbiota in the root canal space.
Infection of the Root Canal Space
The preamble for shedding light on the etiology and pathogenesis of endodontic infections is to understand the oral microbiota as a community of commensals that can switch to opportunistic pathogens. The oral cavity is colonized by a wealth of diverse microorganisms, including bacteria, viruses, fungi, protozoa and archaea, collectively designated the "oral microbiome", which exhibit a considerable redundancy among them.15 Based on phylogenetic analysis of collected 16S rRNA gene sequences, the oral microbiome comprises more than 1000 individual taxa, most of which are as-yet uncultivated. Of note, it is the second most complex microbiome in the human body, following that of the colon.16 Taxa span across 13 individual phyla, namely: Actinobacteria, Bacteroidetes, Chlamydiae, Chloroflexi, Euryarchaeota, Firmicutes, Fusobacteria, Proteobacteria, Spirochaetes, SR1, Synergistetes, Tenericutes, and TM7.17 The oral cavity is a dynamic environment consisting of the "protective microbiota", transient invaders from food and other external sources, and opportunistic inhabitants of specific niches. Most prominent among the latter are the ecological environments created by the teeth, which are the only hard tissue entities in the human body that cross a soft tissue barrier (i.e. the gingival mucosa).
To invade the root canal space, microorganisms present in the oral cavity have to breach dental hard tissues. This they achieve by acidic dissolution of hydroxyapatite,18,19 through cracks in the tooth crown,20 or through exposed dentinal tubules in traumatized teeth with a disconnected nerve-vessel bundle.21 In addition, microorganisms can reach the pulp space via the apical foramen in cases of severe periodontal disease.22 These pathways between the endodontium and the periodontal spaces have been discussed in detail.23 Caries, cracked teeth and dental traumata apparently have long troubled mankind, as these problems are evident in the Neolithic Iceman 3300 BC.24 The pulp contained in the confined space of the root canal system is immunocompetent and capable of fending off infection.25 However, if the infection is not operatively removed or at least arrested, pulp tissue necrosis ensues eventually via the sustained release of proteolytic enzymes by neutrophil granulocytes.26,27 This then leads to infection of the entire root canal space, and the host defense retracts to the tissues surrounding the root tip, i.e., the periapex.28
Given these parameters, dental infections are polymicrobial and opportunistic. This is what renders the task of understanding the different courses of disease particularly difficult. It does not necessarily help in this context that sampling procedures to identify the taxa present at specific sites and in different clinical states are full of methodological pitfalls.29 On the other hand, if these errors are avoided, the root canal space offers a unique opportunity to study opportunistic infections in man.
The Cultivable Root Canal Microbiota
Similar to the early attempts at understanding caries and periodontal disease, root canal infections were first thought to be non-specific.30 Indeed, the sheer number of microorganisms contained in a root canal system appears to influence the severity of the periapical response.31,32 However, the non-specific infection hypothesis was partly driven by the inability to identify the variance in the microbiota found in the infected root canal space. The problem with studies on oral infections was that anaerobic culturing was not possible until the late 1960s,33 and consequently, the facultative taxa were over-estimated in early works on the topic.34 Another problem with investigations on the gradual infection of the root canal space, as indicated above, was and is the collection of samples. Based on the fact that the oral cavity is full of microorganisms, contamination is a problem, and it is not easy to harvest the taxa that form the front of the lesion. This was realized when anaerobic culturing became available to dental researchers, and "biofilm" was still known as “plaque.”35,36 It was also during that time when some ground-breaking observations were made. These studies are known to every endodontist as “the Fabricius studies,” whereas they should actually be attributed to the late Åke Möller (1916–2009). These investigations shaped our biological understanding of root canal infections. A first study in monkey (macaca fascicularis) teeth with controlled infection using different combinations of 11 bacterial species, obtained from autogenously infected root canals in monkeys from earlier work, revealed that these taxa apparently formed multi-species communities, which elicited stronger inflammatory responses when combined.37 Especially one species from the Bacteroidetes phylum, then termed Bacteroides oralis (which most probably corresponds to Prevotella oralis), was able to trigger a strong host response, yet was not able to survive if mono-infected. The concept of positive (and negative) association of different taxa was later confirmed in man,38 and biofilm-like structures residing in the root canal space were described in histologic specimens of extracted teeth with apical periodontitis.39 A further study by the Möller group revealed an important ecological finding: when root canals in monkeys were left open to the oral cavity for one week and then sealed for different time periods up to 3 years (1060 days, to be exact), the facultative anaerobes, which were present at high numbers at first, were gradually out-grown by the obligate anaerobes.40 This study introduced a further methodological ingenuity: samples were harvested from the main canal, the canal wall dentin, and the most apical part of the root canal. Culture results showed that the apical samples were consistently more anaerobic than the counterparts taken from coronal aspects of the same root canal system. Using dark field microscopy in samples from extracted teeth with apical periodontitis, which were sectioned into a coronal, a middle, and an apical root segment, it was later observed that the microbiota differed between these aspects in that there were less coccoid forms in the apical segment compared to the coronal counterpart.41 This investigation also revealed the presence of spirochetes in the root canal system. Spirochetes are not easily cultivable, yet could be highly virulent, which highlights the limitations of microbial culture.42 The idea to use the apical segment of extracted teeth to identify that aspect of the microbiota in necrotic teeth, which is most likely to interact with the immune response of the host was then taken up for culture studies.43 These revealed that the apical microbiota is mostly anaerobic, even in teeth with open caries lesions. However, there was no control group with teeth with intact crowns, only the historic controls of traumatized teeth, which revealed over 90% strict anaerobes,4 as compared to less than 70% in the teeth with carious pulpal exposures.43 On the other hand, a later study comparing cultivable Bacteroidetes in the coronal and the apical segment of necrotic root canals in extracted teeth found no apparent difference between the two environments.44
Culture-Independent Studies
Early authors using microscopic techniques knew already that the picture revealed by culturing the microbial content of the root canal was rather limited.30,45 In recent years, molecular approaches have validated and expanded further the findings derived from culture-dependent methods. The first application was DNA-DNA checkerboard hybridization. This method confirmed that endodontic infections are of mixed microbial etiology; up to 17 previously known taxa were detected in a single root canal,46 including the “red complex” periodontal pathogens.47 Yet, the significant breakthrough in the characterization of the endodontic microbiome came with the use of polymerase chain reaction (PCR)-based approaches, which enabled the detection of the uncultivable microbiota. To this end, the first authors used 16S rDNA-targeted PCR followed by cloning and then sequencing of the PCR products.48 Siqueira & Rocas and co-workers introduced the use of PCR-based denaturing gradient gel electrophoresis (PCR-DGGE) to endodontic research.49,50 Combined with gel band excision, sequencing and sequence analysis, PCR-DGGE can also be used to identify individual taxa. Such studies found unculturable clones of the phyla Spirochaetes and Synergistetes (formerly known as Deferribacteres) in root canals of teeth with apical periodontitis.51-53 Furthermore, the newly identified taxon Dialister was identified to be a frequent member of the endodontic microbiota residing in infected root canals.48 More recent approaches included the use of pyrosequencing of 16S rRNA genes to profile the endodontic microbiome.54,55 These studies revealed a previously unknown high diversity of bacterial communities, also in the apical portion of infected root canals.55
A comprehensive joint analysis of culture and molecular studies showed that over 460 unique bacterial taxa, belonging to 100 genera and 9 phyla, have been identified in different types of endodontic infections.42 The phyla with the highest species richness were confirmed to be Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. Thus, high-throughput nucleic acid-based methods have enabled us to discover a surprisingly high diversity of the microbiota in infected root canals. At least, this diversity proves to be greater than originally appreciated by cultivation or conventional molecular methods. Moreover, the amplitude of uncultivable clones of otherwise well-established taxa, as well as their standpoint within the overall microbiome, was also revealed. Hence, we now have a much broader overview of the mosaic of the complex microbiota associated with endodontic infections.
Koch's Postulates Revisited
Simply assessing the presence of certain taxa in the root canal system may be interesting basic information, yet this does not help us to understand the disease progress from a microbiological point of view. When does a root canal infection become acute, and why? Obviously, the original Koch postulates cannot be used to explain acute forms of apical periodontitis, or any other opportunistic infection, for that matter.56 However, taking into consideration that root canal infections are polymicrobial by nature, it may still be that certain constellations of the endodontic microbiome may be more virulent than others. This was investigated by Göran Sundqvist. In his classical thesis on the cultivable microbiota of traumatized single-rooted teeth with an intact pulp chamber, he noticed that the presence of Bacteroides melaninogenicus (now: Prevotella melaninogenica) in all the acute cases with pus and/or tenderness, but not in counterparts diagnosed with chronic apical periodontitis.4 He also found other taxa to be associated with acute infections, such as Peptostreptococcus spp., Eubacterium spp., and Campylobacter sputorum. Given the fact that the teeth in this investigation were all similar in terms of their ecological conditions in the root canal system, i.e., they all contained a necrotized pulp because of a trauma to the nerve-vessel bundle while their crown was intact, these observations highlighted the possibility that composition of the microbiota in the root canal drives the course of disease. Sundqvist then tested a revised version of Koch's third postulate: he took the 88 culturable bacterial strains, 85 of which were anaerobic, isolated from the traumatized teeth described above.4 Combinations of these taxa found in clinical cases were suspended to a cell density of 108 cells per mL. These suspensions were then injected subcutaneously in guinea pigs. Interestingly, the combinations from the 11 symptomless teeth did not induce transmissible infection. In contrast, 6 of the 7 combinations from the symptomatic teeth did cause abscess formation, and the aspirate from these abscesses again caused an abscess in another animal.57 The transmission was performed 4 times for a combination to be called transmissible. Bacteroides spp. were present in all the abscesses, mostly together with Parvimonas micra (formerly referred to as Peptostreptococcus micros).
The idea to identify virulent combinations of microorganisms in infected root canals was taken up by numerous other investigations, which compared the microbiota in teeth with chronic apical lesions with that of counterparts with exacerbating inflammations of endodontic origin.58 However, the more sophisticated the identification methodologies, the less clear the picture became. Apparently, already within the black-pigmented Bacteroides spp., there are certain taxa associated with acute inflammations, while others are not.59 On the other hand, taxa such as Enterococcus spp. are shown to correlate with periapical health rather than disease.60 Indeed, certain taxa found in recurrent or persistent apical periodontitis after root canal treatment may represent environmental contaminants from food or other extra-oral sources rather than truly therapy-resistant entities.61
Culture-independent techniques again widened the field of possible players in acute endodontic infections.62 A high-throughput multiplexed 16S rRNA gene pyrosequencing analysis of the root canal content of 8 teeth with chronic apical periodontitis was compared with the aspirate from 9 abscesses of endodontic origin.63 This study, while not free from some methodological doubt (the chronic samples were taken from the root canal, the abscess samples from the periapex), revealed that bacteria from the genera Fusobacterium, Atopobium, Parvimonas, Dialister, Porphyromonas, Prevotella, and especially Peptostreptococcus appeared much more frequently in abscesses as compared to chronic root canal infections. This confirmed the earlier observations on transmissible abscess formation by Sundqvist, who already noted the importance of the black-pigmented Bacteroides species and Peptostreptococci. If we accept the concept that Fusobacteria, as their name indicates, are the "bridge-builders" in biofilms, and that apical periodontitis is a biofilm-related disease, then it could be postulated that Fusobacteria are simply present in apical abscesses because they constitute an important part of the apical biofilm, irrespective of virulence properties. A later study taking a similar approach revealed similar findings: Fusobacteria were the most abundant genus in the abscesses.64 In the mouse model, Fusobacterium nucleatum, the most frequently cultured species from teeth with apical periodontitis,58 can induce abscess formation in mono-infection.65 However, its virulence is much greater if administered in combination with Porphyromonas gingivalis or Prevotella intermedia.65 In this same model, strains of Peptostreptococcus anaerobius were pathogenic in pure culture.
So, in summary, results from culture-based studies and culture-independent approaches might not be that different after all, as the main players that drive exacerbation of the inflammation have remained conspicuously similar. However, it remains to be elucidated if any of the as-yet non-cultivable or highly fastidious species play any significant role in clinical endodontics. Among these taxa, spirochetes appear to be among the main candidates that could be of interest.42
Clinical Observations versus Experimental Research
Dentists know that root canal infections can take all kinds of different pathways. They can go unnoticed by the patient, or they can be extremely painful.11 Apparently, there is one form of apical periodontitis that takes an immediate aggressive course. Surprisingly, there is sparse information in the dental literature regarding the dynamics of endodontic disease and what might influence them. In a recent survey performed in the emergency unit of our school, roughly half of the teeth with acute abscesses of endodontic origin did not show any rarefaction on periapical radiographs indicative of a pre-existing chronic condition. This could mean that these teeth had such an aggressive infection that the host could not establish a line of defense leading to a non-painful chronic periapical condition. Indeed, studies on carious teeth have shown that the initial type of infection has a great impact on the host response in the vital pulp.66,67 It is highly conceivable that a form of caries dominated by high content of Gram-negative anaerobic rods results in a root canal microbiota, which can lead to a fast and furious host response in the periapical tissues. Direct evidence for this, however, is missing and should be collected.
What is even more intriguing is the other form of acute exacerbation, which occurs from a pre-existing chronic periapical lesion of endodontic origin. In the developed world with adequate healthcare, this type of acute apical periodontitis is usually restricted to teeth that already received root canal treatment. A radiographic lesion around the root apex may persist or increase in size without any other clinical signs or symptoms. Yet an apparently dormant lesion depicted on the radiograph can suddenly become acute. It should be noted in this context that also after state-of-the-art root canal treatment, periapical radiographic lesions can take up to 4 years to disappear, sometimes more.68 Longitudinal cross-sectional studies suggest that the exacerbation rate of such chronic lesions is relatively low.69,70 In a randomly selected population, from 304 teeth with an apical radiographic lesion on a root-filled tooth, within 6 years 30% of the lesions healed, 60% persisted, and a mere 10% of the teeth were extracted. On the other hand, root-filled teeth with an asymptomatic periapical lesion are routinely re-treated when a patient receives radiation therapy to the jaws or some form of immunosuppressive treatment.71 There is no solid scientific proof to support this clinical concept, but evidently, a reduction in the local or systemic immune defense can trigger the exacerbation of a chronic lesion. Furthermore, clinicians want to avoid any type of intervention in irradiated bone. Patients with acute periapical inflammation on an insufficiently root canal-treated tooth frequently state that their pain started when they had the flu or went through a phase of stress at work or at home. Indirectly, the role of the immunological response may also be revealed by studies that show an association between Herpes viruses infection and acute forms of apical periodontitis.72
Apart from the state of the host, endodontic infections are likely to be driven by changes in the local microenvironment within the tooth, permitting for the establishment of the opportunistically virulent microbiota that has been identified by culture-dependent or by molecular methods. Such conditions are also under-investigated in endodontics. But again, clinical practice may lead to some clues. Dentists often prefer to leave teeth open to the oral cavity in case of an acute periapical lesion of endodontic origin.73 The impact of the endodontic microenvironment on the inflammatory reaction in the periapical tissues was most impressively shown in a case report by John Whitworth.74 A patient with an apical lesion of endodontic origin did not appear for the scheduled appointment. The reason for the necrosis and infection of the pulp was caries. Almost 3 years later, the patient reappeared in the clinic. Now the whole crown was decayed, and the pulp space was open to the oral cavity. The apical lesion in the radiograph had diminished, without any operative intervention. As stated by the author, "the case raises debate on the pathogenesis, diagnosis and monitoring of endodontic lesions, and may stimulate renewed research interest in these most fundamental elements of clinical endodontology".74
Summary and Outlook
The oral microbiota lives in harmony with the host, until an induced micro-environmental change causes disturbances in this symbiotic relationship. This change may result in selective overgrowth of opportunistic pathogens, or in an insufficient capacity of the host to respond to otherwise commensal microorganisms. Hence, either a certain bacterial community may become now more virulent, or the host mounts an immune response that is not sufficient to eliminate its unbalanced growth, eventually resulting in a dysbiotic relationship between the two.75 This view perceives the development of oral infectious disease as an "ecological catastrophe" resulting from an imbalanced cross-talk between the resident oral microbiota and the host response.76,77 A significant number of crucial microecological parameters can dictate the establishment of specific biofilm microbiota in a given niche of the oral cavity. These factors include the local pH, abundance and partial pressure of oxygen, redox potential, availability of selective nutrients, and, last but not least, the state of local host defenses.78 While not much is known about the remaining factors, longitudinal studies have shown that the state of the innate immune system affects the outcome of endodontic treatment.79,80 Along with these properties comes the newly introduced concept of "inflammophilic" bacteria, by Hajishengallis; the selective flourishing of bacteria in an inflammation-rich milieu can perpetuate tissue destruction by setting-off a "vicious cycle" for disease progression, in which dysbiosis and inflammation reinforce each other.81 Nevertheless, the applicability of these emerging concepts of oral ecology awaits further validation in the case of endodontic infections. The revelation of the complete endodontic microbiome is an important step in the direction of identifying the microbial players and understanding the microecological changes that lead to the shift to acute periapical responses. This needs to be investigated not by one, but by several sampling and microbial detection methods. Culture-dependent methods are needed to determine the viability of isolated taxa. Metagenomic approaches, such as high-throughput sequencing, would give qualitative information on the full range of taxa/phylotypes present in a given sample aspirate from the root canal, irrespective of their cultivability.
Furthermore, fluorescence in situ hybridization (FISH) would be ideal to reveal the spatial arrangement of the selected taxa in endodontic biofilms, e.g. in tooth or apicectomy specimens obtained from different clinical conditions. FISH could be used to depict the previously identified virulent taxa in stages of acute apical periodontitis (Fig. 1). Hitherto, FISH has merely been applied in endodontic research to non-selectively identify bacterial cells in apparent biofilms,82 or to specify classes of bacteria in the root canal of teeth with different clinical conditions.83 However, as has been shown by conventional microscopy, in acute states of apical periodontitis, bacteria are found outside the root canal space, while they are curbed inside in chronic apical periodontitis.28,84 To gain further insight into what is really happening when a tooth with a necrotic, infected root canal space becomes acute, it would now be timely and important to repeat such studies, combining FISH and scanning electron microscopy.82,85
Figure 1.
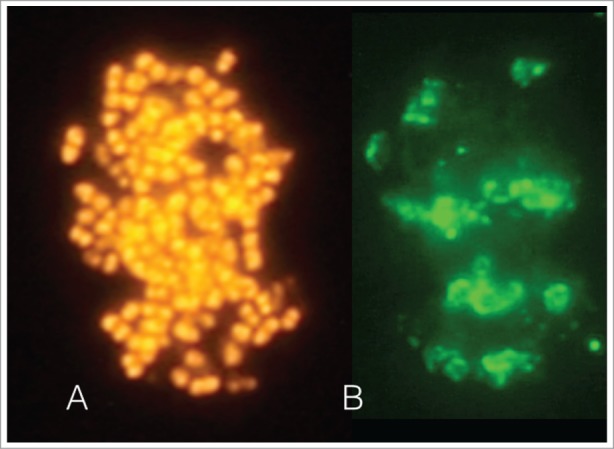
The images depict an intact biofilm-like fraction identified microscopically in a sample obtained from the apical region of tooth with a root canal infection. The sample was further processed in the laboratory. Fluorescence in situ hybridization (FISH) staining by the generic 16S rRNA oligonucleotide probe EUB-338 confirmed that it comprised of eubacteria (A). Immunofluorescence staining with the species-specific monoclonal antibody 326PM2 revealed the presence Parvimonas micra aggregates within this structure (B).
Last but not least, microbial shifts are associated with changes of the immune response in the root canal and periapical region. For this reason, endodontic samples obtained for microbiological analysis should also be evaluated for their inflammatory mediator profile. It is more likely that we will be able to characterize and explain the shift to disease by defining multivariable “signatures” of changes, rather than single parameters alone.
References
- 1. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 1965; 20:340-9; PMID:14342926 [DOI] [PubMed] [Google Scholar]
- 2. Reeves R, Stanley HR. The relationship of bacterial penetration and pulpal pathosis in carious teeth. Oral Surg Oral Med Oral Pathol 1966; 22:59-65; PMID:5220026 [DOI] [PubMed] [Google Scholar]
- 3. Bergenholtz G. Micro-organisms from necrotic pulp of traumatized teeth. Odontol Revy 1974; 25:347-58; PMID:4155793 [PubMed] [Google Scholar]
- 4. Sundqvist G. Bacteriological studies of necrotic dental pulps. [dissertation]. Umeå: University of Umeå; 1976. [Google Scholar]
- 5. Sindet-Pedersen S, Petersen JK, Götzsche PC. Incidence of pain conditions in dental practice in a Danish county. Community Dent Oral Epidemiol 1985; 13:244-6; PMID:3862509 [DOI] [PubMed] [Google Scholar]
- 6. Widström E, Pietila I, Nilsson B. Diagnosis and treatment of dental emergencies in two Finnish cities. Community Dent Health 1990; 7:173-8; PMID:2379092 [PubMed] [Google Scholar]
- 7. Constante HM, Bastos JL, Peres KG, Peres MA. Socio-demographic and behavioural inequalities in the impact of dental pain among adults: a population-based study. Community Dent Oral Epidemiol 2012; 40:498-506; PMID:22607027; http://dx.doi.org/ 10.1111/j.1600-0528.2012.00701.x [DOI] [PubMed] [Google Scholar]
- 8. Shah AC, Leong KK, Lee MK, Allareddy V. Outcomes of hospitalizations attributed to periapical abscess from 2000 to 2008: a longitudinal trend analysis. J Endod 2013; 39:1104-10; PMID:23953280; http://dx.doi.org/ 10.1016/j.joen.2013.04.042 [DOI] [PubMed] [Google Scholar]
- 9. Pau A, Croucher R, Marcenes W, Leung T. Development and validation of a dental pain-screening questionnaire. Pain 2005; 119:75-81; PMID:16297557; http://dx.doi.org/ 10.1016/j.pain.2005.09.016 [DOI] [PubMed] [Google Scholar]
- 10. Miller J, Elwood PC, Swallow JN. Dental pain. An incidence study. Br Dent J 1975; 139:327-8; PMID:1058011 [DOI] [PubMed] [Google Scholar]
- 11. Michaelson PL, Holland GR. Is pulpitis painful? Int Endod J 2002; 35:829-32; PMID:12406376 [DOI] [PubMed] [Google Scholar]
- 12. Dummer PM, Hicks R, Huws D. Clinical signs and symptoms in pulp disease. Int Endod J 1980; 13:27-35; PMID:6935168 [DOI] [PubMed] [Google Scholar]
- 13. Hahn CL, Liewehr FR. Update on the adaptive immune responses of the dental pulp. J Endod 2007; 33:773-81; PMID:17804311; http://dx.doi.org/ 10.1016/j.joen.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 14. Hahn CL, Liewehr FR. Innate immune responses of the dental pulp to caries. J Endod 2007; 33:643-51; PMID:17509400; http://dx.doi.org/ 10.1016/j.joen.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 15. Wade WG. The oral microbiome in health and disease. Pharmacol Res 2013; 69:137-43; PMID:23201354; http://dx.doi.org/ 10.1016/j.phrs.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 16. Structure , function and diversity of the healthy human microbiome. Nature 2012; 486:207-14; PMID:22699609; http://dx.doi.org/ 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol 2010; 192:5002-17; PMID:20656903; http://dx.doi.org/ 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stephan RM. pH and dental caries. J Dent Res 1947; 26:340; PMID:20264622 [PubMed] [Google Scholar]
- 19. Hojo S, Takahashi N, Yamada T. Acid profile in carious dentin. J Dent Res 1991; 70:182-6; PMID:1999556 [DOI] [PubMed] [Google Scholar]
- 20. Cameron CE. Cracked-tooth syndrome. J Am Dent Assoc 1964; 68:405-11; PMID:14128028 [DOI] [PubMed] [Google Scholar]
- 21. Nagaoka S, Miyazaki Y, Liu HJ, Iwamoto Y, Kitano M, Kawagoe M. Bacterial invasion into dentinal tubules of human vital and nonvital teeth. J Endod 1995; 21:70-3; PMID:7714440; http://dx.doi.org/ 10.1016/S0099-2399(06)81098-8 [DOI] [PubMed] [Google Scholar]
- 22. Langeland K, Rodrigues H, Dowden W. Periodontal disease, bacteria, and pulpal histopathology. Oral Surg Oral Med Oral Pathol 1974; 37:257-70; PMID:4520855 [DOI] [PubMed] [Google Scholar]
- 23. Zehnder M, Gold SI, Hasselgren G. Pathologic interactions in pulpal and periodontal tissues. J Clin Periodontol 2002; 29:663-71; PMID:12390561; http://dx.doi.org/ 10.1034/j.1600-051X.2002.290801.x [DOI] [PubMed] [Google Scholar]
- 24. Seiler R, Spielman AI, Zink A, Ruhli F. Oral pathologies of the Neolithic Iceman, c.3,300 BC. Eur J Oral Sci 2013; 121:137-41; PMID:23659234; http://dx.doi.org/ 10.1111/eos.12037 [DOI] [PubMed] [Google Scholar]
- 25. Warfvinge J, Bergenholtz G. Healing capacity of human and monkey dental pulps following experimentally-induced pulpitis. Endod Dent Traumatol 1986; 2:256-62; PMID:3467971 [DOI] [PubMed] [Google Scholar]
- 26. Langeland K. Tissue response to dental caries. Endod Dent Traumatol 1987; 3:149-71; PMID:3326722 [DOI] [PubMed] [Google Scholar]
- 27. Wahlgren J, Salo T, Teronen O, Luoto H, Sorsa T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) in pulpal and periapical inflammation and periapical root-canal exudates. Int Endod J 2002; 35:897-904; PMID:12453017; http://dx.doi.org/ 10.1046/j.1365-2591.2002.00587.x [DOI] [PubMed] [Google Scholar]
- 28. Nair PNR. Apical periodontitis: a dynamic encounter between root canal infection and host response. Periodontol 2000 1997; 13:121-48; PMID:9567926; http://dx.doi.org/ 10.1111/j.1600-0757.1997.tb00098.x [DOI] [PubMed] [Google Scholar]
- 29. Sathorn C, Parashos P, Messer HH. How useful is root canal culturing in predicting treatment outcome? J Endod 2007; 33:220-25; PMID:17320700; 10.1016/j.joen.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 30. Miller WD. The Microorgansims of the Human Mouth. Philadelphia: SS White and Co; 1890 [Google Scholar]
- 31. Korzen BH, Krakow AA, Green DB. Pulpal and periapical tissue responses in conventional and monoinfected gnotobiotic rats. Oral Surg Oral Med Oral Pathol 1974; 37:783-802; PMID:4524385 [DOI] [PubMed] [Google Scholar]
- 32. Byström A, Happonen RP, Sjögren U, Sundqvist G. Healing of periapical lesions of pulpless teeth after endodontic treatment with controlled asepsis. Endod Dent Traumatol 1987; 3:58-63; PMID:3472880 [DOI] [PubMed] [Google Scholar]
- 33. Aranki A, Syed SA, Kenney EB, Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol 1969; 17:568-76; PMID:4890748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoshino E. Predominant obligate anaerobes in human carious dentin. J Dent Res 1985; 64:1195-8; PMID:3861648 [DOI] [PubMed] [Google Scholar]
- 35. Möller ÅJR. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr 1966; 74:Suppl:1-380; PMID:5335186 [PubMed] [Google Scholar]
- 36. Edwardsson S. Bacteriological studies on deep areas of carious dentine. Odontol Revy Suppl 1974; 32:1-143; PMID:4614155 [PubMed] [Google Scholar]
- 37. Fabricius L, Dahlén G, Holm SE, Möller ÅJR. Influence of combinations of oral bacteria on periapical tissues of monkeys. Scand J Dent Res 1982; 90:200-6; PMID:7051261 [DOI] [PubMed] [Google Scholar]
- 38. Sundqvist G. Associations between microbial species in dental root canal infections. Oral Microbiol Immunol 1992; 7:257-62; PMID:1494447 [DOI] [PubMed] [Google Scholar]
- 39. Nair PNR, Luder HU. Wurzelkanal und periapikale Flora: eine licht- und elektronenmikroskopische Untersuchung. Schweiz Monatsschr Zahnmed 1985; 95:992-1003; PMID:3865361 [PubMed] [Google Scholar]
- 40. Fabricius L, Dahlén G, Öhman AE, Moller ÅJR. Predominant indigenous oral bacteria isolated from infected root canals after varied times of closure. Scand J Dent Res 1982; 90:134-44; PMID:6951255 [DOI] [PubMed] [Google Scholar]
- 41. Thilo BE, Baehni P, Holz J. Dark-field observation of the bacterial distribution in root canals following pulp necrosis. J Endod 1986; 12:202-5; PMID:3459804; http://dx.doi.org/ 10.1016/S0099-2399(86)80155-8 [DOI] [PubMed] [Google Scholar]
- 42. Siqueira JFJ, Rocas IN. Diversity of endodontic microbiota revisited. J Dent Res 2009; 88:969-81; PMID:19828883; http://dx.doi.org/ 10.1177/002203-4509346549 [DOI] [PubMed] [Google Scholar]
- 43. Baumgartner JC, Falkler WAJ. Bacteria in the apical 5 mm of infected root canals. J Endod 1991; 17:380-3; PMID:1809801 [DOI] [PubMed] [Google Scholar]
- 44. Dougherty WJ, Bae KS, Watkins BJ, Baumgartner JC. Black-pigmented bacteria in coronal and apical segments of infected root canals. J Endod 1998; 24:356-8; PMID:9641113; http://dx.doi.org/ 10.1016/S0099-2399(98)80134-9 [DOI] [PubMed] [Google Scholar]
- 45. Brown LRJ, Rudolph CEJ. Isolation and identification of microorganisms from unexposed canals of pulp-involved teeth. Oral Surg Oral Med Oral Pathol 1957; 10:1094-9; PMID:13465123 [DOI] [PubMed] [Google Scholar]
- 46. Siqueira JFJ, Rocas IN, Souto R, de Uzeda M, Colombo AP. Checkerboard DNA-DNA hybridization analysis of endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 89:744-8; PMID:10846131 [DOI] [PubMed] [Google Scholar]
- 47. Rocas IN, Siqueira JFJ, Santos KR, Coelho AM. "Red complex" (Bacteroides forsythus, Porphyromonas gingivalis, and Treponema denticola) in endodontic infections: a molecular approach. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001; 91:468-71; PMID:11312465; http://dx.doi.org/ 10.1067/moe.2001.114379 [DOI] [PubMed] [Google Scholar]
- 48. Munson MA, Pitt-Ford T, Chong B, Weightman A, Wade WG. Molecular and cultural analysis of the microflora associated with endodontic infections. J Dent Res 2002; 81:761-6; PMID:12407091; http://dx.doi.org/ 10.1177/154405910208101108 [DOI] [PubMed] [Google Scholar]
- 49. Rocas IN, Siqueira JFJ, Aboim MC, Rosado AS. Denaturing gradient gel electrophoresis analysis of bacterial communities associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004; 98:741-9; PMID:15583550; http://dx.doi.org/ 10.1016/S1079210404006183 [DOI] [PubMed] [Google Scholar]
- 50. Siqueira JFJ, Rocas IN, Rosado AS. Investigation of bacterial communities associated with asymptomatic and symptomatic endodontic infections by denaturing gradient gel electrophoresis fingerprinting approach. Oral Microbiol Immunol 2004; 19:363-70; PMID:15491461; http://dx.doi.org/ 10.1111/j.1399-302x.2004.00170.x [DOI] [PubMed] [Google Scholar]
- 51. Siqueira JFJ, Rocas IN, Cunha CD, Rosado AS. Novel bacterial phylotypes in endodontic infections. J Dent Res 2005; 84:565-9; PMID:15914596; http://dx.doi.org/ 10.1177/154405910508400615 [DOI] [PubMed] [Google Scholar]
- 52. Siqueira JFJ, Rocas IN. Molecular detection and identification of Synergistes phylotypes in primary endodontic infections. Oral Dis 2007; 13:398-401; PMID:17577326; http://dx.doi.org/ 10.1111/j.1601-0825.2006.01301.x [DOI] [PubMed] [Google Scholar]
- 53. Vianna ME, Conrads G, Gomes BP, Horz HP. Quantification and characterization of Synergistes in endodontic infections. Oral Microbiol Immunol 2007; 22:260-5; PMID:17600538; http://dx.doi.org/ 10.1111/j.1399-302X.2007.00353.x [DOI] [PubMed] [Google Scholar]
- 54. Li L, Hsiao WW, Nandakumar R, Barbuto SM, Mongodin EF, Paster BJ, Fraser-Liggett CM, Fouad AF. Analyzing endodontic infections by deep coverage pyrosequencing. J Dent Res 2010; 89:980-4; PMID:20519493; http://dx.doi.org/ 10.1177/002203-4510370026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Siqueira JFJ, Alves FR, Rocas IN. Pyrosequencing analysis of the apical root canal microbiota. J Endod 2011; 37:1499-503; PMID:22000451; http://dx.doi.org/ 10.1016/j.joen.2011.08.012 [DOI] [PubMed] [Google Scholar]
- 56. Fredericks DN, Relman DA. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin Microbiol Rev 1996; 9:18-33; PMID:8665474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sundqvist GK, Eckerbom MI, Larsson AP, Sjögren UT. Capacity of anaerobic bacteria from necrotic dental pulps to induce purulent infections. Infect Immun 1979; 25:685-93; PMID:489126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sundqvist G. Taxonomy, ecology, and pathogenicity of the root canal flora. Oral Surg Oral Med Oral Pathol 1994; 78:522-30; PMID:7800383 [DOI] [PubMed] [Google Scholar]
- 59. Haapasalo M, Ranta H, Ranta K, Shah H. Black-pigmented Bacteroides spp. in human apical periodontitis. Infect Immun 1986; 53:149-53; PMID: 3721577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kaufman B, Spångberg L, Barry J, Fouad AF. Enterococcus spp. in endodontically treated teeth with and without periradicular lesions. J Endod 2005; 31:851-6; PMID:16306816 [DOI] [PubMed] [Google Scholar]
- 61. Zehnder M, Guggenheim B. The mysterious appearance of enterococci in filled root canals. Int Endod J 2009; 42:277-87; PMID:19220511; http://dx.doi.org/ 10.1111/j.1365-2591.2008.01537.x [DOI] [PubMed] [Google Scholar]
- 62. Rocas IN, Siqueira JFJ. Detection of novel oral species and phylotypes in symptomatic endodontic infections including abscesses. FEMS Microbiol Lett 2005; 250:279-85; PMID:16099112; http://dx.doi.org/ 10.1016/j.femsle.2005.07.017 [DOI] [PubMed] [Google Scholar]
- 63. Santos AL, Siqueira JFJ, Rocas IN, Jesus EC, Rosado AS, Tiedje JM. Comparing the bacterial diversity of acute and chronic dental root canal infections. PLoS One 2011; 6:e28088; PMID:22132218; http://dx.doi.org/ 10.1371/journal.pone.0028088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hsiao WW, Li KL, Liu Z, Jones C, Fraser-Liggett CM, Fouad AF. Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics 2012; 13: 345; PMID:;22839737; http://dx.doi.org/ 10.1186/1471-2164-13-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Baumgartner JC, Falkler WAJ, Beckerman T. Experimentally induced infection by oral anaerobic microorganisms in a mouse model. Oral Microbiol Immunol 1992; 7:253-6; PMID:1408362 [DOI] [PubMed] [Google Scholar]
- 66. Hahn CL, Falkler WAJ, Minah GE. Microbiological studies of carious dentine from human teeth with irreversible pulpitis. Arch Oral Biol 1991; 36:147-53; PMID:2059163 [DOI] [PubMed] [Google Scholar]
- 67. Hahn CL, Liewehr FR. Relationships between caries bacteria, host responses, and clinical signs and symptoms of pulpitis. J Endod 2007; 33:213-9; PMID:17320699; http://dx.doi.org/ 10.1016/j.joen.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 68. Ørstavik D. Time-course and risk analyses of the development and healing of chronic apical periodontitis in man. Int Endod J 1996; 29:150-5; PMID:9206419 [DOI] [PubMed] [Google Scholar]
- 69. Kirkevang LL, Vaeth M, Hörsted-Bindslev P, Wenzel A. Longitudinal study of periapical and endodontic status in a Danish population. Int Endod J 2006; 39:100-7; PMID:16454789; http://dx.doi.org/ 10.1111/j.1365-2591.2006.01051.x [DOI] [PubMed] [Google Scholar]
- 70. Kirkevang LL, Vaeth M, Wenzel A. Ten-year follow-up of root filled teeth: a radiographic study of a Danish population. Int Endod J 2014; 47:980-8; PMID:24392750; http://dx.doi.org/ 10.1111/iej.12245 [DOI] [PubMed] [Google Scholar]
- 71. Elad S, Raber-Durlacher JE, Brennan MT, et al. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Support Care Cancer 2014; 23:223-36; PMID:25189149; http://dx.doi.org/ 10.1007/s00520-014-2378-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Slots J, Sabeti M, Simon JH. Herpesviruses in periapical pathosis: an etiopathogenic relationship? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 96:327-31; PMID:12973289; http://dx.doi.org/ 10.1016/S1079210403003524 [DOI] [PubMed] [Google Scholar]
- 73. Tjäderhane LS, Pajari UH, Ahola RH, Backman TK, Hietala EL, Larmas MA. Leaving the pulp chamber open for drainage has no effect on the complications of root canal therapy. Int Endod J 1995; 28:82-5; PMID:7665205 [DOI] [PubMed] [Google Scholar]
- 74. Whitworth JM. Apparent periapical repair without operative intervention: a case report and discussion. Int Endod J 2000; 33:286-9; PMID:11307449 [DOI] [PubMed] [Google Scholar]
- 75. Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol 2012; 27:409-19; PMID:23134607; http://dx.doi.org/ 10.1111/j.2041-1014.2012.00663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology 2003; 149:279-94; PMID:12624191 [DOI] [PubMed] [Google Scholar]
- 77. Marsh PD. Dental plaque: biological significance of a biofilm and community life-style. J Clin Periodontol 2005; 32 Suppl 6:7-15; PMID:16128825; http://dx.doi.org/ 10.1111/j.1600-051X.2005.00790.x [DOI] [PubMed] [Google Scholar]
- 78. Marsh PD, Devine DA. How is the development of dental biofilms influenced by the host? J Clin Periodontol 2011; 38 Suppl 11:28-35; PMID:21323701; http://dx.doi.org/ 10.1111/j.1600-051X.2010.01673.x [DOI] [PubMed] [Google Scholar]
- 79. Fouad AF, Burleson J. The effect of diabetes mellitus on endodontic treatment outcome: data from an electronic patient record. J Am Dent Assoc 2003; 134:43-51; quiz 117-8; PMID:12555956 [DOI] [PubMed] [Google Scholar]
- 80. Marending M, Peters OA, Zehnder M. Factors affecting the outcome of orthograde root canal therapy in a general dentistry hospital practice. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 99:119-24; PMID:15599359; http://dx.doi.org/ 10.1016/j.tripleo.2004.06.065 [DOI] [PubMed] [Google Scholar]
- 81. Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol 2014; 29(6):248-57; PMID:24976068; http://dx.doi.org/ 10.1111/omi.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schaudinn C, Carr G, Gorur A, Jaramillo D, Costerton JW, Webster P. Imaging of endodontic biofilms by combined microscopy (FISH/cLSM - SEM). J Microsc 2009; 235:124-7; PMID:19659906; http://dx.doi.org/ 10.1111/j.1365-2818.2009.03201.x [DOI] [PubMed] [Google Scholar]
- 83. Fernandes Cdo C, Rechenberg DK, Zehnder M, Belibasakis GN. Identification of Synergistetes in endodontic infections. Microb Pathog 2014; 73:1-6; PMID:24837500; http://dx.doi.org/ 10.1016/j.micpath.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 84. Ricucci D, Siqueira JFJ. Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. J Endod 2010; 36:1277-88; PMID:20647081; http://dx.doi.org/ 10.1016/j.joen.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 85. Carr GB, Schwartz RS, Schaudinn C, Gorur A, Costerton JW. Ultrastructural examination of failed molar retreatment with secondary apical periodontitis: an examination of endodontic biofilms in an endodontic retreatment failure. J Endod 2009; 35:1303-9; PMID:19720237; http://dx.doi.org/ 10.1016/j.joen.2009.05.035 [DOI] [PubMed] [Google Scholar]


