Abstract
Prion diseases reflect the misfolding of a self-protein (PrPC) into an infectious, pathological isomer (PrPSc). By targeting epitopes uniquely exposed by misfolding, our group developed PrPSc-specific vaccines to 3 disease specific epitopes (DSEs). Here, antibodies induced by individual DSE vaccines are evaluated for their capacity to neutralize prions in vitro. For both purified antibodies and immunoreactive sera, the PrPSc-specific antibodies were equally effective in neutralizing prions. Further, there was no significant increase in neutralizing activity when multiple DSEs were targeted within an assay. At a low antibody concentration, the PrPSc-specific antibodies matched the neutralization achieved by an antibody that may act via both PrPC and PrPSc. At higher doses, however, this pan-specific antibody was more effective, potentially due to a combined deactivation of PrPSc and depletion of PrPC.
Keywords: antibodies, antibody neutralization, disease specific epitopes, prion, vaccine
Abbreviations
- PrPC
cellular prion protein
- PrPSc
scrapie prion protein
- DSE
disease-specific epitope
- CWD
chronic wasting disease
- YYR
tyrosine-tyrosine-arginine
- YML
tyrosine-methionine-leucine
- RL
rigid loop; mAb, monoclonal antibody
- Lkt
leukotoxin
- SC
subcutaneous
- PBS
phosphate-buffered saline
- AP
alkaline phosphatase
- ANOVA
2-way analysis of variance
- SEM
standard error of the mean
Prion diseases of all animals, including humans, share a molecular mechanism involving misfolding of the self-protein PrPC into the infectious, pathological conformation PrPSc. The “protein-only” hypothesis anticipates that PrPSc functions as a template to promote further misfoldings of PrPC to PrPSc in an autocatalytic, self-propagating reaction.1,2 At the present time, and in all species, prion diseases are fatal, untreatable neurodegenerative disorders.3
Chronic wasting disease (CWD), a prion disease of cervids, is currently the top priority animal prion disease. The progressive spread of CWD throughout North American wild cervids is of considerable concern from wildlife, economic and human health perspectives.4 Given the current inability to manage CWD, in particular in wild animals, there is clear need for additional disease management tools. Historically, vaccines have been the most effective method to manage infectious diseases in both humans and animals.
While developing a vaccine that targets a self-protein faces many challenges, there is promising proof-of-principle evidence that such a breakthrough is achievable for PrPSc.5-12 Notably, previous investigations have focused primarily on overcoming immunological tolerance to achieve immune responses to PrPC.5-9,30 There has been minimal consideration of pathological consequences that may be associated with the induction of PrPC-reactive antibodies. Further, these experimental vaccines often utilize adjuvants, vaccine designs, or vaccination regimes that are inconsistent with commercial vaccine production.10-12,29
While the induction of PrPC reactive antibodies has been shown to be of therapeutic benefit in delaying the onset of prion disease, there is lingering concern that these antibodies may also cause pathology. In particular, antibodies to this ubiquitous cell-surface protein have been shown to cause apoptosis of neurons and disrupt immune cell signaling and function.13-16
The misfolding mechanism that makes prion diseases unique as infectious agents may represent an “Achilles' heel” for the development of a safe and effective immunotherapeutic. Specifically, by targeting regions of the protein that become surface-exposed following misfolding it may be possible to develop vaccines that induce antibody responses specific to the misfolded PrPSc.17,19,23 This conformation-specific approach to immunotherapy offers the potential to target pathological isomers while sparing the function of the natively structured protein.
To date, 3 misfolding-specific vaccine targets, termed disease-specific epitopes (DSEs), have been identified and translated into PrPSc-specific vaccines.17 The initial YYR epitope was identified through experimental misfolding of PrPC and then, based on the localization of the YYR motif to β-strand 2, a YML sequence of the opposing β-strand was proposed and verified as a DSE.17,18 An algorithm that identifies protein regions likely to unfold proposed a DSE between β-strand 2 and α-helix 2.19 This region, termed rigid loop (RL) is noted for its distinctive rigidity in cervids.20,21
Vaccines generated to the YYR, YML and RL DSEs have been characterized for immunogenicity, PrPSc-specificity of the induced antibodies, and safety.17 Thus far only an early-generation, weakly immunogenic version of a YYR-based vaccine has been evaluated for efficacy in a challenge trial. The encouraging outcome of this trial provides impetus for further evaluation of DSE-based vaccine efficacy in clinical trials.22
In this investigation, as a strategy for prioritizing vaccines for future clinical trials, the prion neutralization capacity of antibodies specific to each of the 3 DSEs was evaluated in an in vitro cell-based assay. Antibodies to all 3 DSEs, either purified or in the context of immunoreactive sera, displayed an equivalent capacity to neutralize prions and there was no additive or synergistic effect when antibodies to multiple DSEs were included within the same assay. At low doses, the PrPSc-specific antibodies displayed neutralization activity similar to a monoclonal antibody (mAb) reactive with both PrPC and PrPSc. At higher doses, however, the pan-specific mAb effected greater neutralization, which may be attributed to its potential to both neutralize infectious PrPSc as well as deplete PrPC. This increased neutralization ability is associated, however, with an increased risk of immunopathology due to PrPC reactivity.
Materials and Methods
Construction and Purification of Leukotoxin-Fused Constructs
Genes corresponding to the optimized epitopes were synthesized by Genscript (Piscataway, NJ) and sub-cloned for expression as C-terminal fusions of the Leukotoxin (Lkt) carrier protein.23 The resulting Lkt recombinant fusion proteins were expressed in E. coli BL21 as described.23
Vaccine Formulation and Delivery
Vaccination of Mice
C57Bl6 or Balb/c mice (n=8/group) received 3 subcutaneous (SC) injections of 10 μg of leukotoxin recombinant fusion protein formulated with 30% Emulsigen-D (MVP Technologies, Omaha, NE) in a final volume of 100 μl per vaccine dose. Beginning at 5–6 weeks of age, mice were immunized on days 0, 21 and 42. The SC injections were administered between the shoulder blades to mid back (dorsum) using a 25 gauge 5/8″ long needle. Serum samples were collected on days 0 (pre-immune), 21, 28, 42, 49, and 70.
Vaccination of Sheep
Female and castrated-male Suffolk sheep between 1–2 years of age (n=8/group) were injected SC with 50 μg of Lkt recombinant fusion protein prepared in phosphate-buffered saline (PBS) and 30% Emulsigen-D in an injection volume of 1 mL. Sheep were immunized 3 times at 6-week intervals with the vaccine injected SC by placing a 20 gauge, 1.5 inch needle beneath a tented skin fold on the lateral cervical area, within a triangle bounded by the shoulder, dorsum of the neck, and the lateral processes of the cervical spine. All experimental protocols were approved by the University of Saskatchewan Animal Care Committee following the Canadian Council on Animal Care Guidelines to the Care and Use of Experimental Animals.
ELISAs
Epitope-specific serum antibody titres were quantified by ELISA as previously described using peptides that consisted of a single forward-back-back repeat motif for each DSE sequence.23
Antibody Purification
Prion DSE antibodies were generated by immunizing sheep with Lkt-YML, -YYR, or -RL recombinant fusion proteins. Prion DSE polyclonal antibodies were affinity-purified by Covance (Denver, PA) using individual DSE peptides in affinity columns to isolate antibodies from 110 ml of sheep immune-sera. Affinity-purified antibodies were dialyzed with PBS.
Neutralization Experiments
Prion Neutralization by Olyclonal Antibodies
Prion neutralization by polyclonal antibodies was evaluated using a modified standard scrapie cell assay in both a univalent and trivalent format.25 L929 cells were cultured in 96 well plates, as described previously,24 with individual polyclonal DSE-specific antibodies added at a final concentration of 0.0, 0.01, 0.1, 1.0, or 10 μg/well) or 3x each dose when used in combination. Anti-PrP (Clone SAF83) and anti-β-actin mAbs (mouse monoclonal IgG1, Abcam) were also titrated at 0.01, 0.1, or 1.0 μg/well. For each antibody concentration, cultures were then inoculated with 0.1, 0.01, or 0.001%, RML brain homogenate for 5 days. Exposed cells were passaged 3 times (1:4 and 1:7) in fresh medium without antibody and 20,000 cells were collected at the third passage. Cells were added to individual wells in a MultiscreenHTS IP 96 well, 0.45 μM filter plates (Millipore, Billerica, MA), allowed to attached before being subjected to PK digestion (5 μg/ml), and then denaturation using 3M guanidine thiocyanate. The Elispot reaction was performed using mouse anti-PrP mAb (SAF83, 1:1000) and bound mAb was detected with a goat anti-mouse alkaline phosphatase (AP) conjugated secondary antibody (1:5000). The plates were developed using BCIP/ NBT and spots quantified using an Autoimmun Diagnostika GmbH Elispot plate reader (ELR07).
Prion Neutralization by Murine Immune Sera
L929 cells were co-cultured with or without 15, 1.5, or 0.15 μl murine Lkt-RL immune sera. Cultures were exposed to 0.1% RML brain homogenate for 5 days in 96 well culture plates. Subsequent tissue culture, cell preparation, and Elispot protocols were conducted as previously described.
Statistical Analysis of Data
Data analysis was performed with the Prism 6 statistical software program (GraphPad Software, Inc.). Individual treatment differences were examined by performing 2-way analysis of variance (ANOVA). Tukey's multiple comparison test was used to perform post-hoc tests. Values of P < 0.05, 0.01, 0.001, and 0.0001 are represented as *, **, ***, and ****, respectively.
Results
Prion Neutralization by Pan-Specific PrP Monoclonal Antibodies
To validate and standardize the prion neutralization assay, a dose range of mAb and infectious material was evaluated relative to the RML control (infectious brain material in the absence of added antibodies).
SAF83, a commercially available pan-specific mAb, binds PrPC (amino acids 126–160, based upon linear PrP) and may also act via PrPSc to prevent prion propagation by depleting the cellular substrate, PrPC, and by potentially neutralizing infectious particles. The extent to which SAF83 may favor binding of PrPC over PrPSc in the SSCA is unknown, including the specific extent to which each of these mechanisms may contribute to prion neutralization. While PrPC neutralization is unavailable to PrPSc-specific antibodies, SAF83 provided a positive assay control when measuring prion neutralization by PrPSc-specific antibodies.
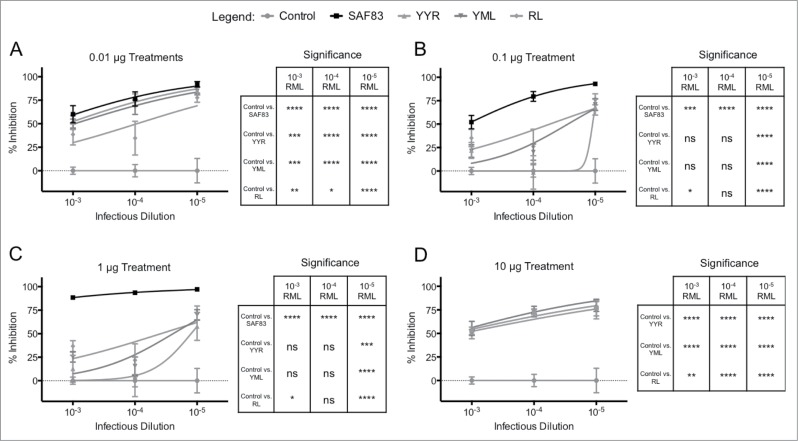
Titration of both infectious brain homogenate (10−3 to 10−5 dilution) and SAF83 mAb (0.01 μg to 1.0 μg) revealed a consistent pattern of dose-dependent prion neutralization (p < 0.0001) (Fig. 1). Titration of the anti-β-actin mAb had no significant effect on PrPSc propagation when compared to cultures infected in the absence of a mAb (Fig. 1). Anti-β-actin neutralization was statistically non-significant when compared to control, and statistically different from SAF83 mAb treatments at 1 μg (p < 0.0001), 0.1 μg (p < 0.001), and 0.01 μg (p < 0.0001). Even at the highest ratio of infectious material to antibody (10−3 RML, 0.01 μg SAF83), there was an approximately 50% reduction of prion propagation (Fig. 1). Increasing the SAF83 mAb concentration to 1 μg/well resulted in nearly complete inhibition of prion propagation across the entire range of infectious doses assayed (Fig. 1C).
FIGURE 1.
In vitro neutralization of prions by mAb SAF83. L929 cells were cultured with 0.01–1 μg SAF83 mAb or 1 μg anti-β-actin prior to adding 0.1%-0.001% RML brain homogenates for 5 days. Exposed cells were passaged 3 times with 20,000 cells collected at the third passage and loaded to filter plates. Exposed cells were passaged 3 times with 20,000 cells collected at the third passage and loaded on to filter plates. Results are expressed as percent inhibition (Control – Treatment / Control *100) + SEM of values from replicate assays.
Prion Neutralization by PrPSc-Specific Antibodies
Antibodies specific to individual DSEs also reduced prion propagation within the in vitro system. For the PrPSc-specific antibodies, at the highest concentration, there was a consistent and significant (p < 0.01 to p < 0.0001) reduction in prion propagation (Fig. 2D). That this neutralization occurs in the absence of an interaction with PrPC supports the conclusion that DSE-specific antibodies have the capacity to directly prevent propagation of infectious particles. The antibodies generated against the 3 DSEs had similar capacities to neutralize PrPSc propagation across a range of infectious concentrations, particularly at the highest and lowest antibody concentrations (Fig. 2A, 2D). The PrPSc-specific antibodies also had no effect on the viability of the L929 when incubated in the absence of RML material such that the reduction in prion formation by treatment of the anti-PrP antibodies is not due to cell death. (data not shown). As such, the current prion neutralization data provides no clear evidence for prioritizing any one of the 3 DSE vaccine targets for further testing.
FIGURE 2.
In vitro neutralization of prions by polyclonal DSE-specific ovine antibodies. Antibody specific for each disease-specific epitope was affinity-purified (pAb) from the immune sera of sheep immunized with either recombinant Lkt-YYR, -YML, or -RL antigen (Hedlin, 2010; Marciniuk, 2014). L929 cells were co-cultured with (A) 0.01 (B) 0.1 (C) 1 or (D) 10 μg pAb or SAF-83 (not 10 μg treatment) and 0.01% – 0.00001% RML brain homogenate for 5 days in 96 well culture plates. Exposed cells were passaged 3 times with 20,000 cells collected at the third passage and loaded on to filter plates. Results are expressed as percent inhibition (Control – Treatment / Control *100) + SEM of values from replicate assays.
At intermediate antibody treatments of 0.1 and 1 ug, PrPSc-specific antibodies displayed prion neutralizing activity similar to the SAF83 mAb at the lowest RML concentration (10−5) (Fig. 2B, 2C). This is a remarkable outcome given the inability of the DSE-specific antibodies to bind and deplete PrPC. Conversely, SAF83 was significantly more effective at neutralizing RML material at higher titers (10−4 and 10−5) in comparison to the PrPSc-specific antibodies (Fig. 2B, 2C).
A distinction between the 2 mechanisms of action (PrPSc neutralization versus combined PrPSc neutralization and PrPC depletion) was evident in the extent to which prion propagation was inhibited by polyclonal antisera and the mAb. Specifically, the pan-specific mAb effected nearly complete inhibition of prion propagation while PrPSc-specific antibodies effected a maximum 80% inhibition of prion propagation (Fig. 2). It is important to note that the inhibitive capacity of these antibodies need to be placed into the context of the reducing infectivity present within the starting RML material, and although the reduction of infectivity is 1–3 log depending upon RML concentration and antibody treatment, substantial infectivity still remains.
The PrPSc-specific polyclonal antibodies demonstrated an unusual dose dependent activity whereby the highest (10 μg) and lowest (0.01 μg) doses resulted in the highest, and equivalent, degrees of prion neutralization (Figs. 2A and 1D). The mid-range antibody doses (0.1 and 1.0 μg) were significantly less effective than the 10-fold higher or 10-fold lower doses. This was consistent over multiple experiments for each PrPSc-specific antibody (although to a lesser extent for RL DSE antibody) at a range of challenge doses (Fig. 2B, 2C). This trend was not apparent for SAF83. It is difficult to speculate on the mechanisms of this dose-dependence or the consequences, if any, to how this would influence the efficacy of these antibodies in vivo. While there is a trend in the extent of neutralization achieved by each DSE-specific antibody, as well as the pan specific antibody, against varying doses of infectious challenge, the extent of neutralization achieved by each antibody across different antibody concentrations is less apparent. As such, interpretation of the data is best limited to comparisons of the relative extent of neutralization achieved by each antibody within each antibody dose.
Prion Neutralization by Multivalent Antibodies
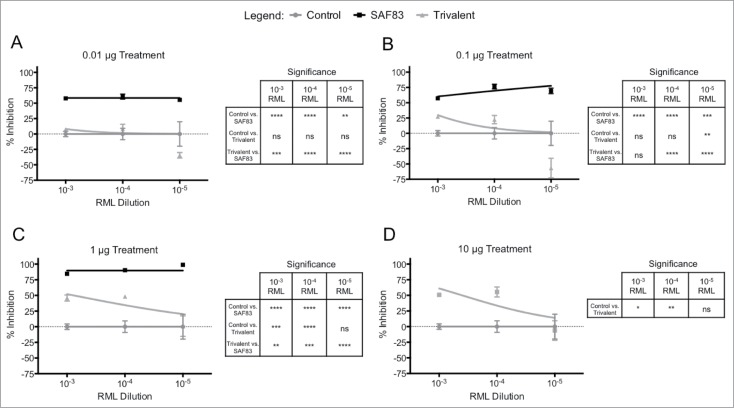
Initial efforts to develop vaccines against the 3 DSEs included consideration of multivalent formulations that induced antibody responses to each DSE.17 Therefore, we investigated whether there may be a functional interaction among neutralizing antibodies targeting distinct DSEs. Prion neutralization assays were performed to compare the activity of polyclonal antibodies targeting individual DSEs vs. antibody mixtures targeting all 3 DSEs.
Relative to the prion neutralization achieved by antibodies targeting individual PrPSc-specific DSEs there was no significant advantage to combining antibodies targeting multiple DSEs (Fig. 3). Instead, for many of the conditions evaluated, there was a decrease in prion neutralization when targeting multiple DSEs (Fig. 2B). Others have reported similar observations where combinations of mAbs achieve lower neutralization than that of the individual antibodies.27
FIGURE 3.
In vitro neutralization efficacy of polyclonal PrPSc-specific ovine antibodies in trivalent formulation. Polyclonal antibody for each disease-specific epitope was purified from immune serum from sheep receiving multiple doses of recombinant Lkt-YYR, -YML, or -RL antigen (Hedlin, 2010; Marciniuk, 2014). L929 cells were co-cultured with (A) 0.01, (B) 0.1, (C) 1.0, or (D) 10 μg of YYR, YML, and RL pAb in combination (Trivalent) and 0.01% – 0.00001% RML brain homogenate for 5 days in 96 well culture plates. Exposed cells were passaged 3 times with 20,000 cells collected at the third passage and loaded on to filter plates. Results are expressed as percent inhibition (Control – Treatment / Control *100) + SEM of values from replicate assays.
Prion Neutralization by Immune Serum from DSE Vaccinated Animals
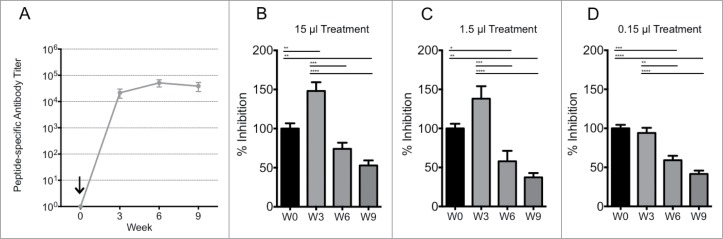
While prion neutralization by affinity-purified PrPSc-specific antibodies is encouraging, it is important to note that this occurred within an in vitro system designed to minimize complexity. There may be additional factors in vivo that influence ligand binding and/or prion propagation. To evaluate prion neutralization in a more biologically relevant system, the prion neutralizing activity of serum samples from animals immunized with the PrPSc-specific RL DSE vaccine were evaluated. Prion neutralization was compared using serum samples collected pre-vaccination and at 3 time points following a single vaccination.
Pre-immune serum did not significantly alter prion propagation and provided a control for the specificity of the post-immunization serum samples. There was no prion neutralization by serum collected 3 weeks after vaccination (Fig. 4B, 4C, 4D) even though the ELISA assay revealed a large DSE-specific antibody response at this time (Fig. 4A). Interestingly, prion neutralization by serum samples increased significantly at 6 and 9 weeks post-vaccination, even though DSE-specific antibody titers detected by ELISA did not change significantly. Since this time-dependent change in prion neutralizing activity was not associated with a significant change in total DSE-specific antibody titer, we hypothesize that maturation in antibody binding avidity may be responsible for the delayed development of prion neutralization by immune serum.
FIGURE 4.
Immunogenicity and in vitro neutralization efficacy of murine Lkt-RL immune sera. Immune sera from C57/BL6 mice (n=8/group) received a single subcutaneous injection with 10 μg Lkt-RL formulated in 30% Emulsigen D. (A) Antibody titers were quantified by capture ELISA using RL peptide, and are reported as mean values ± 1 SD. L929 cells were co-cultured with (B) 15, (C) 1.5, or (D) 0.15 μl of immune sera at 0.1% RML brain homogenate for 5 days in 96 well culture plates. Exposed cells were passaged 3 times with 20,000 cells collected at the third passage and loaded on to filter plates. Neutralization capacity is presented as percent infection (PrPSc-containing cells / 20,000 cells with trivalent treatment versus cells alone), and also as the mean ± SEM of values from replicate assays.
Discussion
The current inability to contain the spread of CWD within wild cervid populations highlights the need for new disease management tools. While vaccination has historically been the most effective approach for the control of infectious diseases, the development of a prion vaccine is complicated by challenges associated with inducing immune responses to a self-protein as well as concerns that PrPC-reactive antibodies could have pathological consequences to otherwise healthy animals. Such concerns take on even greater priority for wildlife vaccines where there is less opportunity to oversee, monitor and regulate vaccinations. Furthermore, there is proof-of-principle that PrPSc-specific antibodies can delay disease onset in a sheep challenge model.22 To date 3 PrP DSEs have been evaluated as vaccines to confirm immunogenicity, induction of PrPSc-specific antibodies, and safety.17,25
Given the cost and time commitments required for large animal prion vaccine trials, it would be useful to identify surrogate markers of disease protection to prioritize testing of PrPSc-specific vaccine candidates. In the current investigation, we evaluated the prion neutralization capacity of antibodies specific to 3 different DSEs. Prion propagation in a cell-based system provided a rapid assay to address a number of questions relevant to vaccine development.
1). Are PrPSc-specific antibodies or a pan-specifc PrP mAb more effective in prion neutralization?
In terms of efficacy, without considering safety, there are arguments in favor of an immunotherapy based on reactivity with both PrPC and PrPSc. This provides a dual mechanism of action through depletion of substrate required for prion propagation and blocking templated protein mis-folding. Both cells and animals lacking PrPC are known to be resistant to prion infection26 and the ability of certain antibodies to “cure” prion-infected cell lines reflects primarily a depletion of PrPC.27 It has been argued that immunotherapy based on PrPC reactivity is the only viable option for development of a prion vaccine.14
Alternatively, it could be argued that limiting antibody responses to PrPSc focuses the immune response on the true culprit of the disease without “wasting” immune effort on non-infectious, non-pathological forms of the protein. Focusing the antibody response on both PrPC and PrPSc may, however, rapidly deplete the therapeutic potential of an antibody response.
In our current in vitro investigations, where safety is not a consideration, then the most effective immunotherapeutic approach was pan-specific antibody that reacts with both PrPC and PrPSc. The SAF83 mAb achieved greater prion neutralization than any of the DSE-specific antibodies. This greater neutralization activity of SAF83 is most likely due to depletion of PrPC, a mechanism unavailable to the PrPSc-specific antibodies.
While the extent to which the pan-specific mAb favor binding PrPC or PrPSc, and which of these interactions has the greater therapeutic benefit is uncertain, it is certain that pan-specific antibodies have a greater number of mechanisms at their disposal for mediating a positive influence of negating prion replication. This difference appears to be of greater significance at higher antibody doses.
To date, vaccine trials using both PrPSc-specific and pan-specific vaccines have provided evidence for a therapeutic benefit. Importantly, the mechanism by which each of these categories of antibodies affords protection is not known and could include the promoted uptake and destruction of PrPSc molecules, blocking the interaction of PrPSc with PrPC to inhibit PrPSc amplification or depletion of PrPC. While any vaccine with a therapeutic benefit is undoubtedly a scientific success, the use of a prion vaccine in wildlife populations will likely need to consider the mechanisms of protection, in particular as they relate to safety.
2). Do individual DSEs differ significantly in the induction of prion neutralizing antibodies?
One objective in the current investigation was to determine whether any of the DSEs should be excluded from future vaccine development based on their capacity to induce antibodies capable of neutralizing infectious prion particles. The positions of these DSEs, within the context of the tertiary PrPC structure, have been presented elsewhere.17 Prion neutralization was considered to be a relevant correlate of immune protection. From this perspective, the similar level of prion neutralization effected by antibodies specific to all 3 DSEs was encouraging. While this observation does not confirm that antibodies induced by all, or any, of these vaccines will be effective in vivo it does suggest that each of these vaccines has sufficient potential to justify further evaluation in an animal trial.
3). Is there functional advantage to using a multivalent DSE vaccine?
The identification of multiple DSEs within the prion protein and translation of these targets into immunogenic PrPSc-specific vaccines provides an opportunity to generate a multivalent vaccine. Such a vaccine may be more efficacious by providing a greater diversity of antibody specificities, providing increased potential to neutralize a wider range of PrPSc structural variants (i.e. strains or misfolding intermediates).
Within the context of the in vitro cell based system there was no significant advantage to targeting multiple DSEs as the mixture of antibodies was, at best, only as effective as antibodies specific to individual DESs. This may, in part, reflect the unusual dose-dependence effects associated with the PrPSc-specific polyclonal antibodies. Alternatively, there may be competitive interference between DSE-specific antibodies. The situation may be very different in vivo if the concentration of DSE-specific antibody is much lower than that used in the cell-based assay. Regardless, the important point remains that while there is considerable theoretical appeal in the development of a DSE-multivalent vaccine these efforts may not be matched by gains in efficacy when compared to a DSE-univalent vaccine.
4). Do DSE-specific antibody titres provide a good surrogate marker for vaccine efficacy?
Given the expense, time, and ethical concerns associated with prion challenge vaccine trials it is desirable to identify in vitro or ex vivo surrogate measures to screen potential vaccine candidates. The first step in the development of a peptide-based vaccine for a self-protein, such as for a prion disease, was to screen a number of vaccine candidates for immunogenicity.23 This screening is based on the assumption that only vaccines inducing robust immune responses will have the capacity to prevent infection or delay disease. There is evidence from other proteinopathy vaccine trials to suggest that protection relates to the magnitude of antibody responses.28 ELISAs, however, simply quantify antibody reactivity with an immobilized peptide representing the DSE. This interaction may not accurately reflect the capacity of antibodies to stably bind to conformationally constrained epitopes within the biological context of a misfolded protein. A marked difference between antibody titre, as measured by ELISA, and prion neutralization, was apparent at 3 weeks post-vaccination (Fig. 4). While these results are encouraging, we must acknowledge the limitations of in vitro prion neutralization assays, where in vitro successes do not always translate to the same degree of in vivo efficacy.31,32 Nonetheless, based on these observations, we propose that in vitro neutralization assays may provide a valuable tool for the high-throughput screening of immune sera to facilitate the selection of vaccine candidates for evaluation in animal challenge studies.
Prion neutralization assays may also provide critical information regarding the onset and duration of a protective antibody response. This information may be critical when designing vaccination protocols for either clinical trials or application in the field. Maintaining sustained levels of prion neutralizing antibody may be critical to effectively prevent CWD infection or transmission throughout the life of an animal. Furthermore, if antibody-mediated neutralization provides a relevant surrogate measure of prion disease protection then it may be informative to further investigate the mechanism responsible for the delayed onset of prion neutralization. The maturation of an antibody response is associated with an increase in both antibody specificity and avidity and a shift in immunoglobulin isotype. Identifying which of these factors contribute to prion neutralizing may provide a way to further increase the value of surrogate measures of vaccine efficacy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 1982; 216:136-44; PMID:6801762; http://dx.doi.org/ 10.1126/science.6801762 [DOI] [PubMed] [Google Scholar]
- 2.Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol 2008; 3:11-40; PMID:18233951; http://dx.doi.org/ 10.1146/annurev.pathmechdis.3.121806.154326 [DOI] [PubMed] [Google Scholar]
- 3.Geshwind MD. Clinical trials for prion disease: difficult challenges but hope for the future. Lancet Neurol (2009) 8:304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haley NJ, Hoover EA. Chronic Wasting Disease of Cervids: Current Knowledge and Future Perspectives. Annu Rev Anim Biosci 2014; 3:205–325; PMID:25387112 [DOI] [PubMed] [Google Scholar]
- 5.Sigurdsson EM, Brown DR, Daniels M, Kascsak RJ, Kascsak R, Carp R, Meeker HC, Frangione B, Wisniewski T. Immunization delays the onset of prion disease in mice. Am J Pathol 2002; 161:13-7; PMID:12107084; http://dx.doi.org/ 10.1016/S0002-9440(10)64151-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanan E, Goren O, Eshkenazy M, Solomon B. Immunomodulation of the human prion peptide 106–126 aggregation. Biochem Biophys Res Commun 2001; 280:115-20; PMID:11162487; http://dx.doi.org/ 10.1006/bbrc.2000.4097 [DOI] [PubMed] [Google Scholar]
- 7.Koller MF, Grau T, Christen P. Induction of antibodies against murine full-length prion protein in wild-type mice. J Neuroimmunol 2002; 132:113-6; PMID:12417440; http://dx.doi.org/ 10.1016/S0165-5728(02)00316-8 [DOI] [PubMed] [Google Scholar]
- 8.Rosset MB, Ballerini C, Grégoire S, Metharom P, Carnaud C, Aucouturier P. Breaking immune tolerance to the prion protein using prion protein peptides plus oligodeoxynucleotide-CpG in mice. J Immunol 2004; 172:5168-74; PMID:15100253; http://dx.doi.org/ 10.4049/jimmunol.172.9.5168 [DOI] [PubMed] [Google Scholar]
- 9.Polymenidou M, Heppner FL, Pellicioli EC, Urich E, Miele G, Braun N, Wopfner F, Schätzl HM, Becher B, Aguzzi A. Humoral immune response to native eukaryotic prion protein correlates with antiprion protection. Proc Natl Acad Sci U S A 2004; 101(Suppl 2):14670-6; PMID:15292505; http://dx.doi.org/ 10.1073/pnas.0404772101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz A, Krätke O, Burwinkel M, Riemer C, Schultz J, Henklein P, Bamme T, Baier M. Immunisation with a synthetic prion protein-derived peptide prolongs survival times of mice orally exposed to the scrapie agent. Neurosci Lett 2003; 350:187-9; PMID:14550926; http://dx.doi.org/ 10.1016/S0304-3940(03)00907-8 [DOI] [PubMed] [Google Scholar]
- 11.Gilch S, Schätzl HM. Promising developments bringing prion diseases closer to therapy and prophylaxis. Trends Mol Med 2003; 9:367-9; PMID:13129701; http://dx.doi.org/ 10.1016/S1471-4914(03)00144-8 [DOI] [PubMed] [Google Scholar]
- 12.Goni F, Mathiason CK, Yim L, Wong K, Hayes-Klug J, Nalls A, Peyser D, Estevez V, Denkers N, Xu J, et al.. Mucosal immunization with an attenuated Salmonella vaccine partially protects white-tailed deer from chronic wasting disease. Vaccine (2015) 33:726-33; PMID:25539804; http://dx.doi.org/ 10.1016/j.vaccine.2014.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cashman NR, Loertscher R, Nalbantoglu J, Shaw I, Kascsak RJ, Bolton DC, Bendheim PE. Cellular isoform of scrapie agent protein participates in lymphocyte activation. Cell (1990) 61:185-192; PMID:1969332; http://dx.doi.org/ 10.1016/0092-8674(90)90225-4 [DOI] [PubMed] [Google Scholar]
- 14.Huang L, Su X, Federoff HJ. Single-Chain Fragment Variable Passive Immunotherapies of Neurodegenerative diseases. Int. J. Mol. Sci. (2013) 14:19109-27; PMID:24048248; http://dx.doi.org/ 10.3390/ijms140919109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solforosi L, Criado JR, McGavern DB, Wirz S, Sánchez-Alavez M, Sugama S, DeGiorgio LA, Volpe BT, Wiseman E, Abalos G, et al.. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science 2004; 303:1514-6; PMID:14752167; http://dx.doi.org/ 10.1126/science.1094273 [DOI] [PubMed] [Google Scholar]
- 16.Sonati T, Reimann R, Falsig J, Baral P, O'Connor T, Hornemann S, Yaganoglu S, Li B, Herrmann U, Wieland B, et al.. The toxicity of antiprion antibodies is mediated by the flexible tail of the prion protein. Nature (2013) 501;102-6; PMID:23903654; http://dx.doi.org/ 10.1038/nature12402 [DOI] [PubMed] [Google Scholar]
- 17.Marciniuk K, Maattanen P, Taschuk R, Airey D, Potter A, Cashman N, Griebel P, Napper S. Development of a Multivalent, PrPSc-Specific Prion Vaccine through Rational Optimization of Three Disease-Specific Epitopes. Vaccine (2014) 32:1988-97; PMID:24486363; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.027 [DOI] [PubMed] [Google Scholar]
- 18.Paramithiotis E, Pinard M, Lawton T, LaBoissiere S, Leathers VL, Zou W-Q, Estey LA, Lamontagne J, Lehto MT, Kondejewski LH, et al.. A prion protein epitope selective for the pathologically misfolded conformation. Nat Med 2003; 9:893-9; PMID:12778138; http://dx.doi.org/ 10.1038/nm883 [DOI] [PubMed] [Google Scholar]
- 19.Guest W, Cashman N, Plotkin S. Structure-Based Prediction of Unstable Regions in Proteins: Applications to Protein Misfolding Diseases. Bull Am Phys Soc 2009; 54. Available at http://meetings.aps.org/link/BAPS.2009.MAR.A40.13 [Google Scholar]
- 20.Gossert AD, Bonjour S, Lysek DA, Fiorito F, Wüthrich K. Prion protein NMR structures of elk and of mouse/elk hybrids. Proc Natl Acad Sci U S A 2005; 102:646-50; PMID:15647363; http://dx.doi.org/ 10.1073/pnas.0409008102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soto C. Constraining the loop, releasing prion infectivity. Proc Natl Acad Sci U S A 2009; 106:10-1; PMID:19118191; http://dx.doi.org/ 10.1073/pnas.0811625106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taschuk R, Marciniuk K, Maaattanen P, Madampage C, Hedlin P, Potter A, Lee J, Cashman N, Griebel P, Napper S. Safety, Specificity and Immunogenicity of a PrPSc-specific prion vaccine based on the YYR Disease-Specific Epitope. Prion February (2014). 7;8(1):51-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedlin PD, Cashman NR, Li L, Gupta J, Babiuk LA, Potter AA, Griebel P, Napper S. Design and Delivery of a cryptic (PrPC epitope for induction of PrP(Sc)-specific antibody responses. Vaccine (2009) 28:981-8; PMID:19925901; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.134 [DOI] [PubMed] [Google Scholar]
- 24.Van der Merwe J, Aiken J, Westaway D, McKenzie D. The Standard Scrapie Cell Assay: Development, Utility and Prospects. Viruses, (2015) 7: 180-98; PMID:25602372; http://dx.doi.org/ 10.3390/v7010180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maattanen P, Taschuk R, Ross L, Marciniuk K, Bertram L, Potter A, Cashman N, Napper S. PrPSc-specific antibodies do not induce prion disease or misfolding of PrPC in highly susceptible Tga20 mice. Prion. (2013) 5:434-9; http://dx.doi.org/ 10.4161/pri.26639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell 1993. 73:1339-47; PMID:8100741; http://dx.doi.org/ 10.1016/0092-8674(93)90360-3 [DOI] [PubMed] [Google Scholar]
- 27.Feraudet C, Morel N, Simon S, Volland H, Frobert Y, Creminon C, Vilette D, Lehmann S, Grassi J. Screening of 145 Anti-PrP Monoclonal Antibodies for Their Capacity to Inhibit PrPSc Replication in Infected Cells. J Biol Chem (2005) 280: 11247-58; PMID:15618225; http://dx.doi.org/ 10.1074/jbc.M407006200 [DOI] [PubMed] [Google Scholar]
- 28.Liu H-N, Tjostheim S, Dasilva K, Taylor D, Zhao B, Rakhit R, et al.. Targeting of monomer/misfolded SOD1 as a therapeutic strategy for amyotrophic lateral sclerosis. J Neurosci (2012) 32: 8791-9; PMID:22745481; http://dx.doi.org/ 10.1523/JNEUROSCI.5053-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Napper S, Cashman N. Immunotherapy for prion diseases: opportunities and obstacles. Immunotherapy (2010) 2:269-82; PMID:20635933; http://dx.doi.org/ 10.2217/imt.10.3 [DOI] [PubMed] [Google Scholar]
- 30.Hanan E, Priola S, Solomon B. Antiaggregating Antibody Raised Against Human PrP 106–126 Recognizes Pathological and Normal Isoforms of the Whole Prion Protein. Cell Molec Neurbiol (2001) 21: 693-703; http://dx.doi.org/ 10.1023/A:1015199904354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pankiewicz J, Prelli F, Sy M, Kascsak R, Kascsak R, Spinner D, Carp R, Meeker H, Sadowski M, Wisniewski T. Clearance and prevention of prion infection in cell culture by anti-PrP antibodies. Eur J Neurosci 23: 2635-47; PMID:16817866; http://dx.doi.org/ 10.1111/j.1460-9568.2006.04805.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadowski M, Pankiewicz J, Prelli F, Scholtzova H, Spinner D, Kascsak RB, Kascsak RJ, Wisniewski T. Anti-PrP Mab 6D11 suppresses PrPSc replication in prion infected myeloid precursor line FDC-P1/22L and in the lymphoreticular system in vivo. Neurobiol Dis. 34: 267-78; PMID:19385058; http://dx.doi.org/ 10.1016/j.nbd.2009.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]