Abstract
Recombinant protein production in microorganisms is one of the most studied areas of research in biotechnology today. In this respect the yeast Pichia pastoris is an important microbial production host due to its capability of secreting the target protein and performing posttranslational modifications. In a recent study, we described the development of a robust bioprocess for a glyco-engineered recombinant P. pastoris strain where the native α-1,6-mannosyltransfrease OCH1 was knocked out (Δoch1 strain). This strain produced the glycosylated enzyme horseradish peroxidase (HRP) with more homogeneous and shorter surface glycans than the respective benchmark strain. However, the recombinant Δoch1 strain was physiologically impaired and thus hard to cultivate. We faced cell cluster formation, cell lysis and consequent intensive foam formation. Thus, we investigated the effects of the 3 process parameters temperature, pH and dissolved oxygen concentration on (1) cell physiology, (2) cell morphology, (3) cell lysis, (4) productivity and (5) product purity in a multivariate manner. However, not only process parameters might influence these characteristics, but also media supplements might have an impact. Here, we describe the effects of different heme-precursors as well as of a protease-inhibitor cocktail on the production of active HRP in therecombinant P. pastoris Δoch1strain.
Keywords: heme precursor, media supplements, OCH1, Pichia pastoris, plant peroxidase, protease inhibitor
Abbreviations
- HRP
horseradish peroxidase
- OCH1, α-1
6-mannosyltransfrease Outer CHain elongation 1
- Δoch1
P. pastoris strain with OCH1 knockout
Introduction
Pichia pastoris, a methylotrophic yeast, is widely used for recombinant protein production due to the possibility of reaching high cell densities during cultivation, protein segregation and the capability of performing posttranslational modifications (e.g.1-3). However, a significant disadvantage of this microorganism is its tendency to perform hyperglycosylation of proteins.4 This represents a significant problem for the production of biopharmaceuticals and for subsequent downstream processing.5,6
Strain engineering of P. pastoris depicts an interesting option to conquer the problem of hyperglycosylation. In a recent study, we described the characterization of a recombinant P. pastoris strain where the native α-1,6-mannosyltransfrease OCH1 was knocked out (Δoch1 strain;4). This Δoch1 strain, which recombinantly produced the heme-enzyme horseradish peroxidase (HRP;6), showed a growth impaired phenotype and considerable rearrangements of cell wall components leading to substrate dependent cell cluster formation and cell lysis. Consequent intensive foam formation made this strain hard to cultivate. In a subsequent study we developed a robust fed-batch process for this recombinant Δoch1 and investigated the effects of the 3 process parameters temperature, pH and dissolved oxygen concentration on (1) cell physiology, (2) cell morphology, (3) cell lysis, (4) productivity and (5) product purity in a multivariate manner.7 We found out that the strain could not be cultivated at 30°C without methanol accumulation and that highest productivity and product purity were reached at 20°C, a pH of 5.0 and a dissolved oxygen concentration of 10%.7 However, not only process parameters can influence productivity and product purity, but also media supplements may affect these parameters. Thus we tested potential effects of (1) different heme precursors and (2) a protease inhibitor cocktail on productivity and product purity by adding these compounds to shake flask cultivations of the recombinant Δoch1 strain.
Effect of heme precursors
The enzyme HRP comprises 4 disulfid-briges, 2 Ca2+- ions as prosthetic group and an iron-protoporphyrin-ring (a heme group) as cofactor in the active site.8,9 It is known that the addition of a heme-precursor supports the production of active heme enzymes.10 In a recent study, we investigated the effect of adding either the heme-precursors Δ-aminolevulinic acid (ALA and ferric sulfate (FeSO4) or hemin on the production of active HRP using a recombinant P. pastoris benchmark strain with active OCH1.11 We found out that medium supplementation with the traditionally used and pricy heme precursor ALA did not increase the yield of active product. FeSO4 and hemin on the other hand turned out to be useful medium supplements to increase the yield of active heme protein.11 Thus, we tested these precursors also for the production of HRP with the recombinant Δoch1 strain.7
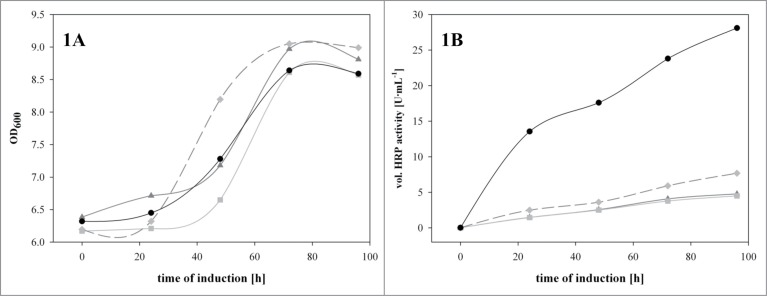
After overnight cultivation in 5 mL BMGY/Zeocin (1% yeast extract; 2% peptone; 100 mM potassium phosphate buffer, pH 6.0; 1,34% YNB; 4·10−5% biotin; 1% glycerol; 50 μg·mL−1 ZeocineTM) in 100 mL baffled shake flasks at 30°C and 230 rpm, the cell suspension was transferred into 20 mL BMMY/Zeocin (1% yeast extract; 2% peptone; 100 mM potassium phosphate buffer, pH 6.0; 1,34% YNB; 4·10−5% biotin; 0.5% methanol; 50 μg·mL−1 ZeocineTM) in 250 mL baffled shake flasks and cultivated at 20°C and 230 rpm. We reduced the cultivation temperature from 30°C to 20°C upon induction since we had seen highest productivity and purity for the recombinant Δoch1 strain at the lower temperature previously.7 The BMMY/Zeocin contained either no heme-precursor or ALA [1 mM], FeSO4 [1 mM] or hemin [30 μM], respectively. Cells were cultivated for 96 hours under these inducing conditions. Every day, samples were taken and analyzed for OD600, extracellular protein content and enzymatic HRP activity and 0.5% (v/v) pure methanol was pulsed. In Figure 1 the time courses of biomass growth (followed by OD600 values; Fig. 1A) and the extracellular HRP activity (Fig. 1B) are shown.
Figure 1.
The recombinant Δoch1 strain was cultivated under inducing conditions in the presence of different heme precursors in shake flasks at 25°C for 96 hours. (A) OD600 values over induction time; (B) volumetric enzyme activity in U·mL−1 over induction time. Dark gray solid line with triangles, no heme precursor; light gray solid line with squares, ALA; dark dashed line with diamonds, FeSO4; back solid line with dots, hemin.
As shown in Fig. 1A, the growth of the recombinant Δoch1 strain was not affected by the presence of either heme precursor. For all the cultivations the strain grew in the first 70 hours of induction, before limitations caused reduced growth. However, in terms of the amount of active extracellular HRP, we observed drastic differences (Fig. 1B). The traditionally used and pricy heme precursor ALA did not have any impact on the amount of active HRP but we observed a slight positive effect of FeSO4 (1.6-times more active enzyme). However, we obtained 7-times more active HRP when we induced the recombinant Δoch1 strain in the presence of 30 μM hemin. In a previous study we observed a boost in the amount of active HRP of up to fold18- when we added 10 μM hemin to minimal media in microscale cultivations of a recombinant P. pastoris benchmark strain.11 In comparison, in the present study performed with the recombinant Δoch1 strain we obtained only a 7-fold boost. This discrepancy might be explained by the different media used in the 2 studies. In our previous study we used minimal media, whereas here we used complex BM(G/M)Y medium, which already contained a certain amount of heme-precursors. Thus the observed boost was not that pronounced. However, we can conclude that the boosting effect by hemin is product related and not strain dependent.
Interestingly, FeSO4 and hemin did not only affect the enzymatic activity, but also the total amount of extracellular protein. In Table 1 the values for OD600, HRP activity, extracellular protein content and specific activity at the end of cultivation are shown. Clearly we obtained the highest amount of active HRP in the presence of hemin, however we also reached the highest extracellular protein concentration under these conditions. In terms of specific activity, we observed a more than 3-fold higher value for HRP produced in the presence of hemin compared to the traditionally used ALA.
Table 1.
Values for OD600, HRP activity, total protein content and specific activity for the recombinant Δoch1 strain induced in the presence of different heme precursors for 96 hours
| Heme precursor | OD600 | HRP activity [U· mL−1] | Protein content [mg· mL−1] | Specific activity [U· mg−1] |
|---|---|---|---|---|
| No precursor | 8.81 | 4.79 | 0.056 | 84.8 |
| ALA [1 mM] | 8.57 | 4.51 | 0.053 | 85.5 |
| FeSO4 [1 mM] | 8.99 | 7.70 | 0.065 | 117.9 |
| hemin [30 μM] | 8.59 | 28.1 | 0.101 | 278.1 |
Furthermore, we investigated if the different heme precursors also influenced cell morphology.12 We analyzed all the samples under the microscope and with a Malvern Mastersizer but could not determine any effect on cell morphology (data not shown). Thus, we conclude that addition of hemin is an effective strategy to obtain more active recombinant peroxidase not only for the benchmark strain, but also for the growth impaired Δoch1 strain.
Effect of protease inhibitor cocktail
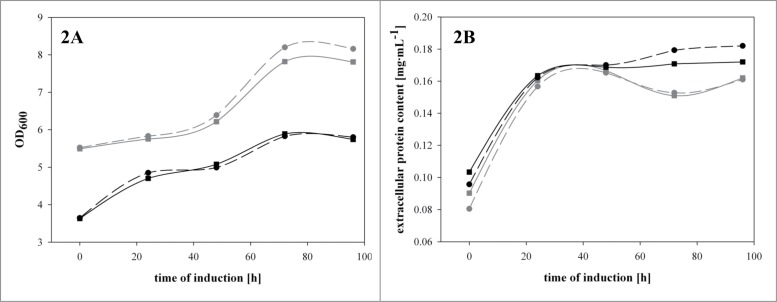
In our previous studies we had shown that the recombinant Δoch1 strain was physiologically impaired and strongly affected by cell lysis.4,7 It is well known that cell lysis also means the release of intracellular proteases, which could potentially degrade the target product.13 Thus, we tested the effect of the presence of a protease inhibitor cocktail (cOmplete Mini EDTA-freeTM; Roche, Switzerland) on the total amount of extracellular protein and active HRP in shake flask experiments. We conducted this study with the P. pastoris benchmark strain with intact OCH1 and with the recombinant Δoch1 strain. The cultivations were again performed in shake flasks, as described above. Both strains showed growth in the first 70 hours of induction, before limitations caused reduced growth at the later phases of cultivation. However, when we analyzed the growth of these 2 strains during the induction phase in more detail, we clearly confirmed the impaired growth of the recombinant Δoch1 strain (Fig. 2A). Interestingly, when we analyzed the total extracellular protein content (Fig. 2B) and the enzymatic HRP activity in the cultivation broths of the 2 strains, cultivated in the presence or absence of the protease inhibitor cocktail, we could not determine any differences. Thus, we concluded that the presence of protease inhibitors does not affect the amount of product for the recombinant benchmark strain nor for the recombinant Δoch1 strain, respectively. As shown in our previous study, cell cluster formation and consequent cell lysis for the recombinant Δoch1 strain are C-source dependent and mainly happen in cultivation phases on glycerol – once the cells switch to methanol cell cluster formation as well as lysis are diminished.7 As we can see from the present data, it is not necessary to add protease inhibitors to the cultivation broth of the recombinant Δoch1 strain to protect the product from the proteases released before.
Figure 2.
A recombinant benchmark strain and the recombinant Δoch1 strain were cultivated under inducing conditions in the presence or absence of a protease inhibitor cocktail in shake flasks at 25°C for 96 hours. (A) OD600 values over induction time; (B) total extracellular protein concentration in mg·mL−1 over induction time. Dark gray solid line with squares, benchmark strain without protease inhibitor; dark gray dashed line with dots, benchmark strain with protease inhibitor; black solid line with squares, Δoch1 strain without protease inhibitor; black dashed line with squares, Δoch1 strain with protease inhibitor.
In conclusion we showed that heme precursors can also be used successfully for the recombinant Δoch1 strain to produce more active HRP. In agreement with our previous study performed with a recombinant benchmark strain,11 we found out that addition of hemin gave the highest amount of active product. Furthermore, we showed that endogeneous proteases released by cell lysis events during glycerol cultivation phases of the recombinant Δoch1 strain do not degrade the product during subsequent induction phases, thus making the addition of protease inhibitors unnecessary.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The authors thank the Austrian Science Fund FWF (project P24861-B19) for funding.
References
- 1. Cregg JM, Cereghino JL, Shi JY, Higgins DR. Recombinant protein expression in Pichia pastoris. Mol Biotechnol 2000; 16:23-52; PMID:11098467; http://dx.doi.org/ 10.1385/MB:16:1:23 [DOI] [PubMed] [Google Scholar]
- 2. Cregg JM, Tschopp JF, Stillman C, Siegel R, Akong M, Craig WS, Buckholz RG, Madden KR, Kellaris PA, Davis GR, et al. . High-Level Expression and Efficient Assembly of Hepatitis-B Surface-Antigen in the Methylotrophic Yeast, Pichia-Pastoris. Nat Biotechnol 1987; 5:479-85; http://dx.doi.org/ 10.1038/nbt0587-479 [DOI] [Google Scholar]
- 3. Zalai D, Dietzsch C, Herwig C, Spadiut O. A dynamic fed batch strategy for a Pichia pastoris mixed feed system to increase process understanding. Biotechnol Prog 2012; 28:878-86; PMID:22505140; http://dx.doi.org/ 10.1002/btpr.1551 [DOI] [PubMed] [Google Scholar]
- 4. Krainer FW, Gmeiner C, Neutsch L, Windwarder M, Pletzenauer R, Herwig C, Altmann F, Glieder A, Spadiut O. Knockout of an endogenous mannosyltransferase increases the homogeneity of glycoproteins produced in Pichia pastoris. Scientific reports 2013; 3:3279; PMID:24252857; http://dx.doi.org/ 10.1038/srep03279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spadiut O, Rossetti L, Dietzsch C, Herwig C. Purification of a recombinant plant peroxidase produced in Pichia pastoris by a simple 2-step strategy. Protein Expr Purif 2012; 86:89-97; PMID:23026679; http://dx.doi.org/ 10.1016/j.pep.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 6. Spadiut O, Herwig C. Production and purification of the multifunctional enzyme horseradish peroxidase: a review. Pharm Bioproc 2013; 1:283-95; http://dx.doi.org/ 10.4155/pbp.13.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gmeiner C, Saadati A, Maresch D, Krasteva S, Frank M, Altmann F, Herwig C, Spadiut O. Development of a fed-batch process for a recombinant Pichia pastoris inverted question mark och1 strain expressing a plant peroxidase. Microb Cell Fact 2015; 14:1; PMID:25567661; http://dx.doi.org/ 10.1186/s12934-014-0183-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith AT, Santama N, Dacey S, Edwards M, Bray RC, Thorneley RNF, Burke JF. Expression of a Synthetic Gene for Horseradish Peroxidase-C in Escherichia-Coli and Folding and Activation of the Recombinant Enzyme with Ca-2+ and Heme. J Biol Chem 1990; 265:13335-43; PMID:2198290 [PubMed] [Google Scholar]
- 9. Veitch NC, Smith AT. Horseradish peroxidase. Adv Inorg Chem 2001; 51:107-62; http://dx.doi.org/ 10.1016/S0898-8838(00)51002-2 [DOI] [Google Scholar]
- 10. Jiang H, Morgan JA. Optimization of an in vivo plant P450 monooxygenase system in Saccharomyces cerevisiae. Biotechnol Bioeng 2004; 85:130-7; PMID:14704995; http://dx.doi.org/ 10.1002/bit.10867 [DOI] [PubMed] [Google Scholar]
- 11. Krainer FW, Capone S, Jager M, Vogl T, Gerstmann M, Glieder A, Herwig C, Spadiut O. Optimizing cofactor availability for the production of recombinant heme peroxidase in Pichia pastoris. Microb Cell Fact 2015; 14:4; PMID:25586641; http://dx.doi.org/ 10.1186/s12934-014-0187-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mellitzer A, Ruth C, Gustafsson C, Welch M, Birner-Grunberger R, Weis R, Purkarthofer T, Glieder A. Synergistic modular promoter and gene optimization to push cellulase secretion by Pichia pastoris beyond existing benchmarks. J Biotechnol 2014; 191:187-95; PMID:25193713; http://dx.doi.org/ 10.1016/j.jbiotec.2014.08.035 [DOI] [PubMed] [Google Scholar]
- 13. Sinha J, Plantz BA, Inan M, Meagher MM. Causes of proteolytic degradation of secreted recombinant proteins produced in methylotrophic yeast Pichia pastoris: case study with recombinant ovine interferon-tau. Biotechnol Bioeng 2005; 89:102-12; PMID:15580575; http://dx.doi.org/ 10.1002/bit.20318 [DOI] [PubMed] [Google Scholar]