Abstract
Background
Recent data obtained in mouse models have initiated a controversy whether basophils are the key antigen-presenting cells (APCs) in allergy. Here, we investigate whether basophils are of importance for the presentation of allergen and the induction of T cell proliferation in allergic patients.
Methods
T cells, basophils, and APCs depleted of basophils were purified from allergic patients. Co-culture systems based on purified major allergens were established to study allergen-specific T cell responses using proliferation assays.
Results
Only co-cultures of T cells with APCs depleted of basophils but not with basophils proliferated in response to allergen. Even addition of IL-3 to T cell–basophil co-cultures failed to induce allergen-specific T cell proliferation.
Conclusions
Our data demonstrate by classical in vitro proliferation assays that basophils are not key antigen-presenting cells that promote T cell proliferation in secondary immune responses to allergen in allergic patients.
Keywords: allergen, allergy, antigen-presenting cells, basophils, Bet v 1, Th2
Internalization and presentation of antigen by professional antigen-presenting cells (APCs) is a crucial step in the initiation and modulation of allergic immune responses (1, 2). The nature and dose of the antigen, its affinity for the T cell receptor (TCR), the presence of co-stimulatory molecules on APCs as well as the cytokine milieu at the time of antigen encounter are important factors determining the differentiation of CD4+ T cells into different T-helper (Th) cell subsets (3). So far, dendritic cells (DCs), Langerhans cells (LCs), macrophages, and B cells have been regarded as the major APCs involved in allergic responses (4, 5).
Basophils have mainly been considered as important effector cells in the induction of allergic inflammation through the release of mediators, proteases, and pro-inflammatory mediators in allergy (6, 7). Recently, an additional function for basophils has been proposed. Employing protease antigens (8), helminthic parasites (9, 10), or antigen–IgE complexes (9) in murine models, three groups concurrently proposed a pivotal role of basophils in antigen presentation and the initiation of Th2 responses in mice. They demonstrated that basophils internalized soluble allergen (8), presented it in the context of MHC II as well as co-stimulatory molecules to T cells, and thus acted as bona fide APCs (8–10).
However, other recent studies that were also performed in murine models questioned the absolute requirement of basophils in the generation of Th2 responses (11, 12). Employing an antibody depletion strategy different from the earlier studies (11) or transgenic mice deficient in basophils (12), it was shown that the absence of basophils did not impair the induction of Th2 responses. Thus, it was demonstrated that DCs and not basophils were mainly involved in the initiation of Th2 responses in murine models of allergy. Therefore, the question whether basophils or DCs are key APCs in human Th2 immunity and allergy remains controversial.
Here, we sought to study whether basophils from allergic patients are capable of inducing allergen-specific T cell proliferation.
Materials and methods
Reagents
See Data S1.
Patients
Birch pollen–allergic patients (n = 16) were included in this study after written informed consent was obtained from all patients before taking the blood. The diagnosis of birch pollen allergy was based on case history, positive skin test results to birch pollen, and IgE serology (13).
Cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood of allergic patients outside the birch pollen season by Ficoll density gradient centrifugation or by Hetasep for experiments where the EasySep human basophil enrichment kit was used. T cells were prepared from PBMCs by negative magnetic separation. PBMCs depleted of T cells by anti-CD3 beads were used for the isolation of basophils using MACS human basophil isolation kit II (antibodies against CD3, CD4, CD7, CD14, CD15, CD16, CD36, CD45RA, HLA-DR, and CD235a) or by EasySep human basophil enrichment kit (antibodies against CD2, CD3, CD14, CD15, CD16, CD19, CD24, CD34, CD36, CD45RA, CD56, and glycophorin A) in certain experiments according to the manufacturer’s instructions. This step yielded two populations: The flow-through contained basophils, while APCs depleted of basophils remained bound to the column and were eluted thereafter. PBMCs, isolated T cells, basophils, or APCs depleted of basophils were tested for purity by flow cytometry and cultured overnight for recovery. In experiments where fixed cells were used, APCs depleted of basophils were fixed in 2% PFA for 10 min at 37°C after overnight culture. Fixed cells were washed twice in phosphate-buffered saline (PBS) before setup of culture.
Thereafter, all cells if not fixed were tested for viability and functionality by flow cytometry. T cells were mixed with either autologous basophils or APCs depleted of basophils at a ratio 1 : 1 or 5 : 1 or 10 : 1 in 96-well plates (200 000 cells/well) as indicated. PBMCs or T cells cultured alone were used as controls. Cells were either left unstimulated in medium or stimulated with Bet v 1, Alt a 1, or Phl p 5 (all at 5 μg/ml unless otherwise indicated) in the presence or absence of IL-3 (50 ng/ml). To investigate the influence of histamine on proliferation, PBMCs were either left unstimulated in medium or stimulated with Bet v 1 or Phl p 5 (5 μg/ml) in the presence or absence of histamine dihydrochloride in certain experiments (100–0.8 ng/well). In certain proliferation experiments, Staphylococcus enterotoxin A (SE-A) was applied at a concentration of 0.5 μg/ml. All experiments were performed in triplicate wells. Cultures were pulsed with 3H-thymidine at 0.5 μCi/well for 16 h before harvest, and 3H-thymidine incorporation was measured using a beta counter (MicroBeta TriLux; PerkinElmer, Waltham, MA, USA). Thymidine incorporation in unstimulated cells was subtracted as background. Proliferation data of patients were included in the analysis, if measurement of thymidine incorporation in PBMCs upon allergen challenge was >0 after subtraction of background in at least one of the triplicates. If measurement of thymidine incorporation within one of the triplicates differed more than 3-fold from the other two, this value was excluded as outlier.
Flow cytometry
See Data S1.
Histamine release
See Data S1.
Immunohistochemistry and electron microscopy
See Data S1.
Statistics
See Data S1.
Results
T cells and basophils isolated from allergic patients are of high purity and functional
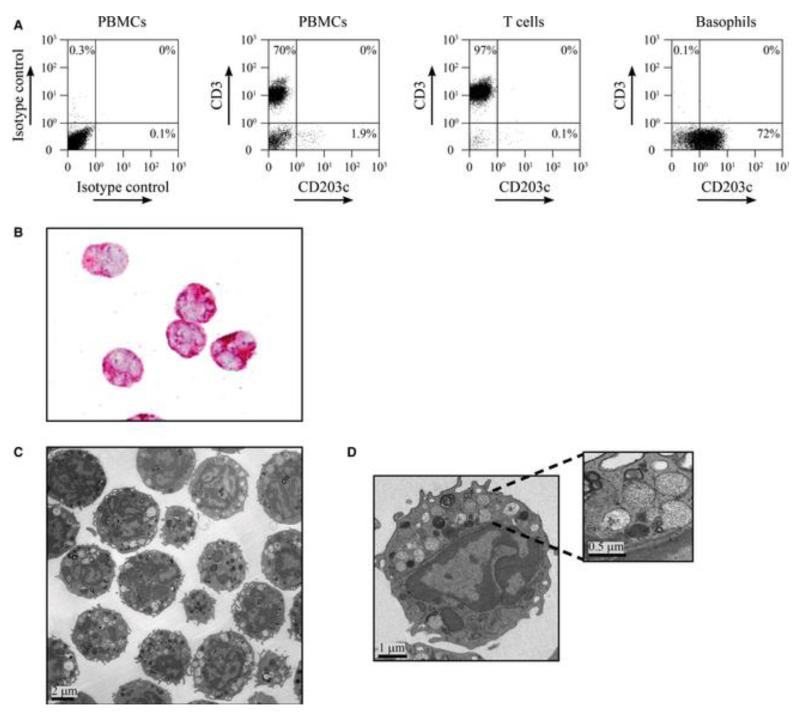
In order to investigate whether basophils can function as allergen-presenting cells and induce allergen-specific T cell proliferation, T cells and basophils were prepared from PBMCs of allergic patients by negative magnetic selection. Flow cytometric analysis of the purification fractions showed that the Ficoll preparation enriched the basophils and that no basophils were lost in the pellet fraction (Fig. S1). Comparison of the Miltenyi basophil isolation kit with the EasySep kit, which does not deplete HLA-DR-expressing cells, showed that both kits yielded basophils of a comparable purity as assessed by double staining for basophil markers CD63 and CD203c (Fig. S2). Expression of HLA-DR on freshly isolated basophils was below 1% regardless of the kit used. The purity of T cells is exemplified in Fig. 1A and, as analyzed for seven donors by anti-CD3 staining, was 94.5 ± 2%. When purified basophils obtained by negative selection were stained with anti-CD203c, only 74.3 ± 16.3% were positive. However, CD203c is expressed only at low levels by non-activated basophils (14). Therefore, we also studied the purity of basophils using anti-CD123 and anti-FcεRI staining and found that the preparations typically contained more than 85% basophils and no DCs as shown by anti-CD1c staining (Fig. S1). The purity of basophil preparations was also confirmed by immunocytochemistry using the basophil-specific antibody 2D7 (Fig. 1B) and by transmission electron microscopy. Basophils in the preparations were identified by their polylobed nuclei with a condensed chromatin pattern and their large secretory granules (Fig. 1C,D) (15). For electron microscopic evaluation of purity, all cells within a section exhibiting a nucleus were analyzed and classified as either basophils, other blood cells, or not definable according to their ultrastructural characteristics. In a typical preparation, 227 cells were assessed, of which 224 (98.7%) were basophils and three cells could not be defined.
Figure 1.
Purity of T cells and basophils isolated from allergic patients. (A) Dot blots of peripheral blood mononuclear cells (PBMCs), purified T cells, and basophils from an allergic patient which were labeled with anti-CD3 PC7 and anti-CD203c PE antibodies and analyzed by flow cytometry. Isotype controls are shown for PBMCs. Blots are representative of results obtained for seven patients. (B) Immunocytochemical detection of purified basophils by staining with 2D7 antibody (red). (C, D) Analysis of purified basophils by electron microscopy. Overview (C) and higher magnifications of one representative basophil (D) showing the typical polylobed nucleus and large cytoplasmic secretory granules (insert).
We also prepared an APC fraction that was depleted of T cells and basophils. This fraction contained CD11c single-positive cells typical for DCs and CD11c, CD14 double-positive cells (data not shown) as well as CD1c-positive DCs (Fig. S1).
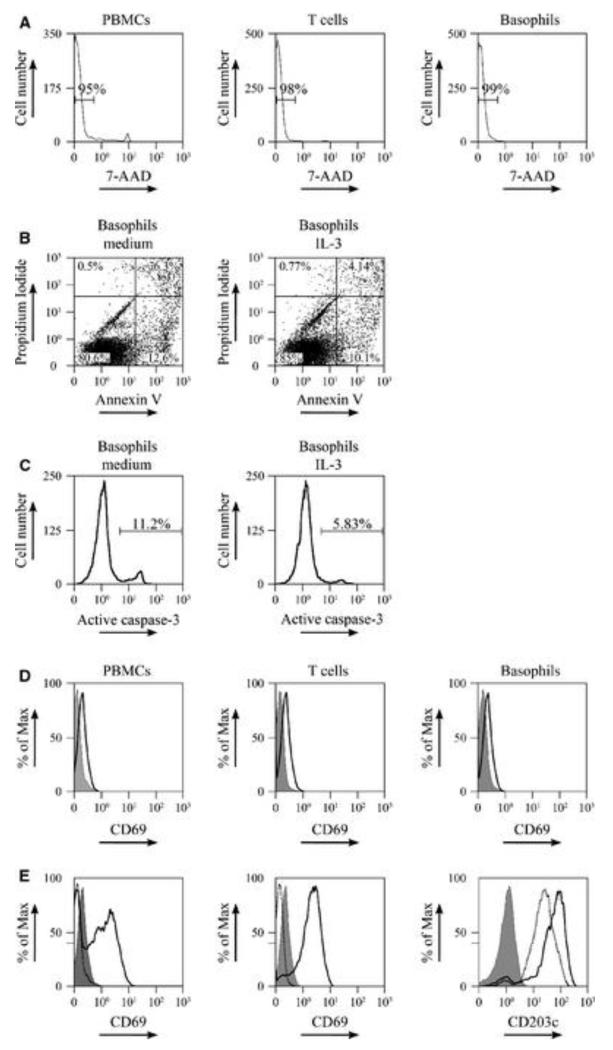
After magnetic separation, T cells, basophils, APCs, and PBMCs were cultured overnight in the absence of any stimulation to allow a recovery of the cells. Thereafter, viability of the cells was determined by staining for 7-amino-actinomycin-D (7-AAD) by flow cytometry. On average, 95.5 ± 2% of the PBMCs, 97 ± 1.1% of the T cells, 94.7 ± 7.1% of the basophils, and 95.7 ± 2.3% of the APCs depleted of basophils were alive (Fig. 2A). Assessment of apoptosis in basophils cultured overnight in the presence or absence of interleukin (IL)-3 showed a more than 80% viability of the basophils for both conditions (Fig. 2B,C). Furthermore, isolated T cells and basophils were not preactivated as they did not express the early activation marker CD69 (Fig. 2D).
Figure 2.
Purified T cells and basophils are viable and can be activated. (A–D) Flow cytometry was performed on peripheral blood mononuclear cells (PBMCs) (left panels in A and D), isolated T cells (middle panels in A and D), and isolated basophils (B, C and right panels in A and D) after 24 h of culture. (A) The percentage of viable cells as determined using 7-amino-actinomycin-D (7-AAD) staining. (B and C) The percentage of apoptotic cells in purified basophils cultured overnight in the absence (left panels) or presence (right panels) of IL-3 (50 pg/ml) was determined by staining for (B) annexin V and propidium iodide or (C) anti-active caspase-3 (D). Expression of the activation marker CD69 on unstimulated cells after overnight culture was determined by flow cytometry using an antibody against CD69 (black open histogram) or matched isotype control antibodies (gray histogram). Isotype staining (solid gray) was used as a control. (E) PBMCs (left panel) and isolated T cells (middle panel) were stimulated overnight with anti-CD3/CD28. Cells were harvested and stained with anti-CD69 (black open histogram) or isotype control (gray histogram) and analyzed by flow cytometry. Unstimulated cells (dotted black open histogram) were used as control. Basophils (right panel) were cultured 48 h in 0.1 ng/ml IL-3, stimulated with anti-IgE, and then stained with anti-CD203c (black open histogram) or the isotype control (gray histogram). Unstimulated basophils were used as control (dotted black open histogram). Data are representative of cell preparations from 7 (A, D) and 2 (B, C, E) allergic patients respectively with comparable results seen in all donors.
Additionally, we studied the functionality of both purified T cells and basophils. To that aim, T cells were stimulated with anti-CD3/CD28 and expression of CD69 was determined by flow cytometry 24 h after stimulation. Isolated T cells upregulated CD69 in response to the anti-CD3/CD28 challenge at a level comparable to PBMCs (Fig. 2E). Functional responsiveness of basophils was assessed by CD203c expression (14) as well as by histamine-release experiments. Cross-linking of FcεRI receptor-bound IgE by anti-IgE up-regulated CD203c on basophils 2.5 ± 0.5% fold (Fig. 2E). Furthermore, basophils proved to be responsive to both anti-IgE and allergen challenge after overnight culture as assessed by histamine release (Fig. S3).
Thus, isolated T cells and basophils were pure, viable, functional, and not preactivated and allowed the setup of co-cultures for allergen stimulation.
Allergen-loaded APCs but not basophils induce allergen-specific T cell proliferation
Next, we set up co-cultures to assess whether basophils of allergic patients are capable of inducing T cell proliferation in secondary responses to the major birch pollen allergen, Bet v 1. To that aim, we mixed T cells from allergic patients either with autologous basophils or with APCs depleted of basophils at a ratio of 1 : 1 in the presence of the Bet v 1 allergen. T cells alone and PBMCs were also incubated with Bet v 1 for control purposes. Following established protocols, cultures were stimulated with the purified allergen Bet v 1 for 1 week, and proliferation was measured thereafter by 3H thymidine incorporation (16).
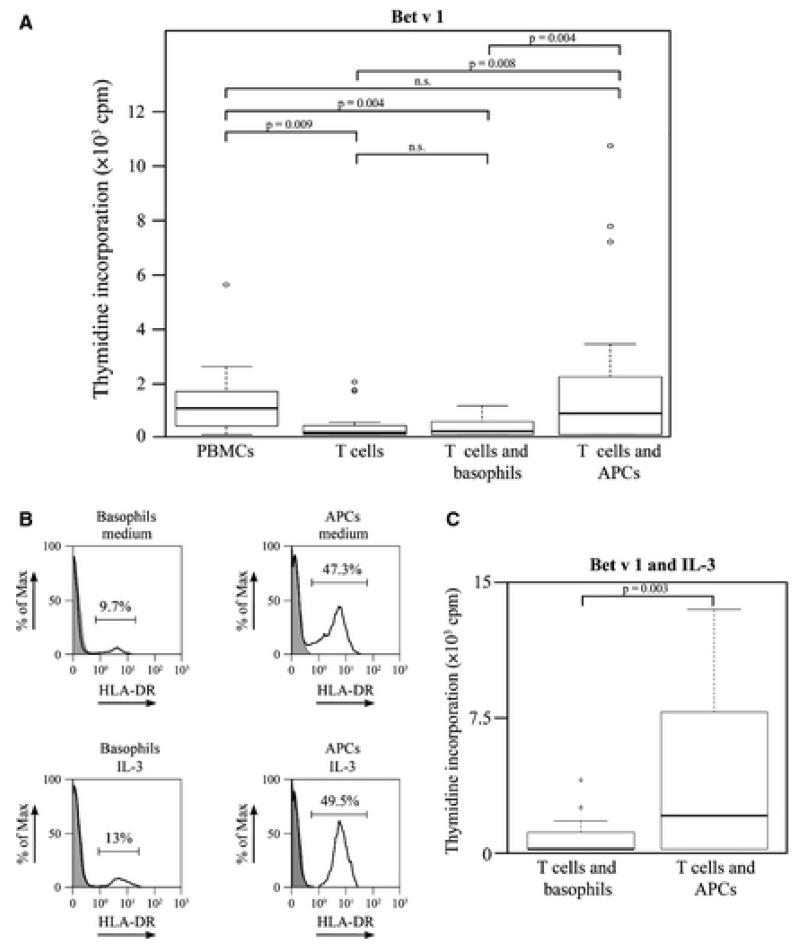
As shown in Fig. 3A, T cells co-cultured with APCs depleted of basophils proliferated in response to Bet v 1 (median = 762.7 cpm) in a similar manner as the unfractionated PBMCs (median = 936.2 cpm). However, if T cells were co-cultured with purified basophils in the presence of Bet v 1, no relevant proliferation (median = 104.7 cpm) was observed similar as when T cells alone were incubated with Bet v 1 (median = 48.7 cpm). Similar results were obtained with the major grass pollen allergen Phl p 5 and the major mold allergen, Alt a 1 (data not shown). Thus in our first set of experiments, basophils did not induce allergen-specific T cell proliferation in allergic patients.
Figure 3.
Allergen-loaded antigen-presenting cells (APCs) but not basophils induce T cell proliferation. (A) Peripheral blood mononuclear cells (PBMCs), purified T cells, and T cells mixed with basophils or APCs depleted of basophils were stimulated with or without Bet v 1 for 1 week, pulsed with 3H-thymidine for 16 h, and 3H-thymidine incorporation was determined. Cpm (counts per minute) values (y-axes) of unstimulated cells were subtracted as background. Data are displayed as box plot summary of experiments performed in triplicates in seven allergic patients. Horizontal lines represent the median. Circles represent outliers. P values shown are representative for unadjusted pairwise comparison. After adjustment for multiple testing, all significant differences remained significant (P values between 0.021 and 0.045, not shown). (B) Isolated basophils and APCs were cultured overnight with medium alone (upper panels) or IL-3 (lower panels). Cells were harvested and stained with anti-HLA-DR (black open histogram) or isotype control (gray histogram) and analyzed by flow cytometry. Blots are representative of cell preparations from 3 allergic patients. (C) T cells mixed with basophils or with APCs depleted of basophils were stimulated with Bet v 1 in the presence of IL-3, and 3H-thymidine incorporation was determined as in (A). Cpm values of cells stimulated only with IL-3 were subtracted as background. Data for seven allergic patients are presented as box plot summary as in (A). P value shown is representative for unadjusted pairwise comparison. After adjustment for multiple testing, the difference remained significant (P = 0.014).
To investigate whether the histamine release of allergen-challenged basophils impairs T cell proliferation in allergen-stimulated basophil–T cell co-cultures, we added histamine to allergen-stimulated PBMC cultures. As shown in Fig. S4, addition of concentrations of histamine up to 500 ng/ml (i.e., 100 ng/well) to the cultures did not have an effect on allergen-induced PBMC proliferation.
Next, we performed co-culture experiments using IL-3 that has been shown to promote the survival as well as the growth of basophils and reportedly increased the expression of MHC II on basophils (9, 17–21). However, overnight culture in medium containing IL-3 did not cause a strong increase in HLA-DR expression on basophils (i.e., increase of 2.6 ± 1.3%) or basophil-depleted APCs (i.e., increase 1.5 ± 0.9%) (Fig. 3B).
Accordingly, the ability of basophils to induce allergen-specific T cell proliferation was not increased when the cells were cultured in the presence of IL-3. Co-cultures of T cells with APCs depleted of basophils proliferated in response to allergen stimulation in the presence of IL-3 (median = 1902.7 cpm), but no relevant proliferation was observed in T cell–basophil co-cultures under the same conditions (median = 0 cpm) (Fig. 3C). Therefore, also basophils cultured in the presence of IL-3 did not induce any relevant allergen-specific T cell proliferation in allergic patients.
Viable APCs without basophils are sufficient for allergen-induced T cell proliferation
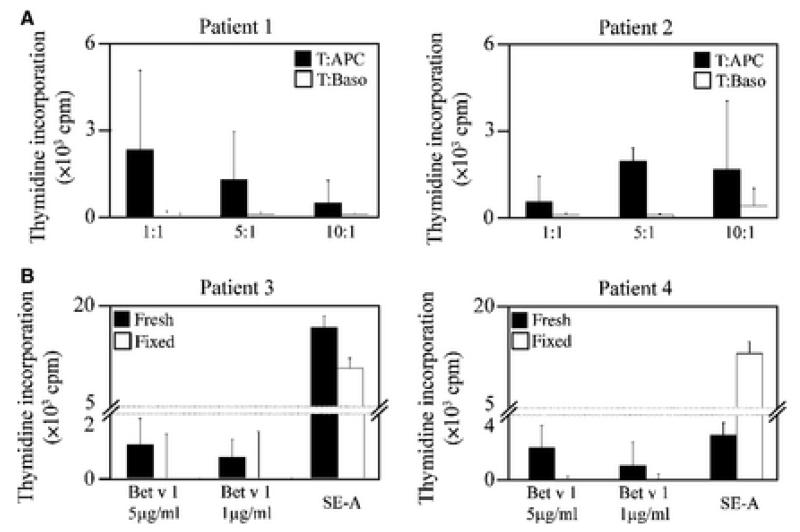
Next, we tested whether the ratio of basophils to T cells may affect the ability of basophils to induce allergen-specific proliferation of T cells. To that aim, we tested different ratios of basophils or APCs depleted of basophils to T cells (1 : 1, 1 : 5, and 1 : 10) and stimulated them with the Bet v 1 allergen. However, also at different cell ratios, basophils did not induce any relevant allergen-specific T cell proliferation, whereas APC depleted of basophils induced allergen-specific T cell proliferation at each of the three tested cell ratios (Fig. 4A).
Figure 4.
Viable APCs without basophils are sufficient for allergen-induced T cell proliferation. (A) T cells from two allergic patients (#1, #2) were cultured either with basophils or with APCs depleted of basophils at a ratio of 1 : 1, 5 : 1, or 10 : 1 with Bet v 1, and 3H-thymidine incorporation was determined. Cpm values (y-axes) represent means of triplicate experiments. (B) T cells from two allergic patients were co-cultured with fresh (filled bars) or fixed APCs (open bars) depleted of basophils at a ratio of 1 : 1 with Bet v 1 or superantigen SE-A. T cell proliferation was determined as in (A), and 3H-thymidine incorporations (means of triplicates) are displayed as counts per minute (cpm) (y-axes) after subtraction of background levels from unstimulated cells.
Finally, we investigated whether viable basophil-depleted APCs are required to induce allergen-specific T cell proliferation. For this purpose, we used viable and formaldehyde-fixed APCs depleted of basophils and cultured them with T cells in the presence of decreasing concentrations of Bet v 1. Bet v 1-specific proliferation was only observed for viable but not fixed APCs (Fig. 4B). However, T cells proliferated if co-cultures of fixed APCs with T cells were stimulated with the superantigen Staphylococcus enterotoxin A (SE-A) that does not require processing and presentation by APCs (Fig. 4B). Thus, viable APCs were required and sufficient to induce T cell proliferation in response to allergen.
Discussion
In contrast to recent studies reporting that basophils were crucial for antigen presentation and initiation of Th2 responses in murine models (8–10), results obtained in our study demonstrate that basophils are unable to induce relevant allergen-specific T cell proliferation in allergic humans. The inability of basophils to induce allergen-specific T cell proliferation could not be overcome by testing different ratios of basophil to T cell or by the addition of IL-3 – a well-established basophil survival and differentiation factor – to the cultures. Only APCs that had been depleted of basophils induced allergen-specific T cell proliferation in allergic humans. Support for our contention that APCs other than basophils are pivotal in promoting allergic immune responses comes from recent studies in mice demonstrating that basophils are dispensable for the induction of Th2 immunity in mice (11, 12, 22, 23). Furthermore, DCs have been shown to be absolutely required for the induction of primary allergic immune responses in several murine models (11, 24, 25). Along the same lines, in secondary immune responses to allergen in humans, Th2 skewing has been described in T cells in vitro if co-cultured with allergen-pulsed mature DCs derived from atopic patients (26–28).
It has also been demonstrated that allergen in complex with IgE but not with IgG1 or IgG4 can increase the capability of DCs as well as monocytes to internalize and present allergen to T cells (29, 30). A similar mechanism of facilitated allergen presentation (FAP) by IgE-mediated uptake is also employed by B cells that express the low-affinity IgE receptor CD23 on their surface. This mechanism may be of crucial importance in allergic disease, as 1000-fold less allergen is required to induce B cell mediated T cell responses if complexed with IgE as compared to allergen alone (31).
There are several possible explanations for the differences observed in our study compared with previously published murine studies (8–10). While we used a clinically relevant allergen (i.e., major birch pollen allergen, Bet v 1) to assess the ability of basophils to act as APCs in human allergy, others have used protease (8) or parasitic antigens (9, 10). The latter might be internalized and processed in a different manner. Furthermore, our study assessed the role of basophils in secondary Th2 responses, where basophils are already armed with allergen-specific IgE and therefore also release histamine upon allergen contact. Although the addition of histamine up to 500 ng/ml did not affect the allergen-specific T cell proliferation, it is possible that histamine may be released in the vicinity of T cells yielding higher local concentrations in vivo, but this cannot be tested in vitro. It is also possible that in vivo other cells or factors may contribute to a possible ability of basophils to induce Th2 responses as has been suggested in the mouse model recently (32).
However, in summary, our data demonstrate in a commonly accepted in vitro test system for allergen-specific T cell activation (i.e., proliferation assay using clinically relevant allergens, avoidance of artificial culture conditions) that various antigen-presenting cells (DCs, monocytes/macrophages) or B cells but not basophil granulocytes are involved in promoting T cell proliferation in allergic immune response in humans.
Supplementary Material
Acknowledgments
Staphylococcus enterotoxin A was kindly provided by Winfried Pickl.
Funding
This study was supported by grants F1815, F1818, F1820, F4605, F4611 and F4613 of the Austrian Science Fund (FWF).
Abbreviations
- APCs
antigen-presenting cells
- BSA
bovine serum albumin
- DC
dendritic cell
- FAP
facilitated antigen presentation
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- IgE
immunoglobulin E
- IL
interleukin
- LC
langerhans cell
- PBMCs
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PC5
phycoerythrin cyanin 5
- PE
phycoerythrin
- SE-A
Staphylococcus enterotoxin A
- TBS
tris–buffered saline
- 7-AAD
7-amino-actinomycin-D
Footnotes
Additional Supporting Information may be found in the online version of this article:
Figure S1. Presence of basophils in different separation fractions.
Figure S2. Comparison of basophils that were purified by two different methods.
Figure S3. Purified basophils respond to anti-IgE- and Bet v 1 by releasing histamine.
Figure S4. Effect of histamine on allergen-induced PBMC proliferation.
Data S1. Methods.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu J, Paul WE. Peripheral CD4+ T cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Bubnoff D, Geiger E, Bieber T. Antigen-presenting cells in allergy. J Allergy Clin Immunol. 2001;108:329–339. doi: 10.1067/mai.2001.117457. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 6.Valent P, Bettelheim P. The human basophil. Crit Rev Oncol Hematol. 1990;10:327–352. doi: 10.1016/1040-8428(90)90009-h. [DOI] [PubMed] [Google Scholar]
- 7.Valent P, Bettelheim P. Cell surface structures on human basophils and mast cells: biochemical and functional characterization. Adv Immunol. 1992;52:333–423. doi: 10.1016/s0065-2776(08)60879-2. [DOI] [PubMed] [Google Scholar]
- 8.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 10.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, et al. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buhring HJ, Streble A, Valent P. The basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell activation and allergy diagnosis. Int Arch Allergy Immunol. 2004;133:317–329. doi: 10.1159/000077351. [DOI] [PubMed] [Google Scholar]
- 15.Dvorak AM. Ultrastructural studies of human basophils and mast cells. J Histochem Cytochem. 2005;53:1043–1070. doi: 10.1369/jhc.5R6647.2005. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira F, Hirtenlehner K, Jilek A, Godnik-Cvar J, Breiteneder H, Grimm R, et al. Dissection of immunoglobulin E and T lymphocyte reactivity of isoforms of the major birch pollen allergen Bet v 1: potential use of hypoallergenic isoforms for immunotherapy. J Exp Med. 1996;183:599–609. doi: 10.1084/jem.183.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valent P, Besemer J, Muhm M, Majdic O, Lechner K, Bettelheim P. Interleukin 3 activates human blood basophils via high-affinity binding sites. Proc Natl Acad Sci U S A. 1989a;86:5542–5546. doi: 10.1073/pnas.86.14.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X, Karsan A, Duronio V, Chu F, Walker DC, Bai TR, et al. Interleukin-3, but not granulocyte-macrophage colony-stimulating factor and interleukin-5, inhibits apoptosis of human basophils through phosphatidylinositol 3-kinase: requirement of NF-kappaB-dependent and -independent pathways. Immunology. 2002;107:306–315. doi: 10.1046/j.1365-2567.2002.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Didichenko SA, Spiegl N, Brunner T, Dahinden CA. IL-3 induces a Pim1-dependent antiapoptotic pathway in primary human basophils. Blood. 2008;112:3949–3958. doi: 10.1182/blood-2008-04-149419. [DOI] [PubMed] [Google Scholar]
- 20.Valent P, Schmidt G, Besemer J, Mayer P, Zenke G, Liehl E, et al. Interleukin-3 is a differentiation factor for human basophils. Blood. 1989b;73:1763–1769. [PubMed] [Google Scholar]
- 21.Valent P, Dahinden CA. Role of interleukins in the regulation of basophil development and secretion. Curr Opin Hematol. 2010;17:60–66. doi: 10.1097/MOH.0b013e328331fae9. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 2010;184:1143–1147. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998;160:4090–4097. [PubMed] [Google Scholar]
- 26.Charbonnier AS, Hammad H, Gosset P, Stewart GA, Alkan S, Tonnel AB, et al. Der p 1-pulsed myeloid and plasmacytoid dendritic cells from house dust mite-sensitized allergic patients dysregulate the T cell response. J Leukoc Biol. 2003;73:91–99. doi: 10.1189/jlb.0602289. [DOI] [PubMed] [Google Scholar]
- 27.Bellinghausen I, Brand U, Knop J, Saloga J. Comparison of allergen-stimulated dendritic cells from atopic and nonatopic donors dissecting their effect on autologous naive and memory T helper cells of such donors. J Allergy Clin Immunol. 2000;105:988–996. doi: 10.1067/mai.2000.105526. [DOI] [PubMed] [Google Scholar]
- 28.Hammad H, Charbonnier AS, Duez C, Jacquet A, Stewart GA, Tonnel AB, et al. Th2 polarization by Der p 1–pulsed monocyte-derived dendritic cells is due to the allergic status of the donors. Blood. 2001;98:1135–1141. doi: 10.1182/blood.v98.4.1135. [DOI] [PubMed] [Google Scholar]
- 29.Lundberg K, Lindstedt M, Larsson K, Dexlin L, Wingren C, Ohlin M, et al. Augmented Phl p 5-specific Th2 response after exposure of dendritic cells to allergen in complex with specific IgE compared to IgG1 and IgG4. Clin Immunol. 2008;128:358–365. doi: 10.1016/j.clim.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Maurer D, Ebner C, Reininger B, Fiebiger E, Kraft D, Kinet JP, et al. The high affinity IgE receptor (Fc epsilon RI) mediates IgE-dependent allergen presentation. J Immunol. 1995;154:6285–6290. [PubMed] [Google Scholar]
- 31.van der Heijden FL, Joost van Neerven RJ, van Katwijk M, Bos JD, Kapsenberg ML. Serum-IgE-facilitated allergen presentation in atopic disease. J Immunol. 1993;150(8 Pt 1):3643–3650. [PubMed] [Google Scholar]
- 32.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.